Abstract
Objective
To determine trends for in-hospital survival and functional outcomes at acute care hospital discharge for severe adult traumatic brain injury (SATBI) patients in Pennsylvania, during 1998–2007.
Methods
Secondary analysis of the Pennsylvania trauma outcome study database.
Main Outcome Measures
Survival and functional status scores of five domains (feeding, locomotion, expression, transfer mobility, and social interaction) fitted into logistic regression models adjusted for age, sex, race, co-morbidities, injury mechanism, extra-cranial injuries, severity scores, hospital stay, trauma center, and hospital level. Sensitivity analyses for functional outcomes were performed.
Results
There were 26,234 SATBI patients. Annual numbers of SATBI increased from 1,757 to 3,808 during 1998–2007. Falls accounted for 47.7% of all SATBI. Survival increased significantly from 72.5% to 82.7% (OR 1.10, 95%CI 1.08–1.11, P<0.001). In sensitivity analyses, trends of complete independence in functional outcomes increased significantly for expression (OR 1.01, 95%CI 1.00–1.02, P=0.011) and social interaction (OR 1.01, 95%CI 1.00–1.03, P=0.002). There were no significant variations over time for feeding, locomotion, and transfer mobility.
Conclusions
Trends for SATBI served by Pennsylvania’s established trauma system showed increases in rates but substantial reductions in mortality and significant improvements in functional outcomes at discharge for expression and social interaction.
Keywords: Hospitalizations, injury, traumatic brain injury, outcomes, functional limitation, disability, trauma system
INTRODUCTION
Traumatic brain injury (TBI) accounts for at least one-third of injury-related deaths and affects more than one million people each year in the United States.1–3 In addition, long-term disability after TBI affects individuals, families, friends, and society. Approximately five million Americans live with TBI-associated long-term disabilities. As a result, the nation spends an estimated $56 billion annually in direct and indirect costs to care for people sustaining TBI.4, 5
Epidemiologic trend reports using the National hospital discharge survey (NHDS)6 found that the incidence of TBI-related hospitalizations in acute care facilities in the US decreased during the 80’s and 90’s,7 but more recent studies reported that the estimated incidence of hospitalized TBI in 2003 in the US increased compared with the annual totals from 1998 to 2002.8 There was a 61% decline in hospitalization for persons with TBI of mild severity but a 90% increase in high severity cases.7 On the other hand, the proportion of in-hospital mortality from severe TBI across the United States has declined from 50% to 25% since 1980, even when adjusted for age and other admission prognostic parameters.9, 10
The delivery of acute care for individuals with severe TBI in the United States in early 1990 varied not only between institutions but also between practitioners at the same institution. Intracranial pressure monitoring was not used routinely and treatment modalities for intracranial hypertension were aggressively used despite the lack of evidence of their efficacy.11 Efforts to promote best practices and to optimize the care of the patient with severe TBI have driven the development of guidelines emphasizing triage to accredited trauma centers with adequate neurosurgical facilities and monitoring and maintaining adequate cerebral perfusion.12–15 The implementation of evidence-based guidelines has resulted in significant improvements in outcomes after severe TBI as compared with management based solely on individual preferences.16 However, there is still no single best way to manage severe TBI. Despite the increased number of randomized controlled trials recently, there is little progress in head injury care suggested by the failure to identify effective therapies to improve outcome.17
The burden and trends in severe TBI focus its importance as a public health problem and raises two questions. First, has survival from severe TBI continued to increase? And second, have the functional outcomes of survivors improved over time? As more patients survive the very acute phase, there should be an increased awareness of the role of rehabilitation medicine for the improvement of patients’ conditions in the community.18 Good baseline information on hospitalization and outcomes is important for planning health care and rehabilitation services.
Pennsylvania trauma centers report that severe TBI was present in more than 40% of all trauma deaths. To better understand the magnitude and trends of this problem in a state with an established trauma system, we aimed to determine if the trends in severe adult TBI (SATBI) in-hospital survival and functional outcomes at acute care hospital discharge of patients discharged alive have changed significantly in Pennsylvania, during 1998–2007.
METHODS
Study design, settings, and study population
We performed secondary data analysis of 10 years of the Pennsylvania Trauma Outcome Study (PTOS) database, a trauma registry that encompassed 31 accredited trauma centers in Pennsylvania, during 1998–2007. Patient information is submitted to the PTOS if the patient was admitted to any accredited trauma center for treatment of trauma and with any of the following: was admitted to an intensive care unit, died on or after arrival, was admitted over 48 hours from the time of arrival to the emergency department, or was transferred from another facility with diagnoses of trauma. Each patient admission recorded in the database is assigned a unique identifier number, which precluded the data from any successive hospital admission of a given patient. Out-of-hospital deaths and injuries treated in non-accredited trauma centers in Pennsylvania were not included.
SATBI case selection
TBI patients were identified using the Barell injury diagnostic matrix,19 a framework that can serve as a practical tool for standardizing injury diagnosis using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes.20, 21 ICD-9-CM injury codes are arranged by the nature of injury and by body regions. The matrix can be used to standardize the patterns of injury, enabling comparisons across time or place. In the matrix, TBI is a specific group of the head and neck body region and is defined based on the TBI case definition of the Centers for Disease Control and Prevention (CDC). The matrix excluded patients with ICD-9-CM codes of adverse effects and complications of medical care, events that are not considered pertinent to injury prevention and surveillance activities.19, 22 Patients who were admitted to the trauma center more than 48 hours after the injury event were excluded to avoid diagnoses of late effects or late consequences of the primary injury event. Adults were defined as patients older than 17 years of age.
TBI severity was classified using the head and neck abbreviated injury scale (AIS), an anatomically based, consensus derived, severity scoring system. It is the most widely used anatomic scale for rating severity of injuries and forms the basis for other scoring systems for risk adjustment and mortality prediction after trauma. The AIS groups injuries in six body regions (head and neck, face, chest, abdomen, extremity, and external) and classifies each injury according to its relative importance on a 6-level scale. AIS scores of 1 are minor injuries and scores of 6 are maximum injury-virtually unsurvivable.23–28 Head and neck AIS scores of 4 through 6 classified a TBI as severe.
The inter-rater and intra-rater reliability of AIS have been assessed. Although clinical raters tend to be more reliable, well-trained non-clinical technicians can code severity accurately and reliably when sufficient information is provided in the charts. However, a person with clinical background may be more cost-effective in terms of time expended to code from the inpatient chart.28 AIS scoring is a process that can take, on average, between 10 and 30 minutes depending on the extent of the injury and the clinical experience of the rater.29–31 This is a significant time commitment that is not always feasible. For large epidemiological research, less resource-intensive coding systems are desired.
In our study, AIS was derived by the computerized algorithm of the International Classification of Disease programs for injury categorization (ICDPIC). The ICDPIC provides inexpensive methods for translating ICD-9-CM codes into standard injury categories and/or scores. The programming code and associated tables are completely open for Stata (statistical software) users.32, 33
The initial development of computerized programs to perform translation of ICD-9-CM into AIS was done at Johns Hopkins School of Hygiene and Public Health and was subsequently marketed as ICDMAP-90. Its validity was assessed by MacKenzie and colleagues. AIS and injury severity scores (ISS) derived using the computerized conversion tables (ICDMAP-90) were compared with those obtained by reviewing the complete medical record for 1,120 consecutive trauma cases treated at two Maryland trauma centers in 1983. It was reported that if the reliability of ICD codes abstracting could be improved, eliminating most of all of the disagreements due to abstracting, the percentage of agreement in maximum AIS would range from 67% for head-neck to 87% for extremities and the percentage of agreement in ISS grouped into ranges of 1–12, 13–19, and ≥20 would be 68%. The researchers concluded that although not perfect, the computerized program provided precise information on injury severity and had the potential for assigning reliable severity scores based on routinely collected hospital discharge data..29
Computerized programs for the translation of ICD-9-CM into AIS or any other severity scoring are less accurate than the direct AIS coding by trained registrants, but achieve good power in discriminating between survivors and non-survivors.31 They can be invaluable when AIS scoring directly from medical charts is expensive or impractical, facilitating research and the application of epidemiologic methods for the evaluation of health services and trauma systems response for geographically defined populations.29
To our knowledge, studies comparing ICDPIC for AIS scoring against direct AIS coding by trained registrants have not been performed using trauma registries from the United States. The help files that come with the ICDPIC program for Stata software explain how the methods were derived and compared against ICDMAP-90, using the National Trauma Data Bank (NTDB) of the American College of Surgeons and the Nationwide inpatient sample (NIS) of the Agency for Healthcare Research and Quality (AHRQ). ISS calculated by ICDPIC was exactly the same as the ISS assigned by ICDMAP-90 in 84.1% of the cases. When ISS was categorized, 92.9% of cases were assigned to the same category and 99.5% to the same or an adjacent category.33 The ICDPIC program could be very attractive to approximate injury severity scoring. It is inexpensive for Stata users and allows exploiting the administrative data banks on hospital admissions that are comprehensive and easy to access in the United States.
Study data measures
De-identified information on demographics (age in years, sex, and race categorized as White, Black and Other), presence of any pre-existing conditions or co-morbidities, injury characteristics (date and mechanism of injury), ICD-9-CM codes, and hospital information (date of admission, length of hospital stay in days, and type of trauma center level) was requested.
PTOS collects information about pre-existing conditions or co-morbidities documented in the medical record of the patient. They are defined as the effect of all other diseases an individual patient might have other than the traumatic injuries. Unfavorable evolution of the traumatic injury or the medical treatment are not considered pre-existing conditions. PTOS allows entering up to 10 pre-existing conditions from cardiac diseases, diabetes, gastrointestinal diseases, hematologic disorders, history of psychiatric disorders, immunosupressed, liver disease, malignancy, musculoskeletal, neurologic, obesity, pulmonary disease, renal disease, substance abuse, pregnancy, trauma history, and endocrine disorders.
Co-morbidities are strongly associated with inpatient death.34 Including information about pre-existing conditions increases the risk of mortality prediction after trauma, independent of age and injury severity. Although there is a well known co-morbidity index for risk adjustment, in trauma patients a comparison of different ways to summarize this information demonstrated similar discrimination for death in prediction models.35 In our study, information about the presence of any preexisting condition or co-morbidity was used for risk adjustment.
PTOS collects information about mechanism of injury using the external cause of injury codes (E-codes) classified by the ICD-9-CM. Based on the recommendations for presenting injury mortality data by the CDC22 and for study purposes, mechanism of injury was grouped into four categories: falls, motor vehicles, firearms, and others.
The new injury severity score (NISS) and the presence of severe extra-cranial injuries were derived by the computerized algorithm of the ICDPIC. The NISS attempts to assess the combined effect of multiple injuries sustained by a given person. With ranges from 1 to 75, it is the sum of the squares of the three most severe AIS, regardless of body region. A patient with an AIS of 6 is automatically given an NISS of 75. NISS were determined and used in the statistical analysis for risk adjustment.24, 25, 27, 28
The presence of severe extra-cranial injuries has been previously identified as prognostic for poor outcome in TBI and was defined as having any injury with AIS score of 4 through 6 in the following body regions: face, chest, abdomen, extremities, and/or external.36
Main outcome measures
The main outcome was status at hospital discharge; either dead or alive. Survivors were evaluated at discharge by a trauma unit nurse who assigned a functional status score for the five domains of feeding, locomotion, expression, transfer mobility, and social interaction.
Functional outcomes were analyzed utilizing the Functional Independence Measure (FIM). The internal consistency, inter-rater reliability, validity, and other psychometrics of the FIM have been reported. FIM distinguishes well among patients with the different types of impairments seen in rehabilitation inpatient, has excellent internal consistency, and provides good inter-rater reliability across a wide variety of raters with different professional backgrounds and levels of training.37, 38
The PTOS adopted the initial version of the FIM that uses a 4-point rating scale, where a score of 1 represents complete dependence in functional status, 2 represents modified dependence, 3 represents modified independence, and 4 represents complete independence.39, 40 Finer gradations of assistance for the modified dependence score (supervision, minimal assistance, and moderate assistance) and for the complete dependence score (maximal assistance and total assistance) are not specified in the PTOS. Recent versions of the FIM use a 7-point scale including these gradations of assistance. However, some recent studies have suggested that the 4-point scale would be more precise in measuring the level of dependence in clinical settings.39, 41
Statistical analysis
Sample size and power calculation for logistic regression42 was performed for discharge status using the Power Analysis and Sample Size (PASS) software. With power of 90% and significance of 5%, it was necessary to have 25,221 SATBI patients to observe a statistically significant change in the regression coefficient less or greater than 0 when the odds of survival changes at least 1% per year over time.
Variables were analyzed descriptively by year. When necessary, comparisons of continuous variables (age, NISS, and length of hospital stay) were performed using non-parametric tests (Wilcoxon Mann-Whitney or Kruskal-Wallis), given their non-normal distributions. Comparisons among categorical variables were performed using Chi-square tests.
The proportion of SATBI patients discharged alive was calculated using the total number of SATBI as the denominator. The proportions of functional status scores for each domain were calculated using the number of SATBI discharged alive as the denominator.
To evaluate the trend of survival associated with SATBI and the trend of complete independence in functional status (FIM equal to 4) for each domain, we built generalized linear mixed effect models. Variations in quality of care among trauma centers have been reported lately.43 Therefore, mixed models were used to account for variations in care between trauma centers and for the random effect fact that patients treated in the same trauma center should be correlated.44 We were not aware of any specific injury prevention effort or any changes to the treatment of brain injuries which would be expected to alter our results.
The odds of survival and the odds of complete independence in functional status (probability of FIM=4/probability of FIM≤3) for each domain were fitted into logistic regressions with the random effect. As we were aware of missing data in functional status scores, sensitivity analysis was performed for each domain, conservatively assigning the missing data to the worst outcome (Missing FIM equal to 1). Trend analyses of the odds of complete independence during 1998–2007 were repeated to evaluate the robustness of the conclusions.
The analyses were performed in Stata (Version 11) software. Models were adjusted for age, sex, race, co-morbidities, injury mechanisms, presence of extra-cranial injuries, NISS, length of hospital stay, and hospital trauma level. Findings are presented as odds ratios (OR) with 95% confidence intervals (CI) and P values.
Subgroup analysis
A high incidence of extremity fractures will result in fewer patients discharged with complete independence scores in mobility domains. A subgroup analysis was performed in adults with isolated severe TBI (isolated SATBI) and those were defined as SATBI patients without other injuries or with extra-cranial injuries with maximum AIS of 1. The proportion of patients discharged alive was calculated using as the denominator the number of isolated SATBI patients. The proportions of functional status scores for each domain were calculated using as denominator the number of isolated SATBI patients discharged alive.
RESULTS
The 26,234 SATBI patients accounted for 30.8% of total adult TBI admitted to accredited trauma centers in Pennsylvania during 1998–2007. Annual SATBI increased from 1,757 in 1998 to 3,808 in 2007 (Table 1). In 1998, SATBI accounted for 28.9% of adult TBI, increasing to 36.2% by 2007.
Table 1.
Descriptive trends of characteristics in SATBI patients
| Characteristics | Total | 1998 | 2007 | Average change per year |
|---|---|---|---|---|
| (N=26,234) | (n=1,757) | (n=3,808) | ||
| Age (years) | ||||
| Mean (SD) | 55.9 (23.6) | 51.1 (23.2) | 59.3 (23.1) | +0.9 years |
| Median [PR] | 57 [35–78] | 47 [30–74] | 63 [41–80] | +1.8 years |
| Sex | ||||
| Female | 34.8 | 31.2 | 36.7 | +2.2 |
| Race | ||||
| White | 80.2 | 78.6 | 80.6 | +0.5 |
| Black | 11.6 | 14.9 | 9.8 | −4.5 |
| Other | 8.2 | 6.5 | 9.5 | +5.4 |
| Pre-existing conditions or co-morbidities | 74.5 | 60.0 | 79.6 | +10.8 |
| Mechanism of injury | ||||
| Falls | 47.7 | 33.5 | 55.7 | +8.9 |
| Motor vehicle | 33.5 | 42.9 | 28.0 | −6.5 |
| Firearms | 6.2 | 9.4 | 4.6 | −7.1 |
| Other | 12.6 | 14.2 | 11.7 | +1.5 |
| Severe extra-cranial injuries* | 5.4 | 8.3 | 5.0 | −3.9 |
| NISS | ||||
| Mean (SD) | 33.9 (11.7) | 36.2 (11.7) | 33.1 (11.6) | −0.22 points |
| Median [PR] | 34 [25–41] | 34 [29–48] | 34 [24–41] | 0 points |
| Length of hospital stay (days) | ||||
| Mean (SD) | 9.9 (13.4) | 10.7 (13.6) | 9.1 (12.7) | −0.22 days |
| Median [PR] | 5 [2–12] | 6 [2–14] | 5 [2–11] | −0.11 days |
| Type of trauma center | ||||
| Level I | 64.2 | 63.7 | 63.8 | +1.8 |
| Level II | 35.8 | 36.3 | 36.2 | −1.8 |
| Discharge status | ||||
| Alive | 77.5 | 72.2 | 82.1 | +6.5 |
Data are percentages unless otherwise indicated. SD, standard deviation; PR, percentile range; NISS, new injury severity score.
Severe extra-cranial injuries are any injury with AIS score of 4 through 6 in the following body regions: face, chest, abdomen, extremities, and/or external.
The age of SATBI increased over the study period. Most patients were males, but the proportion of females increased 2.2% per year during 1998–2007 (Table 1). Males were significantly younger than females (median age 49 vs. 74 years respectively; P<0.001). During 1998–2007, the median age increased from 70 to 76 years for females and from 42 to 54 years for males. Whites comprised 80.2% of the patients and blacks 11.6% (Table 1). Whites were significantly older than blacks and other races (median 62, 42, and 47 years respectively; P<0.001). The proportion of patients with co-morbidities increased during 1998–2007 (Table 1).
The most frequent mechanism of injury was falls (47.7%) followed by motor vehicle crashes (33.5%) and firearms (6.2%). During 1998–2007, the proportion of falls increased 8.9% per year while the proportion of motor vehicle events decreased 6.5% per year. The proportion of firearm related injuries decreased 7.1% per year (Table 1). The median age was 76 years for falls, 39 years for motor vehicles, and 30 years for firearms. Differences in age by mechanism of injury were statistically significant (P<0.001). The median age of patients injured in falls increased over the study period from 75 to 77 years, the median age of patients injured in motor vehicle events increased from 38 to 44 years while the median age of patients injured with firearms decreased from 30 to 27 years.
The proportion of severe extra-cranial injuries decreased 3.9% per year (Table 1). Patients injured in motor vehicle events had a significantly higher proportion of severe extra-cranial injuries (11.8%) than patients injured by firearms (5.4%) and those injured in falls (1.4%) (P<0.001). Median NISS in SATBI patients did not change over the study period (Table 1). Patients injured by firearms demonstrated significantly higher NISS than patients injured in motor vehicles and in falls (median scores of 41, 34, and 32 respectively, P<0.001).
Patients were admitted predominantly to level I trauma centers. The length of hospital stay was significantly higher in level I trauma centers than in level II centers (median of 6 days vs. 5 days respectively, P<0.001). The level I centers’ hospital stay decreased from 6 days in 1998 to 5 in 2007, in level II centers decreased from 7 to 4 days over the 10 years.
Survival at hospital discharge
20,338 (77.5%) patients were discharged alive. Survivors were significantly younger than those who died during hospitalization (median 55 years vs. 63 years respectively; P<0.001). Females were discharged alive in greater proportion than males (79.6% vs. 76.4% respectively; P<0.001). Blacks were discharged alive in lower proportion (72.8%) than whites (78.2%) and other races (77.8%) (P<0.001).
Overall, survival was significantly lower in patients injured by firearms (38.4%) than in patients injured in falls (78.4%), motor vehicles (80%), and other mechanisms (86.5%) (P<0.001). Over time, survival increased among categories of mechanism of injury.
The proportion of patients with severe extra-cranial injuries who were discharged alive was 57.8%. Patients who were discharged alive were less severely injured than those who died, (median NISS 34 and 41 respectively; P<0.001). The median hospital stay for survivors was seven days while in patients who died it was one day (P<0.001).
Trends of survival at hospital discharge associated with SATBI
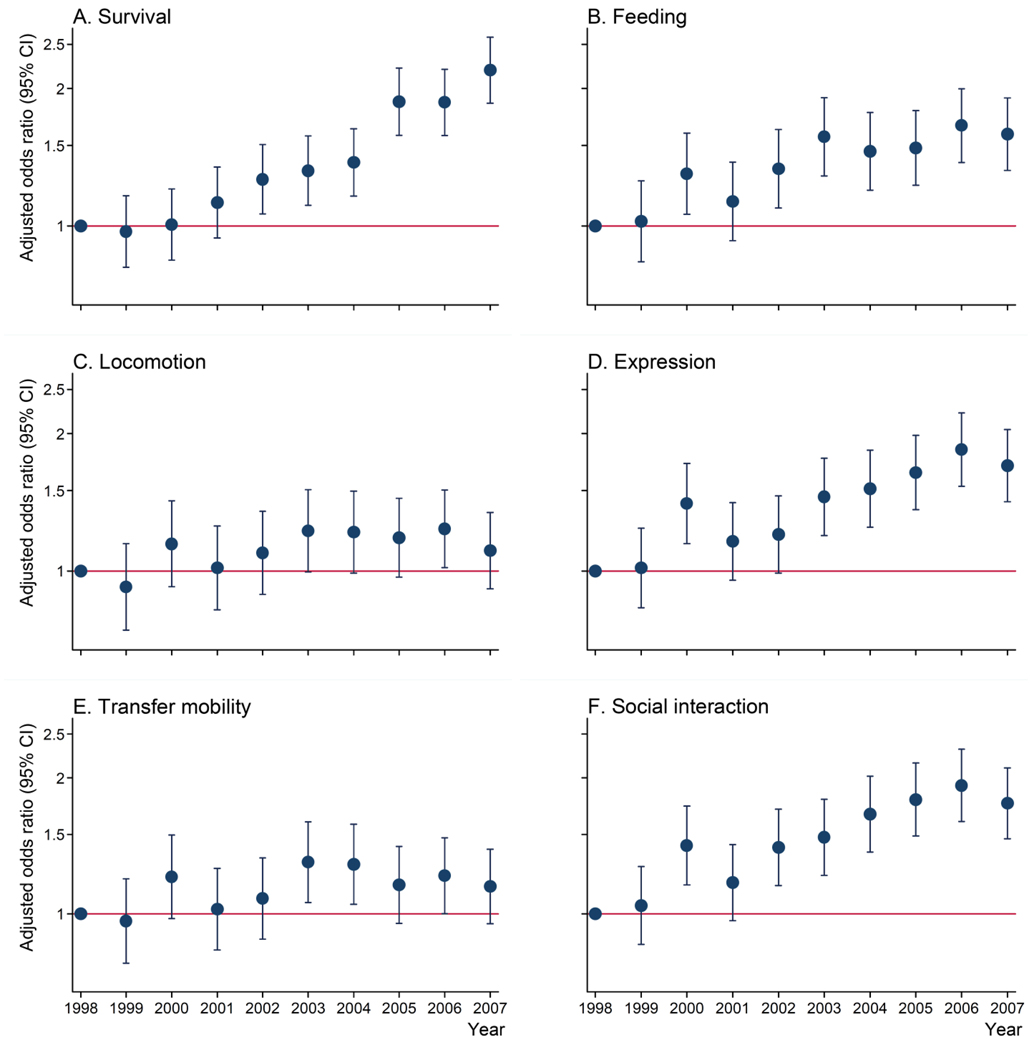
The proportion of survivors increased from 72.2% in 1998 to 82.1% in 2007 (Table 1). We used a logistic regression model with the random effect for trauma center and adjusted for age, sex, race, co-morbidities, injury mechanisms, presence of extra-cranial injuries, NISS, length of hospital stay, and hospital trauma level. The trend analysis showed a significant increase of 10% per year in the odds of survival between 1998 and 2007 (OR 1.10, 95%CI 1.08 to 1.11, P<0.001) (Table 3). All the actual increase in the odds of survival occurred after 2002 when we observed a significant increase of 26% in the odds of survival compared with the baseline year of 1998 (OR 1.26, 95%CI 1.06 to 1.50, P 0.008) and this increase in the odds of survival remained constant until 2007 (OR 2.19, 95%CI 1.85 to 2.58, P<0.001) (Figure 1-A).
Table 3.
Regression analysis* for the trends of survival and complete independence scores in each domain
| Outcome | OR (95%CI) | P value | Sensitivity analysis† | |
|---|---|---|---|---|
| OR (95%CI) | P value | |||
| Severe adult traumatic brain injury | ||||
| Survival | 1.10 (1.08 to 1.11) | <0.001 | ||
| Complete independence score‡ | ||||
| Feeding | 1.05 (1.03 to 1.06) | <0.001 | 1.00 (0.99 to 1.02) | 0.121 |
| Locomotion | 1.01 (1.00 to 1.03) | 0.010 | 0.99 (0.97 to 1.00) | 0.247 |
| Expression | 1.06 (1.05 to 1.08) | <0.001 | 1.01 (1.00 to 1.02) | 0.010 |
| Transfer Mobility | 1.01 (1.00 to 1.03) | 0.018 | 0.99 (0.97 to 1.00) | 0.142 |
| Social Interaction | 1.07 (1.05 to 1.08) | <0.001 | 1.01 (1.00 to 1.03) | 0.002 |
| Isolated severe adult traumatic brain injury§ | ||||
| Survival | 1.11 (1.09 to 1.13) | <0.001 | ||
| Complete independence score‡ | ||||
| Feeding | 1.06 (1.03 to 1.08) | <0.001 | 1.00 (0.98 to 1.02) | 0.598 |
| Locomotion | 1.01 (0.99 to 1.03) | 0.128 | 0.98 (0.96 to 1.00) | 0.073 |
| Expression | 1.06 (1.04 to 1.09) | <0.001 | 1.00 (0.99 to 1.02) | 0.351 |
| Transfer Mobility | 1.01 (0.99 to 1.03) | 0.169 | 0.98 (0.96 to 1.00) | 0.053 |
| Social Interaction | 1.07 (1.05 to 1.09) | <0.001 | 1.01 (0.99 to 1.02) | 0.108 |
OR, Odds ratio; CI, Confidence intervals.
Logistic regressions were performed separately for discharge status and separately for complete independence scores of each domain. Each model was adjusted for age, sex, race, co-morbidities, injury mechanisms, presence of extra-cranial injuries, NISS, length of hospital stay, trauma center, and hospital trauma level.
Sensitivity analyses were performed assigning the missing data in functional status scores to the worst outcome (functional status score equal to 1).
Complete Independence score is the best functional outcome or a functional independence measure score equal to 4 in each domain.
Isolated severe adult traumatic brain injury patients were defined as SATBI patients without other injuries or with extra-cranial injuries with maximum AIS of 1.
Figure 1. Yearly odds of survival and complete independence scores in each domain compared with 1998.
Adjusted odds ratios were calculated using logistic regressions adjusting for age, sex, race, co-morbidities, injury mechanisms, presence of extra-cranial injuries, NISS, length of hospital stay, trauma center, and hospital trauma level. Y axes are in log scale.
Trends of functional status scores at hospital discharge associated with SATBI
Functional status scores are shown in Table 2. The proportion of survivors with complete independence scores at discharge was as high as 47.6% for expression and as low as 28.3% for locomotion. During 1998–2007, the proportion of survivors with complete independence scores increased in all domains. The largest increases were observed in the social interaction and expression domains. The smallest increases were observed in locomotion and transfer mobility (Table 2).
Table 2.
Descriptive trends of functional status scores in SATBI patients discharged alive
| Total | 1998 | 2007 | Average change per year | |
|---|---|---|---|---|
| (N=20,338) | (n=1,269) | (n=3,128) | ||
| Feeding | ||||
| (1) Complete Dependence | 13.9 | 19.1 | 12.2 | −6.5 |
| (2) Modified Dependence | 6.1 | 8.1 | 5.0 | −5.8 |
| (3) Modified Independence | 7.8 | 10.0 | 7.3 | −2.3 |
| (4) Complete Independence | 44.9 | 41.1 | 47.8 | +2.3 |
| Locomotion | ||||
| (1) Complete Dependence | 16.4 | 22.2 | 13.7 | −6.6 |
| (2) Modified Dependence | 13.6 | 16.5 | 14.0 | −1.5 |
| (3) Modified Independence | 14.5 | 12.9 | 15.9 | +2.0 |
| (4) Complete Independence | 28.3 | 26.8 | 28.8 | +0.5 |
| Expression | ||||
| (1) Complete Dependence | 8.9 | 12.9 | 6.9 | −8.5 |
| (2) Modified Dependence | 7.4 | 10.2 | 6.2 | −7.1 |
| (3) Modified Independence | 8.7 | 11.4 | 8.3 | −2.3 |
| (4) Complete Independence | 47.6 | 43.9 | 50.9 | +2.7 |
| Transfer Mobility | ||||
| (1) Complete Dependence | 15.2 | 20.3 | 12.7 | −6.9 |
| (2) Modified Dependence | 14.4 | 17.6 | 14.4 | −1.6 |
| (3) Modified Independence | 12.8 | 12.0 | 14.0 | +2.1 |
| (4) Complete Independence | 30.6 | 28.6 | 31.4 | +0.5 |
| Social Interaction | ||||
| (1) Complete Dependence | 9.2 | 14.0 | 6.9 | −9.7 |
| (2) Modified Dependence | 7.6 | 10.7 | 6.8 | −6.4 |
| (3) Modified Independence | 8.1 | 10.2 | 7.9 | −1.6 |
| (4) Complete Independence | 47.4 | 42.9 | 50.6 | +3.0 |
Data are percentages.
For each domain, logistic regression models with the random effect and adjusted for age, sex, race, co-morbidities, injury mechanisms, presence of extra-cranial injuries, NISS, length of hospital stay, and hospital trauma level were performed separately to evaluate trends of complete independence scores. Initially, statistically significant trends of the odds of complete independence were observed in all domains (Table 3).
FIM missing data in at least one domain was found in 28.5% of SATBI survivors. When missing values were coded as the worst FIM score, the sensitivity analysis indicated a significant trend of 1% increase per year between 1998 and 2007 in the odds of complete independence for the domains expression (OR 1.01, 95%CI 1.00 to 1.02, P 0.011) and social interaction (OR 1.01, 95%CI 1.00 to 1.03, P 0.002). The trends of the odds of complete independence did not reach statistical significance for the domains feeding (OR 1.00, 95%CI 0.99 to 1.02, P 0.121), locomotion (OR 0.99, 95%CI 0.97 to 1.00, P 0.247), and transfer mobility (OR 0.99, 95%CI 0.97 to 1.00, P 0.142), in the sensitivity analysis (Table 3). Figures 1-B to F illustrate the adjusted odds of complete independence scores by year in each domain.
Isolated SATBI
There were 13,959 isolated SATBI patients (i.e., without other injuries or with extra-cranial injuries with maximum AIS of 1). 78.2% were discharged alive. Trend analyses using logistic regression models with random effect and adjusted for age, sex, race, co-morbidities, injury mechanisms, NISS, length of hospital stay, and hospital trauma level are depicted in Table 3. During 1998–2007, survival increased from 72.2% to 83.9%. The trend analysis showed a statistically significant increase of 11% per year in the odds of survival (OR 1.11, 95%CI 1.09 to 1.13, P<0.001).
48% of survivors were discharged with complete independence scores for feeding, 33.2% for locomotion, 49.2% for expression, 35.5% for transfer mobility, and 49.2% for social interaction. The regression analyses indicated statistically significant trends of the odds of complete independence for the domains feeding (OR 1.06, 95%CI 1.03 to 1.08, P<0.001), expression (OR 1.06, 95%CI 1.04 to 1.09, P<0.001), and social interaction (OR 1.07, 95%CI 1.05 to 1.09, P<0.001). The trends of the odds of complete independence did not reach statistical significance for the domains locomotion (OR 1.01, 95%CI 0.99 to 1.03, P 0.128) and transfer mobility (OR 1.01, 95%CI 0.99 to 1.03, P 0.169). FIM missing data in at least one domain was found in 29.5% of the isolated SATBI survivors. In the sensitivity analysis, there were no statistically significant trends of complete independence scores (Table 3).
DISCUSSION
Over the study period, falls became the leading mechanism of SATBI admitted to Pennsylvania’s trauma centers as the population aged. However, the numbers of SATBI related to traffic events also increased and remains an important cause of SATBI hospitalizations and deaths. We found statistically significant trends of increased survival and increased complete independence scores for expression and social interaction. In the sensitivity analysis, variations over time of complete independence scores of feeding, locomotion, and transfer mobility were not statistically significant. In isolated SATBI, the increase of survival was statistically significant. However, the sensitivity analysis showed no statistically significant variations over time of complete independence scores of functional status.
Our study strengths are the use of an established state-wide trauma registry and the large sample size in relation to the number of confounding variables examined. The PTOS is not strictly a population-based registry, but it is still robust due to its coverage of all accredited trauma centers in Pennsylvania over the 10 years of continuous data collection.
There were several limitations. PTOS database is from accredited trauma centers only. TBI admitted and managed elsewhere are not part of our analysis. However, guidelines for pre-hospital management of TBI recommend that, whenever there is a trauma system in place, emergency medical service agencies should transport TBI patients directly from the scene of the event to a trauma center.15 When trauma systems are in place this is what usually occurs. Additionally, SATBI deaths are in hospital deaths only and the reported difference in AIS scoring between ICDPIC and ICDMAP-90 is towards an underestimation of injury severity.33 Therefore, the true incidence of severe TBI in Pennsylvania may be underestimated.
PTOS contains pooled entries from numerous different data entry personnel with varying levels of training and expertise. This might affect accurate classification of TBI and accuracy of outcomes assessments. This misclassification, if differential in relation with the outcomes, may underestimate or overestimate our trends. Nonetheless, PTOS is a requirement for each trauma center in Pennsylvania and each hospital trains data entry personnel according to the standards for trauma center accreditation in the State of Pennsylvania.45 Data assessment and quality controls are performed by the institutions according to the PTOS operations manual. Consistency over time of the accreditation process might reduce misclassification to a minimum. Finally, PTOS database is by definition descriptive. The observational approach used to assess trends of survival and functional outcomes can be biased by trauma care delivered in different trauma centers. The inclusion of a random effect in our analysis reduces this bias.
A report from the Pennsylvania Department of Health indicated that during 2001–2005 the age-adjusted TBI hospitalization rates increased 17.5% for males (from 158.4 to 186.2 per 100,000 population) and 15.5% for females (from 82.2 to 95.0 per 100,000 population).46 This and U.S. hospitalization reports7 are consistent with our findings that SATBI admissions to trauma centers are increasing over time.
Reports of temporal trends of mortality associated with SATBI have shown an overall reduction in the US9 and internationally47 where trauma systems are in place. Others suggest that treatment in neurosurgical centers represents an important strategy in the management of severe head injury, increasing the odds of survival.17 Despite the increased numbers of SATBI admissions to trauma centers, we found statistically significant trends of increased survival and increased complete independence scores for cognitive domains such as expression and social interaction. We hypothesize that the trend of increased good outcomes results from the maturation of the trauma system in Pennsylvania.
The proportion of fall related SATBI injuries increased substantially during the last decade, becoming the leading mechanism of SATBI. Furthermore, the median age of the SATBI increased during 1998–2007. Older adults are the most vulnerable group in terms of injury mortality. With further aging, these long terms trends portend a further increase in trauma center burden.48 Although this may have influenced outcomes over time, age and mechanism of injury were adjusted for in the analysis.
TBI sequelae such as decreased mobility and impaired cognitive function contribute to decreased survival following hospital discharge among more severely injured patients.49, 50 A previous report indicated a 15-month post hospitalization mortality rate of 12% among SATBI.51 The results obtained in our study regarding functional status scores at discharge indicated that at least half of the SATBI patients are discharged with some degree of functional limitation in cognitive domains, and at least two-thirds are discharged with some degree of functional limitation in mobility domains. Nationally, there is an estimated disability rate of 43.3% from hospitalized TBI survivors.4
TBI remains one of the most frequent causes of disability.52 Public awareness has to be raised in Pennsylvania (and elsewhere), where the number of SATBI survivors who likely need rehabilitative or supportive services will increase every year, in greater proportion for functional limitations in their mobility and locomotion. The early identification of these patients is necessary to assist in the development of physical medicine and rehabilitation interventions to improve acute care discharge function and long term rehabilitation in order to maximize their potential.
The importance of measuring the degree of disability for survivors of major trauma has been identified previously as a key element for assessing hospital/system performance. Gabbe and colleagues53 found in a cohort of trauma patients in Australia that the complete independence score for locomotion increased from 22% at discharge to 76% at 6 months, for feeding from 46% to 94%, and for expression from 81% to 92%. The modified Functional Independence Measure at discharge after major trauma was not associated with return to work or study at 6 months post injury, and only the locomotion item was associated with poor recovery at 6 months post injury.53 Equivalent data in severe head injury patients has not been published. Functional outcomes at discharge could be used for assessing hospital/system performance. In the future, it is likely that improvements in measures of long-term disability will be needed as part of the Pennsylvania trauma registry.
Finally, the study results are based on SATBI patients admitted to trauma centers in Pennsylvania. These results cannot be generalized to mild and moderate TBI, to SATBI treated in non-trauma centers or in other inpatient or outpatient settings, and to those untreated. Demographic characteristics of Pennsylvania and trauma systems characteristics may be important to consider for generalizing these results to other communities.
CONCLUSION
SATBI is increasing, and falls have become the leading mechanism in Pennsylvania as its population ages. This brings more trauma victims with pre-existing conditions to the trauma and rehabilitation centers, challenging clinicians as never before. Despite the increase in the numbers of SATBI admissions, inpatient care in Pennsylvania’s established trauma system is substantially improving in-hospital survival rates and cognitive functional status over time. While positive from a clinical perspective, safety programs which use fatal outcomes as evaluation endpoints need to exercise caution about attributing mortality reductions to primary injury prevention interventions. Injury surveillance systems need to examine both mortality and functional status to properly interpret long term trends in recovery from serious trauma. More effective fall prevention programs may reduce the burden of SATBI in trauma centers, while further research is needed to improve treatment and rehabilitation of SATBI. Meanwhile, given the increased survival and the number of patients discharged with functional limitations, access to health care and to rehabilitation services will be an increasing burden on the medical care system.
Acknowledgments
The authors would like to acknowledge the assistance of Dr. Anbesaw Selassie (Medical University of South Carolina), Dr. Mark Roberts (University of Pittsburgh, USA), and Dr Matthew Rosengart (University of Pittsburgh, USA) for their detailed comments and useful suggestions on the analysis and manuscript. We thank the research committee of the Pennsylvania Trauma Systems Foundation for providing the requested data points. We also acknowledge the key role of the hospitals contributing to the Pennsylvania Trauma Outcome Study.
Funding: Fogarty International Center of the National Institutes of Health grant 1 D43 TW007560 01. The funder had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declared that we have no conflicts of interest.
Disclaimers: The Pennsylvania Trauma Outcome Study database was provided by the Pennsylvania Trauma Systems Foundation, Mechanicsburg, Pennsylvania. The foundation specifically disclaims responsibility for any analyses, interpretations, or conclusions. This project was reviewed by the Institutional Review Board at the University of Pittsburgh. Based on the information we provided, this project met all the necessary criteria for an exemption, and was hereby designated as exempt.
Contributor Information
Álvaro I Sánchez, Postdoctoral Associate, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Research Associate, CISALVA Institute, Universidad del Valle, Cali, Colombia.
Robert T Krafty, Assistant Professor, Department of Statistics, University of Pittsburgh, Pittsburgh, PA.
Harold B Weiss, Director and Research Professor, Injury Prevention Research Unit, University of Otago, Dunedin, New Zealand.
Andrés M Rubiano, Assistant Professor, Department of Neurosciences, South Colombian University, Critical Care Unit, Neiva University Hospital, Neiva, Colombia.
Andrew B Peitzman, Professor and Vice-Chair, Department of Surgery, University of Pittsburgh, Pittsburgh, PA.
Juan Carlos Puyana, Faculty Professor, Department of Surgery, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Atkins GG, Drummond JC. Traumatic Brain Injury. In: Willson WC, Grande C, Hoyt D, editors. Trauma: Emergency REsuscitation, Perioperative Anesthesia, Surgical Management. New York, NY: Informa Health Care USA, Inc; 2007. pp. 433–445. [Google Scholar]
- 2.Letarte P. The Brain. In: Feliciano D, Mattox K, Moore E, editors. Trauma. McGraw-Hill Companies, Inc; 2008. pp. 397–415. [Google Scholar]
- 3.Sutcliffe AJ. Traumatic brain injury: Critical Care Management. In: Willson WC, Grande C, Hoyt D, editors. Trauma: Critical Care. New York, NY: Informal Helath Care USA, Inc; 2007. pp. 201–217. [Google Scholar]
- 4.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008 Mar–Apr;23(2):123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 5.Thurman D, Miller L, Hayes R. HEad TRauma: Basic, Preclinical, and Clinical Directions. New York, NY: John Wiley & Son; 2001. The epidemiology and economics of head trauma. [Google Scholar]
- 6.Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000 Dec;(39):1–42. [PubMed] [Google Scholar]
- 7.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282(10):954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 8.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Carney NA, Ghajar J. Guidelines for the management of severe traumatic brain injury. Introduction. J Neurotrauma. 2007;24 Suppl 1:S1–S2. doi: 10.1089/neu.2007.9997. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Marmarou A, Choi S. Mortality from traumatic brain injury. Acta Neurochir. 2005;95 Supplement:281–285. doi: 10.1007/3-211-32318-x_58. [DOI] [PubMed] [Google Scholar]
- 11.Ghajar J, Hariri RJ, Narayan RK, Iacono LA, Firlik K, Patterson RH. Survey of critical care management of comatose, head-injured patients in the United States. Crit Care Med. 1995 Mar;23(3):560–567. doi: 10.1097/00003246-199503000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Guidelines for the Management of Penetrating Brain Injury. Journal of Trauma-Injury Infection & Critical Care. 2001;51(2):S1–S86. [Google Scholar]
- 13.Guidelines for the Surgical Management of Traumatic Brain Injury. Neurosurgery. 2006;58(3):S2-1, S2-62. [Google Scholar]
- 14.Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 Suppl 1:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 15.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12 Suppl 1:S1–S52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 16.Fakhry SM, Trask AL, Waller MA, Watts DD. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma. 2004;56(3):492–499. doi: 10.1097/01.ta.0000115650.07193.66. [DOI] [PubMed] [Google Scholar]
- 17.Patel HC, Bouamra O, Woodford M, King AT, Yates DW, Lecky FE. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet. 2005;366(9496):1538–1544. doi: 10.1016/S0140-6736(05)67626-X. Oct 29–Nov 4. [DOI] [PubMed] [Google Scholar]
- 18.Groswasser Z, Keren O. International Perspectives on TBI Rehabilitation. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice. New York, NY: Demos Medical Publisher, LLC; 2007. [Google Scholar]
- 19.Barell V, Aharonson-Daniel L, Fingerhut LA, et al. An introduction to the Barell body region by nature of injury diagnosis matrix. Inj Prev. 2002 Jun;8(2):91–96. doi: 10.1136/ip.8.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geneva, Switzerland: World Health ORganization; 1977. International Classification of Diseases, 9th Revision. [Google Scholar]
- 21.Thurman DJ, Sniezek JE, Johnson D. Guidelines for Surveillance of Central Nervous System Injury. Atlanta (GA): Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 22.Recommended framework for presenting injury mortality data. MMWR Recomm Rep. 1997 Aug 29;46(RR-14):1–30. [PubMed] [Google Scholar]
- 23.Rating the severity of tissue damage. I. The abbreviated scale. JAMA. 1971;215(2):277–280. doi: 10.1001/jama.1971.03180150059012. [DOI] [PubMed] [Google Scholar]
- 24.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974 Mar;14(3):187–196. [PubMed] [Google Scholar]
- 25.Chawda MN, Hildebrand F, Pape HC, Giannoudis PV. Predicting outcome after multiple trauma: which scoring system? Injury. 2004;35(4):347–358. doi: 10.1016/S0020-1383(03)00140-2. [DOI] [PubMed] [Google Scholar]
- 26.Clark DE, Ahmad S. Estimating injury severity using the Barell matrix. Inj.Prev. 2006;12(2):111–116. doi: 10.1136/ip.2005.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin D, Bachtis C, Acosta JA, et al. Trauma: Emergency Resuscitation, Perioperative anesthesia, Surgical management. New York, NY: Informa Health Care USA, Inc; 2007. Trauma scoring and triage; pp. 59–81. [Google Scholar]
- 28.MacKenzie EJ, Shapiro S, Eastham JN. The Abbreviated Injury Scale and Injury Severity Score. Levels of inter- and intrarater reliability. Med Care. 1985 Jun;23(6):823–835. doi: 10.1097/00005650-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 29.MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412–422. doi: 10.1097/00005650-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie EJ, Steinwachs DM, Shankar BS, Turney SZ. An IC-9-CM to AIS conversion table: Development and application. Proc AAAM. 1986;30:135–151. [Google Scholar]
- 31.Meredith JW, Evans G, Kilgo PD, et al. A comparison of the abilities of nine scoring algorithms in predicting mortality. J Trauma. 2002 Oct;53(4):621–628. doi: 10.1097/00005373-200210000-00001. discussion 628–629. [DOI] [PubMed] [Google Scholar]
- 32.Clark DE, Hahn DR, Osler TM. Programs for injury categorization and scoring based on International Classification of Diseases (ICD) diagnosis codes. Paper presented at: 8th World Conference on Injury Prevention and Safety Promotion; Merida, Mexico. 2008. [Google Scholar]
- 33.ICDPIC: Stata module to provide methods for translating International Classification of Diseases (Ninth Revision) diagnosis codes into standard injury categories and/or scores. Chestnut hill, MA: Boston College Department of Economics; 2009. [computer program] [Google Scholar]
- 34.D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996 Dec;49(12):1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 35.Moore L, Lavoie A, Le Sage N, et al. Using information on preexisting conditions to predict mortality from traumatic injury. Ann Emerg Med. 2008 Oct;52(4):356–364. doi: 10.1016/j.annemergmed.2007.09.007. e352. [DOI] [PubMed] [Google Scholar]
- 36.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996 Dec;77(12):1226–1232. doi: 10.1016/s0003-9993(96)90184-7. [DOI] [PubMed] [Google Scholar]
- 38.Stineman MG, Shea JA, Jette A, et al. The Functional Independence Measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996 Nov;77(11):1101–1108. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- 39.Dijkers M, Greenwald B. Functional Assessment in TBI Rehabilitation. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice. New York, NY: Demos Medical Publishing, LLC; 2007. pp. 225–245. [Google Scholar]
- 40.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 41.Nilsson AL, Sunnerhagen KS, Grimby G. Scoring alternatives for FIM in neurological disorders applying Rasch analysis. Acta Neurol Scand. 2005 Apr;111(4):264–273. doi: 10.1111/j.1600-0404.2005.00404.x. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat.Med. 1998;17(14):1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Shafi S, Stewart RM, Nathens AB, Friese RS, Frankel H, Gentilello LM. Significant variations in mortality occur at similarly designated trauma centers. Arch Surg. 2009 Jan;144(1):64–68. doi: 10.1001/archsurg.2008.509. [DOI] [PubMed] [Google Scholar]
- 44.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. Second ed. College Station, TX: Stata Press; 2008. [Google Scholar]
- 45.Standards for Adult Trauma Center Accreditation. [Accessed December 15, 2009];2009 http://www.ptsf.org/standards_of_accreditation/Adult.
- 46.Injury Deaths and Hospitalizations in Pennsylvania. [Accessed December 15, 2009];2001–2005 www.portal.state.pa.us.
- 47.Cameron PA, Gabbe BJ, Cooper DJ, Walker T, Judson R, McNeil J. A statewide system of trauma care in Victoria: effect on patient survival. Med J Aust. 2008 Nov 17;189(10):546–550. doi: 10.5694/j.1326-5377.2008.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 48.Frankel JE, Marwitz JH, Cifu DX, Kreutzer JS, Englander J, Rosenthal M. A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Arch Phys Med Rehabil. 2006 Jan;87(1):57–62. doi: 10.1016/j.apmr.2005.07.309. [DOI] [PubMed] [Google Scholar]
- 49.Harrison-Felix C, Whiteneck G, DeVivo M, Hammond FM, Jha A. Mortality following rehabilitation in the Traumatic Brain Injury Model Systems of Care. NeuroRehabilitation. 2004;19(1):45–54. [PubMed] [Google Scholar]
- 50.Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study. Arch Phys Med Rehabil. 2009 Sep;90(9):1506–1513. doi: 10.1016/j.apmr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Selassie AW, McCarthy ML, Ferguson PL, Tian J, Langlois JA. Risk of posthospitalization mortality among persons with traumatic brain injury, South Carolina 1999–2001. J Head Trauma Rehabil. 2005 May–Jun;20(3):257–269. doi: 10.1097/00001199-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Gordon WA, Zafonte R, Cicerone K, et al. Traumatic brain injury rehabilitation: state of the science. Am J Phys Med Rehabil. 2006 Apr;85(4):343–382. doi: 10.1097/01.phm.0000202106.01654.61. [DOI] [PubMed] [Google Scholar]
- 53.Gabbe BJ, Simpson PM, Sutherland AM, et al. Functional measures at discharge: are they useful predictors of longer term outcomes for trauma registries? Ann Surg. 2008 May;247(5):854–859. doi: 10.1097/SLA.0b013e3181656d1e. [DOI] [PubMed] [Google Scholar]