Abstract
The purpose our study was to assess mitochondrial biogenesis and distribution in murine primary neurons. Using 5-bromo-2-deoxyuridie (BrdU) incorporation and primary neurons, we studied the mitochondrial biogenesis and mitochondrial distribution in hippocampal neurons from amyloid beta precursor protein (AβPP) transgenic mice and wild-type (WT) neurons treated with oxidative stressors, rotenone and H2O2. We found that after 20 hr of labeling, BrdU incorporation was specific to porin-positive mitochondria. The proportion of mitochondrial area that labeled with BrdU was 40.3 ± 6.3% at 20 hr. The number of mitochondria with newly synthesized DNA was higher in AβPP neuronal cell bodies than in the cell bodies of WT neurons (AβPP, 45.23 ± 2.67 BrdU-positive/cell body; WT, 32.92 ± 2.49 BrdU-positive/cell body; p = 0.005). In neurites, the number of BrdU-positive mitochondria decreased in AβPP cultures compared to WT neurons (AβPP, 0.105 ± 0.008 BrdU-positive/μm neurite; WT, 0.220 ± 0.036 BrdU-positive−/−μm neurite; p = 0.010). Further, BrdU in the cell body increased when neurons were treated with low doses of H2O2 (49.6 ± 2.7 BrdU-positive/cell body, p = 0.0002 compared to untreated cells), while the neurites showed decreased BrdU staining (0.122 ± 0.010 BrdU-positive/μm neurite, p = 0.005 compared to the untreated). BrdU labeling was increased in the cell body under rotenone treatment. Additionally, under rotenone treatment, the content of BrdU labeling decreased in neurites. These findings suggest that Aβ and mitochondrial toxins enhance mitochondrial fragmentation in cell body, and may cause impaired axonal transport of mitochondria leading to synaptic degeneration.
Keywords: Primary neurons, Alzheimer’s disease, Mitochondrial DNA synthesis, 5-bromo-2-deoxyuridine, Cell body mitochondria, Mitochondrial toxins, Mitochondria-targeted antioxidant
Introduction
Increasing evidence suggests that mitochondrial abnormalities are involved in aging and in age-related neurodegenerative diseases, such as Alzheimer’s (AD), Huntington’s (HD, Parkinson’s (PD), and amyotrophic lateral sclerosis (ALS). Age-dependent, mitochondrially generated reactive oxygen species (ROS) have been identified as an important factors that may be responsible for disease progression and cell death in these diseases [1–5]. Treating mitochondria, particularly early on in the disease process or even presymptomatic, is an important option to treat these diseases.
A role for mitochondrial structural changes in mitochondrial dysfunction is emerging from studies of postmortem brain slices from patients with neurodegenerative diseases and of transgenic animal models of these diseases[6–21]. The shape, structure, and distribution of mitochondria are maintained by two important, opposite forces: mitochondrial fission and mitochondrial fusion [22–24]. Fission is controlled and regulated by the dynamin-related protein (Drp1) and mitochondrial fission 1 (Fis1). The increase in mitochondrial free radicals activates Fis1, which is critical for mitochondrial fission. In contrast, mitochondrial fusion is controlled by 3 GTPase proteins: 2 outer-membrane localized proteins Mfn1 and Mfn2, and an inner-membrane localized protein Opa1 [3, 24, 25].
In neurons from AD patients, mitochondrially generated free radicals activated Fis1 and promoted an increase in mitochondrial fragmentation, in turn producing defective mitochondria that ultimately damaged neurons [26,27]. Mitochondrial fusion protected cells from the toxic effects of Aβ by allowing functional complementation of mtDNA, proteins and metabolites.
The imbalance between fission and fusion in the mitochondria appears to lead to functional changes, such as increased ROS production and increased lipid peroxidation; and decreased respiration, decreased ATP production, and decreased membrane potential. This dysfunction and abnormality in mitochondrial dynamics occurs selectively in AD neurons, likely due to high-energy demands and subsequent high oxidative phosphorylation in these neurons [27–28]. Early detection of abnormal mitochondrial dynamics and mitochondrial dysfunction may allow us to intervene in disease processes in order to affect disease progression and to limit disease effects.
One such abnormal mitochondrial dynamics is increased mitochondrial fragmentation and decreased mitochondrial fusion. Mitochondrial fragmentation is the breakup of a single mitochondrion when the cell is exposed to toxins and/or when the cell expresses mutant protein(s). Mitochondrial fragmentation is different from mitochondrial division, which is the natural division of one mitochondrion into two. In both processes, synthesis of mitochondrial DNA (mtDNA) occurs. Mitochondrial synthesis, turnover, and recycling occurs mainly in the cell body because mitochondrial proteins are encoded mostly by nuclear DNA and these nuclear encoded mitochondrial proteins are essential for mitochondrial synthesis [28,29]. However, this process limits mitochondrial biogenesis to the cell body, and delivery of mitochondria to the axons and nerve terminals is dependent on axonal transport.
The hypothesis of “mitochondrial synthesis in cell body” was studied by the earlier work of Davis and Clayton [30]. Using healthy PC12 cells and in situ localization of mtDNA, they found that mtDNA replication mainly occurs in the cell body. However, other studies revealed that mitochondrial proteins were synthesized in the axons [31–33], indicating that mitochondrial replication may occur in the axons as well. This possibility is supported by research in which mitochondrial replications was reported outside the perinuclear region in healthy, non-neuronal cells [34–37]. The issue of where mitochondrial replication occurs becomes even more complicated when it is studied in diseased neurons, such as in neurons that express mutant proteins or that are exposed to toxins. Amiri and Hollenbeck [38] also studied whether mtDNA synthesis can occur in the axons of neurons. Using 11-day primary neurons from the dorsal root ganglia of chickens, and the 5-bromo-2-deoxyuridie (BrdU) incorporation method, they found newly synthesized mtDNA in axons, indicating that mitochondrial division occurs in axons. More recently, Lentz and colleagues [39] used a new sensitive technique to label newly synthesized mtDNA in individual cells by incorporating thymidine analogs such as 5-ethynyl-2′-deoxyuridine (EdU) into mtDNA with a tyramide signal amplification protocol. This technique is a valuable tool for visualizing and measuring mtDNA biogenesis within individual neurons and, importantly, within specific neuronal compartments, such as the somas, dendrites, axons and synapses [39]. BrdU incorporation has also been used to detect cells with new DNA synthesis in flow cytometry analysis [40].
Using mouse hippocampal neurons that were treated amyloid beta (Aβ) peptide 25-35 (a toxic peptide that is involved in AD progression and pathogenesis), we studied axonal transport of mitochondria, including the motility of mitochondria traveling along the axons, the length and size of these mitochondria, and the mitochondrial index per neurite. We also studied synaptic alterations resulting from the axonal transport of mitochondria in the hippocampal neurons [15]. Our results suggest that the Aβ25-35 peptide impairs axonal transport of mitochondria in AD neurons. Further, the average speed of the motile mitochondria was decreased in the Aβ25-35-treated neurons relative to PBS-treated control neurons. Further, synaptic immunoreactivity was also significantly less in the Aβ25-35-treated neurons relative to the PBS-treated neurons, indicating that Aβ25-35 affects synaptic viability [15]. It is still unclear whether, in neurons that express Aβ, mtDNA is synthesized in axons and neurites, or in the cell body and is then transported to axons/neurites and terminals through natural axonal transport mechanisms.
In this study, 1) we evaluated the distribution of BrdU labeled mtDNA in hippocampal neurons from AβPP mice. 2) Further, we also studied BrdU labeled mitochondria from neurons of wild-type mice in response to both acute and chronic toxicity from oxidative stressors, H2O2, and rotenone. 3) Additionally we studied the response of BrdU labeled mitochondria to known neuroprotective molecules such as SS31 and resveratrol.
Materials and Methods
Mice and primary neuronal cultures
The amyloid beta precursor protein (AβPP) transgenic mice (Tg2576 line) and non-transgenic wild-type (WT) littermates are house at the Oregon National Primate Research Center animal facility. The AβPP mice were generated with the mutant human APP gene 695 amino acid isoform and with a double mutation (Lys670Asn and Met671Leu) [41]. Tg2576 mice were gifted by Karen Ashe, PhD, University of Minnesota. All procedures were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee (IACUC) at Oregon Health & Science University. Primary neurons were prepared as previously described [7,15]. Briefly, AβPP and wild-type pups (P0-1) were decapitated, and brains were removed into room temperature HABG (Hibernate A medium; Brain Bits, Springfield IL) supplemented with B-27 (Invitrogen, Carlsbad, CA) and 0.5 mM GlutaMAX (Invitrogen)]. The hippocampus was dissected and reserved for culture, and the cerebellum was used for genotyping. Tissue was minced into pieces and then transferred to a solution of 2 mg/ml papain (Worthington Biochemical Corp., Lakewood, NJ) that was dissolved in Hibernate A without Ca2+ (Brain Bits) and supplemented with 0.5 mM GlutaMAX. Tissue was digested for 30 min at 30°C in a shaking water bath and then removed to 2 ml HABG. Digested tissue was triturated 10 times with a fire-polished, siliconized, 9-in glass pipette. Non-dissociated tissue was allowed to settle for 2 min, and then the supernatant was removed to a fresh tube. An additional 2 ml of HABG was added, and the process was repeated until 6 ml of dissociated cells were collected.
Cells were centrifuged at 200 g for 2 min and then washed with 2 ml HABG. The pellets were resuspended in Neurobasal (Invitrogen) supplemented with B-27 and 0.5 mM GlutaMAX. Live cells were counted using the trypan blue exclusion method and plated at 500 cells/mm2 on poly-D-lysine-coated plates or coverslips. Cells were moved to a 37°C, 5% CO2 incubator and allowed to adhere for one hour. Medium was then changed to Neurobasal, supplemented with B-27 minus antioxidants and 0.5 mM GlutaMAX. One-half the growth medium was changed every 3 days.
Cell treatments
Wild-type cells were treated with SS31 (1 nM, Anaspec, Freemont, CA) or resveratrol (10 μM, Sigma-Aldrich) for 48 hr. At 20 hr prior to harvest, cells were treated with 15 μM 5-bromo-2′-deoxyuradine (BrdU, Sigma-Aldrich, St. Louis, MO). Similarly, wild-type neurons were treated with H2O2 or rotenone for 24 hr with 20 hr BrdU labeling chronologically. Cells were used at 10 DIV.
BrdU Labeling
MtDNA synthesis was measured by BrdU incorporation [39]. Cell cultures grown on 13 mm round coverslips were incubated with a final concentration of 15 μM BrdU for either 4 or 20 hr, after which time the cells were fixed with 4% paraformaldehyde for 10 min at room temperature. Coverslips were washed with PBS and then incubated for 10 min with 0.1% Triton-X100 to permeablize the membranes. The BrdU epitope was exposed by incubating the cells in 2 N hydrochloric acid for 1 hr at 37°C. Then endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in PBS for 30 min at room temperature. After being washed with PBS, cultures were blocked with 1% Bovine serum albumin and 2% normal goat serum in PBS (blocking solution). A primary antibody (anti-BrdU, mouse monoclonal, BD Biosciences, San Jose, CA) was added at a 1:200 dilution in blocking solution, and incubated at room temperature for 1 hr. Cells were washed three times with PBS and then incubated with secondary antibody (anti-mouse-HRP, goat polyclonal, KPL, Gaithersburg, MD) at a 1:200 dilution for 1 hr at room temperature in a blocking solution. BrdU was then detected with Tyramide-488 signal amplification (Invitrogen). Tyramide-488 was diluted 1:100 into assay buffer with 0.15% hydrogen peroxide, and the cells were incubated with the solution for 5 min. Cells were then washed with PBS and either mounted onto slides for image acquisition or co-labeled with mitochondrial or neuronal markers.
Co-labeled cells were then incubated with either a primary antibody for a voltage-dependent anion channel (VDAC/porin) (rabbit polyclonal, Abcam, San Francisco, CA) at 1:200 in blocking solution or MAP-2 (rabbit polyclonal; Millipore, Billerica, MA) at 1:1000 in blocking solution at 4°C overnight. After being washed, coverslips were incubated with secondary antibody (goat anti-rabbit-Alexa 568, Invitrogen) and diluted 1:400 in a blocking solution for 2 hr at room temperature. Coverslips were washed with PBS and mounted onto slides with the Prolong Gold mounting medium.
BrdU Quantification
Images were collected from at least three independent cultures per group. To assess the percentage of mitochondria that took BrdU labeling, we collected images of BrdU-stained and porin-co-labeled neurons. Areas of staining were assessed using ImageJ software. Area of BrdU staining was normalized to porin staining, and the percentage of mitochondria with BrdU-labeled DNA was calculated. To assess the subcellular localization of BrdU-labeled mitochondria, the number of BrdU-stained mitochondria in neuronal cell bodies was assessed and normalized to the number of cell bodies present, and the number of BrdU-positive mitochondria was counted in neuronal projections and was normalized to the total length of neuronal projections. To assess the distribution of BrdU-labeled mitochondria, neurites that were imaged for at least 50 μm from the soma were evaluated. The distance from the soma was calculated for each BrdU-labeled mitochondrion. Data were binned into 5-μm groups, and the percentage of BrdU-positive mitochondria in each bin was assessed. Cumulative probability was calculated as the percentage of BrdU-positive mitochondria within 50 μm of the cell body that were found less than a set distance from the cell body.
Data Analysis and Statistics
All quantitative data are reported as average ± S.E.M. Comparisons of multiple groups were made using ANOVA with Bonferroni post-test. When only two groups were compared, an unpaired student’s t-test was performed. A p<0.05 was considered statistically significant.
Results
BrdU-labeled mitochondria appear in neuronal cell bodies and in neuritic processes
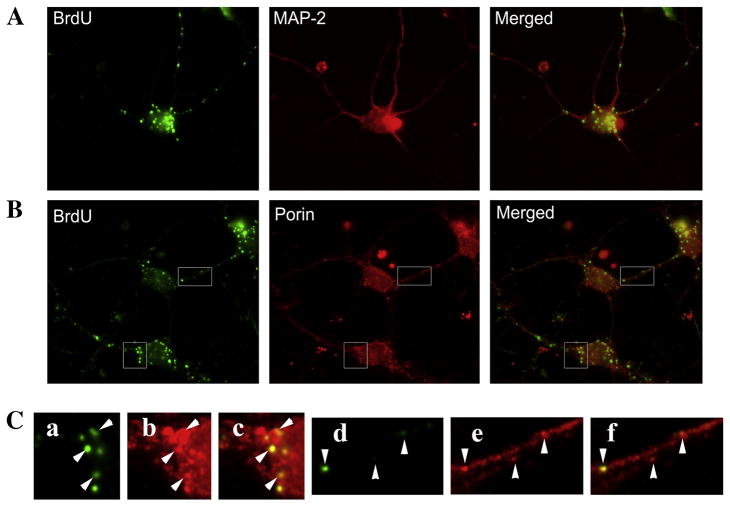
To test whether newly synthesized mtDNA is in neuronal cell processes, we labeled wild-type primary neurons with BrdU for 20 hr and then stained them with neuron-specific protein, MAP-2 (Fig. 1). We found that most of the neuronal projections contained BrdU-labeled mitochondria. Further, we observed that the number of mitochondria labeled with BrdU decreased as their distance increased from the cell body. While we did not assess the site at which mtDNA synthesis occurs, our observation suggests that mtDNA synthesis occurs preferentially in close proximity to the nucleus. After somatic DNA synthesis, the mitochondria would be expected to undergo anterograde transport and populate neurites.
Figure 1. BrdU staining is localized to mitochondria in primary neuronal cultures.
Primary hippocampal neurons were incubated with BrdU for 20 hrs and then stained to visualize BrdU incorporation. Cells were then co-labeled with MAP-2 (neuronal marker) or porin (mitochondrial marker). Primary neurons as identified by MAP-2 staining (A - upper panels) showed robust BrdU labeling as puncta within the cell body and neurites. These BrdU puncta co-localized with porin (B - middle panels), indicating that BrdU was incorporated into the mtDNA. Enlargements (C – lower panels) show co-localization of BrdU is specific to porin-stained organelles (arrowheads). Enlargements represent a, BrdU; b, porin; c, merged; d, BrdU; e, Porin; f, Merged.
BrdU labels newly synthesized mtDNA in primary neuronal cultures
To verify that BrdU labeling was indeed specific to mtDNA, we labeled primary neurons with BrdU and then immunostained the same cells for the mitochondrial outer membrane localized protein, porin. After 20 hr of incubation, BrdU incorporation was specific to the porin-positive mitochondria (Fig. 1). The proportion of mitochondrial area that was labeled with BrdU was about 40.3 ± 6.3% at 20 hr indicating that newly synthesized DNA are present in large numbers of mitochondria within neurons.
Mitochondria with new DNA synthesis exist largely in cell bodies of AβPP neurons
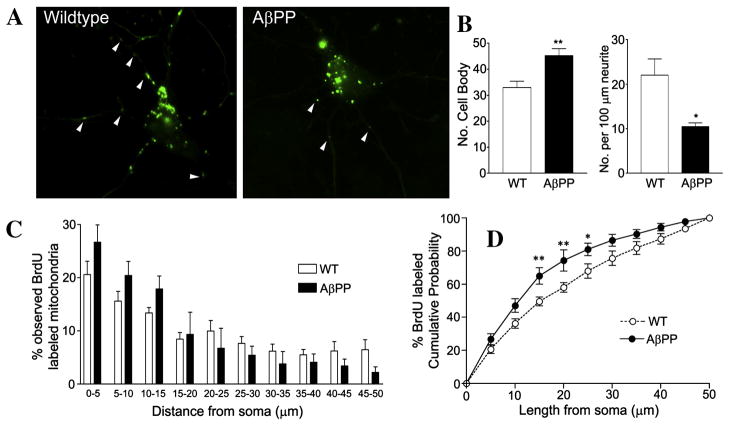
To examine the effects of chronic Aβ exposure on mtDNA synthesis, we monitored mtDNA biosynthesis by counting BrdU-labeled mitochondria in the cell bodies and neurites of primary neurons, from wild-type and AβPP mice at 10 DIV (Fig. 2A). After 20 hr of BrdU incubation, the number of mitochondria with newly synthesized DNA was higher in the cell bodies of AβPP neurons than of the wild-type neurons (Fig. 2B, AβPP: 45.23 ± 2.67 BrdU-positive/cell body; WT: 32.92 ± 2.49 BrdU-positive/cell body; p = 0.005). In neurites, the number of BrdU-positive mitochondria was less in the AβPP cultures compared to the wild-type cultures (Fig. 2B, AβPP: 0.105 ± 0.008 BrdU-positive/μm neurite; WT: 0.220 ± 0.036 BrdU-positive/μm neurite; p = 0.010). We also assessed the distribution of BrdU-labeled mitochondria in neruites up to 50 μm from the cell body. AβPP neurons had a higher percentage of neuritic mitochondria found closer to the cell body, suggesting that the decrease in mitochondria in the AβPP neurites results from deficiencies in mitochondrial transport within these cells (Fig. 2C). AβPP cells had a statistically significant increase in mitochondria within 15 μm, 20 μm, and 25 μm of the cell body, compared to wild-type cells (Fig. 2D). In the wild-type cells, the proportion of BrdU-positive mitochondria within 25 μm of the cell body was 68.0 ± 4.3%, while in the AβPP cells, the proportion of the BrdU-labeled mitochondria within 25 μm of the cell body was 81.5 ± 3.8% (Fig. 2D) (p = 0.043).
Figure 2. AβPP primary hippocampal neurons show increased BrdU staining in the cell body and decreased BrdU staining in neurites.
Primary neurons grown from AβPP and wild-type mice were incubated with BrdU for 20 hr at 10 DIV and then stained for analysis. Representative images of AβPP and WT neurons are shown (A). Quantification of BrdU-labeled mitochondria in the cell body and neruites is shown (B). Quantification of BrdU puncta distribution in relation to the cell soma was performed and analyzed by two methods. The proportion of BrdU-stained puncta in 5-μm bins is shown (C), while the proportion of BrdU puncta within a certain length of neuritic distance is also shown (D). A higher proportion of BrdU-labeled mitochondria were in close proximity to the cell body in the AβPP cultures compared to wild-type cultures.
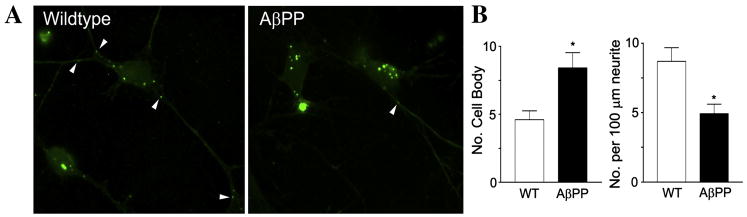
To test whether the distribution of BrdU-labeled mitochondria could be altered over a short time course of BrdU incorporation, cells were incubated with BrdU for 4 hr and then harvested for analysis. BrdU-positive mitochondria were counted separately in neuronal cell bodies and in neurites. In the AβPP cells, BrdU-positive mitochondria were more densely localized in the cell body compared to the wild-type cells (Fig. 3A). Conversely, BrdU-positive mitochondria were less densely localized in the neurites of the AβPP cells compared to wild-type cells. Wild-type neurons exhibited 4.61 ± 0.65 BrdU-positive mitochondria per cell body and 0.087 ± 0.010 BrdU-positive mitochondria per μm of neurite length (Fig. 3B. In comparison, AβPP neurons had 8.42 ± 1.13 BrdU-positive mitochondria per cell body (p = 0.013 compared to wild-type) and 0.049 ± 0.007 BrdU-positive mitochondria per μm neurite length (p = 0.005 compared to wild-type).
Figure 3. Primary neurons from AβPP mice showed altered BrdU distribution even after short-term BrdU incubation.
Representative images of AβPP primary hippocampal neurons that were incubated with BrdU for 4 hr are shown (A). Quantification of cell body and BrdU puncta in neurites are shown (B).
Cells treated with mitochondrial toxins show increased soma mitochondria in BrdU labeling
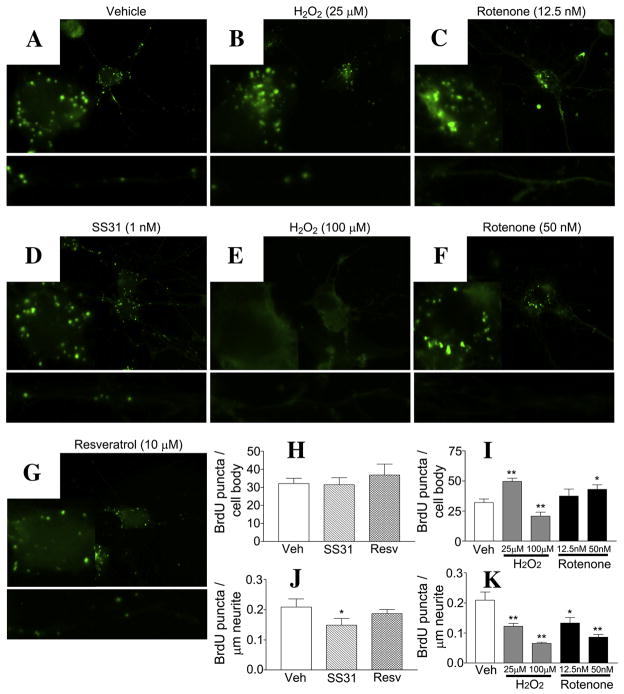
In wild-type cells treated with 25 μM H2O2 for 24 hr (Fig. 4B), BrdU labeling was greater in the cell body (Fig. 4I, 49.6 ± 2.7 BrdU-positive/cell body, p = 0.0002) compared to the untreated cells in the neurites (Fig. 4K, 0.122 ± 0.010 BrdU-positive/μm neurite, p = 0.005 compared to untreated). In the cells treated with 100 μM H2O2, BrdU labeling of both the cell body and neuritic mitochondrial was less than the labeling in the untreated cells (Fig. 4I & K, 20.8 ± 3.3 BrdU-positive/cell body, p = 0.009 compared to the untreated; 0.065 ± 0.004 BrdU-positive/μm neurite, p = 0.0002 compared to the untreated).
Figure 4. Both oxidative toxins and neuroprotective molecules alter the distribution of BrdU-stained mitochondria.
Wild-type cells treated with neuroprotective molecules SS31 (D) and resveratrol (G) for 48 hr, or with H2O2 (B & E) and rotenone (F) for 24 hr were assayed for BrdU incorporation into mitochondria. Representative images for all treatment groups, enlargements of the cell body and selected neurites are shown. SS31 treatment resulted in no change to BrdU staining in the cell body (D & H), but it did result in reduced BrdU staining in the neurites (G,H &J). Images show resveratrol treatment did not alter BrdU staining in the cell body or the neurites. A mildly toxic dose of H2O2 (25 μM) caused an accumulation of BrdU puncta in the cell body and a reduction in the neuritic processes (B,I &K). A higher dose (100 μM) resulted in the loss of BrdU staining in both the cell body and neurites. Two doses of rotenone that did not cause overt toxicity in the culture resulted in an accumulation of BrdU staining in the cell body and a dose-dependent decrease in BrdU staining, in neurites (C,F,I & K). Quantification of images from multiple cultures is shown (H,I,J &K). * p < 0.05 compared to the vehicle-treated group. ** p < 0.005 compared to the vehicle-treated group.
BrdU labeling was also evaluated in wild-type cells treated with rotenone at 12.5 nM and 50 nM for 24 hr (Fig. 4C,F,I &K) BrdU labeling in the cell body progressively increased as the quantity of rotenone increased (12.5 nM rotenone, 37.4 ± 5.9 BrdU-positive/cell body, p = 0.218 compared to the untreated; 50 nM rotenone, 43.0 ± 3.8 BrdU-positive/cell body, p = 0.017 compared to the untreated). BrdU labeling content progressively decreased in the neurites as the quantity of rotenone increased (Fig. 4K, 12.5 nM rotenone, 0.133 ± 0.018 BrdU-positive/μm neurite, p = 0.015 compared to the untreated; 50 nM rotenone, (0.086 ± 0.009 BrdU-positive/μm neurite, p = 0.0005 compared to the untreated). Overall, under mildly toxic conditions, BrdU labeling decreased in neurites with increasingly larger cell bodies. Moreover, BrdU-labeled mitochondria were largely localized in the perinuclear space. Under highly toxic conditions, the incorporation of BrdU into the cell body and neurites was impaired.
Cells treated with mitochondrial antioxidants show alterations in BrdU labeling
We tested whether the mitochondria-targeted antioxidant SS31 (1 nM) altered BrdU labeling of mitochondria in wild-type neurons (Fig. 4D). Cells treated with SS31 for 48 hours and BrdU for 20 hours were stained to evaluate BrdU incorporation. We found that BrdU in the neurites was reduced from 0.209 ± 0.027 BrdU positive/μm neurite in untreated cells to 0.149 ± 0.022 BrdU positive/μm neurite (p = 0.049). Labeling within the cell body was unchanged (Fig. 4H, untreated, 32.1 ± 2.9 BrdU positive/cell body; SS31 treated, 33.5 ± 3.5 BrdU positive/cell body).
Cells were also treated with 10 μM resveratrol, which is thought to induce mitochondrial biosynthesis. We did not see significant changes in BrdU labeling after resveratrol treatment. The neruite content of BrdU labeling was slightly reduced to 0.187 ± 0.014 BrdU positive/μm neurite (p = 0.234 compared to untreated (Fig. 4J). Cell body content was slightly increased to 36.8 ± 6.1 BrdU positive/cell body (p = 0.251 compared to untreated).
Discussion
In the present work, we demonstrated that newly synthesized mtDNA can be labeled using BrdU incorporation in primary murine hippocampal neurons. We found BrdU labeled mitochondria mainly in the soma and distal regions of neurons. However, neurons from AβPP mice and wild-type primary neurons exposed to mitochondrial toxins, rotenone and H2O2, mitochondria are concentrated in the soma and may not be able to transport to distal regions of the neurons. These findings led us to conclude those mitochondrial toxins and Aβ fragment mitochondria excessively and impair distribution of mitochondria to distal regions.
BrdU labeling and distribution of mitochondria in primary neurons
It is well documented that BrdU labeling has been used to identify newly synthesized DNA in mammalian cells and post-mitotic cells such as neurons [reviewed in 42, & 38,39]. In the present study, we investigated mtDNA synthesis in neurons from AβPP mice, and wild-type mice treated with mitochondrial toxins, H2O2 and rotenone, and protective molecules, such as SS31 and resveratrol after incorporating BrdU for 4 and 20 hrs. We identified new synthesized mtDNA in all our experiments for 4 and 20 hrs incorporation. These results were verified by co-labeling analysis using immunostaining of mitochondrial-specific marker, porin and BrdU. We noticed that 20 hrs incorporation of BrdU identifies increased labeling of mitochondria.
The process of mitochondrial biosynthesis is highly interlinked with mitochondrial fission, and therefore BrdU labeled mitochondrial DNA content reflects both mitochondrial biosynthesis and fission. Mitochondrial DNA replication occurs in existing organelles largely within the cell soma. After DNA replication, the organelle will undergo Drp1 mediated fission to produce two daughter mitochondria. Both of these daughter mitochondria are expected to carry the BrdU labeled DNA. Without a fission event to separate the mitochondria, BrdU labeling would occur as a single punctum with high intensity. Under conditions of enhanced Drp1 activation and normal mtDNA synthesis, the number of BrdU puncta would be increased.
Impaired mitochondrial biogenesis in primary neurons from AβPP mice
While measurements of the half-life of mitochondrial proteins has been controversial [43], it has been estimated that the half-life of mitochondria in brain tissue is up to 3 or 4 weeks [44]. An extended lifespan in neurons would not be unexpected, considering the high-energy requirement for the production and distribution in these specialized cells. In the current study, we found that mitochondria with newly synthesized mtDNA comprised about 40% of the total population after 20 hr of BrdU labeling, indicating that newly synthesized mtDNA rapidly distributed throughout the mitochondrial population in neurons. This observation implies that while mitochondrial constituents are long lived, the rate at which new DNA synthesis occurs is relatively high. This new DNA is likely, quickly distributed throughout the mitochondria, through the process of mitochondrial recycling.
Mitochondrial biogenesis may be a generalized compensatory response to mild oxidative toxicity. In support of this hypothesis, increased transcription of mitochondrial-encoded genes was found in AβPP mice (Tg2576 line) early in disease progression [45]. Further, in angiotensin II (ROS inducer)-treated mice, the hearts of mice exhibited an increased upregulation of mitochondrial biogenesis [46], indicating that the increase in mitochondrial biogenesis may be a compensatory response to mitochondrial toxicity caused by angiotensin II. Recent studies have found that mitochondrial fragmentation to be increased in Aβ-treated neurons [7] and to be overexpressed in mutant, in wild-type APP neurons [9,10], and in neurons treated with other toxins, indicating that toxins and mutant proteins are capable of inducing mitochondrial fission.
In a recent electron microscopy study, we [7] found significantly increased numbers of mitochondria in mouse neuroblastoma (N2a) cells treated with Aβ25-35 peptide relative to untreated N2a with Aβ, suggesting that Aβ increased mitochondrial fragmentation. Further, most of these Aβ-induced mitochondria were structurally damaged, and the damaged mitochondria were not able to transport to axons and to distal regions. Those observations are consistent with our present findings of BrdU labeled cells that were treated with mitochondrial toxins concentrate mostly in the soma. These observations led us to conclude that structurally damaged mitochondria (induced by mitochondrial toxins and overexpressed mutant proteins) are not able to transport to terminals.
Impaired mitochondrial biogenesis and defective mitochondrial distribution in neurons exposed to toxins
Our data show that under relatively mild oxidative conditions, BrdU labeling within neurites is reduced with a coincident increase in the cell body. Many of these BrdU labeled mitochondria in the cell body are localized to the perinuclear space. Under a greater oxidative insult, such as 100 μM H2O2, BrdU incorporation into neuronal mitochondria is drastically reduced in both neurites and cell body, as the cells are presumably committed to death. The increase in cell body BrdU puncta may result from one of several mechanisms. First, it is possible that mild oxidative conditions stimulate increased mitochondrial biogenesis. Second, it is possible that increased mitochondrial fission is initiated without concomitant increase in DNA replication. Third, an accumulation in the cell body may be due to decreased ability of the organelles to undergo anterograde transport in neuritic processes. It is also possible that all of these mechanisms play a role.
Impact of protective molecules on mitochondrial biogenesis in primary neurons
Increasing evidence suggests that mitochondrial-targeted antioxidants, such as SS31 and MitoQ, protect mitochondria from toxicity against mutant proteins and other oxidative insults [7,47,48]. The antioxidant molecules enter the mitochondria at several hundred fold and scavenge free radicals, prevent the opening of the mitochondrial permeability transition pore, decrease mitochondrial swelling and protect mitochondria from oxidative insults [49,50,51,52]. In a recent study, we found that SS31 and MitoQ reduced Aβ toxicity, exhibited less mitochondrial damage, and the number of intact mitochondria increased, suggesting that the SS31 and MitoQ balance fission and fusion machinery and enhance neuronal function [7]. Therefore, to further examine the role of fission and fusion on N2a cells, we sought to determine the effect of SS31 on mitochondrial biogenesis. We treated primary neurons with SS31 and the anti-aging agent resveratrol, and we studied newly synthesized mtDNA. As described above, the number of BrdU-labeled mitochondria in the cell body remained unchanged or increased just slightly, indicating that SS31 serve to promote intact mitochondria. These findings are consistent with findings from an earlier study in which N2a cells treated with SS31 and MitoQ showed mitochondrial number is similar to untreated cells [7]. In the cells treated with resveratrol, the number of BrdU labeled mitochondria were unchanged in the cell body and in the neurites, indicating that resveratrol is not involved in fragmenting mitochondria. To increase neuronal function, it is not critical or necessary to have an increased number of mitochondria; rather, what is necessary is that the mitochondria that are present in the neurons need to be healthy and functionally active. Further research is needed to develop drugs/agents that promote healthy and functional active mitochondria, not just to increase the number of mitochondria per se.
Mitochondrial biogenesis is mainly in the soma
mtDNA synthesis is known to occur largely in the cell body [30], but there is evidence that it occurs in neurites as well [38]. Our data showed that the majority of BrdU-labeled mitochondria occur within or very near the cell body, supporting the notion that mitochondrial biogenesis is largely dependent on factors within the soma. In a healthy, normal state, newly formed mitochondria are available for anterograde transport to the distal regions of the neuron, where they will supply the necessary ATP to satisfy the high-energy demands of synaptic activity. However, as in this study, in neurons in a diseased state or in neurons exposed toxins, mitochondria are excessively fragmented and produce large numbers of small, defective mitochondria. These defective mitochondria stay mostly in the soma and do not (or perhaps are unable to transport) to distal regions of the neurons, hence depriving nerve terminals of ATP. This ATP deprivation may ultimately decrease synaptic activities and increase synaptic degeneration. This view is supported by findings from our lab [15] and others [18,53,54], which indicate that mitochondrial anterograde transport is impaired in Aβ-treated cultures. The changes we observed in the BrdU-labeled mitochondrial distribution, as well as the overall decrease in neuritic mitochondria, suggest that transport of newly synthesized mitochondria may be impaired in AβPP cells as well.
In summary, BrdU labeling of mtDNA synthesis allowed us to assess newly synthesized mtDNA in the cell body and in neurites of neurons. We found that Aβ and mitochondrial toxins enhanced mitochondrial fragmentation, and these defective mitochondria were not able to transport to axons, dendrites, and nerve terminals.
Acknowledgments
This research presented was supported by NIH grants AG028072, AG026051, RR00163, S10RR024585 and P30-NS061800, and Alzheimer Association grant IIRG-09-92429 and Medivation, Inc. We also thank Dr. Anda Cornea for her assistance with confocal imaging.
Abbreviations
- AβPP
Amyloid beta precursor protein
- AD
Alzheimer’s disease
- ATP
adenosine triphosphate
- BrdU
5-bromo-2-deoxyuridie
- Drp1
dynamin related protein 1
- Fis1
mitochondrial fission 1
References
- 1.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 2.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–84. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 3.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manczak M, Mao P, Calkins MJ, Cornea A, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics, and abnormal interaction of Amyloid-beta oligomers and the mitochondrial protein Drp1 in neurons from Alzheimer’s disease patients: implications for neuronal damage and cognitive decline. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr139. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 13.Magrané J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;26:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial A beta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 20.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 21.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;15(14 Spec No 2):R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 23.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 24.Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2010 doi: 10.1016/j.brainresrev.2010.11.004. Epub Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20(Suppl 2):S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy PH, Reddy TP. Mitochondria are a therapeutic target for aging and neurodegenerative diseases. Cur Alz Res. 2011 doi: 10.2174/156720511795745401. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 30.Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenig E, Giuditta A. Protein-synthesizing machinery in the axon compartment. Neuroscience. 1999;89:5–15. doi: 10.1016/s0306-4522(98)00282-6. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 33.Twiss JL, van Minnen J. New insights into neuronal regeneration: the role of axonal protein synthesis in pathfinding and axonal extension. J Neurotrauma. 2006;23:295–308. doi: 10.1089/neu.2006.23.295. [DOI] [PubMed] [Google Scholar]
- 34.Larsson NG, Oldfors A, Holme E, Clayton DA. Low levels of mitochondrial transcription factor A in mitochondrial DNA depletion. Biochem Biophys Res Commun. 1994;200:1374–1381. doi: 10.1006/bbrc.1994.1603. [DOI] [PubMed] [Google Scholar]
- 35.Tiranti V, Savoia A, Forti F, D’Apolito MF, Centra M, Rocchi M, Zeviani M. Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the Expressed Sequence Tags database. Hum Mol Genet. 1997;6:615–625. doi: 10.1093/hmg/6.4.615. [DOI] [PubMed] [Google Scholar]
- 36.Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N, Comi G, Morandi L, Santoro L, Toscano A, Fabrizi GM, Somer H, Croxen R, Beeson D, Poulton J, Suomalainen A, Jacobs HT, Zeviani M, Larsson C. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 37.Magnusson J, Orth M, Lestienne P, Taanman JW. Replication of mitochondrial DNA occurs throughout the mitochondria of cultured human cells. Exp Cell Res. 2003;289:133–142. doi: 10.1016/s0014-4827(03)00249-0. [DOI] [PubMed] [Google Scholar]
- 38.Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 2008;68:1348–1361. doi: 10.1002/dneu.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lentz SI, Edwards JL, Backus C, McLean LL, Haines KM, Feldman EL. Mitochondrial DNA (mtDNA) biogenesis: visualization and duel incorporation of BrdU and EdU into newly synthesized mtDNA in vitro. J Histochem Cytochem. 2010;58:207–218. doi: 10.1369/jhc.2009.954701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappella P, Gasparri F, Pulici M, Moll J. A novel method based on click chemistry, which overcomes limitations of cell cycle analysis by classical determination of BrdU incorporation, allowing multiplex antibody staining. Cytometry A. 2008;73:626–636. doi: 10.1002/cyto.a.20582. [DOI] [PubMed] [Google Scholar]
- 41.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 42.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Miwa S, Lawless C, von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- 45.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 46.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates Angiotensin II-induced cardiac hypertrophy and G{alpha}q overexpression-induced heart failure. Circ Res. 2011 Feb 10; doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]