Abstract
Although the importance of adipose tissue (AT) glucose transport in regulating whole-body insulin sensitivity is becoming increasingly evident and insulin resistance (IR) has been widely recognized, the underlying mechanisms of IR are still not well understood. The purpose of the present study was to determine the early pathological changes in glucose transport by characterizing the alterations in glucose transporters (GLUT) in multiple visceral and subcutaneous adipose depots in a large animal model of naturally-occurring compensated IR. AT biopsies were collected from horses, which were classified as insulin-sensitive (IS) or compensated IR based on the results of an insulin-modified frequently-sampled intravenous glucose tolerance test. Protein expression of GLUT4 (major isoform) and GLUT12 (one of the most recently discovered isoforms) were measured by Western blotting in multiple AT depots, as well as AS160 (a potential key player in GLUT trafficking pathway). Using a biotinylated bis-mannose photolabeled technique, active cell surface GLUT content was quantified. Omental AT had the highest total GLUT content compared to other sites during the IS state. IR was associated with a significantly reduced total GLUT4 content in omental AT, without a change in content in other visceral or subcutaneous adipose sites. In addition, active cell surface GLUT-4, but not -12, was significantly lower in AT of IR compared to IS horses, without change in AS160 phosphorylation between groups. Our data suggest that GLUT4, but not GLUT12, is a pathogenic factor in AT during naturally-occurring compensated IR, despite normal AS160 activation.
Keywords: GLUT trafficking; biotinylated photo-affinity label, omental, GLUT12
1. Introduction
Type II diabetes mellitus, an epidemic health problem affecting over 200 million people worldwide, is characterized by insulin resistance (IR, i.e., an impaired ability of insulin to stimulate glucose disposal into muscle and adipose tissue) combined with an inadequate compensatory pancreatic secretory response; this condition is usually associated with obesity. In addition, over 300 million people suffer from the preclinical stages of diabetes (i.e., “prediabetes”), characterized by increased fasting glucose concentration (with value above normal range but below the cutoff for the diagnosis of diabetes), impaired glucose tolerance, or both [1]. Despite intensive research for over 50 years (primarily in rodents), the mechanisms of altered glucose transport and pathogenic factors observed during IR and diabetes remain elusive. One of the challenges is the lack of appropriate animal models that reproduce the preclinical stages of type II diabetes to study the naturally-occurring pathological changes that occur in the tissues of interest as the animal proceeds from normal to impaired glucose tolerance [2]. For example, the majority of both rodent [3] and large animal models [4] display marked hyperglycemia, which does not reflect the prediabetic stage [1]. Thus, there is a need for a well-characterized large animal model of IR that could provide important translational information on the pathophysiology of the preclinical stages of diabetes. Recently there has been increased clinical awareness and recognition of obesity and IR without hyperglycemia in horses from a wide range of breeds, types and performance levels [5]. However, the regulation of glucose transport has not been investigated in adipose tissue (AT) in this novel equine phenotype of naturally-occurring IR.
The central role of AT glucose transport in regulating whole-body insulin sensitivity is becoming increasingly evident, as is the pathogenic function of visceral AT depots compared to subcutaneous sites during IR and prediabetes [6,7]. Glucose uptake into cells is the rate-limiting step in whole-body glucose utilization and is tightly regulated by a family of membrane proteins called glucose transporters (GLUTs), of which GLUT4 is the predominant isoform in insulin-sensitive tissues. Although impairments in the AT glucose transport pathway have been demonstrated during IR and prediabetes [8-11], the initial pathogenic cause of the decreased AT glucose transport during IR is not well elucidated. In addition, although GLUT4 is the predominant isoform in insulin-sensitive tissues, there is new evidence that GLUT12, one of the most recently identified GLUT isoforms, translocates to the cell surface after insulin stimulation and therefore may play a significant role in glucose uptake of insulin-sensitive tissues [12,13]. However, the role of GLUT12 during IR is not well characterized, and other than its original discovery demonstrating its presence in multiple mammalian tissues [12], there have been no studies of GLUT12 in AT under pathophysiologic conditions. In addition, although the downstream insulin signaling pathways regulating glucose transport are not well defined, the Akt substrate protein of 160 kDa (AS160) has recently emerged as a potential key player in GLUT4 translocation in adipose and skeletal muscle [14]. Thus, AS160 could be a potential mechanism for reduced insulin signaling pathways, however, there are no studies investigating AS160 in AT during IR.
Thus, the purpose of the present study was to determine the early pathological changes in key regulatory aspects of AT glucose transport during naturally-occurring IR by determining total and active cell surface GLUT-4 and -12 protein content in multiple different visceral and subcutaneous AT depots in a novel large animal model. We hypothesized that active cell surface GLUT-4 and -12 protein will be altered in AT, independent of its total content, through an AS160 dependent pathway, during naturally-occurring compensated IR.
2. Materials and Methods
2.1 Experimental Protocol
Ten adult light-breed mares (n=5 per group, breed-matched, mean age 14.4 ± 1.9 years) chosen from the Ohio State University research herd were fed forage ad libitum and determined to be free of diseases, based on a clinical examination. This research herd, which received standardized and uniform feeding and management practices, has previously been estimated to contain a significant number of naturally-occurring IR horses [15]. All procedures were approved by The Ohio State University Institutional Animal Care and Use Committee. The animals were classified as either insulin resistant (IR) or insulin-sensitive (IS) based on results of an insulin-modified frequently sampled intravenous glucose tolerance test (FSIGTT) performed as previously described [16, 17]. Briefly, on the morning of the FSIGTT, a baseline blood sample was taken and a dose of 50% dextrose (300 mg kg−1, 50% dextrose, Vedco Inc., St Joseph, MO) was administered intravenously. At 20 min post-dextrose, insulin (20 mIU kg−1 Humulin R, Lilly, Lake Forest, IL) was administered IV and blood sampling continued at 2-30 min intervals until 240 min post-dextrose infusion. All animals were kept in their normal environment during the FSIGTT, and all the needed precautions were taken to ensure the FSIGTT was performed in a quiet environment with no stressful event preceding or occurring during the test [17]. Insulin sensitivity index (SI), acute insulin response to glucose (AIRg, within 20 min after glucose administration), glucose effectiveness (Sg) and disposition index (DI) were determined by the minimal model of glucose and insulin dynamics using specialized software (MinMod Millenium 5.10 [18]). Several weeks following the FSIGTT, the animals were anesthetized for collection of AT samples, as follows: omental, retroperitoneal and mesenteric (visceral) AT was collected via laparotomy, and nuchal ligament and tailhead (subcutaneous) AT was collected (4-5g per site) via incisional biopsy. All samples were flash frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses, with the exception of the photolabeled samples which were processed as described below.
2.2 Tissue Analysis
Total crude extracts of AT for GLUT-4 and -12 analysis were obtained by homogenizing samples in buffer (50mM Tris-Hcl, 50mM sodium pyrophosphate, 5mM sodium orthovanadate, 50mM sodium fluoride, 5mM EDTA, 1% Triton X-100) containing protease and phosphatase inhibitor cocktail (Sigma, St. Louis, MO), centrifuging at 1000g for 25 min, extracting the supernatant and centrifuging at 100,000g for 60 min, after which the pellet was resuspended in buffer and used for analysis. Omental adipose total tissue lysates for analysis of cell surface GLUTs and (total and phospho-) AS160 were obtained by homogenizing samples in buffer, centrifuging at 10,000g for 20 min, and extracting the supernatant.
2.3 Active cell surface GLUT-4 and -12
Visceral (omental and mesenteric) and subcutaneous (tailhead) AT were photolabeled as previously described [17, 19]. Briefly, samples were incubated in Krebs-Henseleit bicarbonate buffer containing the biotinylated bis-mannose photolabeling reagent: N-[2-[2-[2-[(N-Biotinyl-caproylamino)-ethoxy)ethoxyl]-4-[2-(trifluorommethyl)-3H-diazirin-3-yl]benzoyl]-1,3-bis(mannopyranosyl-4-yloxy)-2-propylamine (Toronto Research Chemical, Toronto, ON). The hexose group interacts specifically with the extracellular binding site of GLUT, and upon UV irradiation, the diazrine group loses nitrogen and generates a short-lived carbene, which interacts with the glucose transporter protein by establishing a covalent bond [20]. Adipose samples were then irradiated for 3 × 3 min in a Rayonet RPR-100 photoreactor with 300 nm lamps, flash frozen in liquid nitrogen and stored at −80°C until analysis.
For analysis of active cell surface GLUT-4 and -12 content, extracts of the photolabeled AT samples were obtained by homogenizing as described above and saved as the “total lysate” fraction. To isolate photolabeled GLUTs from the total lysate, samples were incubated with streptavidin agarose beads overnight and the supernatant of the beads isolation, which contained non-photolabeled GLUTs was saved as the “unlabeled” fraction. The “labeled” fraction of GLUT transporters were dissociated from the beads by heating at 95°C, and the labeled, unlabeled and total lysate fractions were then subjected to SDS-PAGE and immunoblotting, as previously described (17).
2.4 Western Immunoblotting
GLUT4 antibody was purchased from AbD Serotec (Raleigh, NC) and has been validated for use in the horse [21]. GLUT12 (against rat), and total and phospho (serine/threonine)- AS160 (against human) antibodies were purchased from Abcam (Cambridge, MA) and Cell Signaling (Beverly, MA), respectively, and were chosen because of their homology (100%) with equine GLUT12 and AS160 [22]; they were also validated against a positive control. AT photolabeled fractions, crude plasma membrane extracts and total tissue lysates were analyzed for protein content by use of electrophoresis and subsequent Western blotting as described previously [17,21]. In brief, equal amounts of protein from samples were resolved on a 7% (AS160) or 12% (GLUTs) SDS-PAGE gel. After electrophoretic transfer, PVDF membranes were incubated with primary polyclonal antibodies overnight (total and phospho-AS160; 1:1000, GLUT12; 1:500, GLUT4; 1:7500) and subsequently an anti-rabbit horseradish peroxidase-linked antibody. Equal protein loading was confirmed by measuring calsequestrin protein expression (Abcam, Cambridge, MA) or MemCode reversible protein staining (Thermo Scientific, Ashville, NC) for GLUTs and AS160, respectively.
2.5 Statistics
Data are presented as mean ± SE. Differences between means were assessed by Student’s t-test, and one-way or two-way ANOVA, as appropriate (Sigmastat, Jandel Scientific). When a significant difference was identified by ANOVA (with group and site as the main effects), post hoc tests were performed using the Student-Newman-Keuls test. Statistical significance was defined as P< 0.05.
3. Results
No significant difference in baseline blood glucose or plasma insulin concentrations was found between groups (Table 1), nor in body weight, body condition score, neck circumference, girth, or ultrasonographic retroperitoneal fat thickness. Insulin sensitivity was significantly lower in IR vs. IS group (P=0.014, Table 1). The peripheral insulin resistance was compensated by a tendency for insulin response (AIRg) to be higher (P=0.063), such that neither glucose effectiveness (Sg) nor secretory function (DI) were significantly lowered in IR compared to IS horses. (Table 1)
Table 1. Baseline parameters in insulin-sensitive and insulin resistant groups.
Data are mean ± SE.
| Insulin Sensitive | Insulin Resistant | |
|---|---|---|
| Body weight (kg) | 534.4 ± 28.8 | 510.2 ± 11.5 |
| Body Condition Score | 6.5 ± 0.7 | 7.0 ± 0.2 |
| Basal [Insulin] (mIU L−1) | 16.4 ± 5.8 | 18.7 ± 4.4 |
| Basal [Glucose] (mg dL−1) | 100.2 ± 4.75 | 101.3 ± 3.76 |
| SIa (L min−1 mU−1) | 2.53 ± 0.60 | 0.62 ± 0.11 * |
| AIRgb (mU L−1 min−1) | 405.6 ± 80.0 | 983.6 ± 256.2 |
| DIc | 874.0 ± 221.6 | 593.4 ± 83.9 |
| Sgd (min−1) | 1.67 ± 0.12 | 1.93 ± 0.28 |
insulin sensitivity
acute insulin response to glucose
disposition index [=SI X AIRg]
glucose effectiveness
denotes significant difference between groups (P<0.05). n=5/group.
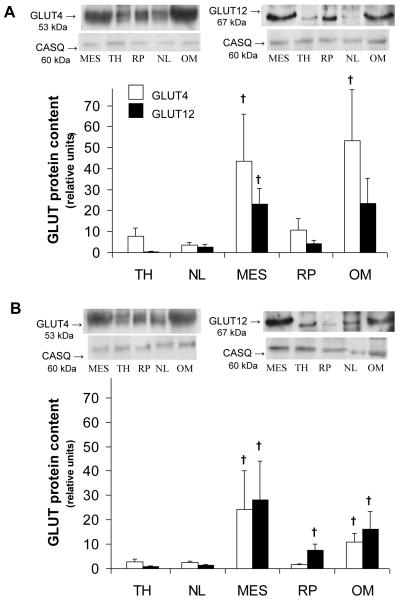
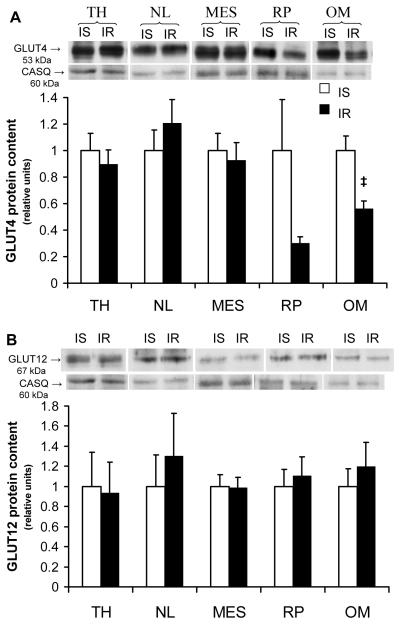
Total GLUT -4 and -12 protein content was measured in plasma membrane enriched fractions across various AT depots of the IS (Fig. 1A) and IR (Fig. 1B) groups, and a significant regional effect (P<0.05) was observed. Total GLUT4 content was significantly greater in visceral (i.e., mesenteric and omental) compared to subcutaneous AT during the IS state. Similarly, total GLUT12 protein content was greater in visceral adipose sites (i.e., mesenteric with a tendency for omental) compared to other sites in the IS group (P<0.05, Fig. 1A). In the IR group, a significantly greater total GLUT-4 and -12 protein content was found in visceral compared to subcutaneous adipose sites. In addition, total GLUT4 content from a membrane-enriched fraction was 44% lower in omental AT of the IR group compared to the IS control group (P=0.027, Fig. 2A). In contrast, there were no significant differences in total GLUT4 protein content between IR and IS groups for all other AT sites sampled. Similarly, no differences were observed in total GLUT-12 content (Fig. 2B) for any of the AT sites sampled between groups.
Figure 1.
Insulin-sensitive (A) and insulin resistant (B) states induces differential protein expression of total GLUT -4 (white bars) and -12 (black bars) across various visceral and subcutaneous adipose tissue. Top panel: Representative Western blot of GLUT from a plasma membrane-enriched preparation of adipose tissue. Calsequestrin (CASQ) protein content was used a loading control. Bottom panel: Mean ± SE total content of GLUT protein in omental (OM), retroperitoneal (RP), and mesenteric (MES) (visceral) sites, and nuchal ligament (NL) and tailhead (TH) (subcutaneous) AT depots (n=5/group). Relative units were expressed in relation to the skeletal muscle of insulin-sensitive (IS) and insulin resistant (IR) subjects, respectively. †: significantly greater than all subcutaneous sites within the same group, P<0.05. Please note that there is a tendency for GLUT12 content to be higher in omental site compared to other sites during IS state (P<0.1).
Figure 2.
Compensated IR induces decreases in omental total GLUT4 protein content. Top panel: Representative Western blot of GLUT from a plasma membrane-enriched preparation of adipose tissue. Calsequestrin (CASQ) protein content was used as a loading control. Bottom panel: Mean ± SE of total GLUT -4 (A) and -12 (B) protein content in various visceral and subcutaneous AT depots during insulin-sensitive and insulin resistant state (n=5 per group). Relative units were expressed in relation to IS horses for each specific depot; ‡: P<0.05. vs. IS group. Please refer to figure 1 for legends.
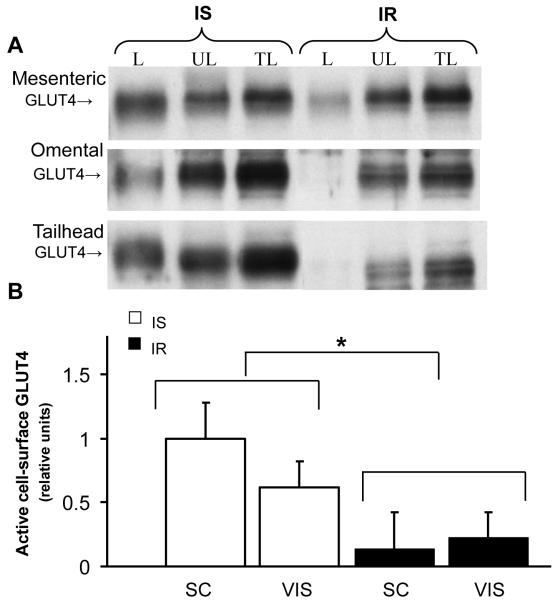
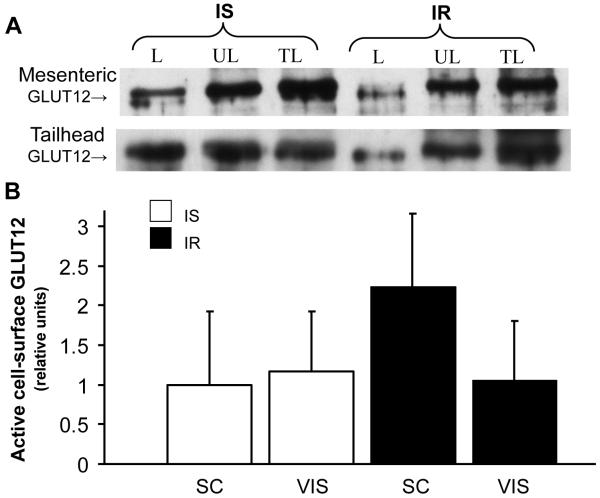
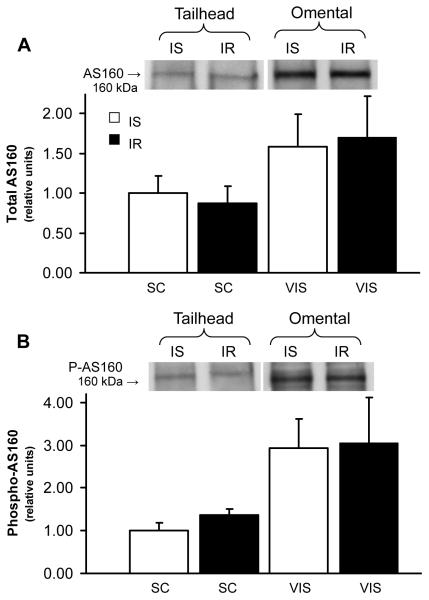
Since alterations in either total GLUT protein or GLUT translocation content could contribute to the pathogenesis of IR, active cell surface GLUT content was measured in photolabeled subcutaneous and visceral AT. An overall group effect was found with significantly lower (P=0.033) active cell surface GLUT4 content in AT of IR compared to IS group (by 63% and 70% in visceral and subcutaneous sites, respectively, Fig 3), suggesting that IR decreased GLUT4 translocation to the cell surface in AT. In contrast, there was no difference in cell surface GLUT12 content between groups for either subcutaneous or visceral sites (P=0.454, Fig 4). In order to characterize potential molecular mechanisms underlying the alterations in GLUT4 trafficking, AS160 was quantified in subcutaneous and visceral AT, and no differences in total AS160 (Fig 5a, P=0.799) or phospo-AS160 (Fig 5b, P=0.896) were found between groups for each site.
Figure 3.
Insulin resistance decreases active cell surface GLUT4 in photolabeled adipose tissue. (A): Representative Western blot of cell surface GLUT4 during insulin-sensitive (IS) and insulin resistant (IR) state; after cell-surface biotinylation of adipose tissue, streptavidin-isolated photolabeled GLUT4 was detected by immunoblotting. L: labeled fraction; UL: unlabeled fraction; TL: total lysate. (B) Mean ± SE of labeled cell-surface content of active GLUT4 in subcutaneous (Sc; tailhead) and visceral (Vis; omental and mesenteric) adipose sites during insulin-sensitive (IS) and insulin resistant (IR) state (n=2-4/group). Relative units were expressed in relation to an internal positive control; ‡: P<0.05. vs. IS group.
Figure 4.
Insulin resistance does not alter active cell surface GLUT12 in photolabeled adipose tissue. (A) Representative Western blot of cell surface GLUT12 in insulin-sensitive (IS) and insulin resistant (IR) state; after cell-surface biotinylation of adipose tissue, streptavidin-isolated photolabeled GLUT12 was detected by immunoblotting. (B) Mean ± SE of labeled cell-surface content of active GLUT12 in subcutaneous and visceral adipose sites (n=2-4/group). Relative units were expressed in relation to an internal positive control. Please refer to figure 3 for legends.
Figure 5.
The decrease in active GLUT4 content in omental tissue occurred despite normal activation of AS160 during insulin resistant state. Total protein content of (A) AS160 and (B) phosphorylated AS160 in adipose tissue during insulin-sensitive (IS) and insulin resistant (IR) state (n=5 per group). Top panel: Representative Western blot of AS160 in adipose total tissue. Bottom panel: Mean ± SE of protein expression of total AS160 (A) and phosphorylated AS160 (B) in subcutaneous (Sc; tailhead) and visceral (Vis; omental and mesenteric) adipose sites.
4. Discussion
The present study investigated the metabolic defects associated with AT glucose transport in a novel large animal model of naturally-occurring compensated IR. We reported that omental GLUT4 content in plasma membrane enriched fractions was lower in IR compared to IS group, while no changes in GLUT4 content were present in other visceral or subcutaneous adipose sites between groups. In addition, using an innovative biotinylated bis-mannose photolabeled technique, we demonstrated that active cell surface GLUT4, but not GLUT12, was lower in AT of horses with compensated IR compared to IS controls. These alterations in GLUT4 trafficking occurred despite normal activation of AS160.
Differential GLUT protein expression across various visceral and subcutaneous AT depots was found in healthy IS subjects in the present study, with greater total GLUT-4 and -12 content in visceral (e.g., omental and mesenteric) adipose tissue. It therefore likely that visceral GLUT-4 and-12 play a substantial role in the regulation of glucose transport, and similar differences in the regulation of glucose transport between several AT have been demonstrated in healthy humans. For example, human omental adipocytes have higher GLUT4 expression and basal- and insulin-stimulated glucose uptake compared with subcutaneous adipocytes [23, 24]. In addition, in healthy non-obese subjects, the total protein levels of multiple insulin signaling intermediates, including the insulin receptor, IRS1/2, p85, GSK3, MEK and ERK1/2, were also found to be higher in omental vs. subcutaneous adipose. In addition, when activation of the insulin signaling cascade was studied following administration of I.V. insulin in vivo, greater and earlier activation levels of the insulin receptor, Akt, GSK3, and ERK1/2 were found in omental than in subcutaneous adipose tissue [24]. Thus, the central role of visceral GLUT in whole-body glucose metabolism provides an important rationale for utilizing this novel large animal model to study the naturally occurring changes in multiple different AT during the initial phase of compensated IR. Although a wide variety of animal models have been used to study Type II diabetes, few of these models mirror the early pathological events that naturally occur in the preclinical stages of non insulin-dependent diabetes [2]. In addition, since rodents rely more on brown AT metabolism compared to the reliance on white AT observed in larger species including horses and humans [25], results from studies of rodent white AT may not translate well to findings in human patients. Finally, this equine model has other advantages over commonly used rodent models, including the ability to obtain repeated measurements of tissue biopsies or samples from an animal over time, the ease of catheterization of horses in protocols requiring it, and the ability to obtain relatively large amounts of tissue for assays. Recently, the horse has been used to establish a large animal model of IR [16, 17], and, similar to other species, obesity, high soluble carbohydrate diets and age have all been associated with equine IR [18,26]. As for humans, it appears that careful dietary management and increasing physical activity promote insulin sensitivity in this model [27]. In addition, the ability to study naturally-occurring IR eliminates problems associated with experimentally-induced IR, such as potential detrimental health consequences associated with feeding a high soluble carbohydrate diet [5]. In the present study, the FSIGTT data indicate an early state of compensatory IR, as there was no significant difference in the disposition index (DI) (a measure of the ability of the hyperbolic relationship between insulin sensitivity and insulin secretion to maintain glucose homeostasis) between groups [28]. To our knowledge, this is the first large animal model of naturally-occurring compensated IR, typical of early prediabetes in humans.
The importance of AT in maintaining peripheral insulin sensitivity has been demonstrated previously, as AT-selective reduction of GLUT4 in mice results in impaired glucose tolerance and peripheral IR [29]. This GLUT4 reduction in AT is characterized by a 50% reduction in whole-body glucose uptake followed by decreased glucose uptake into skeletal muscle (despite preserved total GLUT4 content) [29]. In addition, impairments in the AT glucose transport pathway have been demonstrated during IR and prediabetes [8,9,11]. However, previous studies on GLUT4 regulation in subcutaneous and visceral AT adipose have been equivocal, either demonstrating decreased [30], increased [8] or unchanged gene expression [9]. To our knowledge, this is the first study in which total and cell surface GLUT4 content has been simultaneously quantified in subcutaneous and visceral adipose depots during early stage of IR. Similar to findings in Type II diabetic patients [31,32], total GLUT4 content from a membrane enriched fraction was significantly lower in omental AT but preserved in other visceral and subcutaneous AT sites of IR compared to IS group. In contrast, protein expression of GLUT12 was not impaired in any AT of IR subjects and thus may not be a pathogenic factor during compensated IR. Overall, our data suggest a central role for omental GLUT4 in the pathogenesis of IR and ultimately diabetes, such that a reduction of insulin-stimulated glucose transport in omental adipocytes could secondarily induce IR in other insulin target tissues.
The initial mechanism causing the alteration in omental GLUT4 during IR is unclear, although the development of obesity-induced IR may be due to visceral fat being more sensitive to increases in adipocyte cell size during expansion of adiposity [33], thus resulting in a relative decrease in cellular GLUT4 content as cell size increases. Therefore, in order to determine if alterations in GLUT activity underlie compensated IR, active cell surface GLUT-4 and -12 were quantified in photolabeled AT using a bis-mannose photolabeled compound, which is specific to GLUT transporters and only labels those that are accessible (active) at the cell surface. To our knowledge, we are the first to successfully apply this technique on AT in any species, and we reported that active cell surface GLUT4 was lower in all AT sites (by 63% and 70% in visceral and subcutaneous sites, respectively, P<0.05) in IR compared to IS group. Thus, compensated IR is characterized by altered GLUT4 trafficking, although there were no differences in cell surface content of GLUT12 at any sampling site compared to the IS group. Interestingly, these findings demonstrate impairments in both visceral and subcutaneous AT glucose transport pathway, despite no change in total content of subcutaneous GLUT4, findings which are in agreement with previous studies demonstrating alterations in gene expression of key insulin signaling intermediates [8] and IRS-1 protein expression [11] in subcutaneous AT during prediabetes. In addition, previous studies using membrane fractionation to quantify glucose transporters at the plasma membrane have reported lower GLUT4 protein expression in subcutaneous AT [34, 35] during diabetes, as well as in visceral AT [32]. For example, in subcutaneous adipocytes of Type II diabetics, basal GLUT4 in a plasma membrane fraction was 43-80% of IS subjects [34,35], and gestational diabetes is characterized by the lack of increase in GLUT4 translocation in omental adipocytes [32]. However, it should be noted that, in contrast to the photolabeled technique employed in the present study, the conventional subfractionation techniques used in previous studies cannot differentiate between active GLUTs that have docked and fused with the plasma membrane and inactive GLUTs (i.e., before fusion), as well as GLUT in intracellular membranes [17, 19]. In the present study, the lower basal activity of cell-surface GLUT4 in both subcutaneous and visceral adipose sites of IR compared to IS groups, independent of its total GLUT4 content, suggest an alteration in AT GLUT trafficking, which may constitute an early pathogenic factor in the development of IR.
Although the downstream insulin signaling pathways regulating glucose transport are not well defined, the Akt substrate protein of 160 kDa (AS160) has recently emerged as a potential key player regulating GLUT trafficking [14]. AS160 is mainly unphosphorylated in the basal state and retains GLUT4 vesicles intracellularly, while AS160 phosphorylation by insulin stimulation results in GLUT4 exocytosis [14]. In the present study, the lower active cell surface GLUT4 content in AT of IR compared to IS group was not associated with changes in AS160 phosphorylation, suggesting that AS160 regulation is not a central event in the initial pathogenesis of compensated IR. These findings are supported by previous studies in skeletal muscle of type 2 diabetic subjects, in which neither total nor phospho-AS160 was altered compared to IS subjects [36, 37], although GLUT4 translocation was not reported. In addition, total AS160 was significantly (~15%) higher in the quadriceps muscle of IR rats despite a significantly lower total GLUT4 protein content (~20%) compared to IS controls [38]. Collectively, these results suggest that AS160 regulation is not a central event in the pathogenesis of IR and other downstream targets such as TBC1D1 [39] or other unspecified targets [14] may play a role in the decreased translocation. In addition, GLUT-4 translocation is a complex process involving vesicle trafficking and sorting machinery and there are many potential steps that may be impaired, including regulation by motifs intrinsic to the GLUT4 molecule together with a host of proteins directing vesicle traffic [40]. Of importance, AS160 does not appear to regulate several major steps required for complete GLUT4 translocation, including the recruitment, tethering, and fusion of GLUT4 vesicles with surface membranes and possibly GLUT4 activation [41-42]. Certainly further research is required to characterize the impairments of the downstream signaling pathways responsible for the alteration in GLUT4 translocation observed during the earliest stages of IR and prediabetes.
4.1 Conclusions
In summary, naturally-occurring compensated IR in this large animal model is characterized by selective impairment of the glucose transport pathways, such that active cell-surface GLUT4 content was lower in AT (visceral and subcutaneous depots) of IR compared to IS group, while total GLUT4 content was only reduced in omental tissue. In addition, GLUT12, one of the most recently discovered GLUT isoforms, does not appear to be a pathogenic factor in our model of early stage of IR, since neither its total nor its active content was affected in any adipose depot in the IR state. It is concluded that GLUT-4, but not-12, in AT, particularly in the omental site, contributes to the pathogenesis of naturally-occurring compensated IR.
Acknowledgements
The authors are very grateful to Marie Bevier for excellent technical assistance, Dr. Ray Geor for blood biochemical analyses, and Dr. Kenneth Hinchcliff for advice regarding the development of the initial phase of this research. This research was supported by the National Institutes of Health (K01RR023083-01, VAL), the American Quarter Horse Association (JKB), and the United States Equestrian Federation Equine Health Research Fund (VAL).
Abbreviations
- AIRg
acute insulin response to glucose
- AT
adipose tissue
- DI
disposition index
- FSIGT
frequently sampled intravenous glucose tolerance test
- GLUT
glucose transporter
- IR
insulin resistant
- IS
insulin sensitive
- Sg
glucose effectiveness
- SI
insulin sensitivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Accessed 1/8/11];American Diabetes Association: Prediabetes FAQS. 2010 Available at http://www.diabetes.org/diabetes-basics/prevention/pre-diabetes/pre-diabetes-faqs.html.
- 2.Ionut V, Liu H, Mooradian V, Castro AVB, Kabir M, Stefanovski D, Zheng D, Kirkman EL, Bergman RN. Novel canine models of obese pre-diabetes and of mild type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2010;298:E38–E48. doi: 10.1152/ajpendo.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd PR, Kahn BB. Glucose transporters and insulin action: Implications for insulin resistance and diabetes mellitus. New. Engl. J. Med. 1999;341:258–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Xu Y, Lu L, Bergman B, Leitner JW, 1Greyson C, Draznin B, Schwartz GG. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. Am. J. Physiol. Heart. Circ. Physiol. 2010;298:H310–319. doi: 10.1152/ajpheart.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J. Vet. Intern. Med. 2010;24:467–475. doi: 10.1111/j.1939-1676.2010.0503.x. [DOI] [PubMed] [Google Scholar]
- 6.Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutrition and Metabolism. 2004;1:1–24. doi: 10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: Potential impact of inherent, depot-specific mechanisms. Exp Geront. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLaren R, Cui W, Simard S, Cianflone K. Influence of obesity and insulin sensitivity on insulin signaling genes in human omental and subcutaneous adipose tissue. J. Lipid. Res. 2008;49:308–323. doi: 10.1194/jlr.M700199-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Bajzova M, Kovacikova M, Vitkova M, Climkakova E, Polak J, Kovacova Z, Viguerie N, Vedral T, Mikulasek L, Sramkova P, Srp A, Hejnova J, Langin D, Stich V. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiol. Res. 2008;57:927–934. doi: 10.33549/physiolres.931379. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Eto M, Makino I. Peripheral insulin resistance precedes the onset of hyperglycemia in spontaneously diabetic Chinese hamsters of Asahikawa colony. Diabetes. Res. Clin. Pract. 1993;20:101–109. doi: 10.1016/0168-8227(93)90003-n. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho E, Jansson PA, Axelsen M, Eriksson JW, Huang X, Groop L, Rondinone C, Sjostrom L, Smith U. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. 1999;13:2173–2178. doi: 10.1096/fasebj.13.15.2173. [DOI] [PubMed] [Google Scholar]
- 12.Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT 12. Am. J. Physiol. Endocrinol. Metab. 2002;282:E733–738. doi: 10.1152/ajpendo.2002.282.3.E733. [DOI] [PubMed] [Google Scholar]
- 13.Stuart CA, Howell MA, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J. Clin. Endocrinol. Metab. 2009;94:3535–3542. doi: 10.1210/jc.2009-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008;295:E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muno JD. MSc thesis. The Ohio State University; 2009. Prevalence, risk factors and seasonality of plasma insulin concentrations in normal horses in central Ohio. [Google Scholar]
- 16.Burns TA, Geor RJ, Mudge MC, McCutcheon LJ, Hinchcliff KW, Belknap JK. Proinflammatory cytokine and chemokine gene expression profiles in subcutaneous and visceral adipose tissue depots of insulin-resistant and insulin-sensitive light breed horses. J. Vet. Intern. Med. 2010;24:932–939. doi: 10.1111/j.1939-1676.2010.0551.x. [DOI] [PubMed] [Google Scholar]
- 17.Waller AP, Burns TA, Mudge MC, Belknap JK, Lacombe VA. Insulin resistance selectively alters cell surface glucose transporters, but not their protein expression, in equine skeletal muscle. J. Vet. Intern. Med. 2010 doi: 10.1111/j.1939-1676.2010.0674.x. in press. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman RM, Boston RC, Stefanovski D, Kronfeld DS, Harris PA. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J. Anim. Sci. 2003;81:2333–42. doi: 10.2527/2003.8192333x. [DOI] [PubMed] [Google Scholar]
- 19.Ryder JW, Yang J, Galuska D, Rincon J, Bjornholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:647–654. doi: 10.2337/diabetes.49.4.647. [DOI] [PubMed] [Google Scholar]
- 20.Koumanov F, Yang J, Jones AE, Hatanaka Y, Holman GD. Cell-surface biotinylation of GLUT4 using bis-mannose photolabels. Biochem. J. 1998;330:1209–1215. doi: 10.1042/bj3301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacombe VA, Hinchcliff KW, Devor ST. Effects of exercise and glucose administration on content of insulin-sensitive glucose-transporter in equine skeletal muscles. Am. J. Vet. Res. 2003;64:1500–1506. doi: 10.2460/ajvr.2003.64.1500. [DOI] [PubMed] [Google Scholar]
- 22.BLAST [Accessed 5/21/2010];BLAST protein sequence database. 2010 Available at: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
- 23.Lundgren M, Buren J, Ruge T, Myrnas T, Eriksson JW. Glucocorticoids down regulate glucose uptake capacity and insulin-signalling proteins in omental but not subcutaneous human adipocytes. J. Clin. Endocrinol. Metab. 2004;89:2989–2997. doi: 10.1210/jc.2003-031157. [DOI] [PubMed] [Google Scholar]
- 24.Laviola L, Perrini S, Cignarelli A, Natalicchi A, Leonardini A, De Stefano F, Cuscito M, De Fazio M, Memeo V, Neri V, Cignarelli M, Giorgino R, Giorgino F. Insulin signaling in human visceral and subcutaneous adipose tissue in vivo. Diabetes. 2006;55:952–961. doi: 10.2337/diabetes.55.04.06.db05-1414. [DOI] [PubMed] [Google Scholar]
- 25.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ralston SL, Nockels CF, Squires EL. Differences in diagnostic test results and hematologic data between aged and young horses. Am. J. Vet. Res. 1988;49:1387–1392. [PubMed] [Google Scholar]
- 27.Geor RJ, Harris PA. Dietary management of obesity and insulin resistance: countering risk for laminitis. Vet. Clin. Equine. 2009;25:51–65. doi: 10.1016/j.cveq.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Bergman RN, Ader M, Huecking K, Citters GV. Accurate assessment of β-cell function- the hyperbolic correction. Diabetes. 2002;51:S212. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 29.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Ruiz AG, Milagro FI, Campion J, Martinez JA, Miguel CD. Caveolin expression and activation in retroperitoneal and subcutaneous adipocytes: Influence of a high fat diet. J. Cell. Physiol. 2010;225:206–213. doi: 10.1002/jcp.22241. [DOI] [PubMed] [Google Scholar]
- 31.Kelly KR, Kashyap SR, O’Leary1 VB, Major J, Schauer PR, Kirwan JP. Retinol-binding protein 4 (RBP4) protein expression is increased in omental adipose tissue of severely obese patients. Obesity. 2010;18:663–666. doi: 10.1038/oby.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garvey WT, Maianu L, Zhu JH, Hancock JA, Golichowski AM. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes: heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes. 1993;42:1773–1785. doi: 10.2337/diab.42.12.1773. [DOI] [PubMed] [Google Scholar]
- 33.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2009;297:E495–504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho E, Eliasson B, Wesslau C, Smith U. Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/akt in adipocytes from Type II diabetic subjects. Diabetologia. 2000;43:1107–1115. doi: 10.1007/s001250051501. [DOI] [PubMed] [Google Scholar]
- 35.Maianu L, Keller SR, Garvey WT. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in Type 2 Diabetes Mellitus: Implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 2001;86:5450–5456. doi: 10.1210/jcem.86.11.8053. [DOI] [PubMed] [Google Scholar]
- 36.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn EL, Volpi E, Rasmussen BB. Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during postexercise recovery. J. Appl. Physiol. 2008;105:1967–1974. doi: 10.1152/japplphysiol.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson HKR, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- 38.Lessard SJ, Rivas DA, Chen Z, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB, III, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007;56:1856–1864. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]
- 39.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes. 2009;58:1096–1104. doi: 10.2337/db08-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai L, Wang Y, Fan J, Chen Y, W JI, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal. 2007;19:2209–2217. doi: 10.1016/j.cellsig.2007.06.003. [DOI] [PubMed] [Google Scholar]