Abstract
Background
Genetic and environmental factors are important in the etiology of substance use. However, little is known about the stability of these factors across development. We aimed to answer three crucial questions about this etiology that have never been addressed in a single study: (1) Is there a general vulnerability to substance consumption from early adolescence to young adulthood? (2) If so, do the genetic and environmental influences on this vulnerability change across development? (3) Do these developmental processes differ in males and females ?
Method
Subjects included 1480 twin pairs from the Swedish Twin Study of Child and Adolescent Development who have been followed since 1994. Prospective, self-reported regular smoking, alcohol intoxication and illicit drug use were assessed at ages 13–14, 16–17 and 19–20 years. Structural modeling was performed with the program Mx.
Results
An underlying common factor accounted for the association between smoking, alcohol and illicit drug consumption for the three age groups. Common genetic and shared environmental effects showed substantial continuity. In general, as participants aged, the influence of the shared environment decreased, and genetic effects became more substance specific in their effect.
Conclusions
The current report answers three important questions in the etiology of substance use. The genetic and environmental risk for substance consumption is partly mediated through a common factor and is partly substance specific. Developmentally, evidence was strongest for stability of common genetic effects, with less evidence for genetic innovation. These processes seem to be the same in males and females.
Keywords: Adolescence, development, substance use, twin study
Introduction
Research demonstrates a genetic component in the etiology of substance use and misuse in both sexes (McGue et al. 1992; van den Bree et al. 1998; Kendler et al. 1999, 2000; Agrawal & Lynskey, 2008). However, heritabilities tend to be greater in adult males compared to adult females for substance misuse (Sivkis et al. 1994; van den Bree et al. 1998; Li et al. 2003). Environmental variables such as peer group and family dysfunctions also play a role (Petraitis et al. 1995), particularly at younger ages. In contrast to adults, however, a sex difference in etiology has not been exhibited in adolescence (McGue et al. 2000; Rhee et al. 2003). The results of this research are intriguing and suggest that genetic factors are important in adulthood, shared environmental factors are important at younger ages, and sex differences in these factors are only found in adulthood. Longitudinal twin designs have supported this (Koopmans et al. 1997; Viken et al. 1999; White et al. 2003; Malone et al. 2004; Hicks et al. 2007; Kendler et al. 2008a,b,c) but no study has examined sex differences in this developmental process.
Additionally, recent work has begun to examine whether the genetic and environmental risk factors for substance use are substance specific. In general, the results indicate that risk for substance use is not substance specific but places individuals at risk to use a wide range of substances (Tsuang et al. 1998; Kendler et al. 2003; Young et al. 2006). Moreover, this general vulnerability impacts across substances classes (Han et al. 1999; Young et al. 2006; Kendler et al. 2007). Importantly, this general vulnerability has yet to be examined developmentally.
Overall, there is substantial evidence for both genetic and shared environmental influences on substance use. The importance of these factors changes throughout development, with increasing genetic and decreasing shared environmental effects. These factors also seem to be largely non-specific in nature. In this report we examine, for the first time to our knowledge, three important questions about risk for substance use: (1) Is there a general vulnerability from early adolescence to young adulthood? (2) If so, do the genetic and environmental influences on this vulnerability, and also any substance-specific risks, change across development? (3) Do these developmental processes differ in males and females? We hypothesized that a general vulnerability factor would impact on consumption of alcohol, tobacco and illicit substances across development, with genetic factors increasing and shared environmental factors decreasing in importance, and there would be no sex differences in these developmental processes.
Method
Sample and assessment
The present sample, the Swedish Twin Study of Child and Adolescent Development, began with all twin pairs born in Sweden between May 1985 and December 1986 (Lichtenstein et al. 2007). Twins were recruited through linkage to the Medical Birth Registry and twins and parents identified were sent study questionnaires (Lichtenstein & Svartengren, 1997). Participants had completed four assessment waves and information from waves 2–4 was used for the present study, when twins were aged 13–14, 16–17 and 19–20 years. Responses rates for each wave were 78, 82 and 59% respectively. The Ethics Committee of Karolinska Institutet, Stockholm, Sweden, approved the questionnaires used. In Sweden, responding to the questionnaire constitutes consent.
The current report included 246 female–female and 238 male–male monozygotic (MZ) twin pairs, 181 female–female and 169 male–male dizygotic (DZ) twin pairs, and 392 opposite-sex twin pairs. Zygosity was determined based on computer algorithms of questionnaire responses created from analyses of twin pairs participating in the clinical study with known zygosity (Lichtenstein et al. 2007).
Self-reported alcohol, tobacco and illicit drug consumption was assessed. Unfortunately, the same assessment items were not used at all time waves so categories were created to make questions comparable across waves. Categories were based on wave 2 questions, where specific frequency options were identified as ‘never’, ‘sometimes’ and ‘often ’. In all waves, twins were asked to consider the previous 12 months.
For tobacco consumption, three categories were created to reflect regular smoking: 0=not a regular smoker, 1=smoke sometimes/once in a while (i.e. only sometimes, only at parties, only on weekends), and 2=smoke often (i.e. almost daily, daily). The ‘not a regular smoker’ category includes those individuals who stated they had never smoked and also those who indicated that they had only ‘ tried it ’ or ‘quit ’. This was done because of the phrasing of the question at waves 3 and 4. Participants were asked: ‘Do you smoke?’ with two responses stating: ‘No, I’ve only tried it ’ or ‘No, I quit ’.
Three alcohol consumption categories were generated and reflect frequency of alcohol intoxication. Categories indicate whether the participant (0) had ever been intoxicated (never), (1) had been intoxicated sometimes (i.e. seldom, sometimes when drinking), or (2) had been intoxicated often (i.e. every time drinking, always). Never intoxicated includes non-drinkers and those participants who responded ‘No’ to ‘Have you ever drank so much that you got drunk?’ Categories reflect alcohol intoxication because general alcohol use was not assessed. A binary category was created for any illicit drug consumption reflecting use or no use because wave 2 participants were only asked if they had ever tried marijuana or illicit drugs (e.g. amphetamine, heroin, ecstasy or ‘something similar ’) and were not asked to identify the drugs.
Statistical analysis
To examine whether a general vulnerability exists for substance consumption at each assessment wave, common and independent pathway models were fit to the three substances within each wave, separately in males and females (excluding members of opposite-sex pairs). Both models assume that a common factor influences the observed variables but differ in the way the common factor(s) influence the variables. In the independent pathway model, the common genetic and environmental factors influence the observed variables directly, with separate genetic and environmental components estimated for each of the variable residuals.
The common pathway model asserts that there is a latent factor underlying the observed variables. The variance in this factor is partitioned into genetic and environmental components, and residual genetic and environmental components not encompassed by this factor are also estimated for each variable. This model uses fewer parameters and is more parsimonious than the independent pathway model. Therefore, if this model fits as well as the independent pathway model, it is preferred.
Longitudinal twin model
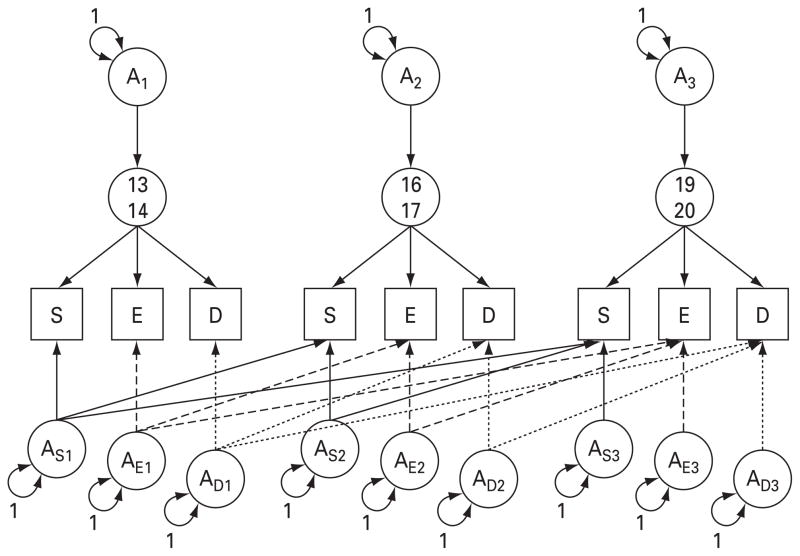
To examine whether the genetic and environmental influences on substance consumption are ‘developmentally stable ’ or ‘developmentally dynamic’, we used the model presented in Fig. 1, which illustrates additive genetic sources only. This model is based on a common pathway model and has three main features. First, the model contains a substance consumption factor for each wave that encompasses those influences that impact on all three substances. There are paths from each of the common factors to the specific substances at each time wave that indicate the degree to which the liability for each specific substance is reflected by the common factor.
Fig. 1.
Genetic components of the full model fitted to self-report measures of smoking (S), alcohol intoxication (E), and illicit drug consumption (D) at ages 13–14, 16–17 and 19–20 years. A1, A2 and A3, additive genetic effects for ages 13–14, 16–17 and 19–20 respectively; As1, Ae1 and Ad1, specific additive genetic effects for smoking, alcohol intoxication and illicit drug use respectively at age 13–14; As2, Ae2 and Ad2, specific additive genetic effects for smoking, alcohol intoxication and illicit drug use respectively at age 16–17; As3, Ae3 and Ad3, specific additive genetic effects for smoking, alcohol intoxication and illicit drug use respectively at age 19–20.
Second, these common factors are influenced by genetic and environmental parameters, which are parameterized as a trivariate Cholesky decomposition. Within this decomposition, the first genetic factor influences the common substance consumption factor at all three waves. The second genetic factor influences the common factor at waves 2 and 3 whereas the third genetic factor only influences the common factor at wave 3. If the genetic liability to the common factors is developmentally stable, we would expect all of the genetic liability to substance consumption to be captured in the first factor, with no evidence for innovation at the latter two. If liability is developmentally dynamic, we would expect to see innovation or new genetic variance in the second and third factors.
Third, this model includes residual genetic and environmental influences that are specific to each substance. These factors are also modeled as a Cholesky decomposition over time and include effects that are time and substance specific and also cross paths within substance cross-time. The magnitude of these paths is interpreted as described above.
Sex differences
The longitudinal model included all five zygosity groups, allowing for the examination of quantitative and qualitative sex differences. Quantitative effects examine whether the magnitude of genetic or environmental effects differs between the sexes (Kendler & Prescott, 2006). Qualitative effects examine whether the same genes are involved in the etiology of substance consumption in the sexes and are measured by the genetic correlation (rg). This correlation reflects the degree of resemblance between the genetic risk factors for males and females and can vary from zero (i.e. entirely distinct set of genes) to one (i.e. identical genetic factors). When examining for sex differences it is assumed there is only one correlation structure for both sexes, but males and females may have different loadings on these factors (Neale et al. 2006). However, the Choleksy decomposition allows for different correlation structures to be estimated for males and females. Thus, a constraint was added to constrain male and female correlation structures to equality (Carey, 2005; Neale et al. 2006).
Given the complexity of the model, the large number of possible simplifications, our moderate sample size and evidence that full models can best capture subtle genetic and environmental effects (Sullivan & Eaves, 2002), we tested only two models. The first assumed no quantitative or qualitative sex effects by constraining path coefficients across males and females and constraining rg to 1.0 at all waves. The second model included both quantitative and qualitative sex effects. The Bayesian information criterion (BIC), which performs well with complex models (Markon & Krueger, 2004), was used to determine the best-fit model. Analyses were conducted using an ordinal, raw data approach in the statistical package Mx (Neale, 1997), which allows data from both incomplete and complete twin pairs to be used. Thresholds were unconstrained across sex and allowed to be freely estimated.
Results
Descriptive statistics
Substance consumption frequency increased across waves. Examining the ‘have consumed’ categories, very few participants endorsed smoking often (2%), becoming intoxicated often (1.1%) or having used an illicit drug (1.1%) at age 13–14. At age 16–17, more participants reported alcohol intoxication sometimes (35.1%) and often (25%) compared to smoking sometimes (14%) or often (8%). Five percent of the sample reported illicit drug use at this age. Finally, at age 19–20, 15% of participants reported smoking sometimes, 12% reported smoking often, 47% reported intoxication sometimes, and 41% reported drinking to intoxication often. Illicit drug use rates more than doubled at this age to 13.3%.
Before beginning the twin analyses we examined whether the categories created for smoking and alcohol consumption were within the same continuum of liability. This was done by estimating the tetrachoric correlations (and thresholds) separately for each of the zygosity groups for these variables. χ2 was not significant for smoking or alcohol consumption at wave 2 (χ2=8.4, p=0.21; χ2=7.0, p=0.32) or wave 4 (χ2=6.4, p=0.37; χ2=6.0, p=0.42), suggesting that these variables satisfy assumptions of multivariate normality. However, χ2 was significant for both substances at wave 3 (χ2=14.6, p<0.05; χ2=19.5, p<0.05). Analyses were repeated separating twins by age (>16.5 or ≤16.5 years) and χ2 was no longer significant. This suggests that wave 3 variables are approximately multivariate normal and the differences detected in our initial analysis are due to age.
Twin analyses
The best-fitting model for all waves for both sexes was the common pathway model, so this model was used in follow-up longitudinal analyses (Table 1). For the longitudinal models, the best BIC value was obtained for the model assuming no quantitative or qualitative sex effects (Table 2).
Table 1.
BIC scores for within-wave common and independent pathway models
| Wave 2 |
Wave 3 |
Wave 4 |
||||
|---|---|---|---|---|---|---|
| −2LL | BIC | −2LL | BIC | −2LL | BIC | |
| Common pathway model | 1379.44 (1319.43) | −5862.50 (−5547.20) | 2516.20 (2132.45) | −5751.76 (−5314.53) | 2431.25 (1583.42) | −4450.53 (−2710.90) |
| Independent pathway model | 1375.00 (1318.85) | −5853.00 (−5843.20) | 2415.50 (2131.80) | −5743.54 (−5305.55) | 2425.82 (1580.58) | −4437.47 (−2699.77) |
BIC, Bayesian Information Criterion; LL, log likelihood.
Male model fits shown in parentheses.
Table 2.
BIC scores for qualitative and quantitative sex-effects models
| Model |
rg |
BIC score | ||
|---|---|---|---|---|
| Age 13–14 | Age 16–17 | Age 19–20 | ||
| Gender invariant | +1.00 | +1.00 | +1.00 | −55 710.0a |
| Gender variant | +1.00 | +0.92 | +0.54 | −55 040.1 |
BIC, Bayesian Information Criterion; rg, genetic correlation, which assesses the degree of qualitative sex effects.
Best-fit model by BIC criterion.
Longitudinal twin analysis
Common genetic and environmental effects
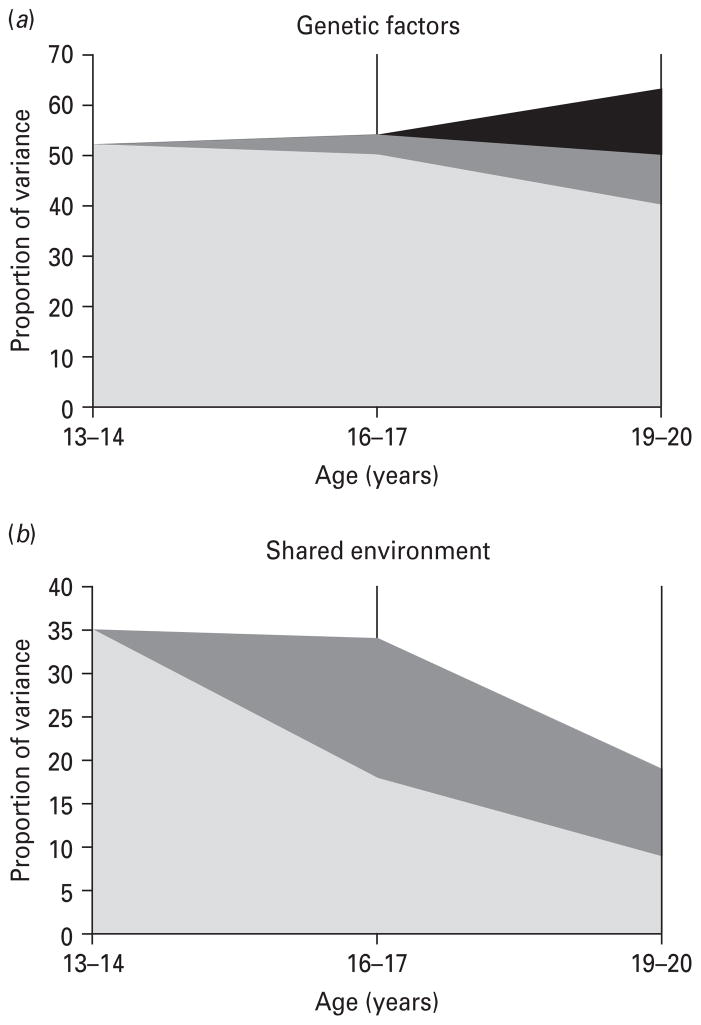
Examining the ‘upper portion’ of the model, six results are noteworthy (Table 3). First, illicit drug use becomes a better representative of the latent factor with increasing age whereas smoking and alcohol intoxication become less representative. Second, heritabilities for the common factors remained stable across development, estimated at 52% for age 13–14, 54% for age 16–17, and 53% for age 19–20. Third, these genetic effects demonstrated evidence for stable and dynamic risk. As illustrated in Fig. 2a, genetic factors at age 13–14 (shown in gray) accounted for a majority of the genetic effects at ages 16–17 and 19–20. However, innovation was also exhibited. Of the total genetic influences at age 16–17, 7% (4% out of a total of 54%) are new genetic factors (shown in dark gray), and at wave 4, 25% (13% out of a total of 53%) of the genetic influences are new factors specific to this age (shown in black).
Table 3.
Path estimates for the best-fit model of substance consumptiona
| Factor (age) | Factor loadings |
Genetic factors |
Shared environmental factors |
Unique environmental factors |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SM | ETOH | DU | Total | Total | Total | ||||||||||
| a2, % | A1 | A2 | A3 | c2, % | C1 | C2 | C3 | e2, % | E1 | E2 | E3 | ||||
| 1 (13–14) | 0.94 | 0.82 | 0.62 | 52 | 0.72 | 35 | 0.59 | 13 | 0.36 | ||||||
| 2 (16–17) | 0.87 | 0.76 | 0.85 | 54 | 0.70 | 0.21 | 34 | 0.43 | 0.40 | 12 | −0.03 | 0.35 | |||
| 3 (19–20) | 0.72 | 0.53 | 0.82 | 53 | 0.63 | 0.32 | 0.36 | 19 | 0.30 | 0.31 | −0.07 | 18 | 0.05 | 0.13 | 0.40 |
Factor loading, depicted in Fig. 1, connects the common factor to self-reported substance use; a2, heritability or proportion of variance in substance use resulting from genetic factors; c2, proportion of variance in substance use resulting from shared environmental factors; e2, proportion of variance in substance use resulting from unique environmental factors; A1, A2 and A3, additive genetic path estimates at ages 13–14, 16–17 and 19–20 respectively; C1, C2 and C3, shared environment path estimates at ages 13–14, 16–17 and 19–20 respectively; E1, E2 and E3, unique environment path estimates at ages 13–14, 16–17 and 19–20 respectively; SM, regular smoking; ETOH, alcohol intoxication; DU, illicit drug use.
Results for ‘ upper portion ’ of the model only (Fig. 1).
Fig. 2.
Proportion of total variance in substance consumption common factors accounted for by (a) genetic and (b) shared environmental effects at ages 13–14 ( , Factor 1), 16–17 (
, Factor 1), 16–17 ( , Factor 2) and 19–20 (■, Factor 3). The y axis represents the total phenotypic variance so the sum of all the factors equals total heritability.
, Factor 2) and 19–20 (■, Factor 3). The y axis represents the total phenotypic variance so the sum of all the factors equals total heritability.
Fourth, shared environmental effects on the latent factors were more important at younger ages, and new effects almost disappeared in young adulthood. Fifth, these effects demonstrated evidence of stability and developmental attenuation. As illustrated in Fig. 2b, shared environmental factors at age 13–14 (shown in gray) accounted for 53% (18% out of a total of 34%) of the variance at age 16–17 and 47% (9% out of a total of 19%) at age 19–20. A small amount of new effects were introduced at age 16–17 (shown in dark gray). Finally, total common unique environmental effects also remained similar across development, being estimated at 13, 12 and 18% respectively, and showed almost no continuity.
Substance-specific effects
Several noteworthy substance-specific results were obtained, as shown in Table 4. For the reader’s reference, genetic and environmental effects for each substance were calculated as follows using smoking at age 16–17 as an example: total common genetic effects were determined by multiplying the factor loading for this age (0.70) by the common genetic estimates for this age (0.87 and 0.21), provided in Table 3, squaring these results, and then adding them [(0.70× 0.87)2+(0.70×0.21)2]. Residual smoking-specific genetic and environmental effects were determined by squaring the residual estimates provided in Table 4. Smoking-specific genetic effects at age 16–17 were calculated as [(−0.012)+(0.332)]. Adding the results of these two calculations provided the total genetic/environmental effects for the substance.
Table 4.
Path estimates for residual genetic and environmental effectsa and total genetic and environmental influences on smoking, alcohol intoxication and illicit drug useb
| Age (years) | Genetic factors |
Shared environmental factors |
Unique environmental factors |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | Ac | As | At | C1 | C2 | C3 | Cc | Cs | Ct | E1 | E2 | E3 | Ec | Es | Et | ||
| Smoking | 13–14 | 0.31 | 46 | 10 | 56 | 0.13 | 31 | 2 | 33 | −0.02 | 11 | 0 | 11 | ||||||
| 16–17 | −0.01 | 0.33 | 40 | 11 | 51 | 0.20 | −0.01 | 26 | 4 | 30 | 0.28 | 0.12 | 9 | 9 | 18 | ||||
| 19–20 | 0.21 | 0.47 | 0.12 | 33 | 27 | 60 | 0.12 | 0.15 | 0.04 | 10 | 3 | 13 | 0.10 | 0.33 | 0.25 | 9 | 18 | 27 | |
| Alcohol intoxication | 13–14 | 0.44 | 35 | 20 | 55 | 0.18 | 23 | 3 | 26 | 0.32 | 9 | 10 | 19 | ||||||
| 16–17 | −0.01 | 0.36 | 31 | 13 | 44 | 0.33 | 0.26 | 20 | 18 | 38 | −0.05 | 0.32 | 7 | 10 | 17 | ||||
| 19–20 | −0.10 | 0.22 | 0.58 | 18 | 40 | 58 | 0.06 | 0.16 | −0.01 | 6 | 3 | 9 | 0.06 | 0.15 | 0.52 | 4 | 30 | 34 | |
| Illicit drug use | 13–14 | 0.70 | 20 | 50 | 70 | −0.04 | 13 | 0 | 13 | 0.68 | 5 | 13 | 18 | ||||||
| 16–17 | 0 | 0.50 | 38 | 25 | 63 | 0.01 | 0.11 | 25 | 1 | 26 | 0.11 | 0.32 | 9 | 2 | 11 | ||||
| 19–20 | 0.02 | 0.37 | 0.41 | 43 | 31 | 74 | −0.01 | 0.01 | 0.11 | 12 | 1 | 13 | −0.05 | 0.03 | 0.05 | 12 | 1 | 13 | |
A1, A2 and A3, additive genetic path estimates at ages 13–14, 16–17 and 19–20 respectively; Ac, percentage of genetic effects due to common factors; As, percentage of genetic effects due to specific factors; At, total genetic effects; C1, C2 and C3, shared environment path estimates at ages 13–14, 16–17 and 19–20 respectively; Cc, percentage of shared environmental effects due to common factors; Cs, percentage of shared environmental effects due to specific factors; Ct, total shared environmental effects; E1, E2 and E3, unique environment path estimates at ages 13–14, 16–17 and 19–20 respectively; Ec, percentage of unique environmental effects due to common factors; Es, percentage of unique environmental effects due to specific factors; Et, total unique environmental effects. Totals may not equal 100% due to rounding error.
Results for ‘ lower portion ’ of the model only (Fig. 1).
Total genetic and environmental influences on smoking, alcohol intoxication, and illicit drug use at waves 2–4, including common and substance-specific/residual influences.
Smoking
There was a minimal increase in total genetic estimates for smoking from age 13–14 to age 19–20 (56% v. 60%). However, these effects decreased from age 13–14 to age 16–17. By contrast, examining the common genetic effects and smoking-specific genetic effects reveals a consistent trend, with common genetic effects decreasing and smoking-specific genetic effects increasing across development. Additionally, of all the substances, the cross-time continuity of the residual genetic effects was highest for smoking, with almost no new specific genetic effects estimated at age 19–20.
Total shared environmental estimates decreased consistently across the age groups. There was also a decrease in common shared environmental effects. Of note, smoking-specific shared environmental effects were minimal yet consistent across the ages. The specific shared environmental effects at age 13–14 accounted for a small amount of the variance of specific effects at age 16–17.
Total unique environmental effects revealed a consistent trend to increase in importance with age. Smoking-specific unique environmental effects also increased in importance whereas common unique environmental effects remained fairly consistent across development. Of note, at age 13–14 all of the unique environmental variance was accounted for by the common factor. A small amount of cross-time continuity for specific unique environmental effects was shown between ages 13–14 and 16–17 and ages 16–17 and 19–20.
Alcohol intoxication
Total genetic effects for alcohol intoxication did not reveal a clear trend of increasing or decreasing across development. Total genetic effects decreased from age 13–14 to age 16–17 (55% v. 44%). However, these effects increased to 58% at age 19–20. Similar to smoking, however, genetic effects became more specific across development, with common genetic estimates decreasing and alcohol intoxication-specific genetic estimates increasing. Finally, examining the cross-time continuity of the alcohol-specific genetic effects indicated that alcohol intoxication had the lowest continuity, with 87% of the specific genetic effects at 19–20 being new.
Total shared environmental effects decreased in importance. This same trend was shown for common shared environmental effects as these effects became almost non-existent by age 19–20. However, the trend was less consistent for alcohol intoxication-specific shared environmental effects. At ages 13–14 and 19–20 specific estimates were equal (3%) whereas there was a spike in these effects at age 16–17 (18%). The cross-time continuity of the residual shared environmental estimates was minimal.
Total and alcohol intoxication-specific unique environmental effects increased consistently across development. In addition, there was no cross-time continuity of the specific effects. Common unique environmental effects were minimal and decreased slightly across age.
Illicit drugs
Total genetic effects for illicit drug consumption decreased slightly from age 13–14 to age 16–17 (70% v. 63%) yet increased at age 19–20 (74%). In contrast to smoking and alcohol intoxication, common genetic effects increased consistently across the age groups whereas specific effects decreased by almost half from age 13–14 (50%) to ages 16–17 (25%) and 19–20 (31%).
The picture for environmental effects was slightly different for illicit drugs. Total shared environmental effects were the same at ages 13–14 and 19–20 (13%). However, these effects doubled (26%) at age 16–17. Additionally, a majority of the shared environmental effects were accounted for by the common factors. Total unique environmental estimates decreased minimally across the ages whereas common estimates increased. Specific unique environmental estimates dropped sharply from age 13–14 (13%) to age 16–17 (2%) and 19–20 (1%) and no cross-time continuity was revealed.
Discussion
As hypothesized, the results show a general vulnerability to substance consumption from early adolescence to young adulthood. However, the representativeness of the factors changes across development. This could indicate that the common genetic factors reflect a more severe liability to substance use, indexed by smoking and alcohol consumption at younger ages, due to the low prevalence of illicit drug use at younger ages.
The results also indicate that the genetic and environmental effects on this general vulnerability are developmentally dynamic and stable, although evidence is stronger for stability. Common genetic effects at age 13–14 accounted for a majority of the common genetic effects at ages 16–17 and 19–20. In contrast to our hypothesis, we did not see common genetic effects increase with age. However, there was a general trend for substance-specific genetic effects to increase with age. This is perhaps our most intriguing finding. That is, with increasing age, genetic influences on substance consumption become more specific in their effect.
Common shared environmental effects also showed substantial continuity and, as hypothesized, decreased in importance across development, on both the common factor and substance-specific residuals. There was, however, a small peak of new effects during adolescence. In addition, for alcohol and illicit drug consumption, shared environmental effects were greatest at this age. This is in contrast to previous studies, which have shown a smooth decline (Koopmans & Boomsma, 1996; Koopmans et al. 1997; Viken et al. 1999). These new effects may be due to a life transition in the lives of participants during this age. For example, at the age of 16, Swedish adolescents move to ‘upper secondary school’, which is the equivalent of high school in the USA. During this time, students choose the type of studies they will pursue (e.g. vocational versus university preparation).
There was almost no continuity in common unique environmental effects. This is not surprising, given these effects are confounded with measurement error, which would have a time-specific impact. The impact of unique environment might also be expected to increase with age as twins spend more time outside of the family, yet the results revealed only a slight increase. This may be reflected in the increase in substance-specific genetic effects as choice of non-familial environments might be genetically influenced (Viken et al. 1999). However, for smoking and alcohol intoxication, specific unique environmental effects did increase with age.
We were also interested in whether the substance-specific genetic and environmental influences were developmentally dynamic or stable. In general, the results suggest that these influences are almost entirely dynamic in nature as genetic and environmental effects at age 13–14 attenuated over time. Additionally, confirming our final hypothesis, we found no evidence for sex differences.
Comparison to prior studies
To our knowledge, no single previous report has examined the same three questions investigated here. However, we are able to make comparisons to previous studies. First, our report confirms a general vulnerability to substance use (Swan et al. 1996; Tsuang et al. 1998; Hettema et al. 1999; Kendler et al. 2003, 2007). A previous examination of the covariation between tobacco, alcohol and other substance use at ages 17–18 also showed that a common factor was responsible for covariance (Han et al. 1999). However, Han et al. (1999) reported the heritability of the substance use factor at 23% whereas shared environment contributed 62%. Our estimates at age 16–17 were 54% and 34% respectively. The reasons for this discrepancy are difficult to determine but may be due to sample characteristics, length of assessment period or differing definitions of substance use.
Comparing results to the developmental processes of specific substances also shows similarities. For example, genetic factors increase whereas shared environmental factors decrease with age for a variety of substances (Koopmans & Boomsma, 1996; Koopmans et al. 1997; Pagan et al. 2006). Our modeling confirms this by showing, in general, a decrease in both common and residual shared environmental effects and an increase in substance-specific genetic effects across age.
No previous report has examined qualitative sex differences in adolescence. However, several reports show no evidence for quantitative sex differences for tobacco initiation, use, problem use and dependence, illicit drug use or abuse, and alcohol initiation, frequency of use, intoxication, problem use and frequency of intoxication during adolescence (Han et al. 1999; Viken et al. 1999; McGue et al. 2000; Rhee et al. 2003; Poelen et al. 2008). Additionally, two studies examined quantitative effects on a ‘ general vulnerability ’ to substance use in adolescence. One report showed no significant sex differences (Han et al. 1999) whereas the second reported increasing heritability estimates for males and increasing environmental variance for females on an ‘externalizing factor ’ (including nicotine, alcohol and illicit drug dependence) from age 17 to 24 (Hicks et al. 2007).
There are several limitations to this report that warrant discussion. First is the sample size and associated modest statistical power, especially for the detection of sex effects (Prescott & Gottesman, 1993). It is possible that we did not have the power to detect these differences. Second are the limitations with the substance use measure. Most notably is that the questions asked were not consistent across waves so we were forced to create comparable categories. A self-report questionnaire was also used that was mailed to the participant’s home. However, participants may be more likely to reveal private information in this manner.
Third, the sample comes from a single birth cohort in Sweden, and substance use rates may differ between the USA and Sweden. Sweden, along with most European countries, has more liberal laws and attitudes with regard to alcohol use, which are thought to foster more responsible drinking in young people. However, European youth in fact drink alcohol and become intoxicated more often than American youth (U.S. Department of Justice, 2005).
Within our own sample, rates of alcohol intoxication (58% of the total sample having been intoxicated) for 16- to 17-year-olds were greater than the annual prevalence of 10th graders in the USA (30%) (Monitoring the Future, 2008). Additionally, the 16- to 17-year-olds had a dramatically lower prevalence of illicit drug use (4.4% of the total sample) compared to the 12-month prevalence of 10th graders in the USA (27%) (Monitoring the Future, 2008). However, 10th-grader lifetime rates (12-month prevalence not assessed) of smoking (31.7%) were similar to the 12-month prevalence (22%) for our 16- to 17-year-olds (Monitoring the Future, 2008). These differences could impact the generalizability of results outside of a Swedish/European population. Importantly, our substance use rates appear in line with rates of use in Sweden. The European School Survey Project on Alcohol and Other Drugs (ESPAD, 2003) reported that 81% of Swedish 17- to 18-year-olds had been intoxicated, 8% had used marijuana, and 2% had used other illicit drugs in the previous 12 months.
Conclusions
This report has important implications for psychiatric genetics. Although the results showed continuity, genetic and environmental changes were also revealed. Psychiatric genetics often assumes a static genome, and the findings of the current study suggest that, to a small extent, this assumption is incorrect. Therefore, gene identification studies need to take into consideration the age of the sample, and that genes (and environments) found to be important within one age group may not be important in another. Another important implication is the increasing specificity of genetic effects. It is unclear what neurobiological or psychological processes are responsible for the increasing specialization of genetic effects.
Acknowledgments
This research was supported by T32-MH-020030 (J.H.B. and H.H.M.). A portion of the analyses conducted was supported by T32-DA-007027 (J.H.B.). This research was also supported by funds from the Swedish Council for Working Life and Social Research (P.L.), the Swedish Research Council (P.L.) and the Virginia Tobacco Settlement Foundation (H.H.M. and K.S.K.), and grants R01-DA018673 (H.H.M.), R01-DA022989 (H.H.M.), U01-DA024413 (H.H.M.), AA-011408 (K.S.K.) and DA-011287 (K.S.K.).
Footnotes
Portions of this paper were presented at the Behavior Genetics Association Conference, June 2008, Louisville, KY, USA.
Declaration of Interest
None.
References
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Carey G. Cholesky problems. Behavior Genetics. 2005;35:653–665. doi: 10.1007/s10519-005-5355-9. [DOI] [PubMed] [Google Scholar]
- ESPAD. Alcohol and drug use among European 17–18 year old students. [Accessed 3 April 2010];European School Survey Project on Alcohol and Other Drugs. 2003 ( www.espad.org/espadreports)
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol, and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug and Alcohol Dependence. 1999;57:69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Lichtenstein P. The development of fears from early adolescence to young adulthood: a multivariate study. Psychological Medicine. 2008a;38:1759–1769. doi: 10.1017/S0033291708002936. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A longitudinal twin study of fears from middle childhood to early adulthood: evidence for a developmentally dynamic genome. Archives of General Psychiatry. 2008b;65:421–429. doi: 10.1001/archpsyc.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski L, Prescott CA. Hallucinogen, opiate, sedative and stimulant use and abuse in a population-based sample of female twins. Acta Psychiatrica Scandinavica. 1999;99:368–376. doi: 10.1111/j.1600-0447.1999.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008c;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI. Familial resemblances in alcohol use: genetic or cultural transmission ? Journal of Studies on Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Doornen LJP, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcoholism: Clinical and Experimental Research. 1997;21:537–546. [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Svartengren M. Genes, environments, and sex: factors of importance in atopic diseases in 7–9-year-old Swedish twins. Allergy. 1997;52:1079–1086. doi: 10.1111/j.1398-9995.1997.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-study. Twin Research and Human Genetics. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Malone SM, Taylor J, Marmorstein NR, McGue M, Iacono WG. Genetic and environmental influences on antisocial behavior and alcohol dependence from adolescence to early adulthood. Development and Psychopathology. 2004;16:943–966. doi: 10.1017/s0954579404040088. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behavior Genetics. 2004;34:593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. Journal of Abnormal Psychology. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Monitoring the Future. [Accessed 3 April 2010];2008 data from in-school surveys of 8th-, 10th-, and 12th-grade students. 2008 ( www.monitoringthefuture.org/data/08data.html#2008data-drugs)
- Neale MC. Mx: Statistical Modeling. Department of Psychiatry, Medical College of Virginia; Richmond, VA: 1997. [Google Scholar]
- Neale MC, Roysamb E, Jacobson KC. Multivariate genetic analysis of sex limitation and G×E interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behavior Genetics. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraitis J, Flay BR, Miller TQ. Reviewing theories of adolescent substance use: organizing pieces in the puzzle. Psychological Bulletin. 1995;117:67–86. doi: 10.1037/0033-2909.117.1.67. [DOI] [PubMed] [Google Scholar]
- Poelen EAP, Derks EM, Engels RCME, van Leeuwe JFJ, Scholte RHJ, Willemsen G, Boomsma DI. The relative contribution of genes and environment to alcohol use in early adolescents: are similar factors related to initiation of alcohol use and frequency of drinking ? Alcoholism: Clinical and Experimental Research. 2008;32:975–982. doi: 10.1111/j.1530-0277.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Gottesman I. Power limitations in detecting heterogeneity of genetic effects: the case of sex-differences in alcoholism. Presented at the annual meeting of the Society for Research on Psychopathology; Chicago, IL. October 1993.1993. [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behavior Genetics. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Velez ML, Pickens RW. Genetic aspects of alcohol use and alcoholism in women. Alcohol Health and Research World. 1994;18:192–196. [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. Journal of Substance Abuse. 1996;8:19–31. doi: 10.1016/s0899-3289(96)90055-3. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Justice. Youth Drinking Rates and Problems: A Comparison of European Countries and the United States. U.S. Department of Justice, Office of Justice Programs. Office of Juvenile Justice and Delinquency Prevention; 2005. [Accessed 3 April 2010]. ( www.udetc.org/documents/CompareDrinkRate.pdf) [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- White VM, Hopper JL, Wearing AJ, Hill DJ. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98:1087–1100. doi: 10.1046/j.1360-0443.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific ? Behavior Genetics. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]