Abstract
Helicobacter pylori resists gastric acidity by modulating the proton-gated urea channel UreI, allowing for pHout-dependent regulation of urea access to intrabacterial urease. We employed pH- and Ca2+-sensitive fluorescent dyes and confocal microscopy to determine the location, rate, and magnitude of pH changes in an H. pylori-AGS cell coculture model, comparing wild-type bacteria with nonpolar ureI-deletion strains (ureI-ve). Addition of urea at pH 5.5 to the coculture resulted first in elevation of bacterial periplasmic pH, followed by an increase of medium pH and then pH in AGS cells. No change in periplasmic pH occurred in ureI-deletion mutants, which also induced a slower increase in the pH of the medium. Pretreatment of the mutant bacteria with the detergent C12E8 before adding urea resulted in rapid elevation of bacterial cytoplasmic pH and medium pH. UreI-dependent NH3 generation by intrabacterial urease buffers the bacterial periplasm, enabling acid resistance at the low urea concentrations found in gastric juice. Perfusion of AGS cells with urea-containing medium from coculture at pH 5.5 did not elevate pHin or [Ca2+]in, unless the conditioned medium was first neutralized to elevate the NH3/NH4+ ratio. Therefore, cellular effects of intrabacterial ammonia generation under acidic conditions are indirect and not through a type IV secretory complex. The pHin and [Ca2+]in elevation that causes the NH3/NH4+ ratio to increase after neutralization of infected gastric juice may contribute to the gastritis seen with H. pylori infection.
Introduction
The neutralophile Helicobacter pylori is a pathogen that resists gastric acidity and colonizes the human stomach (1). No other organism appears to have both capabilities. Urease negative mutants are unable to colonize a variety of animal models (2–4), implicating the large quantities of urease made constitutively by H. pylori in acid resistance of the organism. There are seven genes in the urease gene cluster (5). ureA and ureB encode the structural subunits of urease, and ureE,F,G, and H encode accessory proteins necessary for assembly and Ni2+ insertion to form active urease (6). Knockout of any one of these four accessory genes prevents or retards synthesis of active urease.
The urease produced by H. pylori has a neutral pH optimum and is irreversibly inactivated at pH lower than 4.5 (7, 8). The majority of this urease is intrabacterial, with a minor quantity adhering to the surface after 24–48 hours of culture (9). The pH profile of urease in intact bacteria, in contrast to that of free or surface urease, shows that there is little activity at neutral pH. However, with increasing acidity, urease activity increases between 10- and 20-fold as the pH falls from 6.0 to 5.0, and thereafter remains steady down to pH 2.5 (10, 11). These properties of the surface and intrabacterial urease argue for a greater role of intrabacterial than of surface urease in gastric acid resistance (11).
Acid activation of intrabacterial urease is due to expression of the third gene in the urease gene cluster, ureI, that encodes a H+ gated urea channel, allowing an increase of urea permeability of the bacterial membrane by at least 300-fold as medium pH becomes acidic (12). The presence of this acid-activated urea channel in the inner membrane of the organism is necessary for efficient utilization of the urea present in gastric juice. These data explain the requirement for both urease and UreI for survival at a medium pH of less than 4.0 and for infection of animal models (13, 14).
H. pylori maintains a constant inner membrane potential of –101 mV in the presence of 1–5 mM urea in strong buffer between pH 3.0 and 5.0 (11). At this potential, assuming a constant proton motive force of approximately –220 mV and an internal pH of approximately 8.0, the pH of the periplasm is maintained at a pH of approximately 6.0 by internal urease activity (7). This is within the range of the optimal pH for growth of the organism in vitro in the absence of urea (15). NH3 efflux across the bilayer, and perhaps also through UreI, is predicted to elevate the periplasm to a pH of approximately 6.0 to account for the elevation of membrane potential (7). Buffering of the periplasm needs much less urease activity than does buffering of the external medium immediately surrounding the organism.
All infective H. pylori strains have high levels of urease and most likely UreI, as do other gastric Helicobacter species, such as Helicobacter felis, mustelae, or nemestrinae (11). All patients have gastritis, although only 20% develop symptoms and disease. All infecting organisms, although varying widely in their expression of genes in the pathogenicity island, will generate large quantities of NH3 that converts to NH4+ in gastric acid. Alkalinization of the cytoplasm of many eukaryotic cells results in elevation of intracellular calcium (16) and release of cytokines such as IL-1β (17). H. pylori infection increases cytokine production in stomach and infected cocultures (18, 19). High concentrations of NH3 in the cytoplasm of the organisms could directly alkalinize the cytoplasm of cells to which H. pylori adhere by direct entry from the organism into the cells via the type IV secretory system possessed by the organism (20–24). If NH3 has to leave the organism under the acidic conditions at which it is produced before entry into cells to which it adheres, it is present mainly as impermeant NH4+ in the gastric juice. However, upon entry into the duodenum or at the base of antral glands that absorb, rather than secrete, H+, or during periods of gastric neutrality as occur during the digestive phase, the impermeant NH4+ converts to the permeant NH3. This molecule is able to rapidly enter gastric epithelial cells and the G cell or D cell of pyloric glands with consequent intracellular alkalinization. These latter cells respond to elevation of pHout by increased release of gastrin (G cell) or inhibition of somatostatin release (D cell) (25, 26), effects that would result in increased acid secretion in response to a meal. As a group, duodenal ulcer patients with antral gastritis have a higher rate of acid secretion than do uninfected subjects, thought to be due to the increased gastrin or decreased somatostatin release resulting from H. pylori infection (27, 28).
The work reported here used a coculture system with gastric-derived AGS cells and H. pylori. In this coculture, the organisms adhere to the AGS cells (29). Both confocal and video microscopy were used to define the site(s) at which intrabacterial urease has a buffering action. Microscopy also allowed visualization of the route of NH3 access to the AGS cells contacted by the bacteria. The data show that acidic pHout activation of intrabacterial urease elevates periplasmic and then medium pH. This does not happen or is much slower in the absence of UreI, and all medium pH changes are completely inhibited by 10 μM flurofamide, a urease suicide inhibitor. The generation of NH3 at pH 5.5 had no effect on the intracellular pH of AGS cells to which the bacteria adhered until medium pH increased. In separate experiments, when AGS cells were perfused with medium that had been conditioned by perfusion of the coculture with urea at pH 5.5 (but not at 7.4) and then was titrated to pH 7.4, sufficient NH3 was then present to alkalinize AGS cells, which resulted in an elevation of [Ca2+]in.
Methods
Culture of H. pylori.
The H. pylori strain ATCC 43504 and an ATCC 43504 ureI negative mutant were used in this study. ATCC 43504 was grown on blood agar plates (BBL TSA 5% sheep blood; Becton Dickinson Microbiology Systems, Cockeysville, Maryland,USA) in a microaerophilic atmosphere (5%02, 10%CO2, 85%N2) at 37°C for 24 hours. The ureI- mutant was grown on BHI plates in this atmosphere (Difco Laboratories, Detroit, Michigan, USA) supplemented with 5% FCS and 0.25% yeast extract containing 1% kanamycin. Cells from one plate were harvested into 1 mL of the growth medium used for AGS coculture, and the OD at 600 nm was measured. Aliquots were taken for the different experiments.
Culture of AGS cells.
AGS cells were cultured on round cover slips (No. 1; Fisher Scientific Co., Pittsburgh, Pennsylvania, USA) in a six-well plate to confluence. DMEM/F12 (GIBCO BRL, Rockville, Maryland,USA) was used as the culture medium, containing 10 mg/mL 1% gentamycin (GIBCO BRL), 1% L-glutamine-penicillin-streptomycin solution (Sigma Chemical Co., St. Louis, Missouri, USA), and 10% FCS.
Coculture of AGS cells and bacteria.
AGS cells were washed with the growth media lacking antibiotics. Cover slips were transferred into a new six-well plate with each well containing 2 mL of the same medium. H. pylori was added at an OD600 of 0.4 and cocultured for 1 hour in 95% 02 and 5% C02. The cover slip was removed and mounted in an Attofluor chamber (Molecular Probes Inc., Eugene, Oregon, USA) and washed twice with Hp-medium (see later here). The chamber was placed in a heated stage (Medical Systems Inc., Greenvale, New York, USA) at 37°C and attached to a peristaltic pump for superfusion. Chamber fluid was exchanged at a rate of three times per minute during perfusion. The chambers were viewed either in a Zeiss LSM410 or 510 confocal microscope or imaged in a Zeiss Axiovert TV100 microscope (Carl Zeiss Inc., Thornwood, New York,USA).
Generation of ureI-negative mutants.
Mutants were made according to a published procedure that is only presented in outline here (30). Standard procedures (31) were used for isolation and treatment of plasmid DNA. Escherichia coli DH5α was transformed by electroporation (Gene Pulser II; Bio-Rad Laboratories Inc., Hercules, California, USA). Transformation was carried out according to a standard operation protocol using a 2.5 kV pulse. The electroporated bacteria were plated onto LB plates (LB agar base; GIBCO BRL) supplemented with 50 mg/mL ampicillin. The DNA was generated by PCR of selected primers as detailed previously (30).
For full-length deletion of the ureI gene in the chromosome of H. pylori, a plasmid (pUCureBkanRureE) was designed, carrying a kanR open reading frame with upstream (ureB) and downstream (ureE) sequences from ureI to allow nonpolar replacement of this gene. The template used for PCR amplification of the ureB/E sequences was plasmid pHP808, provided by H. Mobley (Department of Microbiology and Immunology, University of Maryland School of Medicine, Baltimore, Maryland, USA). The DNA template for synthesis of the open reading frame of the kanamycin resistance gene (kanR) was pUC4K (Amersham Pharmacia Biotech, Piscataway,New Jersey, USA). Synthetic DNA oligonucleotides carrying a Shine Dalgarno sequence were used for PCR amplification of the 5′ regions of ureE as well as the 3′ regions of ureB. The resulting plasmid containing the hybrid DNA fragment, (ureB)kanR(ureE), was transformed into E. coli DH5α. Transformants were screened by growth in LB medium (LB broth base; GIBCO BRL) supplemented with 50 mg/mL ampicillin. The presence and orientation of the DNA inserts in the vector plasmids were confirmed by restriction analysis.
Growth and selection of ureI-deletion mutants of H. pylori.
H. pylori was grown on BHI agar plates (Difco Laboratories) supplemented with 10% horse serum (GIBCO BRL) in GasPak System (Becton Dickinson Microbiology Systems) jars under microaerophilic conditions for 24 hours. Cells from one plate were harvested in 1 mL BHI broth (Difco Laboratories) supplemented with 6% FCS (Eurobio, Toulouse, France). After determination of the OD at 578 nm, the cells were diluted to a final OD of 0.1. One milliliter of this suspension was incubated for 4–5 hours at 37°C in 24-well plates in an incubator also in microaerophilic conditions. After addition of 1 mg DNA, the cell suspension was incubated for another 24 hours. The cultures were spread onto BHI agar plates containing 8 mg/mL kanamycin and 10% horse serum for selection and growth. The absence of ureI or its expression in the H. pylori mutant was confirmed by PCR and Western blot analysis using antibodies specific for two of the extracytoplasmic loops of the gene product (11, 12). The nonpolar nature of the mutants was confirmed by showing normal urease activity in the lysates or after permeabilization with 0.01% C12E8 and by complementation using a ureI containing plasmid as detailed elsewhere (32).
Media used for confocal microscopy and pH-metry.
In the experiments reported, a 1 mM sodium phosphate buffer at the required pH values of 5.5 and 7.4 was used containing the following: 138 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 0.5 mM MgSO4, 10.0 mM glucose, and 1.0 mM glutamine. This buffer medium is referred to as Hp medium throughout the text.
pH electrode measurements of pH changes in H. pylori cocultured with AGS cells.
A special pH-sensor for microsamples (PHR-146; Lazar Research Laboratory, Los Angeles, California, USA) was connected to a pH-meter (PHM62; Radiometer Analytical SA, Lyon, France). pH measurement of the chamber medium and simultaneous monitoring of bis-carboxyethylcarboxy-fluorescein–free (BCECF-free) acid fluorescence using confocal microscopy was performed. After 5 mM urea was added to the chamber by perfusion and perfusion stopped, the pH was monitored every 10 seconds for the first minute, every 30 seconds up to 5 minutes, then every minute up to 10 minutes, and finally every 5 minutes up to 30 minutes. In some experiments, the bacteria were preincubated for 2 minutes with 1 μM flurofamide to inhibit external urease while sparing internal urease activity. A total of 10 μM flurofamide was used to inhibit all urease activity. In all experimental situations, the presence of 10 μM flurofamide abolished pH changes with the addition of urea.
Confocal microscopy.
Confocal experiments were done on a Zeiss LSM 410 or 510 microscope (Carl Zeiss Inc.) using a 63× or 100× objective. Higher magnifications were obtained by using the electronic zoom feature provided by the software of the LSM 410/510 as seen in Figure 2b with an electronic zoom factor of 3× (Carl Zeiss Inc.).
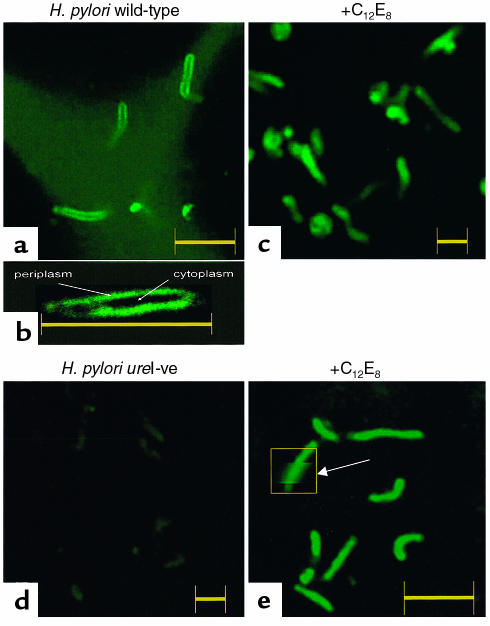
Figure 2.
Localization of pH elevation with urea addition at pHout 5.5. H. pylori cocultured with AGS cells were perfused with medium containing BCECF-free acid and then 5 mM urea added as detailed in the text. Changes of fluorescence were then observed as a function of time using confocal microscopy at ×100 optical magnification and ×3 or ×10 electronic magnification. (a) The addition of 5 mM urea initially resulted in an increase of BCECF fluorescence clearly visible at the periphery of the wild-type organisms. (b) Higher magnification of a single wild-type organism emphasizing the peripheral increase of BCECF fluorescence obtained with urea addition. This restricted region of pH elevation is the periplasm. (c) Pretreatment with 0.01% C12E8 resulted in bacterial cytoplasmic alkalinization after the addition of urea to wild-type organisms. (d) Addition of 5 mM urea resulted in little or no change of fluorescence (pH) in the periplasm of the H. pylori ureI-ve mutant with the addition of 5 mM urea. (e) Pretreatment of the ureI-ve mutants with 0.01% C12E8 followed by urea addition increased cytoplasmic pH as monitored by the rise in BCECF fluorescence within the organism. The arrow points to a region where the image is electronically magnified to show the internal, compared with surface, fluorescence. Each bar corresponds to 3 μm as defined by the confocal software.
Visualizing the bacteria in coculture.
To localize H. pylori on the monolayer, AGS cells were stained with the membrane potential dye dithiocarbocyanine [DiSC3(5)], which was used to define the AGS cell mitochondria and visualized at 546 nm excitation and greater than 650 nm emission. With this dye, the cytoplasm of the AGS cells remains dark. Then BCECF was added to the culture medium and urea added to allow the bacteria to fluoresce. The ATCC 43504 and 43503 strains can be used interchangeably.
Visualizing changes of periplasmic or medium pH in coculture.
BCECF in its free acid form was used at a final concentration of 5 μM in Hp medium. The dye was excited at 488 nm and detected at 515–545 nm. At the start of the experiment, the medium in the coculture chamber was exchanged by superfusion with the Hp-medium containing 5 mM urea at a given pH and then the perfusion was stopped. For the first 2 minutes, images were collected every 0.78 seconds with a scan rate of 0.78 seconds, and then every 30 seconds at the same scan rate up to 30 minutes. In some experiments, the nonionic detergent C12E8 was added to the medium at a concentration of 0.01% to permeabilize the bacterial inner membrane. This concentration of detergent does not disturb bacterial morphology (12). The combination of pH-metry and BCECF fluorescence achieves the goal of defining pH and the site of pH elevation.
Visualizing changes in pH of AGS cells in coculture.
Semi-naphtharhodfluor-acetomethoxy ester (SNARF-AM) at 10 μM was used to load the AGS cells at 37°C for 30 minutes and monitor intracellular pH changes as a function of urea addition at pH 5.5. Simultaneous monitoring of bacterial periplasmic pH and medium pH was carried out using BCECF-free acid fluorescence.
Video microscopy perfusion experiments using BCECF-AM for determining effects on cell pH.
H. pylori-AGS cells cocultured on cover slips for 1 hour (bacteria per cells = 200) were preincubated with BCECF-AM (2 μM) for 30 minutes at 37°C. The cover slip was placed in the microscope chamber and perfused with 5 mM urea added to PBS (Hp buffer, as described earlier) containing 138 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 0.5 mM MgSO4, 10.0 mM glucose, 1.0 mM glutamine (1.0), and 1 mM Na phosphate adjusted to pH 5.5; and the perfusate was collected. HCl was added as necessary to maintain the pH at 5.5, as there was rapid elevation of the pH during perfusion. Then the coculture perfusate, collected at pH 5.5 in the presence of 5 mM urea, was perfused for 5 minutes over AGS cells at 37°C either before or after neutralization with NaOH. The intracellular pH was monitored in ratio mode by alternating the excitation wavelength between 490 and 430 and measuring emitted fluorescence at 530 nm. Intracellular pH changes were expressed as a change in the fluorescence ratio. In experiments not shown, 20 mM NH4Cl was added to the perfusion medium at pH 7.4 and subsequently removed to show that NH3 entry into AGS cells elevated, and exit from AGS cells reduced, pHin.
Measurement of [Ca2+]in of AGS cells.
H. pylori and AGS cells in 1 hour coculture (bacteria per cells = 200) were loaded with 2 μM Fura 2-AM for 30 minutes at 37°C. The cover slip was washed with growth medium and placed in a heated chamber (Medical Systems Inc.). The experimental procedure followed exactly that described earlier here for measurement of intracellular pH. Fura-2 fluorescence was measured by alternating the excitation wavelengths at 340 and 380 nm with emission wavelength at 505 nm. Image pairs were captured under the control of Image-1/FL software (Universal Imaging, West Chester, Pennsylvania, USA) and expressed as the ratio of fluorescence level in the chosen field (33). All data presented in the figures are representative of at least four experiments.
Materials.
Chemicals were at least analytical grade. Biologic material was obtained as outlined above. BCECF, BCECF-AM, Fura2-AM, and SNARF-AM were purchased from Molecular Probes Inc.
Results
Localization of H. pylori on confluent AGS cells.
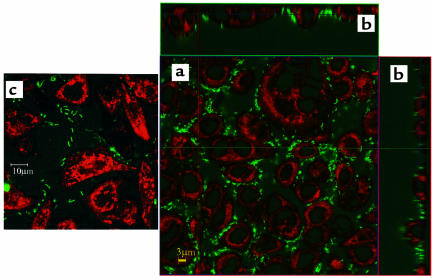
AGS cells grown to confluence have regions of contact but do not form tight junctions. The staining of AGS cell mitochondria by DiSC3(5) is shown as red in Figure 1a (×63) and Figure 1c (×100), and the bacteria stained with BCECF, as green. These images are achieved immediately after the addition of urea. Figure 1c shows that individual bacteria are seen and that the peribacterial region shows pH elevation. The bacteria adhere to the AGS cells, forming a rosette around the cells. When viewed in the z axis, the bacteria are clustered just above the contact regions between the cells (Figure 1b). Further, the bacteria adhering to the AGS cells were immobilized as compared to adherence to a Cell-Tak–coated cover slip (Collaborative Biomedical Products, Bedford, Massachusetts, USA). This specific location and immobilization in one plane are convenient for finding the organism in the confocal microscope and essential for the experiments defining regions of pH change. It was noted in electron micrographs (H. Helander, unpublished observations) that pedestals were formed under the bacteria with distortion of the underlying structures consistent with the ability of H. pylori to carry out type IV secretion when cocultured with these cells (24). The size of the bacteria as determined by the computer in the confocal microscope is of the expected size, approximately 2.5 μm (Figures 1, a and c, and 2).
Figure 1.
Localization of H. pylori in coculture with AGS cells. (a) AGS cells were stained with the dye DiSC3(5) after 1-hour coculture with H. pylori. BCECF-free acid was used to illuminate the bacteria after addition of urea. Both dyes were monitored simultaneously using confocal microscopy. ×63. (b) These images show a perpendicular cut through the same sample as panel a in the X and in the Y plane as shown by the corresponding lines of a. H. pylori are located primarily just above the regions of contact between the AGS cells. (c) A higher magnification view to illustrate that the peribacterial region of individual organisms shows the change in BCECF fluorescence. ×100. The bars correspond to 3 or 10 μm as defined by the confocal software.
Localization of pH elevation with urea addition.
The outer membrane of this Gram-negative organism is permeable, and the inner membrane is impermeable, at pH greater than approximately 5.0 to the pH-sensitive carboxylic acid dye, BCECF. Thus, this dye is able to monitor pH changes outside the bacterial inner membrane (periplasm and medium) when added at pH 5.0. BCECF has a pKa of 6.0 and can monitor pH between about 5.5 and 8.0, its fluorescence increasing with increasing pH.
In wild-type bacteria, the addition of 1 or 5 mM urea initially causes an increase in fluorescence at the periphery of the organisms, with the cytoplasm remaining dark (Figure 2a). At higher magnification, the fluorescence peripheral to the center of the organism is more obvious and the size measurement shows that this image, as in Figure 2a, comes from a single organism (Figure 2b). This restricted region of increased fluorescence (pH) is the periplasm. In Figure 2, a and b, the medium adjacent to the organisms has not yet shown an increase of pH. The addition of 0.01% C12E8 before adding urea results in increased fluorescence in the bacterial cytoplasm because now the BCECF has access to the cell interior. Figure 2, d and e, shows a similar set of experiments in the ureI-negative mutant. There is no change of fluorescence near the ureI mutant with urea addition, showing that neither the surface nor intrabacterial urease of the ureI- mutants enable an initial rapid pH change (12). Detergent treatment results in an increase of pH only inside the organism with urea addition (Figure 2e). The arrow in Figure 2e points to a region of electronic magnification within the highlighted rectangle where the centrally greater fluorescence is evident compared with the lower peripheral fluorescence in contrast to Figure 2, a and b. The regulated or unregulated urea permeability of the inner membrane is bypassed by low concentrations of detergent in both wild-type and mutant organisms.
These data and the previous data for urease activation of intact bacteria and urea transport in Xenopus oocytes show that UreI controls urease activity of the organism by increasing urea permeability of the inner membrane at acidic pH. This allows rapid buffering of the periplasm, as has been deduced from membrane potential measurements (11, 12).
Effects of urea addition on medium pH in coculture
Wild type.
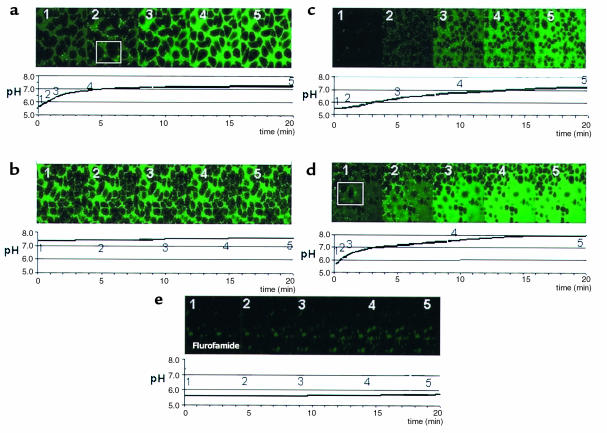
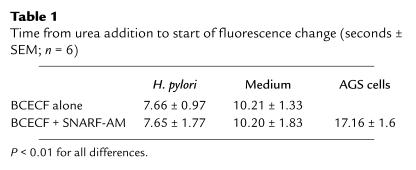
The addition of 5 mM urea at a medium pH of 5.5 produced a rapid exponential rise of medium pH in the first 3–4 minutes as measured by the pH electrode (Figure 3a). Thereafter there was a slow increase of pH to about 7.2 over the next 16 minutes. The image sequence above the pH curve in Figure 3a, from the same experiment, shows the fluorescence increasing markedly first at the bacteria, evident in the first two or three images. In particular, in the second image, the square highlights a region of ×1.5 magnification showing the increase of fluorescence first in the region of the bacteria. The increase in fluorescence then spreads rapidly into the medium, indicative of an increase in medium pH. The fluorescence was close to maximum after about 4 minutes (fourth image), as for pH electrode measurements. A total of 1 μM flurofamide 2 minutes before the experiment did not inhibit the changes of BCECF fluorescence (data not shown). However, 10 μM flurofamide inhibited the pH changes due to urea addition either in the wild-type organism or in the mutant with detergent addition (Figure 3e). Table 1 shows the time taken from urea addition to the start of pH increase in the peribacterial region compared with the medium in several experiments, confirming the visual impressions from the displayed images.
Figure 3.
Bacterial and medium pH effects of urea addition to H. pylori-AGS coculture with either wild-type or ureI mutant strains. (a and b) The effect of urea addition on medium and bacterial pH in coculture with H. pylori expressing UreI. H. pylori and AGS cells in coculture were perfused with medium containing BCECF-free acid at the indicated pH and then 5 mM urea was added. A calibrated pH microelectrode was placed in the chamber to monitor medium pH and confocal microscopy was used to monitor the regions of change of pH after the addition of 5 mM urea using wild-type or ureI-deletion mutants as detailed in Methods. The numbering in the confocal images corresponds in time to the images above the pH tracings. (a) At a medium pH of 5.5, there was a rapid rise of medium pH after the addition of 5 mM urea. Within 2 minutes, the pH had risen to 6.0 and reached a steady-state pH of about 7.0 within 5 minutes. In the image sequence above the pH curve from the same experiment, the fluorescence increased markedly first on or at the bacteria, evident in the first two or three images. The increase in fluorescence then spread into the medium as medium pH increased and is close to maximum at about 4 minutes (see Table 1). (b) In contrast, when urea is added at 5 mM at a medium pH of 7.4, there was little change of medium pH or the pH around the organism. After 20 minutes, the pH had only increased by 0.2 U. (c and d) The effect of 5 mM urea addition on medium and bacterial pH in coculture with H. pylori ureI–negative mutants. (c) When urea was added at pH 5.5 to the ureI-ve strain, there was a slow rise of medium pH, reaching about 6.5 after 5 minutes and then rising slowly to 7.2 at the end of the experiments. There was no obvious change of fluorescence with the addition of urea in the vicinity of the organisms, although they have considerable surface urease activity (11). (d) Urea addition in the presence of 0.01% C12E8 to the ureI-deletion mutant resulted in a rapid change in medium pH, faster than that seen with the wild-type organisms, reaching pH 7.1 within 5 minutes and continuing to increase to more than 8.0, in contrast to the untreated wild-type organisms. In the fluorescence images, there was a similar increase in the fluorescence of the medium reflecting the pH changes monitored with the pH electrode. (e) When 5 mM urea was added in the presence of 10 μM flurofamide at pH 5.5, no change in either bacterial or medium pH could be observed, showing the dependence of these changes on urease activity.
Table 1.
Time from urea addition to start of fluorescence change (seconds ± SEM; n = 6)
BCECF has greater fluorescence at pH 7.4 than at pH 5.5. Therefore, in the experiments beginning with a medium pH at 7.4, the BCECF was already fluorescent. When 5 mM urea was added at a medium pH of 7.4, there was little change of medium pH or organism pH (Figure 3b). After 20 minutes, medium pH had only increased by 0.2 units. This minimal increase is accounted for by the low intrabacterial urease activity due to absence of UreI induced urea permeability at this pH (11, 12).
ureI-ve mutants.
When urea was added at pH 5.5 to H. pylori ureI-ve/AGS coculture, there was a slow increase of medium pH with time (Figure 3c). The medium pH increased to about 6.2 after 5 minutes and to 7.2 after 20 minutes. In contrast to the observations with the wild-type strain of the bacteria, there was no change of fluorescence in the vicinity of the organisms after urea addition, despite having considerable surface urease activity as noted earlier (11). In data not shown, 1 μM flurofamide added 2 minutes before urea addition, which selectively inhibits surface, but not intrabacterial urease, abolished the pH change seen with urea addition to these mutants. This shows that the pH change observed both with the pH electrode and with BCECF is due to mainly to external urease activity in the ureI deletion strain.
To confirm that slow fluorescence changes in the medium or the absence of localized changes of fluorescence at the surface of the organism was due to poor penetration of urea to the intrabacterial urease, a low concentration of the detergent C12E8 was added (Figure 3d). This 0.01% concentration of detergent permeabilizes the inner membrane of the organism with resultant full urease activity (11, 12), as urea penetration is no longer rate limiting. Detergent addition resulted in a rapid increase of medium pH to 7.1 after 3 minutes and to 8.0 after 20 minutes. The rate and magnitude of alkalinization were faster than that seen with the wild-type organisms in the absence of detergent (Figure 3a). In the images, there was consistently an increase first in the fluorescence of bacteria and then the medium as illustrated in the magnified panel in the first image of this sequence.
To exclude an effect of C12E8 in the coculture system, AGS cells were incubated with 0.01% C12E8 without H. pylori and the fluorescence was followed after addition of 5 mM urea. In another experiment, the medium fluorescence in the coculture was monitored after addition of 0.01% C12E8 lacking urea. In both control experiments, no fluorescence change was observed.
Effect of NH3 generation on AGS cell pHin and [Ca2+]in.
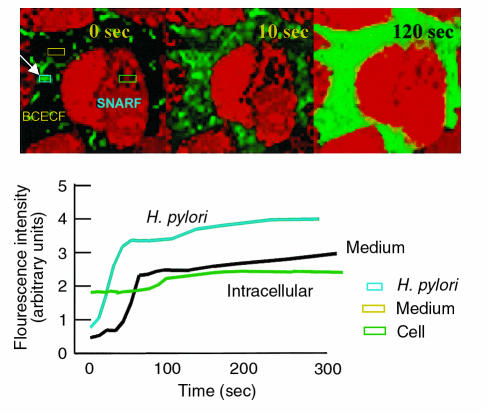
A question that can be answered directly using the coculture model is whether the ammonia produced by the organisms has privileged access to the cells to which they attach. As shown in Figure 4, the addition of urea results first in an increase of fluorescence in or around H. pylori as also illustrated in Figure 3 and Table 1. The blue rectangle is the region of interest chosen for monitoring bacterial fluorescence. This is followed by a change in medium pH spreading from the bacteria visible as increasing fluorescence where the region of interest is highlighted with a yellow rectangle. No change was observed in the AGS cells monitored simultaneously using intracellular SNARF (highlighted with a green rectangle) until the medium pH had changed. The graphs underneath illustrate a typical time course observed in terms of timing of pH changes as shown in Figures 2 and 3 and in this figure.
Figure 4.
Time course of the effect of NH3 generation on peribacterial, medium, and AGS cell pH. SNARF-AM was used to monitor the effect of 5 mM urea addition to the pHin of the AGS cells in bacterial coculture with BCECF-acid present to monitor peribacterial and medium pH changes. Fluorescence changes were analyzed quantitatively on the bacteria, in the medium and in the cells in regions of interest defined by the confocal software (confocal images, rectangles) and displayed as a time course of fluorescence (graphs below). Three time points are illustrated in the confocal images corresponding to the time course shown in the time plot underneath. There was first a large change in periplasmic pH followed by an increase of medium pH. No change was observed in the AGS cells until medium pH had risen. The earliest fluorescence change was seen over the bacteria, and then the increase spread into the medium and then into the AGS cells (see Table 1).
Table 1 also illustrates the time course of the initial changes of fluorescence in the peribacterial region, the medium and inside the AGS cells. Hence, cell pH does not increase until medium pH is elevated.
There is apparently no direct entry of NH3 into the AGS cells from the bacterial cytoplasm in this in vitro model, as cellular alkalinization would happen along with intrabacterial NH3 generation if there were direct transmission of NH3. Rather, the AGS cell alkalinization occurs after the medium pH is elevated by intrabacterial urease activity as illustrated in the graph, color images, and Table 1. This was investigated in more detail by perfusion with conditioned medium at pH 5.5 and 7.4 as discussed later here.
Effects of addition of NH4Cl or cocultured supernatants on AGS cell pHin and [Ca2+]in.
BCECF-AM and Fura-2 AM were loaded into AGS cells and fluorescence measured with video microscopy for changes of pH and intracellular calcium. Because these are ratiometric fluorescent dye probes, the data are independent of microscope focus. When 20 mM NH4Cl was perfused across the AGS cells at pH 7.4, there was a rise in pHin that was maintained during the time of NH4Cl perfusion, as is characteristic of mammalian gastric cells due to NH3 entry (34). There is low permeability of the AGS cell to NH4+, so little equilibration of the NH3/NH4+ couple occurs during the NH4Cl perfusion, maintaining a relatively high intracellular pH. With removal of NH4Cl from the perfusate, there is rapid efflux of NH3, acidifying the cell.
Elevation of cell pH due to NH3 entry increased [Ca2+]in transiently, but there was no increase of steady-state intracellular calcium. This characteristic transient is mainly due to release of calcium from intracellular stores. Acidification of the cell after removal of NH4Cl from the perfusate transiently lowered [Ca2+]in but to a lesser extent than alkalinization had increased [Ca2+]in. The [Ca2+]in increase seen in these experiments is directly dependent on cellular alkalinization because pretreatment with imidazole, a permeant intracellular buffer, abolished the NH4Cl-induced Ca2+ signals (data not shown).
Perfusion of AGS cells with Hp/AGS coculture–derived medium at pH 5.5 had no effect on intracellular pH or calcium. However, when the perfusate collected from the coculture chambers was first adjusted to pH 7.4 before reperfusion, it induced a typical cellular alkalinization shift followed by acidification when washed out. This cellular pH shift was accompanied by Ca2+ release from intracellular stores (Figure 5). The latter effect is similar to that found with the addition of NH4Cl at neutral pH. The supernatant-induced pH changes were absent if the initial perfusion of the coculture had been carried out in the presence of 10 μM flurofamide, showing that it was ammonia/ammonium generation by urease that was responsible for these changes.
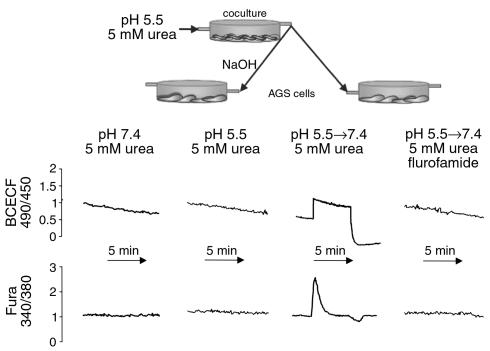
Figure 5.
The effect of coculture perfusate on pHin and [Ca2+]in of AGS cells. These experiments were designed to test the effects of ammonia generated by intrabacterial urease on the AGS cells cocultured with H. pylori either at acidic or neutral pH. Bacteria and cells in coculture were perfused with 5 mM urea at pH 7.4 or 5.5 for 30 minutes, as illustrated in the diagram above the graphs, which shows the protocol for pH 5.5 perfusion without or with 10 μM flurofamide (also see Methods). The AGS cells were loaded in the microscopy chamber with BCECF-AM for pHin measurement or Fura 2-AM for [Ca2+]in measurements. The coculture was reperfused with the conditioned perfusate generated at pH 7.4 or 5.5 either before (2 curves on left) or after (2 curves on right) adjusting the pH to 7.4 with NaOH, and changes of pHin and [Ca2+]in were measured. The perfusion using conditioned medium at acidic pH resulted in no change in cell pH or calcium, whereas if the medium from the coculture was first neutralized to pH 7.4 with NaOH, a robust increase in intracellular pH or a transient increase in intracellular calcium was observed. The presence of flurofamide in the initial perfusion prevented any changes from being observed in the reperfusion at pH 7.4 showing that the effect observed at pH 7.4 reperfusion was due to the urease activity present during the pH 5.5 initial perfusion.
Discussion
Recent data have shown that intrabacterial, rather than surface, urease is the essential compartment of urease required for acid resistance of H. pylori (7, 10, 12, 35). This neutral pH optimum urease is limited in its activity, at a neutral medium pH, by the slow penetration of urea into the organism, the major barrier being the inner membrane. The permeability of urea across unmodified lipid bilayers is ∼4 × 10–6 cm/s (36). This is insufficient to saturate intrabacterial urease at the prevalent gastric juice urea concentration, which lies between 1 and 3 mM urea. The organism overcomes this limitation by expression of an H+-gated urea channel, UreI, which is encoded by one of the genes of the urease gene cluster. This protein has the function of accelerating urea entry at acidic pH. These properties were identified by analysis of the acid activation of urease and by determining properties of UreI when expressed in Xenopus oocytes (7, 10–12). From a comparison of the urea concentration necessary for survival at pH 2.5, it can be estimated that approximately 300 times more urea is required in ureI-ve compared with wild-type organisms to survive this acidity, i.e., UreI expression in H. pylori inner membranes increases urea permeability by a factor of 300 (D. Weeks, unpublished observations). This allows saturation of intrabacterial urease that has a Km,app of approximately 1 mM, close to the normal level of gastric juice urea.
The periplasm is the first site at which an elevation of pH is expected with the addition of urea to wild-type organisms in acidic media. In the absence of UreI, the periplasmic pH is not expected to change dramatically with urea addition, as there is little or no restoration of membrane potential in ureI- mutants with the addition of urea (11). In the wild-type organisms, but not in the mutants, the continuing efflux of NH3 from the cytoplasm to the periplasm and then across the outer membrane should secondarily alkalinize the medium, but the alkalinization will slow as UreI inactivates when periplasmic pH increases to greater than approximately 6.0. In the ureI-deletion mutants, it is predicted that no specific compartment should show a rapid change of pH, but given the presence of finite urea permeability and/or the presence of surface urease, a slow alkalinization of the medium is expected. If the membrane barrier to urea is disrupted in the mutants (or in the wild-type organisms), as can be done by addition of low concentrations of detergent, the addition of urea should result in an even more rapid alkalinization than even in wild-type organisms at acidic pH and should be seen first in the cytoplasm and not slow down when the inactivation pH of UreI is reached. This is indeed what is found as shown in Figures 2 and 3.
These data extend our previous observations on the role of intrabacterial urease and UreI in which we monitored membrane potential or urease activity (11). From these data, we had deduced that the elevation of membrane potential at acidic pH with urea addition was due to a rise in periplasmic pH. Here, this is directly demonstrated in the confocal images.
Accordingly, H. pylori can survive gastric acidity by buffering its periplasm, not the external environment, by stimulation of urease activity at acidic pH due to UreI enhancement of urea entry (12). Maintenance of the proton motive force across the inner membrane of the organism, as well as periplasmic pH, is sufficient for viability and growth. This strategy adopted by these bacteria also minimizes the likelihood of excessive urease activity and toxic alkalinization in the absence of acid, further ensuring maintenance in a gastric environment of varying pH.
Another issue addressed by this work was whether the ammonia generated inside the bacteria diffuses directly into cells to which they adhere. The data presented here show that NH3 does not diffuse directly from organism to infected cell. However, the quantity of NH3 and NH4+ generated at pH 5.5, a level at which urease activity plateaus, is sufficient, when brought back to neutrality, to alkalinize the AGS cells and induce a release of calcium from intracellular stores. Because inhibition of urease activity by flurofamide prevented these effects, they are due to the NH3 generated during incubation. These data, while showing that direct entry of NH3 into the gastric epithelial cells does not occur, also show that, when the NH4+-laden gastric juice is neutralized, as occurs during the digestive phase of acid secretion or upon entry into the duodenum or upon penetration to the base of the antral glands, sufficient NH3 is present to provoke cell alkalinization and initiation of a calcium-signaling cascade. This calcium signal may then provoke release of cytokines and thence gastritis, a universal pathological consequence of H. pylori infection of the human stomach (37).
Acknowledgments
This work was supported by United States Veterans Administration Senior Medical Investigator, and by NIH grants DK-46917, DK-53462, DK-41301, DK-19567, and DK-17294.
Footnotes
Christoph Athmann and Ningxin Zeng contributed equally to this work.
This paper is dedicated to the memory of John Walsh, friend, tennis partner, and colleague.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Andrutis KA, et al. Inability of an isogenic urease-negative mutant stain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun. 1995;63:3722–3725. doi: 10.1128/iai.63.9.3722-3725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton KA, Morgan DR, Brooks CL, Krakowka S. Essential role of urease in the pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuda M, Karita M, Morshed MG, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobley HLT, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott DR, et al. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterol. 1998;114:58–70. doi: 10.1016/s0016-5085(98)70633-x. [DOI] [PubMed] [Google Scholar]
- 8.Dunn BE, Campbell GP, Perez-Perez GI, Blaser MJ. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 9.Hawtin PR, Stacey AR, Newell DG. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990;136:1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- 10.Rektorschek M, Weeks D, Sachs G, Melchers K. The influence of pH on metabolism and urease activity of Helicobacter pylori. Gastroenterol. 1998;115:628–641. doi: 10.1016/s0016-5085(98)70142-8. [DOI] [PubMed] [Google Scholar]
- 11.Scott DR, et al. Expression of the Helicobacter pylori ureI gene is required for acidic pH activation of cytoplasmic urease. Infect Immun. 2000;68:470–477. doi: 10.1128/iai.68.2.470-477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks DL, Eskandari S, Scott DR, Sachs G. A H+ gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485. doi: 10.1126/science.287.5452.482. [DOI] [PubMed] [Google Scholar]
- 13.Skouloubris S, Thiberge J-M, Labigne A, De Reuse H. The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect Immun. 1998;66:4517–4521. doi: 10.1128/iai.66.9.4517-4521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan DR, Freedman F, Depew CE, Kraft WG. Growth of Campylobacter in liquid media. J Clin Microbiol. 1987;25:2123–2125. doi: 10.1128/jcm.25.11.2123-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiegmann TB, et al. Simultaneous imaging of intracellular [Ca2+] and pH in single MDCK and glomerular epithelial cells. Am J Physiol. 1993;265:C1184–C1190. doi: 10.1152/ajpcell.1993.265.4.C1184. [DOI] [PubMed] [Google Scholar]
- 17.El-Omar EM, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 18.Kusugami K, et al. Mucosal chemokine activity in Helicobacter pylori infection. J Clin Gastroenterol. 1997;25(Suppl. 1):S203–S210. doi: 10.1097/00004836-199700001-00032. [DOI] [PubMed] [Google Scholar]
- 19.Maekawa T, et al. Helicobacter pylori induces proinflammatory cytokines and major histocompatibility complex class II antigen in mouse gastric epithelial cells. J Lab Clin Med. 1997;130:442–449. doi: 10.1016/s0022-2143(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 20.Tomb JF, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 21.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 22.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odenbreit S, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 25.Dockray GJ. Gastrin and gastric epithelial physiology. J Physiol. 1999;518:315–324. doi: 10.1111/j.1469-7793.1999.0315p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert ML, Makhlouf GM. Neural regulation of gastrin and somatostatin secretion in rat gastric antral mucosa. Am J Physiol. 1987;253:G721–G725. doi: 10.1152/ajpgi.1987.253.6.G721. [DOI] [PubMed] [Google Scholar]
- 27.Richter-Dahlfors A, Heczko U, Meloche RM, Finlay BB, Buchan AM. Helicobacter pylori infected human antral primary cell cultures: effect on gastrin cell function. Am J Physiol. 1998;275:G393–G401. doi: 10.1152/ajpgi.1998.275.3.G393. [DOI] [PubMed] [Google Scholar]
- 28.Calam J, Gibbons A, Healey ZV, Bliss P, Arebi N. How does Helicobacter pylori cause mucosal damage? Its effect on acid and gastrin physiology. Gastroenterology. 1997;113(Suppl.):S43–S49. doi: 10.1016/s0016-5085(97)80010-8. [DOI] [PubMed] [Google Scholar]
- 29.Segal ED, Falkow S, Tompkins LS. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rektorschek M, et al. Acid resistance of Helicobacter pylori depends on the UreI membrane protein and an inner membrane proton barrier. Mol Cell Biol. 2000;36:141–152. doi: 10.1046/j.1365-2958.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- 31.Ausubel, F.M., et al. 1994. Current protocols in molecular biology. John Wiley and Sons Inc. New York, New York, USA. 1.5.1–1.7.5.
- 32.Heuermann D, Haas R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol Gen Genet. 1998;257:519–528. doi: 10.1007/s004380050677. [DOI] [PubMed] [Google Scholar]
- 33.Zeng N, Walsh JH, Kang T, Wu SV, Sachs G. Peptide YY inhibition of rat gastric enterochromaffin-like cell function. Gastroenterology. 1997;112:127–135. doi: 10.1016/s0016-5085(97)70227-0. [DOI] [PubMed] [Google Scholar]
- 34.Muallem S, Burnham C, Blissard D, Berglindh T, Sachs G. Electrolyte transport across the basal lateral membrane of the parietal cell. J Biol Chem. 1985;260:6641–6653. [PubMed] [Google Scholar]
- 35.Krishnamurthy P, et al. Helicobacter pylori containing only cytoplasmic urease is susceptible to acid. Infect Immun. 1998;66:5060–5066. doi: 10.1128/iai.66.11.5060-5066.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orbach E, Finkelstein A. The non-electrolyte permeability of planar lipid bilayer membranes. J Gen Physiol. 1980;75:427–436. doi: 10.1085/jgp.75.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobley HL, Hu LT, Foxal PA. Helicobacter pylori urease: properties and role in pathogenesis. Scand J Gastroenterol Suppl. 1991;187:39–46. [PubMed] [Google Scholar]