Abstract
Background
Hyperthermic isolated limb perfusion (HILP) and isolated limb infusion (ILI) are utilized to manage advanced extremity melanoma but no consensus exists as to which treatment is preferable and how to monitor patients post-treatment.
Study Design
Using a prospectively-maintained database, we reviewed our experience with melphalan based HILP (that included 62 first time and 10 second time) and ILI (that included 126 first time and 18 second time) procedures performed in 188 patients. PET/CT was obtained 3 months post regional treatment for one year and then every 6 months thereafter.
Results
The overall response rate (complete response (CR) + partial response (PR)) of HILP was 81% (80% CI: 73-87%) while the overall response rate from ILI was 43% (80% CI: 37-49%) for first time procedures only. HILP had a CR rate of 55% with a median duration of 32 months, while ILI had a CR rate of 30% with median duration of 24 months. Patients who experienced a regional recurrence after initial regional treatment were more likely to achieve a CR following repeat HILP (50%, n = 10) compared to repeat ILI (28%, n = 18). Although the spectrum of toxicity was similar for ILI and HILP, the likelihood of rare catastrophic complication of limb loss was greater with HILP (2/62) than ILI (0/122). PET/CT was effective for surveillance after regional therapy to identify regional nodal and pulmonary disease that was not clinically evident, but often amenable to surgical resection (25/49, 51% of cases). In contrast, PET/CT was not effective at predicting complete response to treatment with an accuracy of only 50%.
Conclusions
In the largest single institution regional therapy series reported to date, we found that while ILI is effective, and well-tolerated, HILP is a more definitive way to control advanced disease.
Introduction
After initial appropriate therapy, approximately 2 to 10% of extremity melanoma lesions recur in the extremity as in-transit metastases.1,2 This pattern of recurrence represents multifocal involvement of the extremity’s lymphatic system and local excision of these lesions is frequently followed by rapid recurrence. Treatment of recurrent regional melanoma is important in that at least half of these patients survive for more than 2 years without evidence of distant disease.1 Surgical isolation of an extremity and treatment with regional chemotherapy can deliver cytotoxic agents (usually melphalan) at dosages ten to twenty times higher than can be achieved systemically. This form of therapy has been a relatively effective limb sparing treatment modality for in-transit disease since the 1950s.3 Currently there are two ways drugs are regionally delivered: hyperthermic isolated limb perfusion (HILP) and isolated limb infusion (ILI). HILP, which involves a surgical incision and open cannulation of the extremity’s artery and vein has been associated with single institution complete response rates of 40-80%.4-11 ILI is a less invasive alternative to HILP whereby percutaneous catheters are placed in the artery and vein of the involved extremity and no surgical incision is required. CR rates to ILI have been reported to be between 30-38%.12,13 While response rates to ILI are considered to be less than HILP, ILI remains popular because of the disappointing results of HILP in the multicenter randomized ACOSOG Z0020 trial where significant toxicity and CR rates of only 25% were reported.8,12
The major technical differences between HILP and ILI are that higher doses of melphalan per liter of treated limb volume are used in HILP along with a flow rate that is much higher for HILP (150 to 1,000 mL/min) as compared to ILI (50 to 100 mL/min).14 In addition, HILP uses a higher degree of hyperthermia than ILI and the chemotherapy is circulated for 60 minutes for HILP compared to 30 minutes for ILI. These differences could explain in part why HILP is associated with higher overall response rates. However, HILP often requires blood transfusions (to prime circuit), poses short and long-term risk to vessel patency, and is associated with increased limb loss and mortality.15 Given our large practice involving patients with in-transit disease, we realized that both ILI and HILP, each with their benefits and shortcomings, had potential utility in managing the spectrum of patients with advanced extremity melanoma. We proposed an algorithm in 2008 based upon a review of our experience and that of several other institutions that had the goal of optimizing regional response while minimizing toxicity.9 This algorithm tried to identify the appropriate clinical and patient situations for utilization of either ILI, HILP, or protocol based regional therapies in the management of these patients. Since the review upon which the algorithm is based, we have expanded our regional therapy experience by over 40% and thus wanted to re-evaluate some of the assumptions upon which the treatment algorithm was based to determine if modifications should be constructed. This manuscript summarizes our global experience in managing in-transit extremity melanoma using regional therapy at a single institution over the last 15 years.
Methods
A prospective melanoma surgical database at Duke University Medical Center (DUMC) identified 188 patients who underwent 225 regional procedures for metastatic melanoma (ILI and/ or HILP) from 1995-2010. There were a total of 62 first time HILPs and 126 first time ILIs performed. Several patients underwent a second regional treatment: ILI following ILI in 16 cases, HILP following ILI in 7 cases, ILI following HILP in 2 cases, and HILP following HILP in 3 cases. Nine other procedures were performed as a third or fourth regional treatment in five patients. All patients had advanced extremity melanoma; American Joint Committee on Cancer (AJCC) stage IIIB, IIIC, or IV disease.16 Response was determined at 3 months post-treatment according to Response Evaluation Criteria in Solid Tumors modified for cutaneous lesions.9,12 Overall toxicity was measured using both the Wieberdink limb toxicity scale and the Common Terminology Criteria for Adverse Events (CTCAE version 3) grading scheme. The Wieberdink limb toxicity scale focuses on regional toxicity only and is more commonly utilized in reports of regional therapy currently present in the literature.17 With this scale, toxicity ranges from grade 1 which is no visible effect on the extremity to grade 5 which consists of toxicity resulting in limb amputation. The CTCAE scoring system allowed for documentation and grading of systemic complications in addition to regional skin and soft tissue problems. Serologic toxicity, most notably an elevation of creatine phosphokinase (CPK), could also be assessed according to CTCAE. A CPK of 100 to 250 U/L is considered a grade 1 toxicity, 250 to 500 U/L is a grade 2 toxicity, 500 to 1,000 U/L is a grade 3 toxicity, and CPK>1,000 U/L is a grade 4 toxicity. Post-operative complications including rate of deep venous thrombosis (DVT), wound infection, and limb loss were also examined. Presence of a DVT was defined as evidence and treatment for DVT and/or pulmonary embolus shown by ultrasound, ventilation-perfusion scan, or spiral CT; wound infection was defined as evidence and treatment for wound infection during the initial post-operative hospitalization or requiring readmission for incision and drainage or intravenous antibiotics. Patients who had a partial response (PR) or stable disease (SD) that could not be surgically resected, or developed progressive disease (PD) after their first regional treatment, underwent either a repeat ILI with melphalan plus dactinomycin, HILP with melphalan, or an ILI or HILP with melphalan plus an additional agent as a part of a clinical trial. This study and our prospective regional therapy database were both approved by the institutional review board of DUMC.
Intervention
ILIs were performed as described previously in the literature.9 Briefly, 6 french catheters were percutaneously inserted using the Seldinger technique and fluoroscopic guidance with tips positioned in the involved extremity knee or elbow joint. A warming blanket using circulated heated water was then wrapped around the extremity and kept in place for the duration of the procedure. Once the patient was fully heparinized (ACT greater than 350 seconds) circulation was begun through the infusion circuit using a 20 milliliter (mL) syringe. When adequate circulation through the catheters was achieved, a pneumatic or Esmarch tourniquet was positioned and inflated or tightened around the proximal portion of the extremity. Sixty milligrams (mg) of papavarine was usually given directly into the arterial side of the isolated limb circuit prior to infusion of chemotherapy to theoretically further vasodilate the capillary circulation and aid in the delivery of drug to the subcutaneous tissue. After the extremity reached at least 37.0°C, chemotherapy was rapidly infused (2 to 5 minutes) into the arterial line. Chemotherapy was circulated for approximately 30 minutes. Circuit blood gases were taken at 25 and 30 minutes after initial infusion of chemotherapy to document the degree of hypoxia and acidosis. Following 30 minutes of chemotherapy circulation, the limb was flushed through the arterial catheter with 500 to 1,000 mL isotonic crystalloid solution. Flush was extracted from the venous side manually with a syringe. When the effluent was clearing and 50% to 80% of the flush had been extracted, the tourniquet was deflated/released and arterial and venous catheters were removed. Protamine was used to reverse heparinization in all patients.
A total of 50 patients included in this series underwent ILI as part of phase I/II clinical trials combining melphalan based treatment with a systemic targeted agent. Patients that participated in the dose-escalation sorafenib clinical trial (n = 20) were administered sorafenib 200 mg by mouth in the morning and either 200 mg or 400 mg by mouth in the evening for seven days prior to and following ILI, for a total of 14 days of sorafenib dosing.18 Patients that participated in the dose-escalation ADH-1 clinical trial (n = 30) were administered between 1000 mg and 4000 mg IV at least four hours prior to ILI and on post-operative day 7.19,20
HILPs were performed as described previously.21 Surgical technique included open isolation of the limb circulation with lymphadenectomy to expose the vessels, arterial and venous cannulation, and connection to a membrane oxygenation circuit. An Esmarch tourniquet was positioned and tightened around the proximal portion of the extremity frequently using a Steinman pin. Prior to administration of chemotherapy, vascular isolation was confirmed with less than 2% leak of radiotracer to the systemic circulation. A warming blanket using circulated heated water was wrapped around the extremity and kept in place for the duration of the procedure. After the extremity was warmed to at least 38.5°C, chemotherapy was administered to the circuit. Perfusion of chemotherapy lasted approximately one hour and was followed by a 15-minute washout with isotonic crystalloid solution. Circuit blood gases collected during HILP helped confirm that blood parameters of the isolated extremity were maintained in the physiologic range despite application of the tourniquet. Patients that participated in the HILP plus TNF-alpha trial (n = 10) received regional TNF-alpha (3 mg dose for the upper extremity and 4 mg dose for the lower extremity) just before administration of melphalan.8
Chemotherapy
The standard of care agents used in ILI were melphalan (7.5 mg/L lower extremity, 10 mg/L upper extremity) and dactinomycin (75 μg/L lower extremity, 100 μg/L upper extremity).22 HILP was performed using melphalan alone (10mg/L lower extremity, 13mg/L upper extremity). The volume of the extremity was determined by measuring the circumference of the patient’s arm or leg at 1.5-cm intervals up to the level of planned tourniquet placement, encompassing the entire area to be infused or perfused. Chemotherapy doses were corrected for ideal body weight (IBW) based on evidence that this dosing modification significantly reduced grade 3 and 4 toxicities by 27% (p = 0.027) without altering complete response.23,24
Positron Emission Tomography/Computed Tomography (PET/CT)
Beginning in 2005, we attempted to perform PET/CT scans prior to regional chemotherapy treatment, every 3 months after treatment for one year, and every 6 months thereafter to compare how response to ILI/HILP as assessed by PET/CT correlated with clinical and pathologic response to ILI/HILP. PET/CT was also evaluated as a surveillance tool to detect local and systemic disease recurrence in this population. Complete data was available for 72 patients undergoing ILI and 5 patients undergoing HILP.
Statistical Methods
In-field response at 3 months (CR+PR) was the main outcome variable of interest for model predicting response and toxicity, while CR was the main outcome variable of interest for survival and response duration. Response and toxicity rates and their exact 80% confidence intervals are reported for first and second procedures. Chi-square tests were used to compare the response and toxicity rates of the first ILI and HILP procedures. TTiP, response duration, and overall survival were calculated only for first procedures. TTiP was defined as the time from surgery to in-field disease progression or death, whichever came first. Response duration was defined in CR patients as the time from CR to in-field progression. Overall survival (OS) was defined as the time from surgery to death due to any cause. Log-rank tests were used to compare the two procedures on distribution of TTiP, response duration, and OS. Within the first ILI and HILP procedures separately, the association of response (CR versus all others) with OS is assessed with the log-rank test, with the exception that the baseline time (i.e., time zero) is moved up to 3 months, the time at which response was assessed. Kaplan-Meier curves were used display the results of these tests. A two-sided alpha of 0.05 was used for all tests. All statistical analysis was performed using SAS (SAS Institute, Cary, NC) and plots were created using TIBCO Spotfire S+.
Results
Baseline Characteristics
Between 1995 and 2010, 225 regional chemotherapy treatments in 188 patients were performed at DUMC for the indication of in-transit extremity melanoma. Figure 1 demonstrates the distribution of each type of procedure performed since the regional therapy program was initiated. Table 1 shows the patient and procedure details for all 225 ILI and HILP treatments and represents the surgical practice seen at DUMC with first time and repeat procedures. Table 2 provides procedure details for the first time ILI and HILP treatments. ILI and HILP patients had similar age, gender, and extremity distribution. Correction of melphalan dosing for IBW was initially performed in an attempt to lower toxicity rates for HILP. Forty percent of patients undergoing HILP in this series, primarily those undergoing treatment more recently had their dosing corrected for IBW. After initiating ILI treatments, a similar spectrum of toxicity was observed, and the correction of melphalan dosing for IBW in ILI was adopted relatively quickly, such that 88% of patients who underwent ILI at DUMC had their melphalan dosing corrected for IBW. The stage of melanoma and burden of disease distribution prior to treatment for both ILI and HILP appear similar for both procedures.
Figure 1.
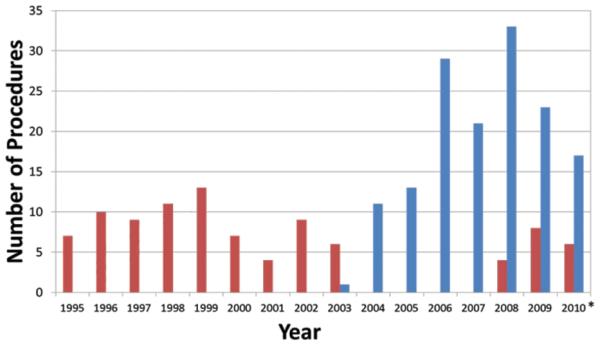
Number of regional chemotherapy treatments performed per year at Duke University Medical Center between 1995 and 2010. *Through August 2010. Red bars, hyperthermic isolated limb perfusion, n=94; blue bars, isolated limb infusion, n=148.
Table 1.
Procedure and Patient Characteristics: Duke University Medical Center Surgical Practice
| HILP | ILI | |||
|---|---|---|---|---|
| n | % | n | % | |
| Total procedures (in 188 patients) | 77 | 34 | 148 | 66 |
|
| ||||
| Age, y | ||||
| < 60 | 36 | 47 | 48 | 32 |
| ≥ 60 | 41 | 53 | 100 | 68 |
|
| ||||
| Men | 32 | 42 | 63 | 43 |
|
| ||||
| Lower extremity | 62 | 71 | 127 | 86 |
|
| ||||
| Disease stage | ||||
| IIIB | 30 | 42 | 70 | 47 |
| IIIC | 41 | 58 | 67 | 45 |
| IV | 0 | 0 | 11 | 7 |
|
| ||||
| Disease burden | ||||
| Low | 26 | 54 | 79 | 53 |
| High | 22 | 46 | 69 | 47 |
|
| ||||
| Correction for IBW | 31 | 40 | 130 | 88 |
|
| ||||
| Repeat procedures | 13 | 36 | 23 | 64 |
HILP, hyperthermic isolated limb perfusion; IBW, ideal body weight; ILI, isolated limb infusion.
Table 2.
Procedure Characteristics: First Time Treatments
| Median | Range | |
|---|---|---|
| ILI | ||
| pH at 30 min | 7.14 | 6.88 - 7.37 |
| PaO2 at 30 min | 6 | 0-37 |
| Base excess at 30 min | −10 | −18.8 - 1.1 |
| Melphalan dose, mg | 48 | 13.7 - 85.8 |
| Peak temperature, °C | 38.6 | 37 - 41.2 |
| HILP | ||
| Melphalan dose, mg | 110 | 24-160 |
| Peak temperature, °C | 40.6 | 39.2 - 42.3 |
HILP, hyperthermic isolated limb perfusion; ILI, isolated limb infusion.
Treatment Response
Of 126 first time ILI procedures performed, 21 (17%) were considered not evaluable for three month response. Patients were not evaluable because they were lost to follow up prior to the three month response evaluation, received an ILI as prophylactic treatment (meaning they had no visible disease at time of treatment), or progressed and went on to have additional therapy prior to the three month response evaluation. Table 3 shows the three month response for all first time treatments. Among the first time ILI procedures, CR was found in 30% (38/126). A CR rate of 55% (34/62) was found for first time HILP procedures, 2 (3%) patients were not evaluable. The response rate (CR+PR) was significantly larger for HILP (81%) compared to ILI (43%) (p < 0.001).
Table 3.
Response at 3 Months: First Time Treatments
| HILP (n = 62) | ILI (n = 126) | |||
|---|---|---|---|---|
| n | % | n | % | |
| CR | 34 | 55 | 38 | 30 |
| PR | 16 | 26 | 16 | 13 |
| SD | 4 | 6 | 14 | 11 |
| PD | 6 | 10 | 37 | 29 |
| NE | 2 | 3 | 21 | 17 |
CR, complete response; HILP, hyperthermic isolated limb perfusion; ILI, isolated limb infusion; NE, ????; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2 summarizes the CR rates observed during first time and repeat procedures. CR rate was larger when HILP was performed after either an ILI (3 of 7 patients) or HILP (2 of 3 patients) (pooled CR of 50%) compared to when ILI was performed after either an ILI (5 of 16 patients) or an HILP (0 of 2 patients) (pooled CR of 28%).
Figure 2.
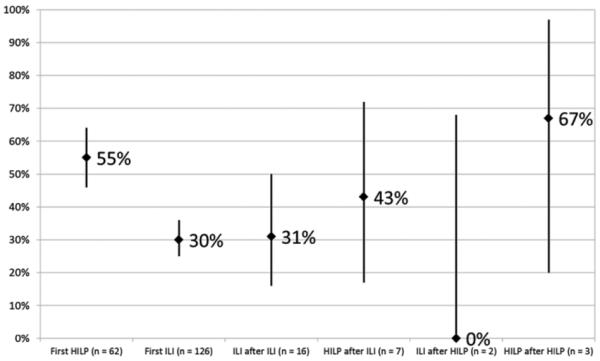
Complete response rates with 80% confidence interval for first time and repeat regional chemotherapy treatments. HILP, hyperthermic isolated limb perfusion; ILI, isolated limb infusion.
Toxicity
CTCAE toxicity was available in 122 (97%) of patients undergoing regional treatment with ILI for the first-time and in all 62 patients undergoing HILP for the first-time. Twenty percent of the ILI patients and 27% of the HILP patients experienced grade 3 or greater toxicity. The rate of treatment-related limb loss was higher for HILP (2/62) than ILI (0/122). The post-operative rate of DVT was 11% for HILP and 4% for ILI. Finally, HILP had a post-operative wound infection rate of 13%. ILI does not involve a surgical incision and thus wound infection rates were not applicable to this procedure.
Table 4 shows the proportion of patients with grade 3 or greater toxicity during first time and repeat procedures. HILP and ILI procedures performed after a first time ILI had larger toxicity rates than the first time procedures. Repeat ILI had a smaller toxicity rate compared to repeat HILP (28% versus 40%, respectively).
Table 4.
Toxicity for First Time and Repeat Treatments
| CTCAE < 3 | CTCAE ≥ 3 | |
|---|---|---|
| First HILP (n = 62) | 45 (73%) | 17 (27%) |
| First ILI (n = 122) | 98 (80%) | 24 (20%) |
| ILI after ILI (n = 16) | 11 (69%) | 5 (31%) |
| HILP after ILI (n = 7) | 3 (43%) | 4 (57%) |
| ILI after HILP (n = 2) | 2 (100%) | 0 (0%) |
| HILP after HILP (n = 3) | 3 (100%) | 0 (0%) |
CTCAE, Common Terminology Criteria for Adverse Events; HILP, hyperthermic isolated limb perfusion; ILI, isolated limb infusion.
Time to in-field progression
TTiP was evaluated in all patients undergoing their first treatment. Fifty of the 62 HILP patients and 85 of the 126 ILI patients had progressed by the time of this analysis. Median TTiP of the HILP procedure was 14 months as compared to 9 months for the ILI procedure (p = 0.122) (Figure 3). The median duration of CR response (in terms of TTiP) was 32 months in HILP and 24 months in ILI (p = 0.156) (Figure 4).
Figure 3.
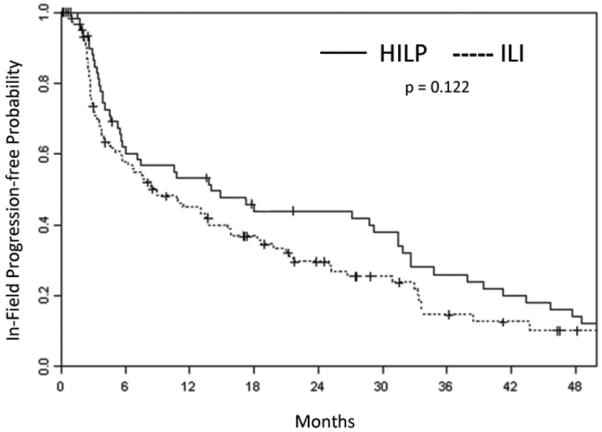
Kaplan-Meier curve demonstrating time to in-field progression for all patients undergoing hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI) for the first time.
Figure 4.
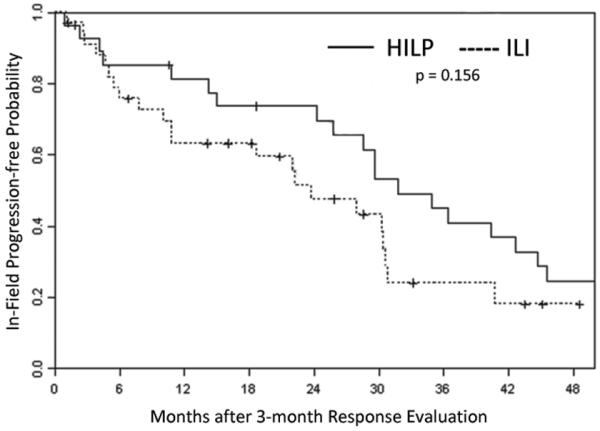
Kaplan-Meier curve demonstrating duration of complete response after undergoing an hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI) for the first time.
Overall Survival
Fifty-two of the 126 ILI patients and 43 of the 62 HILP patients had died by the time of analysis. There was no significant difference between the two procedures in OS (median of 32 months for HILP versus 33 months for ILI, p = 0.647) (Figure 5). The association of OS with response (CR versus other) was assessed with a landmark analysis using three months as the landmark time. Six HILP patients and 17 ILI patients were lost to follow up before three months and one ILI patient died before three months and therefore could not be used in this analysis. For HILP, the 45 patients with a CR had a median survival of 35 months while the 11 patients without a CR had a median of 15 months (Figure 6). For ILI, the 50 subjects with a CR had a median survival of 31 months as compared to the median of 28 months of the 58 patients without a CR.
Figure 5.
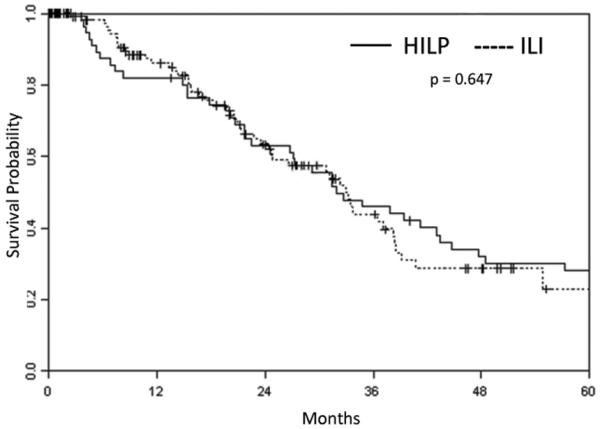
Kaplan-Meier curve demonstrating overall survival in all patients after undergoing either hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI) for the first time.
Figure 6.
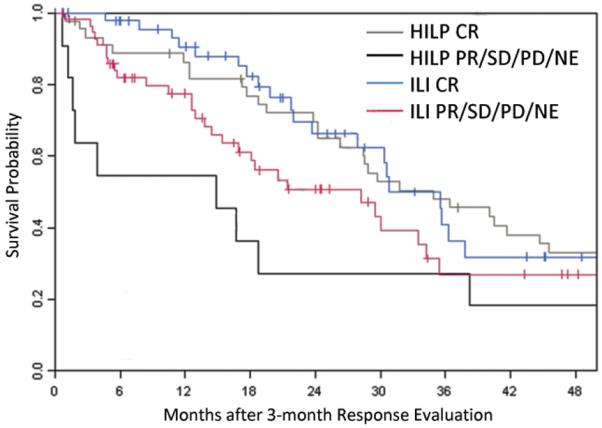
Kaplan-Meier curve demonstrating survival in patients who achieved a complete response and those that did not have a complete response (partial response, stable disease, progressive disease, or not evaluable) after undergoing hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI) for the first time. CR, complete response; NE, ????; PD, progressive disease; PR, partial response; SD, stable disease.
Factors associated with response in-field of treatment
Multivariate analysis was used to determine which patient and procedural variables predict tumor CR+PR response to ILI. A total of 9 variables met criteria for inclusion in the model (Table 5). Variables associated with having a CR+PR included: a lower melphalan dose (44.42 ± 13.39 mg in responders versus 50.96 ± 16.4 mg in non-responders) (p = 0.009), and having an infusion of the lower extremity (p = 0.00). Multivariate analysis of HILP variables (Table 5) included age (p = 0.155) and gender (p = 0.203) as the only variables in the model.
Table 5.
Multivariate Predictors of Response and Toxicity: First Time Treatments
| Response (CR+PR) |
Toxicity (CTCAE ≥ 3) |
|||||
|---|---|---|---|---|---|---|
| Predictor | OR (95% CI) | p Value | Predictor | OR (95% CI) | p Value |
|
| HILP | Age (≥ 60 y) | 0.33 (0.07-1.53) | 0.155 | Age (≥ 60 y) | 4.61 (0.66-32.18) | 0.123 |
| Female sex | 2.60 (0.60-11.33) | 0.203 | Female sex | 0.12 (0.01-1.39) | 0.090 | |
| Lower limb* | Lower limb* | |||||
| Corrected IBW* | Corrected IBW* | |||||
| Disease stage* | Disease stage* | |||||
| High disease burden* | High disease burden | 0.46 (0.07-2.92) | 0.408 | |||
|
| ||||||
| ILI | Age (≥ 60 y) | 2.61 (0.96-7.01) | 0.060 | Age (≥ 60 y)* | ||
| Female sex* | Female sex* | |||||
| Lower limb | 19.32 (2.61-142.50) | 0.004 | Lower limb* | |||
| Melphalan dose | 0.94 (0.90-0.99) | 0.009 | Melphalan dose | 0.95 (0.92-0.99) | 0.010 | |
| Papavarine use* | Papavarine use* | |||||
| Corrected IBW* | Corrected IBW | 13.09 (2.94-58.35) | 0.001 | |||
| Base excess | 0.90 (0.76-1.06) | 0.190 | Base excess* | |||
| PaO2 | 0.88 (0.77-1.00) | 0.044 | PaO2* | |||
| pH* | pH * | |||||
| Ischemia | 1.02 (0.99-1.06) | 0.230 | Ischemia | 1.05 (1.00-1.10) | 0.032 | |
| Limb volume* | Limb volume* | |||||
| Weight* | Weight* | |||||
| Height* | Height | 0.97 (0.91-1.03) | 0.294 | |||
| Peak temperature | 1.53 (0.77-3.03) | 0.220 | Peak temperature* | |||
| Disease stage | 1.51 (0.65-3.50) | 0.330 | Disease stage | 3.68 (1.25-10.84) | 0.018 | |
| High disease burden | 0.51 (0.19-1.39) | 0.190 | High disease burden* | |||
Predictor did not meet inclusion criteria for model.
CR+PR, complete response + partial response; CTCAE, Common Terminology Criteria for Adverse Events; HILP, hyperthermic isolated limb perfusion; IBW, ideal body weight; ILI, isolated limb infusion.
Factors associated with toxicity
Five of the examined variables met inclusion criteria in the multivariate model for ILI factors associated with toxicity (Table 5). Variables associated with having lower toxicity included: a lower melphalan dose (45.75 ± 15.46 mg versus 55.94 ± 14.44 mg) (p = 0.010) and melphalan dosing corrected for IBW (p = 0.001). . Furthermore, patients who had a short hospital stay were more likely to have a CTCAE toxicity < 3 (7.24 ± 2.08 days versus 10.70 ± 6.03 days). CTCAE toxicity ≥ 3 was found in 50% (8/16) of patients who did not have their melphalan dose corrected for IBW versus 15% (16/106) of patients who had their dose corrected for IBW (p = 0.003). Peak CPK was lower for those who had their melphalan dose corrected for IBW compared to those whose dose was not corrected for IBW (1,813.7 ± 3,039.06 U/L versus 2,236.4 ± 3,050.5 U/L).
Univariate analysis for HILP demonstrated three variables that appear to be associated with toxicity. A lower peak in CPK (median values: 418 U/L versus 9,283 U/L) (p = 0.019), earlier postoperative peak in CPK (median values: 1 days versus 2 days) (p = 0.045), and lower dose of melphalan (median values: 94 mg versus 126 mg) (p = 0.018) are associated with CTCAE toxicity < 3. Multivariate analysis included three variables (Table 5) in the model, but failed to show a significant association between HILP variables and toxicity.
PET/CT for response evaluation and surveillance
PET/CT scans were available pre-treatment and every 3 months post-treatment for 72 patients undergoing ILI. Thirty-six percent (26/72) of the patients had a CR at 3 months following ILI as determined by clinical and pathologic exam; however, PET/CT performed 3 months post-treatment identified only 50% (13/26) of these patients as complete responders. For patients with PR/SD/PD after ILI at 3 months, there was agreement between PET/CT performed at that time and clinical/pathologic exam in 98% (44/45) of cases. Only 5 HILP patients had pre-treatment and post-treatment PET/CT scans; three of these patients had CR based on clinical and pathologic exam at 3 months. PET/CT at 3 months post-treatment correctly identified one CR and classified the remaining 4 patients as non-responders. Of those with PR/SD/PD after HILP at 3 months, there was agreement between PET/CT performed at that time and clinical/pathologic exam in 100% (2/2) of cases. During follow up, surveillance PET/CT found 43 of 72 patients who underwent ILI had 49 identified areas of FDG avid activity at a median time of 138 days. FDG activity was seen most often in regional lymph nodes (n = 29) and lung (n = 10). Of the 49 lesions identified, 25 were surgically resected, with lymph node and lung metastasectomy performed in 62% (18/29) and 50% (5/10) of patients, respectively.
Discussion
Over the last several years ILI has gained popularity as a less complex technique to deliver regional chemotherapy as compared to HILP. When comparing the two techniques, both response rates and degree of toxicity must be considered in trying to determine the therapeutic index of the treatments and which treatment should be preferentially utilized. Response is best measured in two ways. Initially the absolute value of response, especially complete responders needs to be defined at three months. Second, the durability of response, especially complete responders, needs to be defined as measured by regional progression free survival. Our single institution experience demonstrates that at 3 months, HILP had a higher complete and overall response rate as compared to ILI. Similar findings, that HILP is associated with higher response rates, were previously reported by the Sydney Melanoma Unit (SMU) and have been seen in multi-center trials.8,12,13,25
For all patients, HILP is associated with a more durable response that is clinically relevant compared to ILI, with median progression free survival of 14 months versus 9 months despite not being statistically significant (p = 0.122). However, once a complete response is achieved, the durability of a complete response appears to be similar between the two techniques. This observation has been suggested by the experience of the Sydney Melanoma Group as well who found that the median durability of a complete response after ILI was 22 months which was similar their reported durability of complete response after HILP (median 19 months).13,26
More controversial is whether any form of regional treatment is associated with improved survival. In a large, multi-center WHO/EORTC trial, improvement in locoregional recurrence after adjuvant HILP did not improve overall survival and there was no impact on prevention of systemic disease.27 However, there does appear to be a survival benefit for those who do achieve a CR to regional treatment. Five year survival after achieving CR to an HILP has been previously reported to be 40% with (95% CI: 30-53%) versus 7% (95% CI: 2.5-17%) in patients with PD.25 Likewise for ILI, median survival was reported as 53 months in those with CR versus 25 months in those with PD.13 Our data also suggests that there may be a benefit of obtaining a CR as compared to any other type of response in that it improves survival for both HILP (35 months versus 15 months) and ILI (31 months versus 28 months). Furthermore, previous data from our group suggested that a CR to HILP was the best predictor of survival in patients with in-transit metastases, outperforming even nodal involvement as a predictor.21 Whether the improved survival seen in patients who achieve a CR to regional therapy is just a manifestation of tumor biology in that more indolent tumors respond better to chemotherapy or that the regional treatment induced some type of systemic immune response that is more marked in patients who have complete destruction of the tumor remains to be determined. Several studies have clearly shown that regional treatments can induce systemic immune responses that improve survival in animal models but whether regional chemotherapy can achieve this in humans is currently under investigation.28
Some reports have questioned whether ILI is associated with a worse survival as compared to HILP because a more distally placed tourniquet is utilized and regional lymph nodes are not routinely removed.29 However, we have demonstrated that Esmarch tourniquets can be utilized very effectively in ILI as an alternative to a pneumatic tourniquet to achieve a higher level of isolation.23 In addition, we have safely performed nodal dissections in conjunction with an ILI after completing the regional treatment and correcting the patient’s coagulation status by reversing the heparinization with protamine. As such, we have found no strong evidence from our own experience of doing both HILP and ILI that overall survival for all treated patients differs between techniques as shown in Figure 5.
Several of the variables that had previously been found to correlate with improved response to regional therapy in our initial report such as a lower limb volume or use of papavarine, were not significant in this review in univariate or multivariate analysis.12 The only clinically relevant variables that were found to be associated with response, was seen in patients undergoing ILI of the lower limb and in patients receiving a lower dose of melphalan. These observations seem to suggest that more optimal drug dosing and delivery may occur in patients with smaller lower limb volumes (i.e., individuals closer to their ideal body weight). This observation was noticed previously in the multicenter ILI study and underscores the need for more work on understanding and optimizing the pharmacokinetics of regional drug therapy with melphalan using ILI.12
Examining the spectrum of toxicity associated with the two procedures, there appears to be a similar side effect profile with the one major exception being catastrophic limb amputation. Review of data from our institution, SMU, and two multi-center trials of regional chemotherapy treatments, found the rate of limb loss after HILP was 2% (6/294) versus ILI with 0.3% (1/313).12 Another large review of HILP series reported in the literature identified a limb loss rate of 0.8%.30 Analysis of variables associated with toxicity in regional therapy found that for ILI, a lower melphalan dose, correcting for IBW, and shorter length of hospitalization were all associated with CTCAE toxicity < 3. Interestingly, on multivariate analysis in this study CPK was not found to be associated with toxicity in those undergoing ILI, although it has been associated with toxicity in several other studies.31,32 The median peak CPK for all the patients in this study undergoing ILI for the first time was 595.5 U/L. We have previously shown that patients that have a melphalan dose corrected for ideal body weight had a lower peak CPK (536.5 U/L versus 1117 U/L).9 Since 87% (110/126) were corrected for IBW this could explain the lower peak in CPK in this study and why it failed to show an association with toxicity. Analysis of toxicity may also have been biased by the 50 patients undergoing ILI who were on trials in which they received systemic targeted agents in conjunction with their regional melphalan treatment. While we were able to demonstrate that the two targeted agent compounds ADH-1 and sorafenib did not affect melphalan pharmacokinetics, we did see a marked increase in toxicity and marked elevations in CPK levels in the context of our phase I study of systemic sorafenib in combination with melphalan ILI.33 We did not observe an increase in either our phase I or II trial of systemic ADH-1 in combination with melphalan ILI.19,20 As new agents are being rapidly developed for treatment of melanoma that may be candidates for combination therapy with melphalan-based ILI or HILP, potential additive toxicity and how to best monitor patients for toxicity is a concern.
For HILP, a lower peak in CPK, earlier postoperative peak in CPK, and lower dose of melphalan were all associated with CTCAE toxicity < 3. The increased CPK and length of stay in hospital being significant seems logical as an increased CPK is indicative of muscle damage which correlates with increased measures on CTCAE grading scale (i.e., pain, edema, etc.). Toxicities in turn keep patients in the hospital longer as we follow daily CPK levels to make sure it is trending back towards normal physiologic range. An increased melphalan dose and not correcting for IBW have also previously been associated with increased toxicity.23
The response and toxicity of repeat procedures is also important to evaluate as patients frequently progress after an initial procedure. The minimally invasive technique of ILI makes it an attractive treatment option for repeat regional therapies, in contrast to HILP which is difficult to repeat in a vascular bed once it has been used. We currently do not know if the patients who respond to one form of melphalan based regional therapy are the same ones who respond to the other, i.e., are complete responders to ILI the same as complete responders to HILP. Overall, repeat HILP had improved response (CR of 50%) compared to repeat ILI procedure (CR 28%); however, repeat HILP did have a higher rate of CTCAE toxicity grade ≥ 3 compared to repeat ILI (40% versus 28%). These numbers, however, suggest that salvaging patients that progress or respond incompletely after an initial regional therapy may be possible. While HILP appeared to have a better response rate in the redo setting, the technique that is ultimately utilized depends upon appropriate patient selection which includes considering: nodal involvement, patient comorbidities, and potential vascular access issues. Decreased response rate and increased toxicity for both repeat ILI and repeat HILP following initial regional chemotherapy treatments have also been reported by other centers.34-36 Larger samples sizes are needed to be able to statistically analyze the repeat procedures data.
Previous studies of the diagnostic performance of PET/CT for melanoma staging and detection of distant metastases have found PET/CT to be accurate; in one study, change of treatment according to PET/CT findings occurred in 48.4% (121 patients).37 Another study of 70 patients found PET/CT to have 87% sensitivity and 98% specificity for detection of metastases.38 Identification of potentially surgical resectable disease is important because complete surgical metastasectomy has been shown to enhance survival.39 In this study, PET/CT scans every 3 months following treatment for one year and every 6 months thereafter identified 49 FDG avid spots of which 51% (25/49) were resected. There is little data examining the role of PET/CT in predicting response after regional chemotherapy treatment. The persistence of fludeoxyglucose (FDG) activity on PET/CT scans in 50% of the patients with a clinical and pathologic CR suggests that PET/CT has limited utility as a surrogate marker of treatment effect. Outside of the treated extremity, we found PT/CT to be more clinically valuable. We are currently examining the individuals who despite complete clinical and pathologic response have remaining FDG activity in the extremity and whether this may be a marker of a beneficial or detrimental outcome.
While one could certainly argue that HILP should be the initial procedure of choice for in-transit melanoma in view of its higher response rates, we have adopted a slightly different approach to patients with regionally advanced disease confined to the extremity based upon our experience presented in this manuscript. Given that there is no difference in overall survival between the two methods of delivering regional therapy and that the durability of a complete response is similar for both procedures, we have opted for utilization of ILI as our initial regional therapy in patients with clinically negative lymph nodes. In patients who have clinically enlarged lymph nodes we generally perform an HILP initially as this allows for dissection of the lymph node basin in which the HILP catheters are placed. For patients who fail to respond appropriately after ILI, we preferentially use HILP as a salvage therapy if their disease is still confined to the extremity. For patients who fail a regional therapy of any type we have phase I protocol based ILI trials using either the novel chemotherapeutic agent temozolomide or a systemic targeted agent in conjunction with a melphalan based ILI as treatment options. While other institutions may not have the volume of patients to be able to follow this approach, our experience suggests that both HILP and ILI have potential utility in this patient population. How one ultimately decides to use these techniques will depend on a number of factors including familiarity with the techniques, volume of patients treated, institutional resources, patients’ disease distribution, and overall functional status of the patient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Dr Tyler received honoraria from Adherex Technologies as a conference attendee and from Bayer Healthcare Pharmaceuticals for participation in an Investigator’s Conference. The ADH-1 trial was supported by a grant from Adherex Technologies. Bayer Healthcare Pharmaceuticals provided study drug (sorafenib, Nexavar) for the phase I trial of systemic sorafenib and regional melphalan. All other authors have nothing to disclose.
Presented at Southern Surgical Association 122nd Annual Meeting, Palm Beach, FL, December 2010.
References
- 1.Balch CM, Houghton AN, Peters LJ. Cutaneous melanoma. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 4th ed. JB Lippincott; Philadelphia: 1993. p. 1612. [Google Scholar]
- 2.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12:587–596. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Creech O, Jr., Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaase JM, Kroon BB, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994;115:39–45. [PubMed] [Google Scholar]
- 5.Minor DR, Allen RE, Alberts D, et al. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55:2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63:2551–2561. doi: 10.1002/1097-0142(19890615)63:12<2551::aid-cncr2820631233>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Kroon BB, Van Geel AN, Benckhuijsen C, Wieberdink J. Normothermic isolation perfusion with melphalan for advanced melanoma of the limbs. Anticancer Res. 1987;7:441–442. [PubMed] [Google Scholar]
- 8.Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;24:4196–4201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 9.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 10.Skene AI, Bulman AS, Williams TR, et al. Hyperthermic isolated perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J Surg. 1990;77:765–767. doi: 10.1002/bjs.1800770716. [DOI] [PubMed] [Google Scholar]
- 11.Fraker DL, Eggermont AM. Cutaneous Melanoma. 14th ed. Quality Medical; St. Louis, MO: 2003. pp. 405–418. [Google Scholar]
- 12.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715-717. [DOI] [PubMed] [Google Scholar]
- 13.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 14.Beasley GM, Kroon HM, Ross MI, et al. Isolated limb infusion. In: Balch CM, Houghton AN, Soper AJ, Soong SJ, Thompson JA, editors. Cutaneous Melanoma. 5th ed. Quality Medical Publishing; St. Louis, Missouri: 2009. pp. 541–562. [Google Scholar]
- 15.Vrouenraets BC, Klaase JM, Nieweg OE, Kroon BB. Toxicity and morbidity of isolated limb perfusion. Semin Surg Oncol. 1998;14:224–231. doi: 10.1002/(sici)1098-2388(199804/05)14:3<224::aid-ssu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 18.McMahon N, Beasley GM, Sanders G, et al. A phase I study of systemic sorafenib in combination with isolated limb infusion with melphalan (ILI-M) in patients (pts) with locally advanced in-transit melanoma. J Clin Oncol. 2009;27 doi: 10.1002/cncr.24509. Abstract no. 9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beasley GM, McMahon N, Sanders G, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009 Jul 27; doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beasley GM, Sanders G, McMahon N, et al. Final results of a prospective multi-center phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion (M-ILI) in patients with advanced extremity melanoma. Ann Surg Oncol. 2010;17:21. [Google Scholar]
- 21.Aloia TA, Grubbs E, Onaitis M, et al. Predictors of outcome after hyperthermic isolated limb perfusion: role of tumor response. Arch Surg. 2005;140:1115–1120. doi: 10.1001/archsurg.140.11.1115. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132:903–907. doi: 10.1001/archsurg.1997.01430320105017. [DOI] [PubMed] [Google Scholar]
- 23.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–961. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng TY, Grubbs E, Abdul-Wahab O, et al. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg. 2003;186:460–467. doi: 10.1016/j.amjsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg. 2007;245:591–596. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lejeune FJ, Deloof T, Ewalenko P, et al. Objective regression of unexcised melanoma in-transit metastases after hyperthermic isolation perfusion of the limbs with melphalan. Recent Results Cancer Res. 1983;86:268–276. doi: 10.1007/978-3-642-82025-0_45. [DOI] [PubMed] [Google Scholar]
- 27.Koops HS, Vaglini M, Suciu S, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol. 1998;16:2906–2912. doi: 10.1200/JCO.1998.16.9.2906. [DOI] [PubMed] [Google Scholar]
- 28.Xing Y, Lu X, Pua EC, Zhong P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochem Biophys Res Commun. 2008;375:645–650. doi: 10.1016/j.bbrc.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reintgen DS, et al. Regional therapy for recurrent metastatic melanoma confined to the extremity: hyperthermic isolated limb perfusion vs. isolated limb infusion. Ann Surg Oncol. 2009;16:S1. doi: 10.3390/cancers20100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taber SW, Polk HC., Jr. Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol. 1997;4:440–445. doi: 10.1007/BF02305559. [DOI] [PubMed] [Google Scholar]
- 31.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009;16:2570–2578. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 32.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 33.Coleman AP, Beasley GM, McMahon N, et al. Augmenting regional therapy with targeted agents: a phase I multi-institutional study of systemic sorafenib in conjunction with regional melphalan for in-transit melanoma of the extremity. 2010 doi: 10.1245/s10434-012-2373-8. In submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932–1940. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 35.Grunhagen DJ, van Etten B, Brunstein F, et al. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol. 2005;12:609–615. doi: 10.1245/ASO.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 36.Noorda EM, Vrouenraets BC, Nieweg OE, et al. Repeat isolated limb perfusion with TNF-alpha and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur J Surg Oncol. 2006;32:318–324. doi: 10.1016/j.ejso.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 38.Aukema TS, Valdes Olmos RA, Wouters MW, et al. Utility of preoperative 18F-FDG PET/CT and brain MRI in melanoma patients with palpable lymph node metastases. Ann Surg Oncol. 2010;17:2773–2778. doi: 10.1245/s10434-010-1088-y. [DOI] [PubMed] [Google Scholar]
- 39.Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919–924. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]


