Abstract
Palladin is an actin associated protein serving as a cytoskeleton scaffold, and actin cross linker, localizing at stress fibers, focal adhesions, and other actin based structures. Recent studies showed that palladin plays a critical role in smooth muscle differentiation, migration, contraction, and more importantly contributes to embryonic development. This review will focus on the functions and possible mechanisms of palladin in smooth muscle and in pathological conditions such as cardiovascular diseases and cancers.
Keywords: Palladin, Smooth muscle, Actin cytoskeleton, Migration, Differentiation
Introduction
Cardiovascular diseases are the world's largest killers taking over 17 million lives a year. Heart disease and stroke, the most common cardiovascular diseases are the top leading causes of death for both men and women in the United States and the World (Hoffman et al. 2004). Cardiovascular disease is a multi-factorial, multi-faceted disease and is fought on all fronts, from basic science to clinical practice, and from genetics to vascular implants and mini-invasive procedures. The interest in vascular smooth muscle cells (SMCs) contribution to cardiovascular disease derives from their principal function to regulate contraction, blood vessel tone, diameter, blood pressure, and blood flow distribution. In addition, as SMCs are not terminally differentiated in the vascular wall, phenotypic switching of SMCs plays a major role in vascular diseases such as post-angioplasty restenosis and atherosclerosis (Kolodgie et al. 2004; Owens et al. 2004). Therefore appreciation of SMC function and its regulation is critical for the understanding of the genesis, progression, diagnosis and treatment of cardiovascular disease as well as the processes involved during development of blood vessels.
Actin is a major cytoskeleton constituent that can polymerize to form helical actin filaments (F-actin), the organization of which supports cellular mechanical strength and movement. Actin architecture is in a dynamic state with regulated assembly, rearrangement and disassembly of F-actin being critical in a variety of complex cellular processes including cell shape and movement, endocytosis, cell division and cellular interactions required for tissue formation and integrity. Major efforts to understand these processes has to date, lead to the identification of multitude of proteins found binding to or associated with the actin cytoskeleton where they function to regulate assembly and disassembly, as scaffolding molecules or signaling mediators. Palladin is a newly discovered actin associated protein that can function as a molecular scaffold and actin cross linker (Boukhelifa et al. 2001; De Kimpe et al. 2009; Dixon et al. 2008; Endlich et al. 2009; Goicoechea et al. 2006). The presence of palladin in the nucleus in the basal state and its ability to translocate to the nucleus upon stimulation suggests that palladin may in addition to its role as a molecular scaffold and actin cross-linking protein have a signaling function in excitation–transcription (Jin et al. 2010; Endlich et al. 2009). In this review I will focus on the role of palladin in SMCs and in the development of diseases such as those of the vasculature and cancers.
Palladin isoforms and structural domains
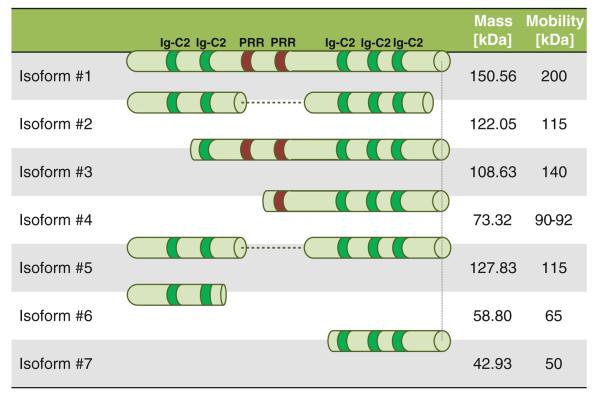
Palladin is a widely expressed protein found in stress fibers, focal adhesions, growth cones, Z-discs, and other actin-based subcellular structures. It belongs to a small gene family that includes the Z-disc proteins myopalladin and myotilin, all of which share similar Ig-like domains (reviewed in Otey et al. 2005, 2009b). Palladin exists as multiple isoforms (Fig. 1) through alternative transcription initiation sites and splicing (Otey et al. 2009b). The three major isoforms are 200, 140, 90–92 kDa. Recently other isoforms have also been reported: 115, 75, 65, and 50 kDa. The expression of different isoforms is likely regulated in a tissue specific manner. The 200 kDa isoform is predominantly expressed in the heart, skeletal muscle, testis and bone (Parast and Otey 2000; Rachlin and Otey 2006; Wang and Moser 2008); the 140 kDa isoform is widely expressed with the exception of liver, muscle, and skin (Parast and Otey 2000; Rachlin and Otey 2006; Wang and Moser 2008); the 90–92 kDa isoform has two isoforms A and B, and is broadly expressed in embryonic tissues and highly expressed in adult smooth muscle tissues (Jin et al. 2007; Wang and Moser 2008); so far the 115 kDa is detected in Hela cells (Parast and Otey 2000), the 65 kDa isoform is detected in the pancreas and uterus (Goicoechea et al. 2010; Wang and Moser 2008), the 75 kDa isoform is described in cancer associated fibroblasts (Salaria et al. 2007), and finally the 50 kDa isoform is found in brain and fibroblasts (Liu et al. 2007a). Whether there are specialized roles for the recently identified isoforms remains to be discovered. The majority of the identified isoforms contains three Ig like domains at the carboxyl-terminus. Most isoforms contain a proline rich N-terminus that interacts with profilin (Boukhelifa et al. 2006), VASP family members (Boukhelifa et al. 2004), Spin90 (Ronty et al. 2007), ArgBP2 (Ronty et al. 2005), Eps8 and Src (Goicoechea et al. 2006; Ronty et al. 2007). The larger isoforms (200 and 140 kDa) have an additional proline rich region that binds to Lasp-1 (Rachlin and Otey 2006), Nebulin and Nebulette (Bang et al. 2001). In addition, the N-terminus of palladin also interacts with α-actinin (Ronty et al. 2004), and lipoma preferred partner (LPP) (Jin et al. 2007). At the C-terminus, palladin interacts with Ezrin (Mykkanen et al. 2001), MRTF (Jin et al. 2010), F-actin (Dixon et al. 2008), and the PDZ motif of enigma family members such as ZASP (von Nandelstadh et al. 2009) and CLP36 (Maeda et al. 2009). The ability to connect with all these molecules suggests that palladin has the potential to serve as a cytoskeleton scaffold and signaling mediator. Palladin's spatial distribution in the cell may also define its roles. For example, it interacts with LPP solely at focal adhesions and with α-actinin periodically localized along stress fibers and at the Z line of cardiac muscles (Jin et al. 2007; Ronty et al. 2004) (Fig. 3).
Fig. 1.
Scheme showing multiple palladin isoforms and their domain organization. PRR proline rich region, Ig immunoglobulin domain. The schema was modified from Dr. Otey (Otey et al. 2009b)
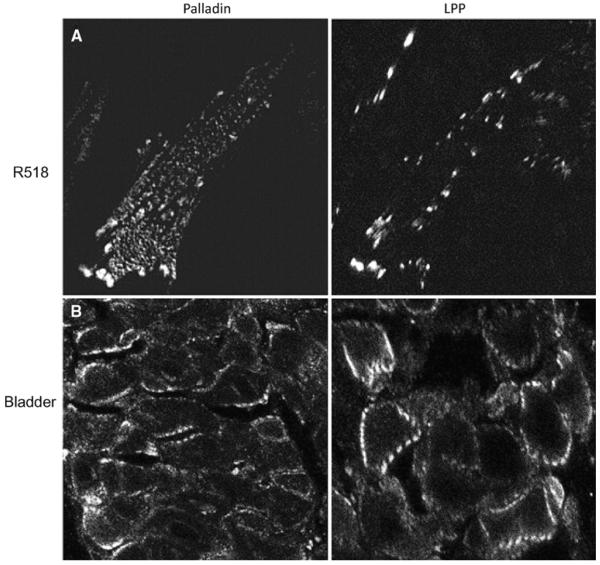
Fig. 3.
Palladin localizes at actin attachment sites at cell membrane dense bodies in SM tissue and their equivalent, focal adhesions in cultured rat aortic SMCs (Jin et al. 2007). a Both palladin and LPP localize in focal adhesions of cultured SMCs (R518, rat aortic smooth muscle cells). Unlike LPP, palladin also localizes periodically along the stress fibers with α-actinin (not shown) in cultured SMCs. b Note the periodic localization of both palladin and its partner protein, LPP around the periphery of bladder SMCs at the plasma membrane dense bodies. Cultured rat aortic SMC and Guinea pig bladder were fixed and immunostained with antibodies against palladin (monoclonal) and LPP (polyclonal). The signals were detected with corresponding secondary IgG conjugated with Alexa fluor 488 or 594 and visualized with a confocal microscope
Palladin and SMC differentiation
Vascular SMCs are not terminally differentiated and can undergo phenotype switching upon the alteration of local environmental cues such as occurs in vascular injury. This allows SMCs to carry out an array of functions in different SMC-containing organs during normal development and maturation, as well as to permit repair of injured smooth muscle tissues resulting from mechanical trauma or inflammation (Carmeliet 2000; Owens et al. 2004). A large amount of evidence suggests that an impaired SMC phenotype during development results in defects in vascular remodeling of great arteries and congenital cardiovascular anomalies (Gerthoffer and Gunst 2001; Kawai-Kowase and Owens 2007; Owens 1995; Owens et al. 2004), but a full understanding of the complex processes underlying SMC differentiation is far from complete. SMC differentiation is highly dependent on the complex local environmental cues including mechanical forces, extracellular matrix components, neuronal and cellular factors that change the expression of SMC marker genes. During the process, SMCs acquire a different SMC marker gene expression profile. Changes include the structure of the contractile actin cytoskeleton and expression of actin associated proteins. The differentiation of SMCs is characterized by the up-regulation of SM markers that include smooth muscle alpha-actin (SMA), smooth muscle myosin heavy chain (SM MHC) and smooth muscle 22 (SM22), which are associated with the contractile phenotype.
Several lines of evidence suggest that palladin plays an important role in regulating SMC phenotypic switching. In an in vitro embryoid body differentiation system we have found that palladin mRNA was induced in embryoid bodies at day 20 (Jin et al. 2009b). The spontaneously contracting SMCs formed during the development process from both palladin null and wild type embryonic stem cells (Jin et al. 2009b). However, in palladin null embryoid bodies at day 28, the expression of SMC marker genes SMA, SM22, and SM MHC was significantly decreased compared with wild type. The expression of these genes was also partially decreased in heterozygote palladin embryoid bodies. The expression of myosin, SMA, calponin, and h-caldesmon mRNA and protein was markedly decreased in palladin null SMCs (Jin et al. 2009b). This surprising observation was confirmed in another in vitro SMC differentiation model, A404 cells. A404 cells were derived from multipotential P19 embryonic carcinoma cells with a SMA promoter-enhancer-driven puromycin resistant gene (Manabe and Owens 2001). The undifferentiated A404 cells have no detectable SMC gene expression but express all known SMC marker genes upon treatment with retinoic acid. After 48 h treatment with retinoic acid, the 90–92 kDa isoform of palladin protein was readily and significantly induced in A404 cells as well as the expected SMCs marker genes SMA and SM22. The induction of palladin preceded the expression of SMA (Jin et al. 2010). The induction of palladin was also reported in acute promyelocytic leukemia cells treated with retinoic acid (Liu et al. 2000). Overexpression of palladin in the absence of retinoic acid can also significantly induce SMA and SM22 gene expression in undifferentiated A404 cells (Jin et al. 2010). In contrast, knock down of 90–92 kDa palladin with siRNA attenuates the retinoic acid-induced expression of SMC marker genes. In palladin knockout embryos, the expression of SMA and SM22 proteins was decreased. In the knockout model, the 90–92 and 50 kDa isoforms were not detected, while the expression of other isoforms is not clear. Due to palladin deficient animals dying at embryo day E15.5 (Liu et al. 2007a), precluding harvesting of adult blood vessels, the expression of SM marker genes was measured in umbilical vessels. In umbilical vessels from palladin knockout embryos E11.5, the SMC marker proteins were also decreased both at the mRNA and protein levels (Jin et al. 2010). All these observations suggest that palladin somehow regulates SMC marker gene expression.
Regulation by palladin during differentiation also occurs in the other cell types. For example, it has been shown that palladin plays a role in the differentiation of Rcho-1 stem cells into trophoblast giant cells (Parast and Otey 2000). Carpen's laboratory showed that palladin was upregulated during the process of myofibroblast differentiation in response to TGF-β1 and skin injury (Ronty et al. 2006). After treatment with TGF-β1, human skin fibroblasts had more robust stress fibers and increased expression of the 90–92 kDa isoform of palladin. The 140 kDa isoform of palladin was abundantly induced after 24 h of TGF-β1 treatment, which preceded the induction of SMA. In rat dermal wounds palladin colocalized with neo formed SMA in the stress fibers, as the proto-myofibroblasts differentiated into myofibroblasts (Ronty et al. 2006). Similarly, palladin was newly expressed in the hypertrophic and migratory astrocytes that assemble strong actin filament bundles in response to nerve injury, while there was no detectable palladin expression in static astrocytes (Boukhelifa et al. 2003). More recently, a study revealed that palladin was down regulated in a metastatic colon cancer cell line E1 (Tay et al. 2010). This E1 cell line derived from a colon cancer cell line HCT116, showed a mesenchymal/fibroblastic morphology (epithelial-mesenchymal transition, EMT), dissociated adheren junctions, and more invasive features. Suppression of EMT with Erk inhibitor restored the expression of palladin in E1 cells. Yet knockdown of palladin with siRNA in HCT115 cells suppressed the E-cadherin junction localization and cell migration. The author suggested that palladin plays a role in EMT in transforming HCT116 cells to E1 cells (Tay et al. 2010).
A major question is what is the molecular mechanism underlying palladin's ability to regulate the expression of SMC marker genes and whether it involves actin dynamics? One possible mechanism is through myocardin-related transcription factors (MRTFs) which are widely expressed, known to be important in regulating expression of SMC specific marker genes. These potent transcriptional factors associate with serum response factor (SRF), a transcription factor that binds to CArG boxes typically found in the promoter region of SMC marker genes (Cen et al. 2004; Han et al. 2006; Miralles et al. 2003; Wang et al. 2004, 2007). Binding of G-actin to the amino-terminus of MRTF inhibits MRTF activity by preventing nuclear accumulation of MRTFs and by repressing transcriptional activation by the MRTF–SRF complex (Medjkane et al. 2009; Mouilleron et al. 2008; Posern and Treisman 2006; Vartiainen et al. 2007). In response to RhoA signaling or other stimuli that promote actin polymerization, MRTFs are released from G-actin and translocate to the nucleus, where they associate with SRF to drive the expression of SMC genes and other cytoskeletal proteins. Knockout of palladin in SMCs and fibroblasts results in decreased and disordered stress fibers associated with the decreased F- to G-actin ratio (Goicoechea et al. 2006; Jin et al. 2009b; Liu et al. 2007b).
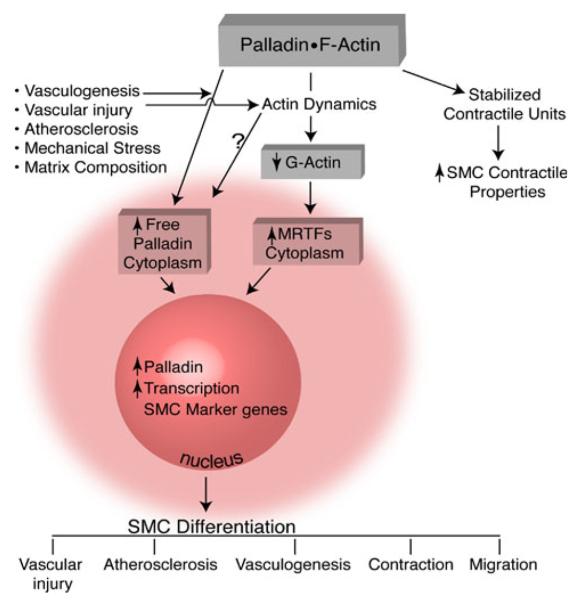
On the other hand, overexpression of palladin induces strong stress fibers in cultured cells. Palladin's ability to increase the F:G actin ratio in SMCs would free MRTF from cytosolic G-actin. In addition, it was found that the C-terminus of palladin interacts and colocalizes in the nucleus with MRTFs (Jin et al. 2010). The endogenous palladin was readily detected in the nucleus in rat aortic SMCs (Jin et al. 2010) and podocytes in kidney derived cells (Endlich et al. 2009; Mykkanen et al. 2001). The nuclear localization of palladin and MRTF may promote chromatin remodeling and initiate the MRTF–SRF transcriptional activation of SMCs marker genes. Investigation is needed to determine whether palladin interacts with MRTFs in the cytoplasm and then translocates to the nucleus, or they interact in the nucleus directly. Another possible mechanism for palladin's ability to positively influence the expression of SMC marker proteins is that palladin can directly interact with the promoters of SMC marker genes and initiate transcription of SMC marker genes. It was shown that mutation of CArG elements in the SMC gene promoters significantly decreased the responses to palladin (Jin et al. 2010). In addition, ChIP assays suggested that palladin can bind to SMA promoter within intact chromatin. Thus, it is also possible that palladin can shuttle to the nucleus either alone or with a partner and directly bind to SMC gene promoters to modulate chromatin structure and regulate transcription of SMC marker genes. However, no known nuclear localization signal is identified in the palladin protein sequence, raising the possibility that another yet to be identified protein shuttles palladin to the nucleus. There are also other possibilities that palladin may regulate SMC gene expression through other pathways such as Smad3 and MAPK as demonstrated in myofibroblast cells (Ronty et al. 2006). Based on the evidence of nuclear localization of endogenous palladin and interaction with MRTFs we favor the hypothesis that palladin regulates SMC differentiation both directly by binding to the SMC marker gene promoters and indirectly by interacting with transcription factors such as SRF and MRTFs (Fig. 2).
Fig. 2.
Possible mechanisms for the roles of palladin in regulating SMC differentiation and contraction (Jin et al. 2010)
Palladin and SM contractile force development
F-actin transduces the mechanical force and biochemical signals between the contractile apparatus and the extracellular matrix coupling adjacent SMCs by attaching to cell membrane dense bodies in SM tissues and their equivalent focal adhesions in cultured SMCs. These in turn associate with transmembrane integrins and adhesion receptors. The barbed ends of actin filaments insert at membrane and cytosolic dense bodies (Bond and Somlyo 1982) and based on the Z-discs of striated muscles, this would be expected to serve as the major site for rapid filament growth and control of filament length by capping proteins. The details of how SMCs carryout their contractile work while simultaneously refreshing their filament network and maintaining its architecture is poorly understood, but this could contribute to measured changes in actin dynamics. Studies in canine tracheal SM showed that actin dynamics somehow impacts contractility in response to external environmental cues (Ding et al. 2009; Gunst et al. 2003). However, the generality of this phenomenon as well as the structural and mechanical basis of these intriguing observations is yet to be revealed. Palladin is well positioned in cells to affect matrix-cytoskeleton signaling by virtue of both binding to filamentous actin and by localizing to focal adhesions and dense bodies (Fig. 3). In palladin null mouse embryonic fibroblasts, the cell-ECM interaction was found to be weakened (Liu et al. 2007b) and the actin cytoskeleton architecture was disordered. In addition the ECM receptor, β1-integrin, was decreased, and this reduction could be rescued by treatment with the proteinase inhibitor MG-132. These findings suggest that palladin not only regulates the actin cytoskeleton structure, but can also stabilize the β1-integrin expression level by influencing the proteolysis process (Liu et al. 2007b). We have recently reported that force generation was impaired in palladin deficient embryoid body derived SMCs (Jin et al. 2009b). Force generation in these cells was measured by incorporating SMCs into a collagen gel, which following polymerization was cut into small strips and attached to a force transducer (Sinha et al. 2006; Woodsome et al. 2006). It was found that the myosin phosphatase inhibitor, Calyculin A, used to induce maximum myosin light chain phosphorylation and force, generated significantly less force in palladin null SMCs than that of wild type SMCs (Jin et al. 2009b). Endothelin-1, sphingosine 1-phosphate, and thrombin stimulated tension was also reduced by about 70% in palladin null muscle collagen fibers compared to wild type fibers. Myosin light chains were phosphorylated by lysophosphatidic acid to the same extent in palladin null and wild type SMCs indicating that the Ca2+, calmodulin/myosin light chain kinase signaling was not perturbed in these cells. However, there was a higher G:F actin ratio and less total myosin in palladin null cells, resulting in fewer actin sites for myosin crossbridges for force development (Jin et al. 2009b). Thus loss of palladin significantly compromised the force generating capacity of SM, at least in this experimental paradigm. The findings suggest that the decrease in force of agonist stimulated palladin deficient muscle fibers may be caused by a loss of myosin as well as disruption of the assembly of the actin cytoskeleton and its association and insertion at plasma membrane associated dense bodies. The observed decrease in calponin and caldesmon may also influence force output but this is less clear. Furthermore, palladin has been reported to interact with Ezrin and VASP and this may also contribute to SM contraction (Boukhelifa et al. 2004, 2006). Ezrin and other ERM proteins are known to function as upstream and downstream effectors of RhoA activity. In SMCs VASP localizes to microfilaments and focal adhesions and interacts with palladin. The ability of palladin to interact with both Ezrin and VASP may coordinate the Rho and VASP mediated control of the cytoskeleton in SMC migration or contraction.
Palladin regulates SMC migration
Vascular smooth muscle cell migration plays an important role during vascular development and contributes to lesion formation and repair in the adult vasculature in response to vascular injury, and during atherosclerosis. Coordinated remodeling of the cytoskeleton within the cell occurs in time and space to bring about cell migration. As an actin associated protein, palladin thus impacts cell migration. We have shown that overexpression of palladin significantly increased cell migration of human iliac vein SMCs and also in migration-defective FAK null fibroblasts (Jin et al. 2007). In a wound healing assay, palladin deficient SMCs showed a significant delay in wound repair (Jin et al. 2009b). In addition, we found that palladin was recruited to lamellipodia (Jin et al. 2007), podosome, and dorsal ruffles (Goicoechea et al. 2006; Jin et al. 2009a) in vascular SMCs supporting its importance in SMC migration and its involvement in the invasion of SMCs in vascular disease. Numerous studies showed that podosomes play a critical role in cell migration. Podosomes are highly dynamic actin based structures. Many actin associated adhesion molecules, including palladin, surround the actin filament core of podosomes. Podosomes have been found in a variety of cells including SMCs (Goicoechea et al. 2006; Jin et al. 2009a), macrophages (Cortesio et al. 2010; Labernadie et al. 2010), and invasive cancer cells. They often form as a ring on the ventral surface of highly invasive cells such as SMCs invading the vascular intima during the development of atherosclerosis or the neointima following injury. In palladin knockdown SMCs, the formation of podosomes was markedly decreased (Goicoechea et al. 2006). Similarly, in breast cancer cells, knockdown of palladin reduced podosome formation, cell motility and invasiveness (Goicoechea et al. 2009). The abundance of palladin was found to be associated with the invasiveness in breast cancer tissue and cell lines (Goicoechea et al. 2009). The impact of palladin on migration was also verified in a colon cancer cell line (Tay et al. 2010). However, another study showed an opposite role of palladin on cell migration under 3D culture conditions: down-regulation of palladin in breast cancer cell lines MDA-MB-231, SUM-159-PT, MCF-7 resulted in increased cell motility (Chin and Toker 2010). The discrepancies among experiments may be explained by different assay conditions, or the expression of different isoforms of palladin in these cell lines. Based on the abundant expression of palladin in SMCs and on its ability to enhance migration in in vitro assays, we hypothesized a role of palladin in cytoskeleton remodeling during the transition of SMCs to migratory cells during disease states. In a rat aortic injury model, both palladin and its partner protein, LPP, like SMA, were expressed in the media of the vessels, and appeared in the neointima 7 days after injury (Jin et al. 2007). In human coronary atherosclerotic vessels, palladin also presents in the artheroma. These data suggest that palladin may play a role in the disease progression and repair of the lesion. The detailed role of palladin isoforms in SM disease states needs to be investigated further in conditional knockout mice. Other cell models have also strongly implicated a role for palladin in cell migration. For example, loss of palladin retards motility of primary neurons and neuroblastoma cells, astrocytes, and cultured fibroblasts (Boukhelifa et al. 2001, 2003; Liu et al. 2007b).
Palladin in embryonic development
As palladin plays a critical role in the architecture of the actin cytoskeleton and also affects cell motility, which is both essential for embryonic development and tissue homeostasis, one would expect a severely compromised palladin null phenotype. Indeed, palladin deficient animals are embryonic lethal at E15.5 due to defects in the neural tube and body wall closure resulting in exencephaly and herniation of the intestine and liver (Liu et al. 2007a; Luo et al. 2005). These authors also reported that palladin null embryos had defects in erythropoiesis (Liu et al. 2007a). These palladin null embryos had decreased red blood cells generated from fetal liver. The attenuation of erythrocytes is accompanied by increased apoptosis of erythroblasts, partial blockage of erythroid differentiation, and erythroblast formation. The SMC marker proteins were decreased in whole embryo homogenates as well as in isolated umbilical vessels of palladin null embryos (Jin et al. 2010). As indicated above, in an in vitro embryoid body differentiation model, we also found that palladin deficient embryonic stem cells can form embryoid bodies containing SMCs, but these cells showed marked reductions in the expression of multiple SMC markers (Jin et al. 2009b). In a comparative expression level analysis (Wang and Moser 2008), the 90–92 kDa isoforms, which is highly expressed in smooth muscle rich tissues, is expressed as early as E8.5 suggesting that vascular development may be impaired in palladin null mice. This remains to be explored in conditional palladin knockout mice targeted to smooth muscle.
Palladin and signaling pathways
Rho and actin dynamics
It has been shown that palladin expression is regulated by actin dynamics (Jin et al. 2007). In rat aortic SMC, the expression of palladin was increased upon treatment with jasplakinolid, an actin stabilizer; while the expression was decreased with latrunculin B treatment, an actin dissociation chemical (Jin et al. 2007). By immunofluorescence analysis, loss of palladin periodicity along stress fibers was observed after 5–10 min treatment with latrunculin B; by 30 min palladin associated with large bundles or clumps of F-actin at the cell periphery or at the perinuclear region. Palladin localized at focal adhesions at early time points but at later time points, palladin was diffusely distributed in the cytoplasm and occasionally at small focal adhesions at the cell periphery.
The Rho family of small GTPases also regulates the expression of palladin. Treatment of rat aortic SMCs with the Rho kinase inhibitor, H1152 resulted in a loss of stress fibers and focal adhesions as expected. This was accompanied by a loss of palladin periodicity along stress fibers (Jin et al. 2007). Thus palladin expression and localization is regulated by RhoA signaling and by actin dynamics but it is not clear whether the RhoA-ROCK pathway acts directly on palladin or indirectly through its modulation of actin dynamics or perhaps both.
FAK, Prx1 and Tenascin C
Focal Adhesion Kinase (FAK) plays an important role in promoting cell invasion. FAK was used to remodel focal adhesions to facilitate SMC proliferation and migration. FAK null embryonic cells display reduced cell motility with enhanced focal adhesion contact formation (Ilic et al. 1995). Both palladin and its partner LPP were decreased in FAK null cells while inducible expression of FAK in FAK null cells led to a marked increase in expression of palladin and LPP (Jin et al. 2007). To understand how FAK regulates palladin and LPP, Prx1, a paired-related homeobox gene that has been shown to promote cells migration and regulate angiotension II-induced expression of SMC differentiation marker genes by increasing SRF binding to CArG elements was studied (Jones et al. 1997, 1999; Yoshida et al. 2004). Expression of Prx1 in FAK null cells increased the expression of palladin and LPP (Jin et al. 2007). Similarly, an increase in palladin and LPP was observed when SMCs were cultured on denatured collagen or on a substrate with increased stiffness. Under these conditions the increase in palladin was accompanied by an increase of phosphorylated FAK and Tenascin C (Jin et al. 2009a). Tenascin C is an extracellular matrix glycoprotein, which plays a critical role in SMC proliferation, migration, angiogenesis, and its expression is regulated by the transcription factor, Prx1 (Jones et al. 2001; Jones 2007). The expression of Tenascin C is also regulated by matrix metalloproteinase (MMPs), since the expression of Tenascin C is suppressed with the MMP inhibitors (Jones et al. 1997), and the deletion of MMP 19 prevents tenascin C accumulation in the airway wall (Gueders et al. 2010). Recent studies also showed that Tenascin C promotes cancer cell invasion by upregulating MMP 9 (Kalembeyi et al. 2003), 3 and 13 (Hancox et al. 2009), and stimulates Glioma cell invasion by upregulating MMP 12 (Sarkar et al. 2006). Furthermore, in a vessel injury model, palladin and LPP as well as SMA were expressed in medial SMCs and also in the neointima induced by injury (Gorenne et al. 2006; Jin et al. 2009a). In this model, FAK and phosphorylated FAK were detected in the media and enhanced expression occurred in the neointima. The Prx1 transcription factor was found in SMC nuclei. Interestingly, the intensity of labeling of media nuclei was variable suggesting the possibility of different populations of SMCs at day 0. At day 7, the Prx1 signal shifted to the neointima; at day 14 Prx1 staining occurred intensively in the neointima. Tenascin C was first detected in the outer media at day 3 and moved over time to the fibrous cap. In the ApoE model of atherosclerosis, palladin and LPP as well as phosphorylated FAK were decreased compared to wild type cross sections of the aortic arch media. Palladin and phosphorylated FAK also appeared in the lesion plaque but not LPP. Upregulated Tenascin C expression was also observed in the plaque (Jin et al. 2009a). All of these findings demonstrate that palladin expression is correlated with expression of FAK, Prx1 and Tenascin C in a time and spatial manner and that Prx1 promotes expression of palladin. The findings also suggest that FAK and Prx1 not only respond to changes in cell adhesion within developing and diseased blood vessels, but that these transcription factors form part of a dynamic loop which controls cell behavior at the level of cell adhesion.
Src and Rac
Ronty et al. (2007) reported that palladin interacts with SH3 domains of Spin90 and Src and is required for Src induced cytoskeletal remodeling. Src is a non-receptor kinase, which regulates cell adhesion, motility, and invasion by remodeling of the actin cytoskeleton (Brunton et al. 2004; Frame et al. 2002). These authors also show that palladin is phosphorylated in cells expressing active Src and that active Src kinase resulted in coordinated translocation of both palladin and Spin90 to membrane protrusions. Palladin was also involved in subcellular targeting of Src. Furthermore, the study showed that knock down of palladin leads to subsequent inhibition of this relocation of active Src and Spin90 raising the possibility that targeting of Src by palladin may be a mechanism to control Src dependent events. While the role of Src in adhesion, invasion and motility and the possibility that palladin functioning as a scaffold protein targets Src to its site of action is interesting, the exact underlying mechanism needs to be explored.
It was also shown that knockdown of palladin in cells using siRNA caused a decrease in the activity of the small GTPase Rac (Goicoechea et al. 2006). While RhoA is known to regulate stress fiber formation through Rho kinase and mDia, Rac is known to regulate lamellipodia and membrane ruffles through P21 activated kinase (Hall 1998; Otey et al. 2009a). Palladin is regulated by Rho as Rho kinase inhibitor decreases the expression and alters the localization of palladin in SMCs (Jin et al. 2009b). Palladin was also found to interact with Eps8 and colocalize with Eps8 in podosomes and dorsal ruffles upon induction by platelet-derived growth factor (PDGF) or phorbol easter in SMCs (Goicoechea et al. 2006). Eps8, a receptor tyrosine kinase substrate, is a signaling molecule that was originally identified as a substrate for the epidermal growth factor receptor (EFGR) (Fazioli et al. 1993). It plays an important role in activation of Rac by participating in the formation of a trimetric complex that also includes the scaffold protein Abi-1 and the Sos-1 (Innocenti et al. 2003). The interaction of palladin and Eps8 is essential for the Abi1–Sos1–Eps8 complex formation by possibly regulating the actin capping activity of Eps8 or stabilizing the Abi1–Sos1–Eps8 complex, whereby palladin may contribute to the regulation of Rac activity and the formation of lamellipodia, podosomes, and membrane ruffles.
Role of Palladin in cardiovascular diseases and cancer
The family members of palladin have been shown to be associated with human diseases. This small family includes palladin, myopalladin, and myotilin. All of them have similar Ig like domains at the C-terminus. Myopalladin localizes at the Z-line, I-band, and nucleus of skeletal and cardiac muscle (Bang et al. 2001). Myotilin is found in the Z-disc of skeletal muscle and cardiac muscle (Godley et al. 1999; Salmikangas et al. 1999). Mutations in several sarcomeric proteins have been shown to result in muscular dystrophy and cardiomyopathy. Mutations of the myopalladin gene were found associated with dilated cardiomyopathy (Duboscq-Bidot et al. 2008). Transfection of myopalladin P1112L and V1195M mutations into rat neonatal cardiomyocytes led to sarcomere disorganization and premature cell death (Duboscq-Bidot et al. 2008). Mutations in the family member, Myotilin have recently been associated with a late onset autosomal dominant distal limb girdle muscular dystrophy (McNeill et al. 2009), spheroid body myopathy (Foroud et al. 2005), and myofibrillar myopathy (Sanger et al. 2009). While myopalladin and myotilin are restricted to skeletal and cardiac muscle, palladin is more widely expressed and as it is also expressed in skeletal and cardiac muscle it may also contribute to cardiomyopathies and muscular dystrophies. Palladin is associated with other diseases. In fact, palladin has been shown to be potentially associated with human cardiovascular diseases and cancers (Shiffman et al. 2005; Goicoechea et al. 2010; Pogue-Geile et al. 2006; Tay et al. 2010).
Palladin may also play a role in myocardial infarction that occurs when thrombosis, induced by a ruptured fibrous cap or eroded atherosclerotic plaque occludes coronary blood flow leading to ischemia and eventually to necrosis of the myocardium. Myocardial infarction is a complex disease with a strong genetic component (Lusis et al. 2004). To identify gene variants associated with myocardial infarction, one study analyzed 11053 single nucleotide polymorphisms (SNPs) in 340 cases and 346 control subjects using three stage genome wide screen methods (Shiffman et al. 2005). There were 637 SNPs associated with myocardial infarction in the first round. To limit false positive associations generated by multiple testing, two more rounds of testing were performed. Finally four gene variants were found associated with myocardial infarction. Palladin was one of the genes. The SNP of palladin locates at the intron with a G to A change. This implicates a role for palladin in the pathology of myocardial infarction. Later, however, another group tried to validate the SNPs with myocardial infarction, but was not successful (Koch et al. 2009). The inconsistency between two groups may be caused by samples sizes, and different population backgrounds. A large population case matched study will be necessary to confirm the correlation of palladin with myocardial infarction.
A role for palladin in pancreatic cancers is highly suggestive. Otey's group showed that the 90 kDa isoform of palladin is highly upregulated in tumor associated fibroblasts in both primary and metastatic pancreatic tumors, in lymph node and liver metastases (Goicoechea et al. 2010). The palladin binding partner, Eps8 was also increased in pancreatic cancer (Welsch et al. 2007). Palladin and Eps8 together may contribute to the invasiveness of cancer cells. In 2006, one study found that a palladin mutation, P239S, was linked to a familial pancreatic cancer in family X from USA. The mutation existed in affected family members and was absent in the other members. Moreover, Hela cells presented a disorganized actin cytoskeleton when P239S mutated palladin was transfected (Pogue-Geile et al. 2006). Later, other studies showed that the palladin mutation P239S was not likely the reason for the pancreatic cancer in the European families (Earl et al. 2006; Slater et al. 2010; Zogopoulos et al. 2007). These results suggest that the mutation may be specific for the single family. Even so, a definite relationship between palladin mutation and pancreatic cancer merits a further investigation. Pancreatic cancer is unlikely to reflect a single defective protein but rather multiple molecules that display different patterns in different patients. In view of the role of palladin in SMC migration it may contribute to the vasculogenesis of tumors.
It has also been shown that palladin plays a role in colon cancer cell invasiveness (Tay et al. 2010). Evidence supports a connection between palladin and breast cancer (Chin and Toker 2010; Goicoechea et al. 2009; Parast and Otey 2000) as described above in the migration section. The discrepancy between different investigators concerning the role of palladin in breast cancer (Goicoechea et al. 2009; Chin and Toker 2010) may be due to the different experimental conditions and the stage of the disease. Thus, further in vivo and in vitro studies are necessary to validate these potentially important findings.
Conclusion and future directions
The insights into the role of palladin have developed significantly since the discovery of palladin as an actin associated protein in 2000 (Parast and Otey 2000). Palladin has been found as a molecular scaffold by linking actin to a number of anchor proteins; as an actin organizer by stabilizing actin stress fibers; as a sensor for environmental changes; and as a modulator for cell differentiation. The studies have been focused on a wide range of functions, from embryonic development to disease. All these studies have greatly improved our understating of the protein but have also raised important issues.
The number of interacting proteins is still increasing. Only a few interaction partners to the carboxyl- and amino-terminus Ig domains of palladin have been identified to date. Future studies are expected to identify additional interaction partners. The role of alternative splice variants and different tissue expression profiles needs to be solved. It is likely that many important binding partners and functions of palladin will be identified. This could explain certain functional discrepancies between different studies. Although the conventional knockout mice model helps understanding the role of palladin in embryonic development and disease progression, this has proven to be a challenge as the mice die at the embryonic stage. It is important to have conditional knockout mice for further understanding the function of palladin and the palladin family members. Post-translation modification has received less attention. In depth understanding of post translational modifications such as phosphorylation and nuclear translocation will likely reveal new functions of palladin. Polymorphism of the palladin gene may cause human disorders. Whether these disorders are caused by structural changes or by alterations in the interactions with other proteins need to be investigated. A role for palladin in the ongoing maintenance of the actin cytoskeleton as SM tissues contract and maintain basal tone is a fascinating area that needs further exploration.
Acknowledgments
I deeply appreciate Drs. Avril Somlyo and Carol Otey for the enthusiastic discussion and comments on the manuscript, Bartek Zieba for assistance in art work. I apologize for not citing all of the contributions to this field due to space limitations. This work was supported by PO1 HL19242-33 to Dr. Avril Somlyo.
References
- Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M, Somlyo AV. Dense bodies and actin polarity in vertebrate smooth muscle. J Cell Biol. 1982;95:403–413. doi: 10.1083/jcb.95.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell. 2001;12:2721–2729. doi: 10.1091/mbc.12.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhelifa M, Hwang SJ, Valtschanoff JG, Meeker RB, Rustioni A, Otey CA. A critical role for palladin in astrocyte morphology and response to injury. Mol Cell Neurosci. 2003;23:661–668. doi: 10.1016/s1044-7431(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Moza M, Johansson T, Rachlin A, Parast M, Huttelmaier S, Roy P, Jockusch BM, Carpen O, Karlsson R, Otey CA. The proline-rich protein palladin is a binding partner for profilin. FEBS J. 2006;273:26–33. doi: 10.1111/j.1742-4658.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem. 2004;93:74–82. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio CL, Wernimont SA, Kastner DL, Cooper KM, Huttenlocher A. Impaired podosome formation and invasive migration of macrophages from patients with a PSTPIP1 mutation and PAPA syndrome. Arthritis Rheum. 2010;62:2556–2558. doi: 10.1002/art.27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kimpe L, Janssens K, Derua R, Armacki M, Goicoechea S, Otey C,, Waelkens E, Vandoninck S, Vandenheede JR, Seufferlein T, Van Lint J. Characterization of cortactin as an in vivo protein kinase D substrate: interdependence of sites and potentiation by Src. Cell Signal. 2009;21:253–263. doi: 10.1016/j.cellsig.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Ding HL, Ryder JW, Stull JT, Kamm KE. Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem. 2009;284:15541–15548. doi: 10.1074/jbc.M900888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, Otey CA. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- Duboscq-Bidot L, Xu P, Charron P, Neyroud N, Dilanian G, Millaire A, Bors V, Komajda M, Villard E. Mutations in the Z-band protein myopalladin gene and idiopathic dilated cardiomyopathy. Cardiovasc Res. 2008;77:118–125. doi: 10.1093/cvr/cvm015. [DOI] [PubMed] [Google Scholar]
- Earl J, Yan L, Vitone LJ, Risk J, Kemp SJ, McFaul C, Neoptolemos JP, Greenhalf W, Kress R, Sina-Frey M, Hahn SA, Rieder H, Bartsch DK. Evaluation of the 4q32-34 locus in European familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1948–1955. doi: 10.1158/1055-9965.EPI-06-0376. [DOI] [PubMed] [Google Scholar]
- Endlich N, Schordan E, Cohen CD, Kretzler M, Lewko B, Welsch T, Kriz W, Otey CA, Endlich K. Palladin is a dynamic actin-associated protein in podocytes. Kidney Int. 2009;75:214–226. doi: 10.1038/ki.2008.486. [DOI] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoska V, Castagnino P, Miki T, Wong WT, Di Fiore PP. Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 1993;12:3799–3808. doi: 10.1002/j.1460-2075.1993.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Pankratz N, Batchman AP, Pauciulo MW, Vidal R, Miravalle L, Goebel HH, Cushman LJ, Azzarelli B, Horak H, Farlow M, Nichols WC. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–1940. doi: 10.1212/01.wnl.0000188872.28149.9a. [DOI] [PubMed] [Google Scholar]
- Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src's hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Godley LA, Lai F, Liu J, Zhao N, Le Beau MM. TTID: a novel gene at 5q31 encoding a protein with titin-like features. Genomics. 1999;60:226–233. doi: 10.1006/geno.1999.5912. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–3324. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, Garcia-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, Stack C, Cowan DW, Volmar K, Thorne L, Cukierman E, Rustgi AK, Brentnall T, Hwang RF, McCulloch CA, Yeh JJ, Bentrem DJ, Hochwald SN, Hingorani SR, Kim HJ, Otey CA. Isoform-specific upregulation of palladin in human and murine pancreas tumors. PLoS One. 2010;5:e10347. doi: 10.1371/journal.pone.0010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenne I, Jin L, Yoshida T, Sanders JM, Sarembock IJ, Owens GK, Somlyo AP, Somlyo AV. LPP expression during in vitro smooth muscle differentiation and stent-induced vascular injury. Circ Res. 2006;98:378–385. doi: 10.1161/01.RES.0000202802.34727.fd. [DOI] [PubMed] [Google Scholar]
- Gueders MM, Hirst SJ, Quesada-Calvo F, Paulissen G, Hacha J, Gilles C, Gosset P, Louis R, Foidart JM, Lopez-Otin C, Noel A, Cataldo DD. Matrix metalloproteinase-19 deficiency promotes tenascin-C accumulation and allergen-induced airway inflammation. Am J Respir Cell Mol Biol. 2010;43:286–295. doi: 10.1165/rcmb.2008-0426OC. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Tang DD, Opazo Saez A. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151–168. doi: 10.1016/s1569-9048(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han Z, Yi P, Li X, Olson EN. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development. 2006;133:1175–1182. doi: 10.1242/dev.02285. [DOI] [PubMed] [Google Scholar]
- Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res. 2009;11:R24. doi: 10.1186/bcr2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Frittoli E, Ponzanelli I, Falck JR, Brachmann SM, Di Fiore PP, Scita G. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J Cell Biol. 2003;160:17–23. doi: 10.1083/jcb.200206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Kern MJ, Otey CA, Wamhoff BR, Somlyo AV. Angiotensin II, focal adhesion kinase, and PRX1 enhance smooth muscle expression of lipoma preferred partner and its newly identified binding partner palladin to promote cell migration. Circ Res. 2007;100:817–825. doi: 10.1161/01.RES.0000261351.54147.de. [DOI] [PubMed] [Google Scholar]
- Jin L, Hastings NE, Blackman BR, Somlyo AV. Mechanical properties of the extracellular matrix alter expression of smooth muscle protein LPP and its partner palladin; relationship to early atherosclerosis and vascular injury. J Muscle Res Cell Motil. 2009a;30:41–55. doi: 10.1007/s10974-009-9173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yoshida T, Ho R, Owens GK, Somlyo AV. The actin-associated protein Palladin is required for development of normal contractile properties of smooth muscle cells derived from embryoid bodies. J Biol Chem. 2009b;284:2121–2130. doi: 10.1074/jbc.M806095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Gan Q, Zieba BJ, Goicoechea SM, Owens GK, Otey CA, Somlyo AV. The actin associated protein palladin is important for the early smooth muscle cell differentiation. PLoS One. 2010;5:e12823. doi: 10.1371/journal.pone.0012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL. Move on!: smooth muscle cell motility paired down. Circ Res. 2007;100:757–760. doi: 10.1161/01.RES.0000263446.33849.ed. [DOI] [PubMed] [Google Scholar]
- Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Jones FS, Zhou B, Rabinovitch M. Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a beta3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. J Cell Sci. 1999;112(Pt 4):435–445. doi: 10.1242/jcs.112.4.435. [DOI] [PubMed] [Google Scholar]
- Jones FS, Meech R, Edelman DB, Oakey RJ, Jones PL. Prx1 controls vascular smooth muscle cell proliferation and tenascin-C expression and is upregulated with Prx2 in pulmonary vascular disease. Circ Res. 2001;89:131–138. doi: 10.1161/hh1401.093582. [DOI] [PubMed] [Google Scholar]
- Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor beta1. Int J Cancer. 2003;105:53–60. doi: 10.1002/ijc.11037. [DOI] [PubMed] [Google Scholar]
- Kawai-Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59–C69. doi: 10.1152/ajpcell.00394.2006. [DOI] [PubMed] [Google Scholar]
- Koch, W, Hoppmann P, Schomig A, Kastrati A. Variations of specific non-candidate genes and risk of myocardial infarction: A replication study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.07.028. doi:10.1016/ j.ijcard.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charriere GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci USA. 2010;107(49):21016–21021. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH, Lanotte M, Waxman S, Chen SJ, Mao M, Hu GX, Zhu L, Chen Z. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. [PubMed] [Google Scholar]
- Liu XS, Li XH, Wang Y, Shu RZ, Wang L, Lu SY, Kong H, Jin YE, Zhang LJ, Fei J, Chen SJ, Chen Z, Gu MM, Lu ZY, Wang ZG. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007a;110:870–876. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, Wang ZG. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing beta1-integrin. J Cell Biochem. 2007b;100:1288–1300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, Xu G, Sun X, Kong H, Gu M, Chen S, Chen Z, Wang Z. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–515. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, Fogelman AM, Fonarow GC. Genetic basis of atherosclerosis: part II: clinical implications. Circulation. 2004;110:2066–2071. doi: 10.1161/01.CIR.0000143098.98869.F8. [DOI] [PubMed] [Google Scholar]
- Maeda M, Asano E, Ito D, Ito S, Hasegawa Y, Hamaguchi M, Senga T. Characterization of interaction between CLP36 and palladin. FEBS J. 2009;276:2775–2785. doi: 10.1111/j.1742-4658.2009.07001.x. [DOI] [PubMed] [Google Scholar]
- Manabe I, Owens GK. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ Res. 2001;88:1127–1134. doi: 10.1161/hh1101.091339. [DOI] [PubMed] [Google Scholar]
- McNeill A, Birchall D, Straub V, Goldfarb L, Reilich P, Walter MC, Schramm N, Lochmuller H, Chinnery PF. Lower limb radiology of distal myopathy due to the S60F myotilin mutation. Eur Neurol. 2009;62:161–166. doi: 10.1159/000227266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11:257–268. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Mouilleron S, Guettler S, Langer CA, Treisman R, McDonald NQ. Molecular basis for G-actin binding to RPEL motifs from the serum response factor coactivator MAL. EMBO J. 2008;27:3198–3208. doi: 10.1038/emboj.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- Otey C, Goicoechea S, Garcia-Mata R. Roles of the small GTPases RhoA and Rac1 in cell behavior. F1000 Biol Rep. 2009a;1:4. doi: 10.3410/B1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Dixon R, Stack C, Goicoechea SM. Cytoplasmic Ig-domain proteins: cytoskeletal regulators with a role in human disease. Cell Motil Cytoskeleton. 2009b;66:618–634. doi: 10.1002/cm.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile KL, Chen R, Bronner MP, Crnogorac-Jurcevic T, Moyes KW, Dowen S, Otey CA, Crispin DA, George RD, Whitcomb DC, Brentnall TA. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Rachlin AS, Otey CA. Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- Ronty M, Taivainen A, Moza M, Otey CA, Carpen O. Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett. 2004;566:30–34. doi: 10.1016/j.febslet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ronty M, Taivainen A, Moza M, Kruh GD, Ehler E, Carpen O. Involvement of palladin and alpha-actinin in targeting of the Abl/Arg kinase adaptor ArgBP2 to the actin cytoskeleton. Exp Cell Res. 2005;310:88–98. doi: 10.1016/j.yexcr.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Ronty MJ, Leivonen SK, Hinz B, Rachlin A, Otey CA, Kahari VM, Carpen OM. Isoform-specific regulation of the actin-organizing protein palladin during TGF-beta1-induced myofibroblast differentiation. J Invest Dermatol. 2006;126:2387–2396. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- Ronty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, Carpen O. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–2585. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaria SN, Illei P, Sharma R, Walter KM, Klein AP, Eshleman JR, Maitra A, Schulick R, Winter J, Ouellette MM, Goggins M, Hruban R. Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed in neoplastic cells. Cancer Biol Ther. 2007;6:324–328. doi: 10.4161/cbt.6.3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmikangas P, Mykkanen OM, Gronholm M, Heiska L, Kere J, Carpen O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Wang J, Holloway B, Du A, Sanger JM. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil Cytoskeleton. 2009;66:556–566. doi: 10.1002/cm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Nuttall RK, Liu S, Edwards DR, Yong VW. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006;66:11771–11780. doi: 10.1158/0008-5472.CAN-05-0470. [DOI] [PubMed] [Google Scholar]
- Shiffman D, Ellis SG, Rowland CM, Malloy MJ, Luke MM, Iakoubova OA, Pullinger CR, Cassano J, Aouizerat BE, Fenwick RG, Reitz RE, Catanese JJ, Leong DU, Zellner C, Sninsky JJ, Topol EJ, Devlin JJ, Kane JP. Identification of four gene variants associated with myocardial infarction. Am J Hum Genet. 2005;77:596–605. doi: 10.1086/491674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Wamhoff BR, Hoofnagle MH, Thomas J, Neppl RL, Deering T, Helmke BP, Bowles DK, Somlyo AV, Owens GK. Assessment of contractility of purified smooth muscle cells derived from embryonic stem cells. Stem Cells. 2006;24:1678–1688. doi: 10.1634/stemcells.2006-0002. [DOI] [PubMed] [Google Scholar]
- Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos J, Greenhalf W, Bartsch DK. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78(5):490–494. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- Tay PN, Tan P, Lan Y, Leung CH, Laban M, Tan TC, Ni H, Manikandan J, Rashid SB, Yan B, Yap CT, Lim LH, Lim YC, Hooi SC. Palladin, an actin-associated protein, is required for adherens junction formation and intercellular adhesion in HCT116 colorectal cancer cells. Int J Oncol. 2010;37:909–926. doi: 10.3892/ijo_00000742. [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-disc myopathies. Mol Cell Biol. 2009;29:822–834. doi: 10.1128/MCB.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HV, Moser M. Comparative expression analysis of the murine palladin isoforms. Dev Dyn. 2008;237:3342–3351. doi: 10.1002/dvdy.21755. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- Wang J, Li A, Wang Z, Feng X, Olson EN, Schwartz RJ. Myocardin sumoylation transactivates cardiogenic genes in pluripotent 10T1/2 fibroblasts. Mol Cell Biol. 2007;27:622–632. doi: 10.1128/MCB.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Endlich K, Giese T, Buchler MW, Schmidt J. Eps8 is increased in pancreatic cancer and required for dynamic actin-based cell protrusions and intercellular cytoskeletal organization. Cancer Lett. 2007;255:205–218. doi: 10.1016/j.canlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–1780. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hoofnagle MH, Owens GK. Myocardin and Prx1 contribute to angiotensin II-induced expression of smooth muscle alpha-actin. Circ Res. 2004;94:1075–1082. doi: 10.1161/01.RES.0000125622.46280.95. [DOI] [PubMed] [Google Scholar]
- Zogopoulos G, Rothenmund H, Eppel A, Ash C, Akbari MR, Hedley D, Narod SA, Gallinger S. The P239S palladin variant does not account for a significant fraction of hereditary or early onset pancreas cancer. Hum Genet. 2007;121:635–637. doi: 10.1007/s00439-007-0361-z. [DOI] [PubMed] [Google Scholar]