Abstract
Isoindoline nitroxides are potentially useful probes for viable biological systems, exhibiting low cytotoxicity, moderate rates of biological reduction and favorable Electron Paramagnetic Resonance (EPR) characteristics. We have evaluated the anionic (5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl; CTMIO), cationic (5-(N,N,N-trimethylammonio)-1,1,3,3-tetramethylisoindolin-2-yloxyl iodide, QATMIO) and neutral (1,1,3,3-tetramethylisoindolin-2-yloxyl; TMIO) nitroxides and their isotopically labeled analogues (2H12- and/or 2H12-15N-labeled) as potential EPR oximetry probes. An active ester analogue of CTMIO, designed to localize intracellularly, and the azaphenalene nitroxide 1,1,3,3-tetramethyl-2,3-dihydro-2-azaphenalen-2-yloxyl (TMAO) were also studied. While the EPR spectra of the unlabeled nitroxides exhibit high sensitivity to O2 concentration, deuteration resulted in a loss of superhyperfine features and a subsequent reduction in O2 sensitivity. Labeling the nitroxides with 15N increased the signal intensity and this may be useful in decreasing the detection limits for in vivo measurements. The active ester nitroxide showed approximately 6% intracellular localization and low cytotoxicity. The EPR spectra of TMAO nitroxide indicated an increased rigidity in the nitroxide ring, due to dibenzo-annulation.
Keywords: nitroxide, aminoxyl, oximetry, EPR, ESR, isoindoline, azaphenalene, isotopic labeling
1. Introduction
Nitroxides are persistent, stable free radicals, in which the unpaired electron is delocalized across the N-O moiety, occupying p orbitals of the nitrogen and oxygen atoms. Nitroxides readily react with carbon-, sulfur- and phosphorous-centered radicals to form diamagnetic adducts at close to diffusion controlled rates. They also readily undergo single-electron reduction and oxidation processes to form, hydroxylamine and oxoammonium cation derivatives respectively. In more complex interactions with oxygen-centered radicals, cycling between these oxidation states can occur, providing a catalytic mechanism for the dismutation of reactive oxygen species [1].
The potential applications of EPR (Electron Paramagnetic Resonance) spectroscopy using nitroxides include biophysical and biochemical studies, such as the analysis of membrane fluidity, pH, thiols, temperature, assays for reactive free radicals, oximetry and measurement of redox interactions with antioxidants and oxidants [2-9]. With the development of in vivo EPR, it is now possible to perform non-invasive studies of the pharmacokinetics of nitroxides, providing an effective approach to understand the fundamental aspects of the metabolism (redox status) and distribution of the nitroxides in vivo. Nitroxides and their hydroxylamine analogues have been suggested as potential therapeutic or diagnostic drugs based on their function as superoxide dismutase mimics and their interactions with free radicals such as superoxide and peroxynitrite [7]. In vivo EPR spectroscopy of nitroxides provides a non-invasive method to measure the presence of such reactive free radicals through their effects on the concentration of the nitroxides. It is possible to obtain images that reflect these processes by combining Magnetic Resonance Imaging (MRI) with the oxygen dependent metabolism of nitroxides [10-12].
The measurement of oxygen concentration in vivo using EPR spectroscopy has considerable potential, as numerous deleterious pathologies are associated with low concentrations of oxygen (hypoxia), including tumors, rheumatoid arthritis and tissues undergoing an ischemic episode (e.g. myocardial infarct, ischemic stroke). EPR oximetry relies on the use of paramagnetic probes with oxygen-dependent EPR spectra, and a number of particulate and soluble materials have been utilized in this role [13]. Of the soluble probes, nitroxide and triarylmethyl (trityl) radicals are those most widely investigated. While the narrow single-line EPR spectra of triarylmethyl radicals are sensitive to O2 concentration, the hydrophobicity of these probes restricts their biological application, although the use of micro- and nano-scale delivery systems and preparation of new derivatives is promising [14-19]. Nitroxides, in contrast, offer greater structural diversity and are readily synthetically modified (including isotopic labeling) to enhance biocompatibility.
With such high chemical versatility and a broad range of potential applications , a large number of different nitroxides have been synthesized for specific applications [1, 7]. An important goal of this work is the ability to selectively accumulate a nitroxide probe in a tissue of choice and this is a major aspect of our quest to develop improved imaging and oximetry agents. Contrast in EPR imaging and Magnetic Resonance Imaging (MRI) may be obtained on the basis of both the paramagnetic nature of nitroxides and their differential localization in vivo. Hepatic targeting has been demonstrated by Gallez et al, who used nitroxide-labelled arabinogalactan [20] and lipids [21, 22] as contrast agents for hepatic MRI.
More recently, the pyrrolidine nitroxide 3-acetoxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidin-1-yloxyl (CxP-AM) has emerged as one of the most promising compounds to partially address the goal of selective tissue accumulation, having been shown to selectively accumulate in brain tissue [23-26]. In contrast to most water-soluble nitroxides, CxP-AM is membrane-permeable and capable of crossing the blood-brain barrier. Intracellular esterases hydrolyze the compound to the corresponding carboxylate which is resistant to bioreduction and accumulates to millimolar concentrations within the cells. Selective localization and retention of this compound in the brain, has seen this compound utilized for oximetry in mouse brains [27, 28]. Additionally, this nitroxide could potentially be used to study the redox status of the cerebellum under physiological and pathological conditions including primary or secondary brain tumors.
In previous work, we evaluated a number of isoindoline nitroxides, including anionic (5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl; CTMIO), cationic (5-(N,N,N-trimethylammonio)-1,1,3,3-tetramethylisoindolin-2-yloxyl iodide, QATMIO) and neutral species (1,1,3,3-tetramethylisoindolin-2-yloxyl; TMIO), and an isoindoline-based hydroxylamine, as probes for EPR oximetry in viable biological systems [29]. Structurally, isoindoline nitroxides are benzo-annulated analogues of the pyrroline and pyrrolidine nitroxides and are known to exhibit superior chemical and physical stability in a range of environments [30-33], as well as narrow EPR line-widths [34-36]. The investigated compounds exhibited low cytotoxicity and moderate rates of metabolism in the CHO cells. The EPR spectra of the isoindoline nitroxides were also found to be more sensitive to oxygen concentration than the piperidine and pyrrolidine nitroxides, TEMPONE and PCA respectively, due to the presence of resolved superhyperfine splitting [29]. This suggested them as potentially useful oximetry probes and here, we have furthered these investigations by evaluating isotopically labeled (2H12- and/or 2H12-15N) CTMIO, QATMIO and TMIO for this purpose. The three nitrogen-manifolds (14N; I = 1) observed in the EPR spectra of the nitroxides are typically broadened by unresolved hyperfine coupling, primarily due to protons on the four β methyl groups. Deuteration of these methyl groups can significantly narrow the nitroxide EPR line-widths with a concomitant increase in signal intensity. Isotopic labeling of the nitroxide moiety with 15N (I = 1/2) further simplifies the spectrum, producing two resonances rather than three, with a corresponding increase in the signal intensity.
We have also investigated two new nitroxides; 1,1,3,3-tetramethyl-2,3-dihydro-2-azaphenalen-2-yloxyl (TMAO) [37] and 5-acetoxymethoxycarbonyl-1,1,3,3-tetramethylisoindolin-2-yloxyl (AMCTMIO) [38]. The latter is an isoindoline nitroxide analogue of CxP-AM and has consequently been studied with regard to its biological localization and the effect of O2 on its EPR spectrum. TMAO is a dibenzo-annulated analogue of the piperidine nitroxides such as TEMPO, in the same way that the isoindoline nitroxides are benzo-annulated analogues of the pyrroline and pyrrolidine nitroxides. The structural rigidity afforded by the fused rings in the structure of TMAO promises improvements in the spectroscopic (EPR) characteristics of the compound, relative to TEMPO and its analogues.
2. Results and Discussion
2.1. EPR characteristics and the effects of the oxygen concentration
We have previously reported the potential of isoindoline nitroxides as EPR oximetry probes in viable biological systems [29]. At low oxygen concentrations these nitroxides exhibit EPR spectra with well-resolved superhyperfine coupling due to the twelve protons of the methyl groups. In contrast, the superhyperfine coupling is unresolved in spectra of piperidine and pyrrolidine type nitroxides such as Tempone and PCA. The isoindoline nitroxides display high sensitivity to oxygen, exhibiting large relative changes in the line-width with increasing oxygen concentration.
The nitroxides utilized in this study are shown in Figure 1. The isotopically labeled isoindoline nitroxides were synthesized according to established procedures [39-41], using appropriately labeled reactants and/or reagents. The EPR nitrogen manifolds of the deuterated compounds are free of the superhyperfine features that are readily observed in the spectrum of TMIO at 0% O2 (but unresolved at high O2 concentrations). Consequently, the overall manifold width is narrowed, with a concomitant increase in signal amplitude. Labeling with 15N (I = 1/2) gives two nitrogen manifolds, rather than the usual three, again with an increase in the signal amplitude. These changes are illustrated for the parent isoindoline nitroxide, TMIO, in Figure 2.
Figure 1.
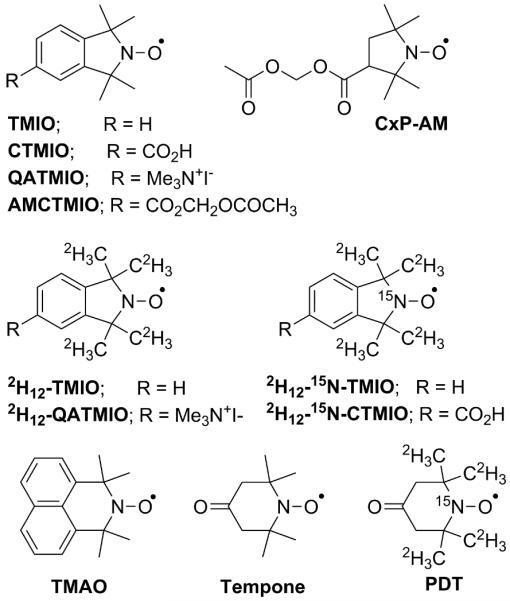
Nitroxides used in this study
Figure 2.
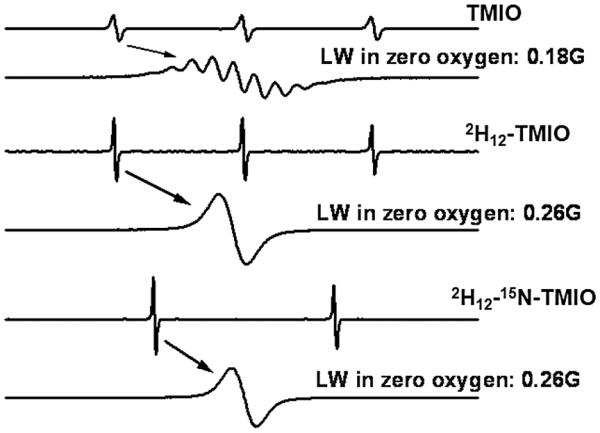
The effect of isotopic labeling on the EPR spectrum of TMIO (1 mM in water) in the absence of O2
Similar changes were noted in the EPR spectrum of CTMIO upon deuteration and 15N labeling (Figure 3). Relative to 2H12-15N-TMIO, the spectrum of 2H12-15N-CTMIO shows a greater discrepancy in the signal intensity between the high and low field lines, suggesting that its motion in solution is more anisotropic in character. This has been previously observed for unlabelled CTMIO and attributed to the formation of hydrogen bonding dimers in solution [36, 39].
Figure 3.
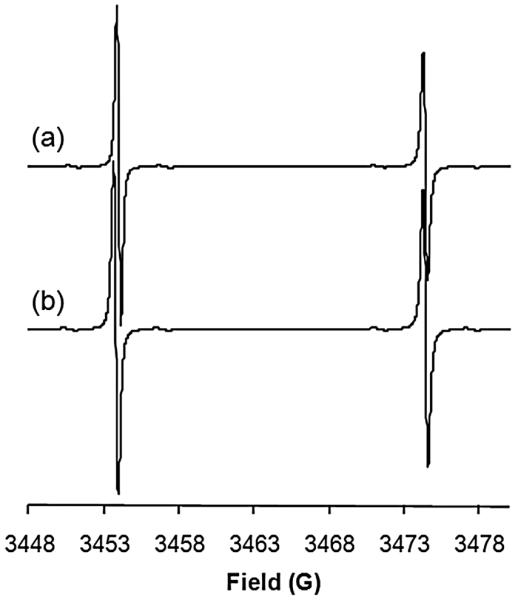
X-band EPR spectra of (a) 2H12-15N-CTMIO and (b) 2H12-15N-TMIO in CHCl3 (0.05 mM) at 298 K
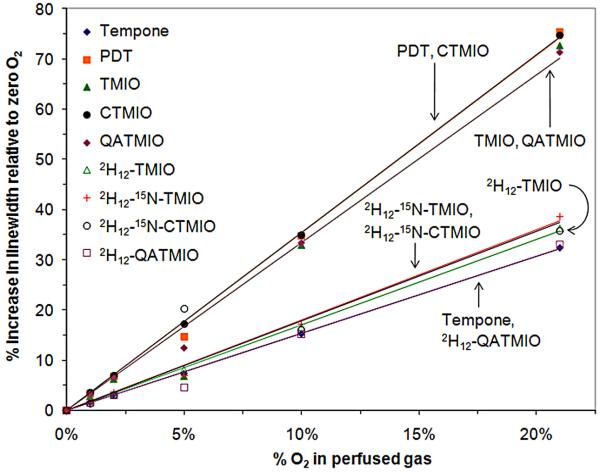
As expected, the EPR spectra of the nitroxides investigated here displayed oxygen-induced line broadening, with absolute increases in line-width of approximately 0.1 G as the oxygen concentration increased from 0 to 21%. Table 1 lists the fitted line-width of the low field (N = 1) hyperfine manifold for each nitroxide. The standard deviation in the line-widths was <1% in all cases, and the fitting procedure is described in detail in the Experimental section. While the absolute EPR line-width changes with oxygen concentration, the relative change in the line-width provides a better measure of the sensitivity of a given probe, as it is easier to accurately measure a given change in line-width on a narrower line. Figure 4 shows the relative change in the line-width for the nitroxides and these results indicate that the nitroxides can be potentially categorized into two classes on the basis of their relative sensitivity to oxygen.
Table 1.
Line-width of the low field EPR manifold of the nitroxides at different concentrations of perfused O2 in water. The standard deviation in the fitted line-widths was <1% in all cases.
| Nitroxides | Line-width (G) at concentration of perfused O2 of: | |||||
|---|---|---|---|---|---|---|
| 0% | 1% | 2% | 5% | 10% | 21% | |
| Tempone | 0.315 | 0.320 | 0.325 | 0.338 | 0.363 | 0.417 |
| PDT | 0.150 | 0.155 | 0.160 | 0.172 | 0.202 | 0.263 |
| TMIO | 0.176 | 0.181 | 0.187 | 0.188 | 0.234 | 0.304 |
| CTMIO | 0.146 | 0.151 | 0.156 | 0.171 | 0.197 | 0.255 |
| QATMIO | 0.153 | 0.158 | 0.163 | 0.172 | 0.204 | 0.262 |
| 2H12-TMIO | 0.260 | 0.264 | 0.268 | 0.281 | 0.303 | 0.354 |
| 2H12-15N-TMIO | 0.262 | 0.266 | 0.271 | 0.280 | 0.307 | 0.363 |
| 2H12-15N-CTMIO | 0.238 | 0.241 | 0.245 | 0.286 | 0.276 | 0.323 |
| 2H12-QATMIO | 0.263 | 0.267 | 0.271 | 0.275 | 0.303 | 0.350 |
Figure 4.
Increase in the EPR line-widths of the nitroxides relative to that at 0% O2 in the presence of different perfused O2 concentrations in water
Significantly, the oxygen sensitivity of the unlabelled isoindoline nitroxides is comparable to PDT, which is one of the most sensitive nitroxide oximetry probes available. CTMIO and PDT are essentially indistinguishable, while TMIO and QATMIO are slightly less sensitive than PDT. Thus, the isoindoline nitroxides offer oxygen sensitivity which matches that of PDT, but without the expense and synthetic complexity of isotopic labeling, and with the potential for more specific localization. Notably it is the superhyperfine resolution of the EPR spectra of the isoindoline nitroxides that provides the basis of the high sensitivity to oxygen. Changes in the line-width of the narrow superhyperfine features are readily measured and the resulting relative line-width changes are high. For Tempone, line-width changes must be measured across the whole of the nitrogen manifold, as the superhyperfine interactions are unresolved and relative line-width changes are consequently smaller. Notably, the unlabelled isoindoline nitroxides TMIO, CTMIO and QATMIO represent sensitive neutral, anionic and cationic oximetry probes respectively, with significantly varied partitioning coefficients [36].
Deuteration of the nitroxides results in the narrowing of the nitrogen manifolds due to a significant reduction in magnitude of the superhyperfine coupling. For Tempone, this leads to increased oxygen sensitivity (as observed for PDT) as relative line-width changes are increased. The consequence for the isoindoline nitroxides however, is that the useful superhyperfine lines are unresolved, so that the changes in the line-width must now be monitored for the whole nitrogen manifold, as for the piperidine nitroxides. The narrowing of the manifold does not compensate for the loss of hyperfine structure, and the relative changes in line-width with oxygen content are smaller than those observed for the unlabelled compounds. Labeling with 15N results in a two-line EPR spectrum but has essentially no effect on oxygen sensitivity, with the 2H12-15N-labeled compounds exhibiting essentially identical sensitivity to the 2H12-14N compounds. The 15N-labeled compounds do however possess a signal-to-noise advantage, due to the greater signal intensity of their two-line spectrum, which could be significant for in vivo oximetry.
The X-band EPR spectra of active ester nitroxides CxP-AM and AMCTMIO were acquired in solution at 37 °C. Nitroxide CxP-AM exhibited a typical 3-line spectrum, with no resolution of superhyperfine coupling across the studied range of oxygen concentration. In contrast, the spectrum of nitroxide AMCTMIO featured resolved superhyperfine lines as observed for other isoindoline nitroxides (Figure 5). The spectrum of AMCTMIO was simulated by assuming four equivalent methyl groups and notably, the superhyperfine lines broadened linearly with increases in perfused oxygen concentration. The oxygen sensitivity of AMCTMIO is similar to the parent CTMIO [29]. While the sensitivity of nitroxide CxP-AM is similar to that of the isoindoline nitroxides, in previous work its parent nitroxide 3-carboxy-2,2,5,5-tetramethylpyrrolidin-1-yloxyl (PCA) was found to be considerably less sensitive than the isoindoline nitroxides [29]. It must be noted that in a biological environment, esters CxP-AM and AMCTMIO are expected to be hydrolyzed to generate the parent nitroxides PCA and CTMIO, and it is most likely that these will be the actual species detected by in vivo EPR oximetry.
Figure 5.
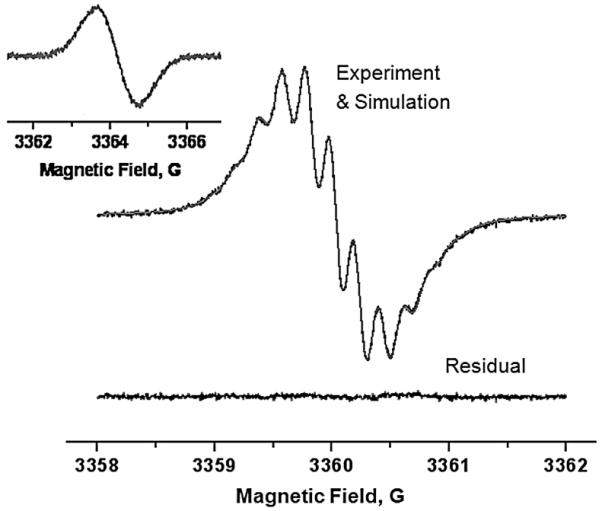
Simulation of the low field manifold of the X-band EPR spectrum of active ester nitroxide AMCTMIO perfused with N2. Inset shows corresponding manifold for pyrrolidine nitroxide CxP-AM under identical conditions
The azaphenalene nitroxide TMAO is essentially insoluble in water and, while this complicates direct delivery to a biological system, the probe may instead be delivered by utilizing a lipophilic micro- or nano-scale delivery vehicle. This has the added potential of increasing its sensitivity to oxygen concentration as previously demonstrated for liposome [42], microsphere [16, 43] and nano-emulsion [15] systems containing nitroxide and trityl-radical oximetry probes. Encapsulation has the added advantage of protecting nitroxide probes from bioreduction. Here, the EPR characteristics of TMAO and its sensitivity to O2 concentration were studied in ethanol. The room-temperature X-band EPR spectra feature resolved superhyperfine interactions at very low oxygen concentrations (Figure 6). Resolution of the superhyperfine splittings rapidly decreases with increasing O2 content of the perfusing gas and the superhyperfine features are completely broadened at 2% O2. In contrast, these features are resolved in the spectra of isoindoline nitroxides at O2 concentrations of up to 5% - 10%. Additionally, an extremely large relative change in line-width is observed in going from 0% to 21% perfused O2 (Table 2).
Figure 6.
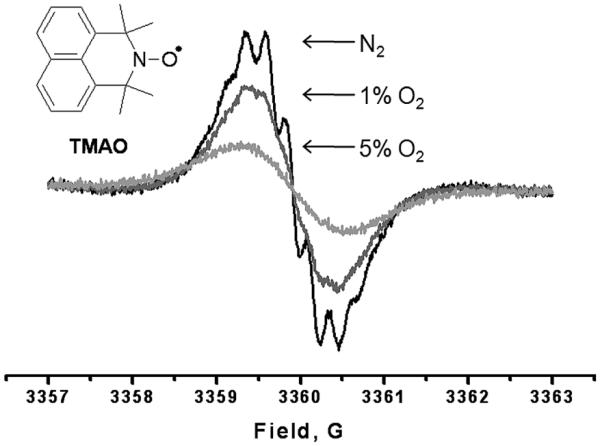
Low field manifold of the X-band EPR spectrum of TMAO perfused with various concentrations of O2
Table 2.
Line-width and percent change of the low field EPR manifold of TMAO in ethanol (0.1 mM) at different concentrations of perfused O2
| % O2 | Line-width (G) | % changea |
|---|---|---|
| 0% | 0.241 | 0.00 |
| 1% | 0.304 | 26.1 |
| 2% | 0.387 | 60. 6 |
| 5% | 0.665 | 176 |
| 10% | 1.179 | 389.2 |
| 21% | 2.398 | 895.0 |
relative to line-width at 0% O2
The pronounced sensitivity to oxygen of TMAO is likely to be a consequence of the high solubility of O2 in ethanol. The mole fraction solubility of O2 (at 101.325 kPa partial pressure of O2 and 298.15 K) in ethanol (5.83 × 10−4) is more than 25 times greater than that in water (2.293 × 10−5) [44]. Consequently the dynamic range of EPR line widths of the nitroxide in ethanol, with changing O2 concentration, will be much greater than in water, resulting in increased sensitivity [43]. As mentioned above, this again suggests that the encapsulation of TMAO (or other probes) in a non-aqueous or lipophilic vehicle could have significant benefits in terms of sensitivity.
The resolution of the superhyperfine interactions in the spectra of TMAO at low O2 concentrations illustrates one of the significant effects of benzo-annulation. For normal piperidine nitroxides, such as TEMPO, the superhyperfine interactions are unresolved under identical experimental conditions. Crystallographic studies [37] show that the geometric parameters of TMAO and TEMPO are very similar. The compounds also share very similar steric environments around the nitroxide moieties. It is most likely that the improved resolution in the TMAO spectra is due to the increased rigidity in the 6-membered ring. The motion that occurs in the TEMPO ring on the EPR timescale is precluded in TMAO due to the dibenzo-annulation of the nitroxide containing ring, and consequently narrower EPR manifolds are observed.
2.2. Intracellular localization of nitroxides CxP-AM and AMCTMIO
The localization of nitroxides in various intravascular compartments could potentially provide a direct measure of oxygen concentration and related parameters non-invasively using in vivo EPR spectroscopy and imaging. Utsumi et al have recently demonstrated this approach for simultaneous molecular imaging of redox processes using Overhauser-enhanced MRI with 14N- and 15N-labelled nitroxides [45-47]. Nitroxides that exhibit different EPR and partitioning properties could be used simultaneously to monitor intra and extra-cellular oxygen concentrations in cell suspensions or in vivo. As shown in Figure 7, an intracellularly localized 14N nitroxide (anionic CTMIO generated by intracellular metabolism of AMCTMIO) and an extracellularly localized 15N-labelled nitroxide (cationic 15N-QATMIO) may be interrogated simultaneously by EPR to provide localized measures of oxygen concentration. In a similar approach, the combination of an insoluble particulate probe, such as lithium phthalocyanine (LiPc), localized in tissue and a circulating nitroxide probe can simultaneously report tissue and intravascular oxygen concentrations, respectively. The well-resolved EPR spectra of LiPc and 15N-QATMIO shown in Figure 8 were recorded in PBS buffer at X-band and demonstrate the potential feasibility of this approach. Further control over localization can be achieved according to the size and placement of the external loop resonator used for the in vivo measurements.
Figure 7.
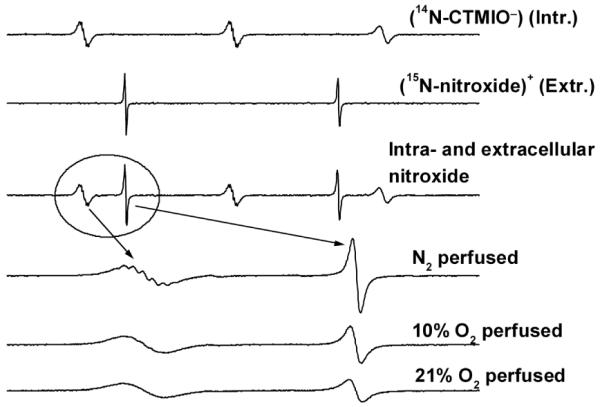
Simultaneous measurement of intra- and extra-cellular oxygen concentration in cells using two charged nitroxides
Figure 8.
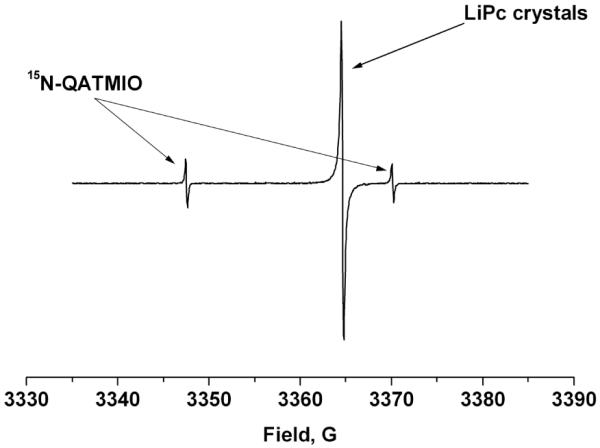
The well resolved X-band spectra of LiPc and 15N-QATMIO in PBS demonstrate the feasibility of simultaneous measurement of tissue and intravascular oxygen concentration using particulate LiPc and a charged nitroxide
The intracellular localization of active ester nitroxides AMCTMIO and CxP-AM was assessed in Chinese Hamster Ovary (CHO) cell suspensions (1 × 106 cell/100 μl). Results indicate that, with an initial nitroxide concentration of 0,2 mM, approximately 6% of AMCTMIO and 19% of CxP-AM was localized in the intracellular compartment within 2 hours and no significant increase in intracellular signal intensity was observed at 4 and 6h time points. Importantly, >90% cell viability was maintained over the course of these experiments.
2.3. Cytotoxicity of active ester nitroxide AMCTMIO in CHO cells
While the non-toxicity of CxP-AM [23-26] and CTMIO [29, 38, 48, 49] (the hydrolysis product of AMCTMIO) have been established in cell-based assays and live animal studies, the effects of AMCTMIO on biological systems were unknown. Subsequently, three different methods were used to determine the toxicity of the nitroxide; (i) the Trypan blue assay which provides a measure of cell membrane integrity, (ii) the Colony formation test, a more rigorous test to determine the ability of cells to multiply and (iii) the effect of nitroxides on the oxygen consumption rate of the cells.
According to the Trypan blue test, nitroxide AMCTMIO exhibited no significant toxicity at concentrations up to 0.5 mM for exposures of up to 4 hours. At 1 mM however, cell viability decreased by approximately 60% after 1 hour exposure, and no viable cells were present after 4 hours exposure. Colony formation by CHO cells exposed to 0.2 mM AMCTMIO for 6 hours was similar to that of the control, but at higher concentrations (0.5 and 1 mM) the nitroxide completely inhibited colony formation. Exposure to nitroxide AMCTMIO for 16 hours resulted in complete inhibition of colony formation at all tested concentrations >0.1 mM. The oxygen consumption rates of the CHO cells were determined immediately and 2 hours post-treatment with different concentration of the nitroxides (0.05, 0.1, 0.2 and 0.5 mM). Biologically significant decreases (>50% compared to control) in the oxygen consumption rates were observed only at the concentration of 0.5 mM, both immediately and 2 hrs after treatment with AMCTMIO (Figure 9).
Figure 9.
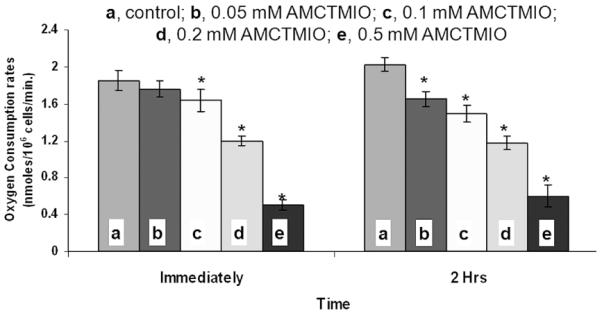
Oxygen consumption rates of CHO cells treated with nitroxide AMCTMIO, immediately and 2 hours post-treatment.
3. Conclusions
The EPR spectra of the isoindoline nitroxides CTMIO, TMIO and QATMIO exhibit sensitivities to oxygen concentration, in terms of relative change in line-width, which are equivalent to that of PDT. Significantly, in contrast to PDT, this sensitivity is achieved without isotopic labeling (deuteration or 15N). Isoindoline nitroxides with a range of partitioning characteristics and charges are available synthetically, and the compounds are non-toxic at the concentrations required for oximetry [29, 38, 48, 49]. The compounds may consequently prove useful in EPR oximetry for simultaneously measuring intra- and extracellular oxygen concentration in cell suspensions or in vivo. Deuteration of the isoindoline nitroxides fails to increase their sensitivity to O2 concentration due to the loss of narrow superhyperfine features in the EPR spectra. 15N-labelling has essentially no effect on O2 sensitivity, but does increase the EPR signal intensity which may be important for in vivo applications. The active ester isoindoline nitroxide AMCTMIO exhibits low toxicity and evidence of localizing partially in the intracellular compartment. The EPR spectra of AMCTMIO and its hydrolysis product (CTMIO) exhibit high sensitivity to O2 concentration. The isoindoline nitroxides investigated here are potentially useful probes for in vitro and in vivo EPR oximetry. For the isoindoline nitroxides, the ideal combination of O2 sensitivity and signal intensity may be achieved with non-deuterated 15N-labeled compounds. Notably, refinements in spectral fitting methodology, such as the development of efficient procedures based on molecular structure, are expected to aid the derivation of line-width data from the experimental spectra and further improve the reliability of oximetry measurements. This is especially true when utilizing unresolved, or poorly resolved, superhyperfine features.
Superhyperfine resolution in the EPR spectrum of the azaphenalene nitroxide TMAO at low O2 concentration appears to be a consequence of increased structural rigidity (with respect to piperidine nitroxides such as TEMPO) due to dibenzo-annulation. The spectrum of TMAO is sensitive to O2 concentration, but its poor solubility in water means that, for biological applications, it may be more readily utilized in association with a lipophilic nanoscale delivery vehicle.
4. Experimental
4.1. General
All reactions were conducted in oven- or flame–dried glassware. All solvents and reagents were used as commercially supplied. TLC was carried out on pre-coated silica gel 60 F254 plates. Chromatography refers to flash chromatography on silica gel 60, 230-400 mesh (eluants are given in parentheses). 3-Acetoxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidin-1-yloxyl (CxP-AM) [23, 24], 1,1,3,3-tetramethylisoindolin-2-yloxyl (TMIO) [40], 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) [39], 5-(N,N,N-trimethylammonio)-1,1,3,3-tetramethylisoindolin-2-yloxyl iodide (QATMIO) [41], 1,1,3,3-tetramethyl-2,3-dihydro-2-azaphenalen-2-yloxyl (TMAO) [37] and 5-acetoxymethoxycarbonyl-1,1,3,3-tetramethylisoindolin-2-yloxyl (AMCTMIO) [38] were prepared according to previously published procedures. 4-Oxo-2,2,6,6-tetramethylpiperidin-d16-1-15N-oxyl (PDT) was purchased from MSD Isotopes (St. Louis, MD). Tempone was obtained from Molecular Probes (Junction City, OR). The gadolinium complex (Magnevist-GdDTPA) was obtained from Berlex Imaging (Wayne, NJ).
4.2. 15N labeled isoindoline nitroxides
2H12-15N-TMIO was synthesized according to published procedures [40, 50]. Similarly, 2H12-15N-CTMIO was synthesized from 15N-N-benzylphthalimide according to procedures established for the unlabelled analogue [39, 41]. 15N-N-benzylphthalimide was synthesized from potassium 15N-phthalimide and benzyl bromide according to the method of Betley et al [51].
4.3. Deuterated TMIO and derivatives
The deuterated TMIO derivatives were synthesized from 2H12-2-benzyl-1,1,3,3-tetramethylisoindoline or 2H12-15N-2-benzyl-1,1,3,3-tetramethylisoindoline, according to procedures established for the unlabelled analogues [39-41, 50]. Deuterated 2-benzyl-1,1,3,3-tetramethylisoindolines were obtained via the method described by Griffiths et al [40], utilizing perdeuterated methyl iodide in the Grignard reaction [34]. Notably, the yield of 2H12-2-benzyl-1,1,3,3-tetramethylisoindolines is significantly higher (~60 %) than that obtained for the unlabelled analogue (~30 %). The yields of the subsequent synthetic steps were unaffected by deuteration.
4.4. Cell Culture
Chinese hamster ovary (CHO) cells were seeded in McCoy’s 5A medium supplemented with 10% fetal bovine serum, 20 mM HEPES, and 1% penicillin/streptomycin, and cultured in a humidified incubator at 37 °C with 95% air and 5% CO2. The cells were maintained as monolayers and subcultured three times before each experiment.
4.5. Cell Localization Studies
The intracellular concentration of the nitroxides was determined by comparing the EPR signal intensities with/without the broadening agent. Briefly, the nitroxides (0.2 mM) were added in the cell suspension containing 1 × 106 CHO cells/100 μl and the EPR spectra were collected at 2 h, 4 h and 6 h time points at X-band EPR spectrometer. The suspension was kept in the incubator to maintain cell viability during the experiments. An extracellular broadening agent (Magnevist-GdDTPA, 20μl) was added to broaden the EPR signals of the extracellular nitroxide present in the cell suspension. The final concentration of the broadening agent was 20 mM. The remaining EPR signal provided a direct measure of the nitroxide localized in the intracellular compartments of the CHO cells.
4.6. Clonogenicity Assay
The CHO cells were collected by trypsinization (0.25% trypsin), centrifuged (200 g, 5 min), and then seeded into 12-well, round-bottom sterile plates at a concentration of 200 cells/well. Different concentrations (0.1, 0.2, 0.5, 1 and 2 mM final concentration) of the nitroxides were added into the culture media. After incubation for 36 hours, the cells were washed 3 times with HEPES and cultured with fresh media. After being cultured for 7 days, the cells were fixed and observed under a microscope to count visible colonies. The effects of the nitroxides on colony formation were calculated by comparing with untreated controls.
4.7. Trypan Blue Exclusion Test
The effects of the nitroxides on cell integrity were measured by the trypan blue exclusion test. Different concentrations (0.1, 0.2, 0.5 and 1 mM final concentration) of the nitroxides were added to cultured media and incubated at 37 °C in a humidified incubator with 95% air and 5% CO2 for 24 hours. The ability to exclude 0.4% trypan blue was determined using a hemocytometer under a light microscope.
4.8. Oxygen Consumption Measurements
The effects of the nitroxides on cell function were assessed by measuring the rate of consumption of oxygen. Each 100 μl sample of cells (2.5 × 106 cells/ml) was mixed with 10% dextran (to retard settling of the cells) and 0.5 mM 15N-PDT. Different concentrations (0.05, 0.1, 0.2 and 0.5 mM final concentration) of the nitroxides were added into the system. The resulting solution was drawn into a 1 mm (inner diameter) quartz capillary tube that was then sealed at both ends. The EPR spectra were recorded at 30 s intervals and the rates of oxygen consumption by CHO cells were calculated from the slope of the change in line-width of 15N-PDT with time. During the time required for the assay, the concentration of PDT did not change significantly and therefore the changes in line-width could be attributed entirely to changes in [O2].
4.9. Measurement of Sensitivity to Oxygen
Solutions of the various nitroxides (0.1 mM) were prepared and their EPR spectra recorded in different concentrations of perfused gas. The relative sensitivity to oxygen was determined in terms of the change in line-width with respect to that in 0 % oxygen.
4.10. EPR Spectroscopy
The EPR spectra were recorded on a Varian E-109 EPR spectrometer, equipped with a Varian gas-flow temperature controller. Representative spectroscopic parameters were: field centre, 3362 Gauss; frequency, 9.05 GHz; and non-saturating microwave power. The modulation amplitude was kept one third of the line-width to avoid signal distortion due to over modulation. To derive the line-width, the lower field component (N = 1) of the EPR signal was fitted using the EWVoigt program (Scientific Software, IL), which utilizes a convolution of Lorentzian and Gaussian functions to describe EPR line shape. To fit the line shape of EPR signals without superhyperfine structure several parameters such as Lorentzian line-width, signal intensity, centre field, and signal phase were adjusted while keeping the Gaussian function constant as approximately 10% of the total line-width. To derive the line-width of EPR signals with well resolved superhyperfine structure the signal was fitted using the superhyperfine splitting of 12 equivalent protons as an additional adjustable parameter. To fit the spectra of these radicals with unresolved superhyperfine structures at 21% oxygen, the superhyperfine splitting derived at 0% perfused oxygen was used as a non-adjustable parameter to derive the line-width. For EPR lines with superhyperfine splittings, the line-width of each superhyperfine line was assumed to be the same and the fitting gave the mean line-width of the superhyperfine splittings of the N = 1 hyperfine component. All line-widths are expressed as width at half height.
The EPR spectrum shown in Figure 8 was recorded from sample consisting of LiPc crystals suspended in a PBS solution of 15N-QATMIO.
The EPR spectra of 2H12-15N-CTMIO and 2H12-15N-TMIO were recorded in CHCl3 (0.05 mM) at 298 K on a Bruker Elexsys E580 EPR spectrometer (X-band, ~9.2 GHz) using a Bruker microwave frequency counter and a Bruker ER036M teslameter for microwave frequency and magnetic field calibration. The resulting spectra were simulated using the XSophe-Sophe-XeprView computer simulation software suite [52] running on a personal computer with Mandriva Linux 2008 as the operating system.
Research highlights.
Isoindoline nitroxides are promising in vivo and in vitro EPR oximetry probes
Isoindoline nitroxides exhibit high oxygen sensitivity without isotopic labelling
Active ester isoindoline nitroxide is non-toxic and partially localises in cells
Rigid structure of azaphenalene nitroxide results in highly-resolved EPR spectra
5. Acknowledgements
J.P.B., S.E.B., K.H. and A.S.M. thank the Australian Research Council Centres of Excellence Funding Program CE0561607 for financial support. NK and HMS will like to acknowledge financial support from NIH grants PO1EB2180 and CA120919.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References and Notes
- [1].Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radical Biol. Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gallez B, Mader K, Swartz HM. Noninvasive measurement of the pH inside the gut by using pH-sensitive nitroxides. An in vivo EPR study. Magn. Reson. Med. 1996;36:694–697. doi: 10.1002/mrm.1910360507. [DOI] [PubMed] [Google Scholar]
- [3].Eckburg JJ, Chato JC, Liu KJ, Grinstaff MW, Swartz HM, Suslick KS, Auteri FP. The measurement of temperature with electron paramagnetic resonance spectroscopy. J Biomech. Eng. 1996;118:193–200. doi: 10.1115/1.2795959. [DOI] [PubMed] [Google Scholar]
- [4].Gallez B, Bacic G, Goda F, Jiang J, O’Hara JA, Dunn JF, Swartz HM. Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: in vivo EPR and MRI studies. Magn. Reson. Med. 1996;35:97–106. doi: 10.1002/mrm.1910350113. [DOI] [PubMed] [Google Scholar]
- [5].Glockner JF, Norby SW, Swartz HM. Simultaneous measurement of intracellular and extracellular oxygen concentrations using a nitroxide-liposome system. Magn. Reson. Med. 1993;29:12–18. doi: 10.1002/mrm.1910290105. [DOI] [PubMed] [Google Scholar]
- [6].Roshchupkina GI, Bobko AA, Bratasz A, Reznikov VA, Kuppusamy P, Khramtsov VV. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Radical Biol. Med. 2008;45:312–320. doi: 10.1016/j.freeradbiomed.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox Signaling. 2007;9:1731–1743. doi: 10.1089/ars.2007.1722. [DOI] [PubMed] [Google Scholar]
- [8].Swartz HM, Khan N, Khramtsov VV. Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid. Redox Signaling. 2007;9:1757–1771. doi: 10.1089/ars.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamada KI, Kuppusamy P, English S, Yoo J, Irie A, Subramanian S, Mitchell JB, Krishna MC. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol. 2002;43:433–440. doi: 10.1080/j.1600-0455.2002.430418.x. [DOI] [PubMed] [Google Scholar]
- [10].Hyodo F, Soule BP, Matsumoto K.-i., Matusmoto S, Cook JA, Hyodo E, Sowers AL, Krishna MC, Mitchell JB. Assessment of tissue redox status using metabolic responsive contrast agents and magnetic resonance imaging. J. Pharm. Pharmacol. 2008;60:1049–1060. doi: 10.1211/jpp.60.8.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Matsumoto K.-i., Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, Krishna MC. High-resolution mapping of tumor redox status by Magnetic Resonance Imaging using nitroxides as redox-sensitive contrast agents. Clin. Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- [12].Swartz HM. Metabolically Responsive Contrast Agents. In: Feig E, editor. Advances in Magnetic Resonance Imaging. Ablex Publishing Company; Norwood, NJ: 1989. pp. 49–71. [Google Scholar]
- [13].Springett R, Swartz HM. Measurements of Oxygen In Vivo: Overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid. Redox Signaling. 2007;9:1295–1302. doi: 10.1089/ars.2007.1620. [DOI] [PubMed] [Google Scholar]
- [14].Ardenkjær-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J. Magn. Reson. 1998;133:1–12. doi: 10.1006/jmre.1998.1438. [DOI] [PubMed] [Google Scholar]
- [15].Charlier N, Driesschaert B, Wauthoz N, Beghein N, Préat V, Amighi K, Marchand-Brynaert J, Gallez B. Nano-emulsions of fluorinated trityl radicals as sensors for EPR oximetry. J. Magn. Reson. 2009;197:176–180. doi: 10.1016/j.jmr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [16].Sostaric JZ, Pandian RP, Bratasz A, Kuppusamy P. Encapsulation of a highly sensitive EPR active oxygen probe into sonochemically prepared microspheres. J. Phys. Chem. B. 2007;111:3298–3303. doi: 10.1021/jp0682356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu Y, Villamena FA, Sun J, Wang T.-y., Zweier JL. Esterified trityl radicals as intracellular oxygen probes. Free Radical Biol. Med. 2009;46:876–883. doi: 10.1016/j.freeradbiomed.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. Trityl radicals as persistent dual function pH and oxygen probes for in vivo electron paramagnetic resonance spectroscopy and imaging: concept and experiment. J. Am. Chem. Soc. 2007;129:7240–7241. doi: 10.1021/ja071515u. [DOI] [PubMed] [Google Scholar]
- [19].Dhimitruka I, Bobko AA, Hadad CM, Zweier JL, Khramtsov VV. Synthesis and characterization of amino derivatives of persistent trityl radicals as dual function pH and oxygen paramagnetic probes. J. Am. Chem. Soc. 2008;130:10780–10787. doi: 10.1021/ja803083z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gallez B, Lacour V, Demeure R, Debuyst R, Dejehet F, De Keyser JL, Dumont P. Spin labelled arabinogalactan as MRI contrast agent. Magn. Reson. Imaging. 1994;12:61–69. doi: 10.1016/0730-725x(94)92353-1. [DOI] [PubMed] [Google Scholar]
- [21].Gallez B, Debuyst R, Demeure R, Dejehet F, Grandin C, Van Beers B, Taper H, Pringot J, Dumont P. Evaluation of a nitroxyl fatty acid as liver contrast agent for magnetic resonance imaging. Magn. Reson. Med. 1993;30:592–599. doi: 10.1002/mrm.1910300510. [DOI] [PubMed] [Google Scholar]
- [22].Gallez B, Demeure R, Debuyst R, Leonard D, Dejehet F, Dumont P. Evaluation of nonionic nitroxyl lipids as potential organ-specific contrast agents for magnetic resonance imaging. Magn. Reson. Imaging. 1992;10:445–455. doi: 10.1016/0730-725x(92)90516-3. [DOI] [PubMed] [Google Scholar]
- [23].Sano H, Naruse M, Matsumoto K.i., Oi T, Utsumi H. A new nitroxyl-probe with high retention in the brain and its application for brain imaging. Free Radical Biol. Med. 2000;28:959–969. doi: 10.1016/s0891-5849(00)00184-2. [DOI] [PubMed] [Google Scholar]
- [24].Utsumi H, Sano H, Naruse M, Matsumoto K-I, Ichikawa K, Oi T. Nitroxyl probes for brain research and their application to brain imaging. Methods Enzymol. 2002;352:494–506. doi: 10.1016/s0076-6879(02)52043-7. [DOI] [PubMed] [Google Scholar]
- [25].Kao JPY, Rosen GM. Esterase-assisted accumulation of 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl into lymphocytes. Org. Biomol. Chem. 2004;2:99–102. doi: 10.1039/b310467b. [DOI] [PubMed] [Google Scholar]
- [26].Rosen GM, Burks SR, Kohr MJ, Kao JPY. Synthesis and biological testing of aminoxyls designed for long-term retention by living cells. Org. Biomol. Chem. 2005;3:645–648. doi: 10.1039/b415586f. [DOI] [PubMed] [Google Scholar]
- [27].Miyake M, Shen J, Liu S, Shi H, Liu W, Yuan Z, Pritchard A, Kao JPY, Liu KJ, Rosen GM. Acetoxymethoxycarbonyl nitroxides as electron paramagnetic resonance proimaging agents to measure O2 levels in mouse brain: a pharmacokinetic and pharmacodynamic study. J. Pharmacol. Exp. Ther. 2006;318:1187–1193. doi: 10.1124/jpet.106.106245. [DOI] [PubMed] [Google Scholar]
- [28].Shen J, Liu S, Miyake M, Liu W, Pritchard A, Kao JPY, Rosen GM, Tong Y, Liu KJ. Use of 3-acetoxymethoxycarbonyl-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl as an EPR oximetry probe: potential for in vivo measurement of tissue oxygenation in mouse brain. Magn. Reson. Med. 2006;55:1433–1440. doi: 10.1002/mrm.20894. [DOI] [PubMed] [Google Scholar]
- [29].Shen J, Bottle S, Khan N, Grinberg O, Reid D, Micallef A, Swartz H. Development of isoindoline nitroxides for EPR oximetry in viable systems. Appl. Magn. Reson. 2002;22:357–368. [Google Scholar]
- [30].Adam W, Bottle SE, Finzel R, Kammel T, Peters EM, Peters K, Von SHG, Walz L. Trapping of cyclopentanediyl and trimethylenemethane triplet diradicals with the nitroxide 1,1,3,3-tetramethyl-1,3-dihydroisoindolin-2-yloxyl. J. Org. Chem. 1992;57:982–988. [Google Scholar]
- [31].Moad G, Rizzardo E, Solomon DH. Selectivity of the reaction of free radicals with styrene. Macromolecules. 1982;15:909–914. [Google Scholar]
- [32].Griffiths PG, Rizzardo E, Solomon DH. Quantitative studies on free radical reactions with the scavenger 1,1,3,3-tetramethylisoindolinyl-2-oxy. Tetrahedron Lett. 1982;23:1309–1312. [Google Scholar]
- [33].Bottle SE, Micallef AS. Synthesis and EPR spin trapping properties of a new isoindole-based nitrone: 1,1,3-trimethylisoindole N-oxide (TMINO) Org. Biomol. Chem. 2003;1:2581–2584. doi: 10.1039/b300642e. [DOI] [PubMed] [Google Scholar]
- [34].Bolton R, Gillies DG, Sutcliffe LH, Wu X. An EPR and NMR study of some tetramethylisoindolin-2-yloxyl free radicals. J. Chem. Soc., Perkin Trans. 2. 1993:2049–2052. [Google Scholar]
- [35].Gillies DG, Sutcliffe LH, Wu X. NMR determination of EPR hyperfine coupling constants of some 5-(n-alkyl)-1,1,3,3-tetrakis(trideuteriomethyl)isoindolin-2-yloxyls. J. Chem. Soc., Faraday Trans. 1994;90:2345–2349. [Google Scholar]
- [36].Bottle SE, Gillies DG, Micallef AS, Reid DA, Sutcliffe LH. ESR measurements of the partitioning of some new spin probes in n-octanol-water. Magn. Reson. Chem. 1999;37:730–734. [Google Scholar]
- [37].Blinco JP, McMurtrie JC, Bottle SE. The first example of an azaphenalene pro-fluorescent nitroxide. Eur. J. Org. Chem. 2007:4638–4641. [Google Scholar]
- [38].Hosokawa K, Chen P, Lavin FM, Bottle ES. The impact of carboxy nitroxide antioxidants on irradiated ataxia telangiectasia cells. Free Radical Biol. Med. 2004;37:946–952. doi: 10.1016/j.freeradbiomed.2004.06.035. [DOI] [PubMed] [Google Scholar]
- [39].Bottle SE, Gillies DG, Hughes DL, Micallef AS, Smirnov AI, Sutcliffe LH. Synthesis, single crystal X-ray structure and W-band (95 GHz) EPR spectroscopy of a new anionic isoindoline aminoxyl: synthesis and characterization of some derivatives. J. Chem. Soc., Perkin Trans. 2. 2000:1285–1291. [Google Scholar]
- [40].Griffiths PG, Moad G, Rizzardo E, Solomon DH. Synthesis of the radical scavenger 1,1,3,3-tetramethylisoindolin-2-yloxyl. Aust. J. Chem. 1983;36:397–401. [Google Scholar]
- [41].Reid DA, Bottle SE, Micallef AS. The synthesis of water soluble isoindoline nitroxides and a pronitroxide hydroxylamine hydrochloride UV-VIS probe for free radicals. Chem. Commun. (Cambridge, U.K.) 1998:1907–1908. [Google Scholar]
- [42].Glockner JF, Chan H-C, Swartz HM. In vivo oximetry using a nitroxide-liposome system. Magn. Reson. Med. 1991;20:123–133. doi: 10.1002/mrm.1910200113. [DOI] [PubMed] [Google Scholar]
- [43].Liu KJ, Grinstaff MW, Jiang J, Suslick KS, Swartz HM, Wang W. In vivo measurement of oxygen concentration using sonochemically synthesized microspheres. Biophys. J. 1994;67:896–901. doi: 10.1016/S0006-3495(94)80551-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Battino R, Rettich TR, Tominaga T. The solubility of oxygen and ozone in liquids. J. Phys. Chem. Ref. Data. 1983;12:163–178. [Google Scholar]
- [45].Utsumi H, Yamada K.-i., Ichikawa K, Sakai K, Kinoshita Y, Matsumoto S, Nagai M. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1463–1468. doi: 10.1073/pnas.0510670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Benial AMF, Ichikawa K, Murugesan R, Yamada K.-i., Utsumi H. Dynamic nuclear polarization properties of nitroxyl radicals used in Overhauser-enhanced MRI for simultaneous molecular imaging. J. Magn. Reson. 2006;182:273–282. doi: 10.1016/j.jmr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- [47].Benial AMF, Utsumi H, Ichikawa K, Murugesan R, Yamada K-I, Kinoshita Y, Naganuma T, Kato M. Dynamic nuclear polarization studies of redox-sensitive nitroxyl spin probes in liposomal solution. J. Magn. Reson. 2010;204:131–138. doi: 10.1016/j.jmr.2010.02.016. [DOI] [PubMed] [Google Scholar]
- [48].Gueven N, Luff J, Peng C, Hosokawa K, Bottle SE, Lavin MF. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radical Biol. Med. 2006;41:992–1000. doi: 10.1016/j.freeradbiomed.2006.06.018. [DOI] [PubMed] [Google Scholar]
- [49].Chen P, Peng C, Luff J, Spring K, Watters D, Bottle S, Furuya S, Lavin MF. Oxidative stress is responsible for deficient survival and dendritogenesis in Purkinje neurons from ataxia-telangiectasia mutated mutant mice. J. Neurosci. 2003;23:11453–11460. doi: 10.1523/JNEUROSCI.23-36-11453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bolton R, Sutcliffe LH, Wu X. Synthesis of isotopically-labeled nitroxyl radicals for use as spin probes. J. Labelled Compd. Radiopharm. 1994;34:663–668. [Google Scholar]
- [51].Betley TA, Peters JC. A Tetrahedrally Coordinated L3Fe-Nx Platform that Accommodates Terminal Nitride (FeIV≡N) and Dinitrogen (FeI-N2-FeI) Ligands. J. Am. Chem. Soc. 2004;126:6252–6254. doi: 10.1021/ja048713v. [DOI] [PubMed] [Google Scholar]
- [52].Hanson GR, Gates KE, Noble CJ, Griffin M, Mitchell A, Benson S. XSophe-Sophe-XeprView. A computer simulation software suite (v. 1.1.3) for the analysis of continuous wave EPR spectra. J. Inorg. Biochem. 2004;98:903–916. doi: 10.1016/j.jinorgbio.2004.02.003. [DOI] [PubMed] [Google Scholar]