Summary
GTPases function as intracellular, bimolecular switches by adopting different conformational states in response to binding GDP or GTP. Their activation is mediated through cell-surface receptors. Rho GTPases act on several downstream effectors involved in cellular morphogenesis, cell polarity, migration and cell division. In neurons, Rho GTPases regulate various features of dendritic and axonal outgrowth during development and regeneration mainly through their effects on the cytoskeleton. This review summarizes the main functions of Rho, Rac and Cdc42 GTPases as key regulators of morphological neuroplasticity under normal and pathological conditions.
Abbreviations: C3, Clostridium botulinum ribosyltransferase; cAMP, cyclic adenosine monophosphate; Cdc42, cell division control protein 42 homolog; CRIB, Cdc42/Rac interactive binding; DRG, dorsal root ganglia; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; FGF-2, fibroblast growth factor 2; GAP, GTPase-activating protein; GDI, guanine nucleotide dissociation inhibitor; GEF, guanine nucleotide exchange factor; GSK-3β, glycogen synthase kinase-3β; JNK, Jun N-terminal kinase; LPA, lysophosphatidic acid; m-Dia, formin mammalian diaphanous; MAP, mitogen-activated protein; MLC, myosin light chain; N-WASP, neural Wiskott–Aldrich syndrome protein; NGF, nerve growth factor; NT3, neurotrophin-3; PAK, partitioning defective-6; PAR6, p21-activated kinase; PI3K, phosphatidylinositol-3 kinase; PIP, phosphatidylinositol 4,5-bisphosphate; PIP-5kinase, phosphatidylinositol-4 phosphate-5 kinase; PKN, protein kinase N; Rac, Ras-related C3 botulinum toxin substrate; Ras, Rat sarcoma; Rho, Ras homologous; ROCK, Rho-associated coiled-coil-containing protein kinase; SAPK, stress-activated protein kinase; Smurf, Smad ubiquitination regulatory factor
Keywords: Rac, Cdc42, GEF, GAP, Axon, Dendrite, Growth cone, Branching, Sprouting, Elongation
1. Introduction
Outgrowing neuronal processes utilize an intrinsic, actin-based treadmilling mechanism to advance the leading edge of their growth cones, which represent highly specialized structures localized at the tips of axons and dendrites. These cones extend finger-like filopodia and veil-like lamellipodia highly enriched in actin polymer chains (Gallo and Letourneau, 2000; Smith and Li, 2004; Letourneau, 2009). An appropriate substratum is required for the generation of traction and tension within the growth cone. Thereby, poorly attached processes are removed while adherent ones are stabilized or move. Cell adhesion molecules, in particular members of the integrin family, are central to our understanding of the underlying molecular mechanisms of these processes which are under tight regulation by neuronal growth factors and guidance cues. Over the recent years, several small GTPases have been identified as key mediators of the interactions between cell adhesion molecules and the cytoskeleton constituting axonal and dendritic morphology. Among them, Rho GTPases are now regarded as major regulators of axonal and dendritic growth.
Rho proteins act as molecular switches integrating signals from the extracellular environment. They cycle between two conformational states (from an active GTP-bound state to an inactive GDP-bound state) by hydrolyzing GTP to GDP. Eukaryotic cells contain hundreds of such low molecular mass GTPase switches (appr. 21 kDa). The Ras superfamily of GTPases falls into five major groups (Ras, Rho, Rab, Arf and Ran). The Rho and Ras families are particularly relevant for cell biology by regulating morphogenesis, polarity, migration and division at the cellular level. Moreover, at the molecular level they are involved in cytoskeletal dynamics, vesicular transport and gene expression (Etienne-Manneville and Hall, 2002). Rho GTPases were first found in fibroblasts as mediators of filopodia and lamellipodia-formation (Nobes and Hall, 1995). Subsequently, RhoA, Rac, and Cdc42 were identified as key regulators of axonal and dendrite morphogenesis in tissue culture and in vivo (Sebok et al., 1999; Hall and Lalli, 2010). The functional relevance of these three GTPases is underscored by gene knockouts. Mice lacking RhoB or RhoC exhibit no major developmental defects, but global deficiency of RhoA, Rac1 or Cdc42 is embryonically lethal (Heasman and Ridley, 2008).
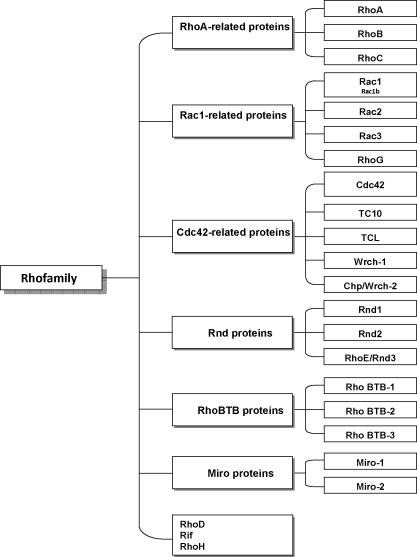
In this overview, we focus on the mammalian Rho family that comprises over 20 proteins and can further be divided into several subgroups (Fig. 1). The main members of four of these subgroups, Rho (RhoA, -B, and -C), Cdc42 (Cdc42, TC10, TCL), Rac (Rac1, -2 and -3, RhoG) and Rif (Rif, RhoD), belong to the classical Rho GTPases. These switches are regulated by their GTPase activities and under the control of many switch activators (guanine nucleotide exchange factors, GEFs) and inactivators (GTPase-activating proteins, GAPs). Some of the GEFs and GAPs have been identified as regulators of assembly, disassembly and dynamic rearrangements of the actin and microtubule cytoskeleton (Linseman and Loucks, 2008; Marx et al., 2005; Ng and Luo, 2004; Gad and Aspenstrom, 2010). A recent genome-wide analysis of GAP functions in Drosophila identified p190 RhoGAP as essential stabilizer of axons involved in olfactory learning and memory (Billuart et al., 2001). In addition, GDI proteins (guanine nucleotide dissociation inhibitors) prevent binding of Rho GTPases to plasma membranes by stabilizing the GDP-bound form (Siderovski and Willard, 2005). Furthermore, GDIs dissociate from Rho proteins in response to the activation of adhesion receptors of the integrin family (del Pozo et al., 2004).
Fig. 1.
Structure of the Rho protein family.
1.1. Regulation
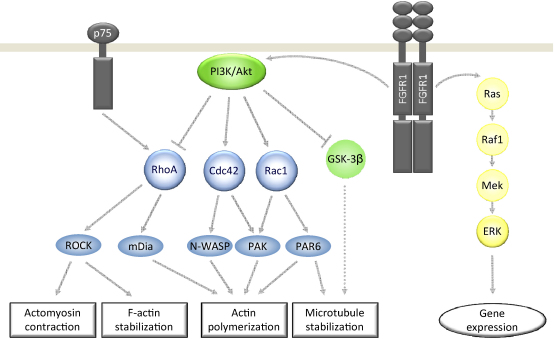
Activation of Rho GTPases is mediated predominantly through cell surface receptors (cytokine-dependent, tyrosine kinase- or G-protein-coupled). Receptor tyrosine kinases (RTKs) are activated by their respective ligands, which lead to the dimerization and autophosphorylation of the receptor and to the stimulation of various signaling pathways including small Rho GTPases (Fig. 2). Most RTKs influence more than one Rho GTPase in a time course similar to the activation of the Ras/Raf/ERK (extracellular signal-regulated kinase) signaling cascade, i.e., within minutes (Schiller, 2006). Some of these Rho proteins, in turn, activate MAP kinase pathways, e.g. c-Jun N-terminal kinase (JNK). Rac1 is activated by various RTKs and induces phosphorylation of Raf (Coles and Shaw, 2002). RhoB is involved in growth factor stimulated RTK trafficking, thus playing a role in modulating RTK signaling from endosomes (Gampel et al., 1999). The link between RTKs and Rho switches is often constituted by Rho GEFs. Some of them mediate signals from several RTKs, while other Rho GEFs appear to be more specific for certain RTKs.The Rnd proteins represent atypical Rho family members that lack intrinsic GTPase activity. Therefore, they remain constitutively active and probably represent another link between RTKs and Rho GTPases. For example, activated fibroblast growth factor receptor (FGFR) type 1 phosphorylates FRS2β, which recruits Shp2 and releases Rnd1 from FRS2. Liberated Rnd1 then inhibits RhoA and promotes neurite outgrowth in FGF-stimulated PC12 (pheochromocytoma) cells (Greene and Tischler, 1982; Harada et al., 2005).
Fig. 2.
Regulation and downstream effectors of Rho GTPases RhoA, Cdc42 and Rac1 involved in shaping neuronal morphology. The PI3K/Akt and Ras/Raf/ERK signaling pathways are activated by RTKs upon ligand binding. Both pathways are indispensable for neurite outgrowth during development and regeneration in response to growth factors. The activation of the MAPK-machinery is implicated in elongative axon growth after injury. PI3K/Akt signaling is central to the regulation of cytoskeletal proteins and linked to axonal branching by adult neurons. The p75 neurotrophin receptor is activating RhoA as part of a receptor complex binding myelin-derived ligands like myelin-associated glycoprotein (MAG), reticulon family member Nogo-A or oligodendrocyte myelin glycoprotein (OMG). Effector proteins downstream of small GTPases include ROCK, mDia, N-WASP, PAK, and PAR6, all of which are involved in restructuring the cytoskeleton.
1.2. Effectors
Rho proteins act on several downstream effectors involved in the stabilization, contraction, polymerization and capture of cytoskeletal building blocks. Among the critical associations are RhoA binding to mDia (formin mammalian diaphanous), Rac1 binding to WAVE (WASP-family verprolin-homologous protein) and Cdc42 binding to N-WASP (neural Wiskott–Aldrich syndrome protein) which all induce protein assemblies required for actin polymerization (Fig. 2). Microtubule stabilization is regulated by RhoA, Rac1 and Cdc42 through the actions of mDia, PAK (p21-activated kinase) or PAR6 (partitioning defective-6) (Iden and Collard, 2008). Moreover, RhoA activates several other effector proteins, among them the Rho-associated coiled-coil-containing protein kinases, ROCKI and ROCKII, which in turn phosphorylate myosin light chain (MLC) and its phosphatase resulting in enhanced actomyosin-based contractility (Kimura et al., 1996; Amano et al., 1996). Inhibition of ROCK in semaphorin-treated embryonic hippocampal neurons reverses the stimulatory effect on axonal branching and increases axonal length. In contrast, dendritic branching is not markedly altered by ROCK inhibition (Hunt et al., 2003; Vodrazka et al., 2009). Other downstream signaling molecules of Rho proteins are not directly related to the cytoskeleton, such as p38α, which is required for calcium-dependent excitotoxic cell death (Semenova et al., 2007).
1.3. Degradation
Ubiquitylation and proteasomal degradation of Rho GTPases has been demonstrated as the decisive mechanism to limit and spatially restrict GTPase signaling. The E3 ubiquitin ligase Smurf1 is responsible for the elimination of RhoA-GDP. Its overexpression reduces RhoA protein levels in Neuro2a cells during dibutyric cyclic AMP (cAMP) induced neurite outgrowth suggesting that localized regulation of different subsets of Rho GTPases regulates neurite outgrowth and guidance (Bryan et al., 2005). Furthermore Smurf is required for neuronal polarity. Smurf2 ubiquitinates the small GTPase Rap1B, which is under the control of PI3K (phosphatidylinositol-3 kinase). Degradation of Rap1B results in restriction to a single neurite and thereby ensures that neurons extend a single axon only (Schwamborn et al., 2007).
2. Axon elongation, sprouting and collateralization
Axon branches are formed by two different ways. Terminal branching is characterized by the bifurcation of the growth cone that gives rise to two or more separate axons shafts. Alternatively, interstitial branching occurs by the de novo initiation of axon branches from previously quiescent regions of the axon (Bastmeyer and O’Leary, 1996; Schmidt and Rathjen, 2010). During transition from a dynamic filopodium to a stable branch, unbundling of axonal microtubules is necessary to enable microtubule ends to interact with actin bundles to form the emanating branch (Letourneau, 2009; Yu et al., 1994). Microtubules then splay apart and invade actin-rich filopodial-like structures on the axon shaft (Kalil et al., 2000). Subsequently, microtubules become bundled again, and the generation of new actin protrusions stops in the plasma membrane lateral to the bundled microtubules, thereby stabilizing the newly formed branch (Bastmeyer and O’Leary, 1996; Gallo, 1998). Terminal branching is considered to occur in the process of axonal outgrowth only, whereas interstitial branching may take place after axonal development and target contact as well (Schmidt and Rathjen, 2010).
In response to axotomy, it is particularly important to separate regenerative axon elongation from axon sprouting (branching) and both processes from axon collateralization. Collateral axons branch from neighboring, uninjured axons which is distinct from sprouting of lesioned axons (Blesch et al., 2005). Furthermore, axonal sprouting and elongation are regulated by different signaling mechanisms. Inhibitors of axon elongation have almost no effect on collateral branch formation indicating that there are different mechanisms for microtubule transfer to axon shafts or to collateral branches (Gallo, 1998). Axons often stop elongating before they start to develop branches. For example, growth cone collapse precedes terminal branching in retinal ganglion cells of Xenopus embryos (Campbell et al., 2001). Furthermore, enlargement and pausing behavior of growth cones is observed before axon branching starts (Tang and Kalil, 2005). Neurotrophic growth factors like FGF-2 or NGF induce interstitial branching (Blesch et al., 2005; Heffner et al., 1990) which is accompanied by an accumulation of actin (Gallo, 1998; Kalil et al., 2000; Willis et al., 2007).
The Ras/Raf/ERK and PI3K/Akt signaling pathways (Fig. 2) are both required for axon outgrowth, and each pathway induces distinct axonal morphologies (Atwal et al., 2000; Markus et al., 2002b; Gallo, 1998; Jones et al., 2003). Overexpression of Ras and Raf stimulates elongative axon growth by embryonic DRG neurons, whereas overexpression of Akt or PI3K enhances axon calibre and branching. Furthermore, neurotrophin-3 (NT3) induces more highly branched and thicker axons than NGF (nerve growth factor) in different types of embryonic neurons (Lentz et al., 1999; Markus et al., 2002b; Ulupinar et al., 2000). Accordingly, NT3 activates Akt more strongly than NGF in embryonic DRG cultures (Markus et al., 2002a). Moreover, NGF induces a stronger activation of Akt in adult DRG cultures than FGF-2, and only FGF-2 but not NGF promotes elongative axon growth in neuronal cultures obtained from animals with a preconditioning sciatic nerve lesion (Hausott et al., 2009; Klimaschewski et al., 2004) suggesting that FGF-2, but not the neurotrophins, promotes long-distance regeneration in response to nerve injury at the lesion site.
PI3K is activated at the leading edge of the growth cone (Zhou et al., 2004). This enzyme is pivotal in regulating the cytoskeleton dynamics of actin filaments through Rac and Cdc42 and of microtubules via GSK-3β (Zhou and Snider, 2006). Activation of PI3K is necessary for branch formation induced by NGF (Gallo, 1998). The link between the main neurite outgrowth signaling pathways and Rho GTPases has been established primarily for PI3K/Akt. Downstream of PI3K, Akt regulates the actin cytoskeleton via Rac1 (Kwon et al., 2000) and microtubule dynamics through the inactivation of glycogen synthase kinase-3β (GSK-3β; Zhou et al., 2004; Yoshimura et al., 2005; Cross et al., 1995; Fukumoto et al., 2001). PI3K-induced activation of Rac1 inhibits RhoA transiently during NGF-induced neurite outgrowth in PC12 cells (Nusser et al., 2002). Inactivation of GSK-3β leads to enhanced axon growth by adult DRG neurons (Jones et al., 2003) and by hippocampal neurons (Jiang et al., 2005; Yoshimura et al., 2005). In addition, the ERK pathway is involved in the regulation of gene expression underlying axon maintenance (Zhou and Snider, 2006). However, ERK is required for local axon assembly and regulates axonal microtubules and actin filaments as well (Atwal et al., 2000; Atwal et al., 2003; Goold and Gordon-Weeks, 2005). Inhibition of ERK results in depolymerization of actin and growth cone collapse (Atwal et al., 2003), a phenomenon induced by the activation of RhoA, too.
3. Specific functions of Rho GTPases in neuronal and glial cells
Rho proteins are decisive in establishing intracellular asymmetry in response to environmental cues. This function becomes particularly obvious in neurons due to their complex polarity. One of the several cytoplasmic processes develops into an axon, whereas the others become dendrites that later establish synaptic contacts with axons through the formation of actin-rich projections (spines). In general, Rho proteins inhibit neurite extension, whereas Cdc42 and Rac act as positive regulators of neurite outgrowth and dendritic spine formation by promoting membrane protrusion through actin filament assembly (Luo, 2000). However, as discussed in this section, recent work has challenged the overly simplistic view that repulsion is mediated by Rho, whereas Rac and Cdc42 promote attraction (O’Donnell et al., 2009).
Other novel aspects relate to the functions of Rho GTPases in glial cells, which enfold multiple layers of plasma membrane around the axon to form myelin. Ablation of Cdc42 in cells of the oligodendrocyte lineage results in a stage-specific myelination phenotype characterized by an enlargement of the inner tongue of the oligodendrocyte process. Similarly, knockout of Rac1 results in abnormal accumulation of cytoplasm in oligodendrocytes as well. In Schwann cells, the lack of Rac1 produces a delay in the process of radial sorting of axons and arrests myelination (Thurnherr et al., 2006). It is conceivable that cortical actomyosin contraction pushes cytoplasm out as the membranes extend around the axon. Cdc42 deficient Schwann cells are defective in axon myelination, and perturbation of RhoA activity inhibits glial migration and defasciculation of sensory axons (Feltri et al., 2008).
3.1. RhoA
RhoA is critically involved in the growth cone response to collapsing guidance cues by promoting membrane retraction (Jin and Strittmatter, 1997; Kuhn et al., 1999; Thies and Davenport, 2003). Slit, ephrins, netrins, and semaphorins have all been demonstrated to influence Rho GTPases (O’Donnell et al., 2009). Guidance receptors like plexin-B inhibit Rac directly probably by sequestering Rac from its effector PAK. Within the growth cone, Rac1 and Cdc42 activities are high in the peripheral domain (P-domain), whereas Cdc42 activity increases gradually towards the growth cone edge. RhoA activity is apparently high in the P-domain as well (Nakamura et al., 2005). As expected, suppression of RhoA activity leads to a loss or deformation of actin bundles in the growth cone. It is likely that RhoA-GTP is required in the P-domain to retain the spread morphology of axonal growth cones, but leads to the retraction of neuronal processes when activated within the shaft. Accordingly, RhoA has been proposed to mediate cytoskeletal changes implicated in limiting dendrite branching (Georges et al., 2008).
Although studies concerning axonal branching are less numerous, they indicate that RhoA affects elongation and branching of axons differently as compared to dendrites. Dominant negative RhoA decreases axonal branching in hippocampal neurons obtained from embryonic mice (Ahnert-Hilger et al., 2004). However, constitutively active RhoA negatively influences dendritic but not axonal branching. In contrast, RhoA facilitates branching dynamics and the formation of small branches of upper cortical layer axons in slice cultures, thereby acting as a mediator of activity-dependent branching (Ohnami et al., 2008). In this context it is noteworthy that RhoA mRNA is directed to axons via a targeting element located in the 3′ untranslated region of its mRNA. Semaphorin 3A-mediated growth cone collapse is apparently due to the local translation of the RhoA mRNA within the growth cone (Wu et al., 2005).
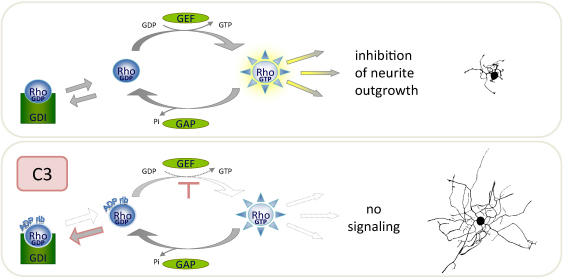
On the other hand, data from animal models lacking molecules involved in the regulation of Rho further complicate our understanding of Rho function in neuronal morphogenesis and plasticity. For example, the lack of an adapter protein (FAK) activating Rho via p190RhoGEF leads to enhanced axonal branching by Purkinje cells and hippocampal neurons (Rico et al., 2004). Corroborating these findings it was demonstrated that Rho-inactivating Rnd2 promotes branching of PC12 cell neurites (Kakimoto et al., 2004; Fujita et al., 2002; Riou et al., 2010). In line with these observations are results showing that overexpression of constitutively active RhoA activates GSK-3β, a key enzyme for regulating axonal elongation which is itself regulated by inhibition (Sayas et al., 2002). However, Sayas et al. demonstrated that GSK-3β is activated by lysophosphatidic acid during neurite retraction in rat cerebellar granule neurons. Since the activation of GSK-3β is blocked by coexpression with C3 transferase (Fig. 3), Rho appears to play a role in the regulation of GSK-3β as well, thereby limiting axon outgrowth in most primary neuron models.
Fig. 3.
C3 exoenzymes exclusively mono-ADP-ribosylate RhoA, B and C resulting in a high affinity complex with GDI. Non-complexed ADP-ribosylated Rho is inhibited from the interaction with Rho GEF, a guanine-nucleotide exchange factor. C3, therefore, stabilizes the inactive GDP-bound form of Rho and, thereby, effectively blocks the interaction of Rho with its effectors such as mDia or ROCK.
Differential effects of various manipulations of the Rho/ROCK pathway on neurite outgrowth may be due to different developmental stages of the animal models applied. Moreover, effects on axonal or dendrite growth and branching are not necessarily identical throughout maturation. Nerve growth factor (NGF), for example, induces growth of sparsely branched sensory axons in vitro at embryonic day 13 (Lentz et al., 1999), but highly branched axons in DRG neurons cultured from adult animals (Cafferty et al., 2001; Klimaschewski et al., 2004).
3.2. Cdc42
With regard to Cdc42, studies applying dominant negative and constitutively active mutants have demonstrated an important function of this GTPase in filopodium extension by growth cones and in multiple aspects of neuronal development, including axon elongation and guidance by diffusible factors such as neurotrophins (Watabe-Uchida et al., 2006; Yuan et al., 2003). Cdc42 is required for filopodia formation and for concomitant activation of the actin regulator cofilin which is inactivated by phosphorylation. Mice with a conditional knockout of Cdc42 in the brain die at birth and exhibit decreased axon numbers and a reduced size of the brain cortex with striking defects in the formation of axonal tracts (Garvalov et al., 2007). Neurons from the Cdc42 null mice extend minor neurites but have a strongly suppressed ability to generate axons. At the cellular level, the cytoskeletal organization is disrupted, the growth cones are enlarged and filopodial dynamics inhibited. The mutant mice exhibit a specific increase in the phosphorylation (i.e., inactivation) of the Cdc42 effector cofilin, and the reduction of this actin regulator results in polarity defects.
3.3. Rac
Rac1, Rac3 and RhoG all promote neurite outgrowth. Loss of Rac1 and Rac2 results in reduced branching of Drosophila mushroom body neurons (Ng et al., 2002), whereas overexpression of Rac1 enhances axon branching by chicken retinal neurons (Albertinazzi et al., 1998). Expression of dominant negative mutants of Rac1 causes a reduction in the number of primary dendrites in cortical neurons. Conversely, the expression of active mutants of Rac results in higher numbers of dendrites (Threadgill et al., 1997). Constitutively active Rac1 increases the proportion of collapsed growth cones in cultures of embryonic chick DRG neurons, whereas dominant negative Rac1 inhibits collapsin-1 induced growth cone collapse and inhibition of neurite outgrowth (Jin and Strittmatter, 1997). In contrast, studies in a conditional knockout of Rac1 in telencephalic neurons only reveal normal axon outgrowth. However, a failure of axons to cross the midline together with defasciculation defects implies that Rac1 is more relevant for axon guidance in the CNS (Chen et al., 2007). A similar phenotype was demonstrated in mice lacking the RhoGEF Trio (triple functional domain protein) (Estrach et al., 2002). Axon growth is decreased in the absence of Trio probably due to the loss of Rac1 activation. Since Trio has two GEF domains which activate at least three GTPases, the effect of Trio might be a combination of Rac, RhoG and RhoA signaling. The opposing functions of RhoA and Rac1 are probably balanced by the GEF T-cell lymphoma invasion and metastasis-1 (TIAM1) which regulates Rac-dependent actomyosin disassembly (van Leeuwen et al., 1999).
4. The role of RhoA in myelin-dependent inhibition of axon regeneration
In contrast to axons in the adult central nervous system (CNS), peripheral axons are capable of regrowth after axotomy. Whereas CNS axon tracts contain myelin-associated neurite growth inhibitors (Schwab et al., 1993), the micro-environment of the peripheral nerve is regarded to lack these inhibitors and provides sufficient support to stimulate and maintain axon regeneration into the denervated muscle or skin. It is generally accepted that peripheral glia (Schwann cells) secretes neurotrophic factors required for survival and regeneration as well as appropriate extracellular matrix molecules (laminins) that function as substrate for integrin receptors to stimulate the axonal growth machinery (Fu and Gordon, 1997). Moreover, the formation of scars or cysts, which act as major barriers for regeneration in the injured CNS (Fitch and Silver, 2008), is greatly reduced in the lesioned peripheral nervous system. However, complete and functionally sufficient long distance regeneration is rarely observed, although sophisticated microsurgical techniques have been developed for nerve repair and nerve grafting (Maggi et al., 2003; Fawcett and Keynes, 1990). Possible mechanisms underlying these deficits in peripheral nerve regeneration are currently under investigation including the possible presence of myelin-associated axon growth inhibitors.
The myelin-associated sialic acid-binding Ig-like glycoprotein (MAG) and the reticulon family member Nogo are among the most potent blockers of axon growth in the CNS. MAG is found in glial cells and inhibits regeneration by adult sensory neurons in vitro (Mukhopadhyay et al., 1994) and in vivo (Torigoe and Lundborg, 1998). Nogo, which is expressed in three different splice variants (Nogo-A, -B, and -C), is detected in neurons and glial elements. Nogo-A is synthesized in oligodendrocytes and in several neuronal populations particularly during development (Huber et al., 2002). In contrast to MAG, however, Nogo-A is apparently absent from Schwann cells (GrandPre et al., 2000). Nogo-B is ubiquitously expressed, whereas Nogo-C is predominantly found in muscle (Schwab, 2004). All three Nogo isoforms exhibit the C-terminal 66-residue inhibitory loop between two putative transmembrane domains (Nogo-66). Nogo-B lacks the N-terminally located NiG domain that consists of several discrete regions with inhibitory characteristics, the most potent of which is the 181-residue NiG-D20 (Niederost et al., 2002). Both Nogo-66 and NiG cause growth cone collapse (Fournier et al., 2001; GrandPre et al., 2000). Nogo-66 requires binding to the glycosyl-phosphatidylinositol (GPI)-linked, leucine-rich repeat glycoprotein, NgR, while the inhibitory effects of MAG and NiG are also detected in cells that lack NgR expression (Niederost et al., 2002; Schweigreiter et al., 2004). NgR mRNA and protein are expressed in neurons projecting into peripheral nerves, in spinal motor neurons and in a subpopulation of peripheral sensory neurons (Hunt et al., 2002; Kim et al., 2003), both in rodents and humans (Josephson et al., 2002).
Downstream from their receptor binding, MAG, Nogo-66 or NiG induce a rapid increase in the amount of cellular GTP-bound RhoA, at least partly mediated by the neurotrophin receptor p75 (Bandtlow, 2003; Schweigreiter et al., 2004; Fournier et al., 2003). Trituration of cerebellar granule cells with the Rho-specific ADP-ribosyltransferase C3 (Fig. 3) or application of the ROCK inhibitor Y-27632 (Bito et al., 2000) significantly improves neurite outgrowth on substrate-bound Nogo-66, NiG or MAG (Niederost et al., 2002). The latter study revealed that C3 effectively suppresses RhoA activity and inhibits MAG- and Nogo-induced RhoA activation. Numerous axons of the optic nerve regenerate beyond the lesion site in response to crush injury following C3 treatment (Lehmann et al., 1999). Moreover, DRG neurons loaded or treated with C3 do not exhibit growth cone collapse upon contact with oligodendrocytes (Niederost et al., 2002) and reveal enhanced neurite outgrowth on myelin, MAG, and Nogog-66 substrates (Fournier et al., 2003). Interestingly, non-steroidal anti-inflammatory drugs (NSAIDs) apparently promote DRG axon regeneration via RhoA inhibition as well (Fu et al., 2007). Furthermore, the Rho kinase (ROCK) inhibitor fasudil (HA-1077) increases the number of large diameter axons and enhances the amplitudes of distally evoked compound muscle action potentials in the mouse sciatic nerve lesion model corroborating the in vivo relevance of the RhoA/ROCK-signaling pathway during peripheral nerve regeneration (Hiraga et al., 2006; Cheng et al., 2008). Another ROCK inhibitor, Y-27632, promotes axonal growth on both myelin and adult rat spinal cord sections (Borisoff et al., 2003), and enhances sprouting of the corticospinal tract accompanied by improved locomotor recovery in adult rodents in vivo (Fournier et al., 2003; Dergham et al., 2002). Taken together, RhoA is induced in motor and sensory neurons after axotomy and plays a central role in mediating the effects of myelin-derived inhibitors on axon regeneration.
5. Conclusions and outlook
Rho family GTPases act as molecular switches that couple changes in the extracellular environment to various intracellular signal transduction pathways. They are influenced by cell surface receptors and regulate distinct aspects of the cytoskeletal protein machinery, such as actin polymerization and depolymerization, anchoring and cross-linking, myosin motor activities and microtubule stabilization. From a morphologist's point of view the recent development of fluorescent probes that non-invasively report the changing subcellular locations of each Rho GTPase activity in single living neurons is particularly promising (Pertz and Hahn, 2004; Pertz et al., 2006). These studies reveal that Rho GTPase signaling dynamics occur on micrometer length scales and subminute timescales to regulate a wide variety of morphogenetic events, among them membrane protrusion and ruffling underlying axonal and dendritic outgrowth as well as growth cone advance and turning during development and regeneration. In all likelihood, we will soon learn how the specific interactions of one GTPase with different guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and their effectors finally determine the details of morphological plasticity in the nervous system.
Acknowledgments
Maria Auer is a student of the PhD graduate program ‘Signal processing in neurons’ (SPIN) and supported by the Austrian Science Fund (FWF). We would like to apologize to all authors whose original papers could not be cited in this review due to space constraints.
References
- Ahnert-Hilger G., Holtje M., Grosse G., Pickert G., Mucke C., Nixdorf-Bergweiler B., Boquet P., Hofmann F., Just I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J. Neurochem. 2004;90:9–18. doi: 10.1111/j.1471-4159.2004.02475.x. [DOI] [PubMed] [Google Scholar]
- Albertinazzi C., Gilardelli D., Paris S., Longhi R., de Curtis I. Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J. Cell Biol. 1998;142:815–825. doi: 10.1083/jcb.142.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Atwal J.K., Massie B., Miller F.D., Kaplan D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and PI3-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Atwal J.K., Singh K.K., Tessier-Lavigne M., Miller F.D., Kaplan D.R. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J. Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C.E. Regeneration in the central nervous system. Exp. Gerontol. 2003;38:79–86. doi: 10.1016/s0531-5565(02)00165-1. [DOI] [PubMed] [Google Scholar]
- Bastmeyer M., O’Leary D.D. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J. Neurosci. 1996;16:1450–1459. doi: 10.1523/JNEUROSCI.16-04-01450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P., Winter C.G., Maresh A., Zhao X., Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Bito H., Furuyashiki T., Ishihara H., Shibasaki Y., Ohashi K., Mizuno K., Maekawa M., Ishizaki T., Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Blesch A., Conner J., Pfeifer A., Gasmi M., Ramirez A., Britton W., Alfa R., Verma I., Tuszynski M.H. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol. Ther. 2005;11:916–925. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Borisoff J.F., Chan C.C., Hiebert G.W., Oschipok L., Robertson G.S., Zamboni R., Steeves J.D., Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol. Cell. Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Bryan B., Cai Y., Wrighton K., Wu G., Feng X.H., Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005;579:1015–1019. doi: 10.1016/j.febslet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Cafferty W.B., Gardiner N.J., Gavazzi I., Powell J., McMahon S.B., Heath J.K., Munson J., Cohen J., Thompson S.W. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J. Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.S., Regan A.G., Lopez J.S., Tannahill D., Harris W.A., Holt C.E. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J. Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liao G., Waclaw R.R., Burns K.A., Linquist D., Campbell K., Zheng Y., Kuan C.Y. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J. Neurosci. 2007;27:3884–3893. doi: 10.1523/JNEUROSCI.3509-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Webber C.A., Wang J., Xu Y., Martinez J.A., Liu W.Q., McDonald D., Guo G.F., Nguyen M.D., Zochodne D.W. Activated RHOA and peripheral axon regeneration. Exp. Neurol. 2008;212:358–369. doi: 10.1016/j.expneurol.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Coles L.C., Shaw P.E. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002;21:2236–2244. doi: 10.1038/sj.onc.1205302. [DOI] [PubMed] [Google Scholar]
- Cross D.A.E., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Alderson N.B., Kiosses W.B., Chiang H.H., Anderson R.G., Schwartz M.A. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- Dergham P., Ellezam B., Essagian C., Avedissian H., Lubell W.D., McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S., Schmidt S., Diriong S., Penna A., Blangy A., Fort P., Debant A. The human Rho-GEF Trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fawcett J.W., Keynes R.J. Peripheral nerve regeneration. Annu. Rev. Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Feltri M.L., Suter U., Relvas J.B. The function of RhoGTPases in axon ensheathment and myelination. Glia. 2008;56:1508–1517. doi: 10.1002/glia.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch M.T., Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A.E., GrandPre T., Strittmatter S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier A.E., Takizawa B.T., Strittmatter S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Hue J., Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J. Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S.Y., Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Fujita H., Katoh H., Ishikawa Y., Mori K., Negishi M. Rapostlin is a novel effector of Rnd2 GTPase inducing neurite branching. J. Biol. Chem. 2002;277:45428–45434. doi: 10.1074/jbc.M208090200. [DOI] [PubMed] [Google Scholar]
- Fukumoto S., Hsieh C.M., Maemura K., Layne M.D., Yet S.F., Lee K.H., Matsui T., Rosenzweig A., Taylor W.G., Rubin J.S., Perrella M.A., Lee M.E. Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Gad A.K., Aspenstrom P. Rif proteins take to the RhoD: Rho GTPases at the crossroads of actin dynamics and membrane trafficking. Cell. Signal. 2010;22:183–189. doi: 10.1016/j.cellsig.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Gallo G. Involvement of microtubules in the regulation of neuronal growth cone morphologic remodeling. J. Neurobiol. 1998;35:121–140. [PubMed] [Google Scholar]
- Gallo G., Letourneau P.C. Neurotrophins and the dynamic regulation of the neuronal cytoskeleton. J. Neurobiol. 2000;44:159–173. doi: 10.1002/1097-4695(200008)44:2<159::aid-neu6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gampel A., Parker P.J., Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr. Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- Garvalov B.K., Flynn K.C., Neukirchen D., Meyn L., Teusch N., Wu X., Brakebusch C., Bamburg J.R., Bradke F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges P.C., Hadzimichalis N.M., Sweet E.S., Firestein B.L. The yin-yang of dendrite morphology: unity of actin and microtubules. Mol. Neurobiol. 2008;38:270–284. doi: 10.1007/s12035-008-8046-8. [DOI] [PubMed] [Google Scholar]
- Goold R.G., Gordon-Weeks P.R. The MAP kinase pathway is upstream of the activation of GSK3 beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol. Cell. Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- GrandPre T., Nakamura F., Vartanian T., Strittmatter S.M. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Greene L.A., Tischler A.S. PC12 pheochromocytoma cultures in neurobiological research. Adv. Cell. Neurobiol. 1982;3:373–414. [Google Scholar]
- Hall A., Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Katoh H., Negishi M. Direct interaction of Rnd1 with FRS2 beta regulates Rnd1-induced down-regulation of RhoA activity and is involved in fibroblast growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 2005;280:18418–18424. doi: 10.1074/jbc.M411356200. [DOI] [PubMed] [Google Scholar]
- Hausott B., Vallant N., Auer M., Yang L., Dai F., Brand-Saberi B., Klimaschewski L. Sprouty2 down-regulation promotes axon growth by adult sensory neurons. Mol. Cell. Neurosci. 2009;42:328–340. doi: 10.1016/j.mcn.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Heasman S.J., Ridley A.J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Heffner C.D., Lumsden A.G., O’Leary D.D. Target control of collateral extension and directional axon growth in the mammalian brain. Science. 1990;247:217–220. doi: 10.1126/science.2294603. [DOI] [PubMed] [Google Scholar]
- Hiraga A., Kuwabara S., Doya H., Kanai K., Fujitani M., Taniguchi J., Arai K., Mori M., Hattori T., Yamashita T. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J. Peripher. Nerv. Syst. 2006;11:217–224. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Huber A.B., Weinmann O., Brosamle C., Oertle T., Schwab M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D., Coffin R.S., Prinjha R.K., Campbell G., Anderson P.N. Nogo-A expression in the intact and injured nervous system. Mol. Cell. Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hunt D., Mason M.R., Campbell G., Coffin R., Anderson P.N. Nogo receptor mRNA expression in intact and regenerating CNS neurons. Mol. Cell. Neurosci. 2002;20:537–552. doi: 10.1006/mcne.2002.1153. [DOI] [PubMed] [Google Scholar]
- Iden S., Collard J.G. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Jiang H., Guo W., Liang X.H., Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3 beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jin Z., Strittmatter S.M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.M., Tucker B.A., Rahimtula M., Mearow K.M. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J. Neurochem. 2003;86:1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Josephson A., Trifunovski A., Widmer H.R., Widenfalk J., Olson L., Spenger C. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J. Comp. Neurol. 2002;453:292–304. doi: 10.1002/cne.10408. [DOI] [PubMed] [Google Scholar]
- Kakimoto T., Katoh H., Negishi M. Identification of splicing variants of Rapostlin, a novel RND2 effector that interacts with neural Wiskott–Aldrich syndrome protein and induces neurite branching. J. Biol. Chem. 2004;279:14104–14110. doi: 10.1074/jbc.M312763200. [DOI] [PubMed] [Google Scholar]
- Kalil K., Szebenyi G., Dent E.W. Common mechanisms underlying growth cone guidance and axon branching. J. Neurobiol. 2000;44:145–158. [PubMed] [Google Scholar]
- Kim J.E., Bonilla I.E., Qiu D., Strittmatter S.M. Nogo-C is sufficient to delay nerve regeneration. Mol. Cell. Neurosci. 2003;23:451–459. doi: 10.1016/s1044-7431(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L., Nindl W., Feurle J., Kavakebi P., Kostron H. Basic fibroblast growth factor isoforms promote axonal elongation and branching of adult sensory neurons in vitro. Neuroscience. 2004;126:347–353. doi: 10.1016/j.neuroscience.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Kuhn T.B., Brown M.D., Wilcox C.L., Raper J.A., Bamburg J.R. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of Rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Kwon D.Y., Chun J., Kim J.H., Kang S.S. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J. Biol. Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Fournier A., Selles-Navarro I., Dergham P., Sebok A., Leclerc N., Tigyi G., McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz S.I., Knudson C.M., Korsmeyer S.J., Snider W.D. Neurotrophins support the development of diverse sensory axon morphologies. J. Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P.C. Actin in axons: stable scaffolds and dynamic filaments. Results Probl. Cell Differ. 2009;48:65–90. doi: 10.1007/400_2009_15. [DOI] [PubMed] [Google Scholar]
- Linseman D.A., Loucks F.A. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front. Biosci. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Maggi S.P., Lowe J.B., III, Mackinnon S.E. Pathophysiology of nerve injury. Clin. Plast. Surg. 2003;30:109–126. doi: 10.1016/s0094-1298(02)00101-3. [DOI] [PubMed] [Google Scholar]
- Markus A., Patel T.D., Snider W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Markus A., Zhong J., Snider W.D. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Marx R., Henderson J., Wang J., Baraban J.M. Tech: a RhoA GEF selectively expressed in hippocampal and cortical neurons. J. Neurochem. 2005;92:850–858. doi: 10.1111/j.1471-4159.2004.02930.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G., Doherty P., Walsh F.S., Crocker P.R., Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Aoki K., Matsuda M. FRET imaging in nerve growth cones reveals a high level of RhoA activity within the peripheral domain. Brain Res. Mol. Brain Res. 2005;139:277–287. doi: 10.1016/j.molbrainres.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Ng J., Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Ng J., Nardine T., Harms M., Tzu J., Goldstein A., Sun Y., Dietzl G., Dickson B.J., Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Niederost B., Oertle T., Fritsche J., McKinney R.A., Bandtlow C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nusser N., Gosmanova E., Zheng Y., Tigyi G. Nerve growth factor signals through TrkA, phosphatidylinositol 3-kinase, and Rac1 to inactivate RhoA during the initiation of neuronal differentiation of PC12 cells. J. Biol. Chem. 2002;277:35840–35846. doi: 10.1074/jbc.M203617200. [DOI] [PubMed] [Google Scholar]
- O’Donnell M., Chance R.K., Bashaw G.J. Axon growth and guidance: receptor regulation and signal transduction. Annu. Rev. Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnami S., Endo M., Hirai S., Uesaka N., Hatanaka Y., Yamashita T., Yamamoto N. Role of RhoA in activity-dependent cortical axon branching. J. Neurosci. 2008;28:9117–9121. doi: 10.1523/JNEUROSCI.1731-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O., Hodgson L., Klemke R.L., Hahn K.M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Pertz O., Hahn K.M. Designing biosensors for Rho family proteins – deciphering the dynamics of Rho family GTPase activation in living cells. J. Cell Sci. 2004;117:1313–1318. doi: 10.1242/jcs.01117. [DOI] [PubMed] [Google Scholar]
- Rico B., Beggs H.E., Schahin-Reed D., Kimes N., Schmidt A., Reichardt L.F. Control of axonal branching and synapse formation by focal adhesion kinase. Nat. Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou P., Villalonga P., Ridley A.J. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays. 2010;32:986–992. doi: 10.1002/bies.201000060. [DOI] [PubMed] [Google Scholar]
- Sayas C.L., Avila J., Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 2002;22:6863–6875. doi: 10.1523/JNEUROSCI.22-16-06863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M.R. Coupling receptor tyrosine kinases to Rho GTPases – GEFs what's the link. Cell. Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Rathjen F.G. Signalling mechanisms regulating axonal branching in vivo. Bioessays. 2010;32:977–985. doi: 10.1002/bies.201000054. [DOI] [PubMed] [Google Scholar]
- Schwab M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Schwab M.E., Kapfhammer J.P., Bandtlow C.E. Inhibitors of neurite growth. Annu. Rev. Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- Schwamborn J.C., Muller M., Becker A.H., Puschel A.W. Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 2007;26:1410–1422. doi: 10.1038/sj.emboj.7601580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schweigreiter R., Walmsley A.R., Niederost B., Zimmermann D.R., Oertle T., Casademunt E., Frentzel S., Dechant G., Mir A., Bandtlow C.E. Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Mol. Cell. Neurosci. 2004;27:163–174. doi: 10.1016/j.mcn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Sebok A., Nusser N., Debreceni B., Guo Z., Santos M.F., Szeberenyi J., Tigyi G. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J. Neurochem. 1999;73:949–960. doi: 10.1046/j.1471-4159.1999.0730949.x. [DOI] [PubMed] [Google Scholar]
- Semenova M.M., Maki-Hokkonen A.M., Cao J., Komarovski V., Forsberg K.M., Koistinaho M., Coffey E.T., Courtney M.J. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat. Neurosci. 2007;10:436–443. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- Siderovski D.P., Willard F.S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.G., Li R. Actin polymerization: riding the wave. Curr. Biol. 2004;14:R109–R111. [PubMed] [Google Scholar]
- Tang F., Kalil K. Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J. Neurosci. 2005;25:6702–6715. doi: 10.1523/JNEUROSCI.0871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies E., Davenport R.W. Independent roles of Rho-GTPases in growth cone and axonal behavior. J. Neurobiol. 2003;54:358–369. doi: 10.1002/neu.10135. [DOI] [PubMed] [Google Scholar]
- Threadgill R., Bobb K., Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Thurnherr T., Benninger Y., Wu X., Chrostek A., Krause S.M., Nave K.A., Franklin R.J., Brakebusch C., Suter U., Relvas J.B. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J. Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K., Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Exp. Neurol. 1998;150:254–262. doi: 10.1006/exnr.1997.6775. [DOI] [PubMed] [Google Scholar]
- Ulupinar E., Jacquin M.F., Erzurumlu R.S. Differential effects of NGF and NT-3 on embryonic trigeminal axon growth patterns. J. Comp. Neurol. 2000;425:202–218. doi: 10.1002/1096-9861(20000918)425:2<202::aid-cne4>3.0.co;2-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F.N., van Delft S., Kain H.E., van der Kammen R.A., Collard J.G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Vodrazka P., Korostylev A., Hirschberg A., Swiercz J.M., Worzfeld T., Deng S., Fazzari P., Tamagnone L., Offermanns S., Kuner R. The semaphorin 4D-plexin-B signalling complex regulates dendritic and axonal complexity in developing neurons via diverse pathways. Eur. J. Neurosci. 2009;30:1193–1208. doi: 10.1111/j.1460-9568.2009.06934.x. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M., Govek E.E., Van Aelst L. Regulators of Rho GTPases in neuronal development. J. Neurosci. 2006;26:10633–10635. doi: 10.1523/JNEUROSCI.4084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D.E., van Niekerk E.A., Sasaki Y., Mesngon M., Merianda T.T., Williams G.G., Kendall M., Smith D.S., Bassell G.J., Twiss J.L. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.Y., Hengst U., Cox L.J., Macosko E.Z., Jeromin A., Urquhart E.R., Jaffrey S.R. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Yu W., Ahmad F.J., Baas P.W. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J. Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.B., Jin M., Xu X., Song Y.Q., Wu C.P., Poo M.M., Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- Zhou F.Q., Snider W.D. Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.Q., Zhou J., Dedhar S., Wu Y.H., Snider W.D. NGF-induced axon growth is mediated by localized inactivation of GSK-30 and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]