Abstract
Aquaporin (AQP) 1 and AQP0 water channels are expressed in lens epithelial and fiber cells, respectively, facilitating fluid circulation for nourishing the avascular lens to maintain transparency. Even though AQP0 water permeability is 40-fold less than AQP1, AQP0 is selectively expressed in the fibers. Delimited AQP0 fiber expression is attributed to a unique structural role as an adhesion protein. To validate this notion, we determined if wild type (WT) lens ultrastructure and fiber cell adhesion are different in AQP0−/−, and TgAQP1+/+/AQP0−/− mice that transgenically express AQP1 (TgAQP1) in fiber cells without AQP0 (AQP0−/−). In WT, lenses were transparent with ‘Y’ sutures. Fibers contained opposite end curvature, lateral interdigitations and hexagonal shape, and were arranged as concentric growth shells. AQP0−/− lenses were cataractous, lacked ‘Y’ sutures, ordered packing and well-defined lateral interdigitations. TgAQP1+/+/AQP0−/− lenses showed improvement in transparency and lateral interdigitations in the outer cortex while inner cortex and nuclear fibers were severely disintegrated. Transmission electron micrographs exhibited tightly packed fiber cells in WT whereas AQP0−/− and TgAQP1+/+/AQP0−/− lenses had wide extracellular spaces. Fibers were easily separable by teasing in AQP0−/− and TgAQP1+/+/AQP0−/− lenses compared to WT. Our data suggest that the increased water permeability through AQP1 does not compensate for loss of AQP0 expression in TgAQP1+/+/AQP0−/− mice. Fiber cell AQP0 expression is required to maintain their organization, which is a requisite for lens transparency. AQP0 appears necessary for cell-to-cell adhesion and thereby to minimize light scattering since in the AQP0−/− and TgAQP1+/+/AQP0−/− lenses, fiber cell disorganization was evident.
Keywords: AQP0, lens cataract, cell-to-cell adhesion, analogous function, unique function, lenticular architecture and transparency
1. Introduction
The incessantly growing ocular lens is avascular and normally transparent to aid in the process of focusing light on to the retina. Vertebrate lenses have two major cell types, a single layer of cuboidal epithelial cells on the anterior surface and numerous layers of elongated fiber cells in the bulk of the lens. Cells in the equatorial region undergo remarkable morphological, biochemical, and physiological transformations as they differentiate to form a new layer of secondary fiber cells, covering the older fibers. Ends of lens fiber cells flare and curve away from the poles in opposite directions and meet the opposite fibers at their flared ends. End-to-end they form the lens suture branches [1]. In the mouse lens, the ‘Y’-shaped suture is formed by the meeting of three anterior fibers that are oriented at 120° to each other; the opposite ends of the curvatures of the three posterior ends of the fibers form an ‘inverted Y’ suture.
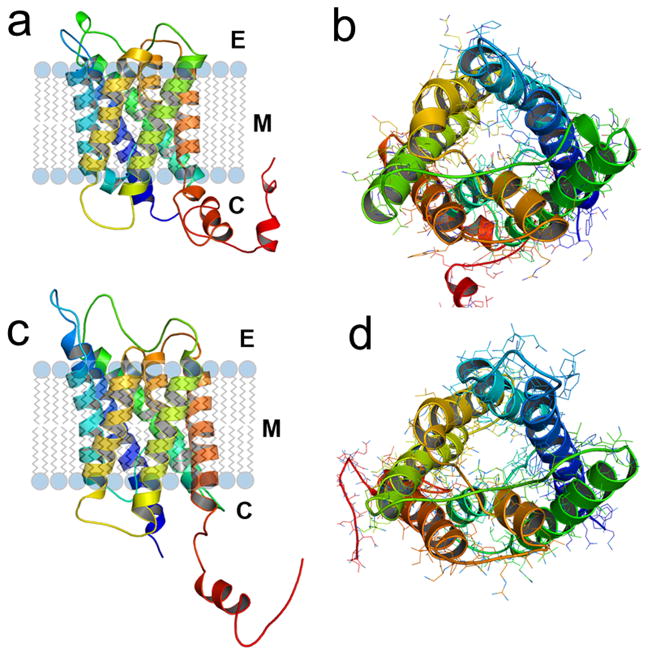
Proteins present in the lenticular cells play an important role in lens transparency. Two structurally similar aquaporins, Aquaporin 0 (AQP0, Fig. 1a,b) and Aquaporin 1 (AQP1, Fig. 1c,d), are predominantly expressed in the lens. Even though they both have appreciable water permeability, AQP1 permeability is 40-fold higher than that of AQP0. One major difference between them is that AQP0 has a narrower pore (Fig. 1b) than AQP1 (Fig. 1d). Interestingly, AQP0 expression is localized to the fiber cells, whereas AQP1 is found only in the epithelial cells. The expression of AQP1 is down-regulated and replaced by AQP0 during secondary fiber cell differentiation from the equatorial epithelial cells. Aquaporin 0 (AQP0) is the most abundant membrane protein in the fiber cells, constituting ~45% of the total lens membrane proteins. A trace amount of AQP5 (~0.4%) has also been reported as being expressed in the fiber cells [2–4]. Natural mutations thus far reported for AQP0, six in humans and three in mice, have been associated with congenital autosomal dominant lens cataracts [5,6]. Knockout of AQP0 leads to severe lens cataract in mouse [7]. Under diabetic conditions, AQP0 gets glycated causing lens cataractogenesis [8,9]. These investigations highlight the significant role of AQP0 for lens transparency and homeostasis. Additionally, lack of functional AQP1 in human [10] or mouse [11] did not produce any detectable adverse effect on lens transparency under normal conditions.
Fig. 1.
Three dimensional simulations of wild type mouse AQP0 (a) and AQP1 (b) proteins predicted using 3D-JIGSAW version 2.0. The cartoons were created using PyMOL. Monomers of mouse AQP0 and AQP1 are rendered in cartoon showing the folds, helix assignment, and location in the membrane. Modeled structures of mouse AQP0 and mouse AQP1 are in the same orientation. (a) and (c), ribbon diagrams of AQP0 and AQP1, respectively, with transmembrane helices embedded in the lipid bilayer. (b) and (d) AQP0 and AQP1, respectively; channels viewed across the cell membrane. In the middle, AQP0 water channel (b) is occupied by more side chains compared to AQP1 (d) that exhibits a wider aqueous pore.
Several roles have been proposed for AQP0 such as being a gap junction channel, ion channel, water channel and a cell-to-cell adhesion protein (review [5]). While the first two roles have been experimentally ruled out, water channel function has been thoroughly investigated. In vitro expression studies demonstrated that AQP0 could form water channels in Xenopus oocytes [12–18]. Ex-vivo lens model studies further supported the water permeability of AQP0 [19–20]. The channel function may be regulated by zinc [21], C-terminal Ca2+/Calmodulin interaction, and phosphorylation [20,22–26]. In vivo cell-to-cell adhesion function of AQP0 remains an open question. Nevertheless, several indications support the hypothesis of a structural role for AQP0, viz., (1) presence of AQP0 in the lateral fiber cell interdigitations, which are specialized interlocking units existing as ball and socket configuration in the outer cortex and tongue and groove in the inner cortex and nucleus, (2) presence of square arrays of AQP0 in the membrane [27–32], (3) protein structural studies [33,34] indicating extracellular space reduction by AQP0 through cell-to-cell adhesion, and (4) in vitro experimental evidence for AQP0 cell-to-cell adhesion elicitation [35].
There is substantive evidence that fiber cell organization is not only dependent on the level of water permeability provided by AQP expression. The fact that cell-to-cell adhesion is critical was shown in our recent study using a transgenic mouse model (TgAQP1+/+/AQP0−/−) that expresses a non-native water channel, AQP1 (normally expressed in lens epithelial cells) in the fiber cells of AQP0 deficient mouse that develops lens cataract. Despite such replacement in the fiber cells, the lenses were only partially transparent [36]. The requirement of AQP0 expression in the fiber cells suggested that this AQP elicited some other essential function needed for lens transparency. To gain insight into the fiber cell AQP0 requirement, we used light microscopy (LM) to study lens transparency and histology, scanning electron microscopy (SEM) for fiber surface morphology and transmission electron microscopy (TEM) to examine the cross-sectional ultrastructure of the fiber cells in wild type, AQP0-deficient (AQP0−/−), and TgAQP1+/+/AQP0−/− mice.
Analysis of our results shows that the disorganization of the fiber cells in TgAQP1 +/+/AQP0−/− lens could be an effect of the absence of AQP0 expression. AQP0 has been shown previously [35] to possess cell-to-cell adhesion property in vitro. The current study corroborates that cell-to-cell adhesion is a unique AQP0 function. In contrast, AQP1 lacks the property of cell-to-cell adhesion, both in vitro and in vivo.
2. Materials and methods
2.1. Animals
Wild type (FVB strain; Charles River Inc.), AQP0 knockout (AQP0−/−) [7], and AQP0 knockout mice expressing transgene AQP1 in the lens fiber cells (TgAQP1+/+/AQP0−/−) [36] were used. Even though a CP49 mutation has been reported [37] for the FVB mouse, the strain we have been breeding and rearing in our animal facility for the past eight years, did not develop lens opacity or cataract [38].
2.2. Lens transparency
Mouse eyes were removed and transferred to mammalian physiological saline prewarmed to 37°C. Lenses were dissected out, transferred to glass petri dishes containing prewarmed lens physiological saline, and photographed.
2.3. Histological analysis of lens
2.3.1. Light microscopy
Eyes from one-month-old mice were dissected out, fixed overnight in 10% neutral buffered formalin, rinsed briefly in phosphate-buffered saline (PBS), and cryosectioned serially at 18 mm thickness. Sections were stained with hematoxylin and eosin (H&E) for standard histological studies.
2.3.2. Lens cortex fiber cell-to-fiber cell adhesion strength assay
Lens capsules from wild type, AQP0−/−, and TgAQP1+/+/AQP0−/− mice, three months of age, were removed and briefly fixed in 2% paraformaldehyde or 1% glutaraldehyde at room temperature. The outer cortex was peeled off from the inner cortex and nucleus. Bundles of outer cortex fiber cells were subjected to manual teasing, using fine forceps to pull them apart, in order to assess the adhesive strength. The teased fiber cell suspensions were imaged under a Zeiss phase contrast microscope.
2.3.3. Scanning electron microscopy (SEM)
Lenses were washed several times in PBS (pH 7.2), kept overnight in the buffer, divided through polar axis using a sharp blade to reveal fiber organization in radial fiber cell columns and post-fixed overnight at room temperature in 1% aqueous OsO4. After washing several times in PBS and dehydrating through a graded ethanol series, lenses were immersed for 40 min in a 50:50 mixture of acetone and hexamethyldisilazane (HMDS) followed by an hour immersion in pure HMDS. Excess HMDS was blotted off and lenses were transferred to desiccators to avoid moisture-associated contamination. After drying, lens specimens were attached to aluminum stubs or plates with conductive silver paint or tape and were mounted on their convex surfaces so that the exposed radial fiber cell columns were oriented at ~90° to the direction of the electron beam. Specimens were sputter-coated with gold under vacuum and examined in a SFEG-SEM model LEO1550 with Robinson backscatter detector (RBSD) Scanning Electron Microscope. Images were taken with a digital camera system, saved as TIFF files and compiled using Adobe Photoshop version 8.
2.3.4. Transmission electron microscopy (TEM)
Lenses were enucleated and fixed in 4% paraformaldehyde overnight. The fixative was replaced with 2.5% glutaraldehyde mix in PBS overnight at 4°C and was changed daily for 5 days. After washing several times in PBS, lenses were post-fixed in 2% OsO4 in PBS for 4 h at room temperature, dehydrated in a graded series of ethyl alcohol and embedded in spur resin. Ultra-thin sections of 80 nm were cut with a Reichert-Jung UltracutE ultramicrotome and placed on formvar-coated slot copper grids. Sections were counterstained with uranyl acetate and lead citrate and viewed with a FEI Tecnai12 BioTwinG2 electron microscope. Digital images were acquired with an AMT XR-60 CCD Digital Camera system and compiled using Adobe Photoshop version 8.
3. Results and Discussion
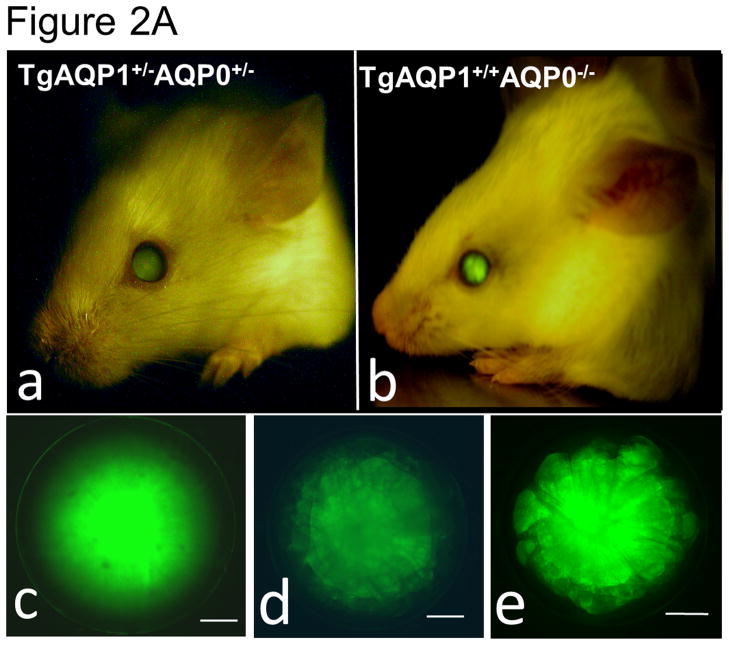
As there is suggestive accumulating evidence that AQP0 expression is required for cell-to-cell adhesion, we directly tested such conjecture by using genetically engineered animal models. This was done by determining the impact of substituting AQP0 with AQP1, on the fiber cell organization [36]. Two mouse models were developed: a transgenic model (TgAQP1+/+) expressing AQP1-EGFP chimeric transgene in the lens fiber cells of the wild type mouse to rule out the incidence of undesired effects, and another transgenic model (TgAQP1+/+/AQP0−/−) using AQP0 knockout mouse (AQP0−/−) to study whether AQP0 performs unique function(s) besides the analogous role of being a water channel. If AQP1 is able to replace AQP0 functionally and rectify the cataract in AQP0 knockout mice, then presumably, the provision of water permeability function by AQP1 is sufficient for lens transparency maintenance. However, if cataract persists even after the water permeability function has been compensated, then, AQP0 may be performing at least one unique function. Figure 2A shows TgAQP1+/−/AQP0+/− ((a); heterozygous) and TgAQP1+/+/AQP0−/− ((b); homozygous) mice expressing transgenic AQP1-EGFP in the lens. EGFP fluorescent lenses were dissected out, photographed, and shown for TgAQP1+/+ (Fig. 2A,c), TgAQP1+/−/AQP0+/− (d) and TgAQP1+/+/AQP0−/− (e) mice. Intensity of EGFP fluorescence of the homozygous mouse (Fig. 2A,e) was roughly twice that of the heterozygous (Fig. 2A,d) lens, as shown by quantification (Fig. 2B). Figure 2C shows the osmotic water permeability (Pf) of fiber cell membrane vesicles from WT (36 ± 5 μm/sec), AQP0−/− (8 ± 2 μm/sec), TgAQP1+/+ (125 ± 19 μm/sec), and TgAQP1+/+/AQP0−/− (97 ± 16 μm/sec) lenses, as well as that of lipid vesicles (1.7 ± 0.2 μm/sec) from WT lenses. The presence of higher fiber cell membrane water permeability in WT and transgenic mouse models is due to aquaporin expression. Comparatively, water permeability in the AQP0−/− and lens lipids is significantly low. Membrane water permeability of TgAQP1+/+/AQP0−/− lenses increased significantly by 2.6-fold compared to that of WT lenses, due to the expression of transgenic AQP1 in the lens fiber cells (Fig. 2C). Development of the transgenic models as well as the physiological and functional impacts of the transgenic expression of AQP1, has been described [36]. The major focus of the present paper is to analyze the morphology and ultrastructure of the lenses from the different mouse genotypes in order to assess whether AQP0 performs a unique function in the lens besides the analogous water channel function.
Fig. 2.
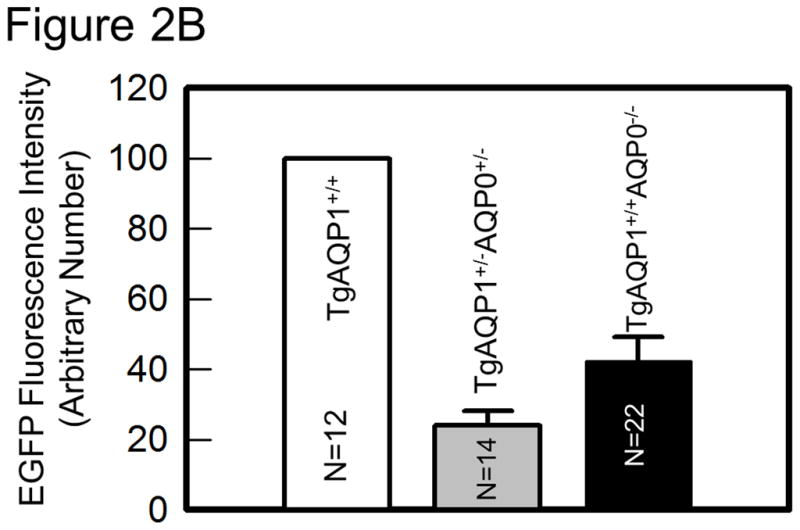
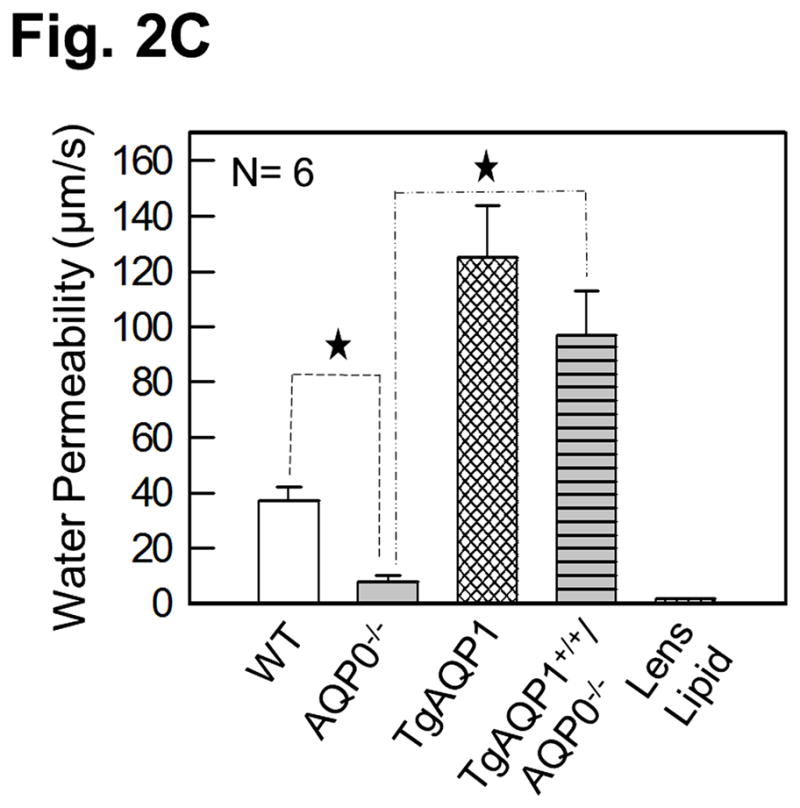
Expression of transgene AQP1-EGFP. A. Fluorescent eyes of AQP1 transgenic AQP0 knockout mice exhibiting different levels of chimeric AQP1-EGFP expression in the lenses: a) heterozygous (TgAQP1+/−/AQP0+/−) and b) homozygous (TgAQP1+/+/AQP0−/−). Special light and filter device called Dark Reader was used to observe the fluorescence. c–e) Fluorescent lenses of adult mice expressing chimeric AQP1-EGFP: c) Wild type expressing AQP1-EGFP (TgAQP1+/+), d) heterozygous (TgAQP1+/−/AQP0+/−) and e) homozygous (TgAQP1+/+/AQP0−/−) transgenic lens. Scale bars represent 250 μm. B. Quantification of the intensity of EGFP fluorescence. C. Fiber cell membrane and lipid vesicle water permeability of WT, AQP0−/−, TgAQP1 and TgAQP1+/+/AQP0−/− mouse lenses. N, number of lenses used. Star represents the degree of significance in comparison, P < 0.0001.
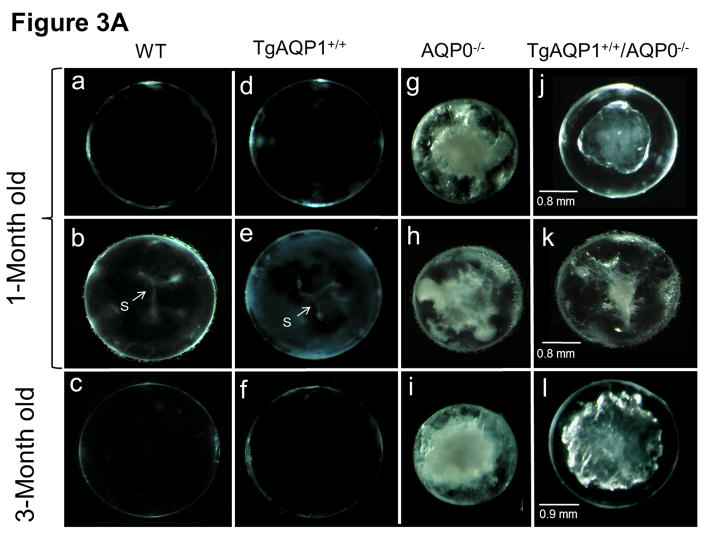
Figure 3A shows some images of the impact of the transgenic expression of AQP1 on lens transparency, and gross morphology of lenses of WT, AQP0−/−, TgAQP1+/+, and TgAQP1+/+/AQP0−/− mice from postnatal day 30 (P30) to 3 months of age. Wild type (Fig. 3A, a–c) and transgenic AQP1 (TgAQP1+/+; Fig. 3A, d–f) lenses were transparent from P30 to 24-months of age (maximum age at which observations were made). TgAQP1+/+ did not cause any deleterious effect on ‘Y’ suture formation (Fig. 3A, e), and lenses were transparent with no macroscopic changes (Fig. 3A, d–f). At birth, AQP0−/− mouse lenses showed lens cataract whereas TgAQP1+/+/AQP0−/− lenses showed a reduction in the cataract. As lens development progressed, cataractous lenses of AQP0−/− mice showed increase in lens opacity (Fig. 3A, g–i); there was a gradual decrease in the anterior to posterior streamlined architecture that is characteristic of normal fiber cells (data not shown). TgAQP1+/+/AQP0−/− lenses exhibited improvement in lens transparency compared to AQP0 knockout lenses (Fig. 3A, compare (j) with (g) and (l) with (i)). With progression in age, the level of lens transparency gradually decreased, as observed for AQP0 knockout lenses (Fig. 3A compare (g) with (i) and (j) with (l)). Lens transparency of the TgAQP1+/+/AQP0−/− lens improved significantly relative to AQP0−/− lens; however, it did not attain the level of the WT or TgAQP1+/+ lens (Fig. 3A, c,f). Lenses of WT and TgAQP1+/+ mice showed typical ‘Y’ suture (Fig. 3A, b,e indicated with ‘S’ and arrow) at one month of age whereas there was no suture formation in the age-matched AQP0−/− and TgAQP1+/+/AQP0−/− lenses as the fiber cells became distorted and disorganized. Quantification of lens transparency (Fig. 3B) revealed comparable clarity between the WT and TgAQP1+/+ lenses, as well as significant improvement in transparency in TgAQP1+/+/AQP0−/− compared to the AQP0−/− lens.
Fig. 3.
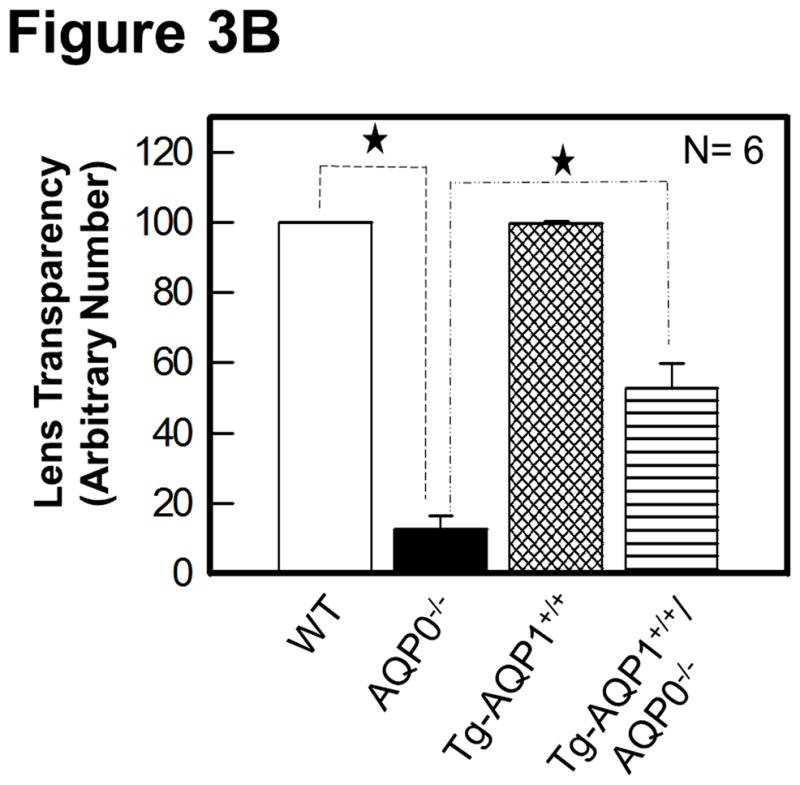
Lens transparency and morphology. A. Lens transparency and cataract in relation to aquaporin expression. (a, b, and c), WT; (d, e, and f), TgAQP1+/+; (g, h, and i), AQP0−/−; (j, k, and l), TgAQP1+/+/AQP0−/−; (b and e), light microscopic images showing ‘Y’ shaped suture lines (indicated by arrow, S); (a, b, d, e, g, h, j, and k), 1-month old mouse lens; (b, e, h, and k) anterior pole in focus to show the suture lines; (c,f,i,l), 3-month old mouse lens. B. Quantification of lens transparency in 1-month-old mice in presence or absence of AQP0 or AQP1 in the fiber cells. N, number of lenses used. Star represents degree of significance in comparison, P < 0.0001.
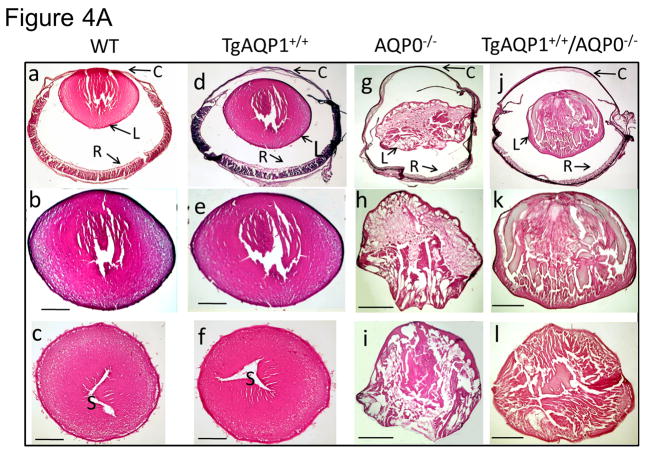
To assess the overall internal organization of the lens, we performed Hematoxylin and Eosin-staining of sagittal and cross-sections of eyes and lenses. WT (age, 1 month; Fig. 4A, a–c) and TgAQP1+/+ (Fig. 4A, d–f) eyes and lenses were normal without any developmental defects or cataracts; there were no obvious morphological changes in the lens epithelial cells, cornea or retina of the TgAQP1+/+ mice compared to the WT. In comparison to WT (Fig. 4A, a–c) and TgAQP1+/+ (Fig. 4A, d–f), eye and lens sections of the AQP0−/− mouse were smaller without sutures (Fig. 4A, g); fiber cells contained large vacuoles and were disorganized with severe alterations in uniformity and ordered arrangement along the polar axis of the lens (Fig. 4A, g–i). Eye and lens sections of TgAQP1+/+/AQP0−/− mouse showed improvement in size, fiber cell uniformity, and ordered arrangement along the polar axis, as well as a reduction in vacuoles (Fig. 4A, j–l) in comparison to AQP0−/− lenses, but remained less transparent with limited fiber cell uniformity and architectural organization compared to the WT or TgAQP1+/+ mouse lenses. TgAQP1+/+/AQP0−/− lens epithelial cells were cuboidal in shape, ~5 μm high, and the fiber cells resembled those of the wild type at the early stages of the secondary fiber cell differentiation.
Fig. 4.
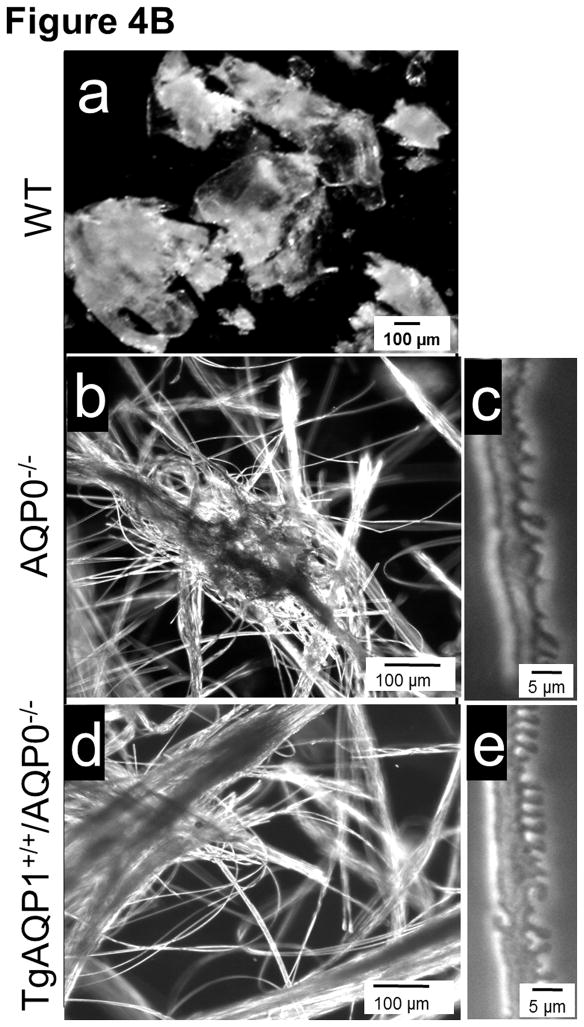
Light microscopic analysis of eye and lens. A. Hematoxylin and eosin (HE) staining of 1 month old WT (a–c), TgAQP1+/+ (d–f); AQP0−/− (g–i) and TgAQP1+/+/AQP0−/− (j–l) eyes and lenses. a, d, g, and j are sagittal sections (polar plane) through the eye (anterior pole facing up); b,e,h, and k are sagittal sections (polar plane) through the lens (anterior pole facing up); c, f, i and l are cross-sections (equatorial plane) through the lens. Scale bar represents 250 μm. C — cornea, L — lens, R — retina, S — suture. B. Characterization of mouse lens fiber cell-to-cell adhesion by manual teasing using sharp forceps: (a) Wild type (holds firmly fiber-to-fiber and breaks apart as lumps when teased); (b, c), AQP0−/−, (d, e) TgAQP1+/+/AQP0−/− and (c, e) individual fiber cells can be teased apart with ease).
We tested the WT fiber-to-fiber physical holding strength by assessing how difficult it is to manually tease them apart from one another. WT fiber cells were hard to separate indicating firm fiber-to-fiber adhesion. The cortex tissue broke into uneven pieces of fiber cell mass due to the fibers being held firmly to each other (Fig. 4B, a). In contrast, both AQP0−/− and TgAQP1+/+/AQP0−/− lens cortex fiber cells were easily separable (Fig. 4B, b,c and d,e, respectively). This difference further suggests that AQP0 might have a role in holding the fiber cells together by promoting fiber cell-to-fiber cell adhesion. Such a possibility is consistent with our finding that it was relatively easier to separate fiber cells from the TgAQP1+/+/AQP0−/− than from the WT, even though, with the homozygous AQP0−/− fibers, the least resistance was encountered. Using the fiber cell teasing technique, Shiels et al. [39] have shown that Membrane Protein 20 (MP20), facilitates fiber-to-fiber cohesion; MP20 knockout mouse lens showed loss of fiber cell aggregation. We observed a similar loss of fiber cell-to-cell adhesion/aggregation in AQP0−/− and TgAQP1+/+/AQP0−/− lenses. Our data suggest that AQP0 could be involved in performing a unique function of cell-to-cell adhesion in order to hold the cells close to each other, as well as to reduce the extracellular space and light scattering.
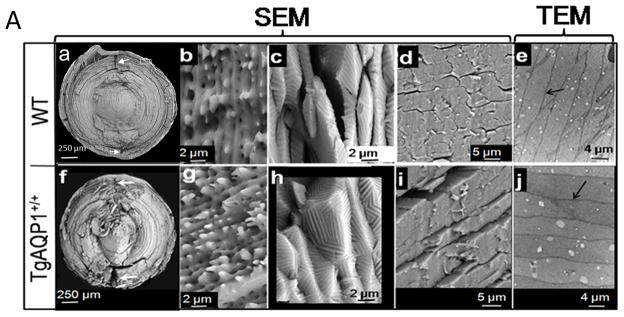
Since hematoxylin and eosin-staining revealed disorganization of the fiber cells and the manual teasing of fiber cells demonstrated weak cell-to-cell adhesion in the AQP0−/− and TgAQP1+/+/AQP0−/− lenses, we characterized the lens ultrastructure (Fig. 5A,B). Analysis by scanning electron microscopy (SEM) revealed the customarily crescent shaped fiber cells in WT and TgAQP1+/+ (Fig. 5A, a,f) lenses, with opposite end curvature and typical ‘Y’ sutures. Fibers were uniform in the cortex and irregular at the nucleus, arranged in ordered concentric rings with lateral interdigitations along their length. Ball and socket interdigitations (b,g) of the outer cortex and tongue and groove interdigitations (c,h) of the inner cortex are hallmark structures in a normal mammalian lens, and were very well pronounced in the TgAQP1+/+ lens, as in the WT. Cross sections (d,i) showed tight packing of the fibers. Transmission electron micrographs (e,j) substantiated the tight cell-to-cell fiber adherence revealing minimal extracellular space (indicated with arrows). Kuszak et al. [40], Taylor et al. [41], and Al-Ghoul et al. [42] reported similar observations for WT lenses. Epithelial cells of TgAQP1+/+ lenses were cuboidal, ~5 μm high, and at the equator, differentiated into secondary fiber cells ranging in length from ~100 – ~2500 μm as in the WT mouse lens (data not shown). At the equator, the differentiating epithelial cells rotated on their axes and elongated toward the poles in both directions, as in the case of WT lens. Duke-Elder and Wybar [43] made similar observations for WT. Overall, there were no significant structural differences between WT and TgAQP1+/+ mice lenses at low or high magnification in embryonic (E11.25 – E19.5), as well as postnatal lenses (up to 24 months; data presented for one month-old lenses). The results indicate that expression of transgenic AQP1 did not create any adverse effects on WT lens ultrastructure.
Fig. 5.
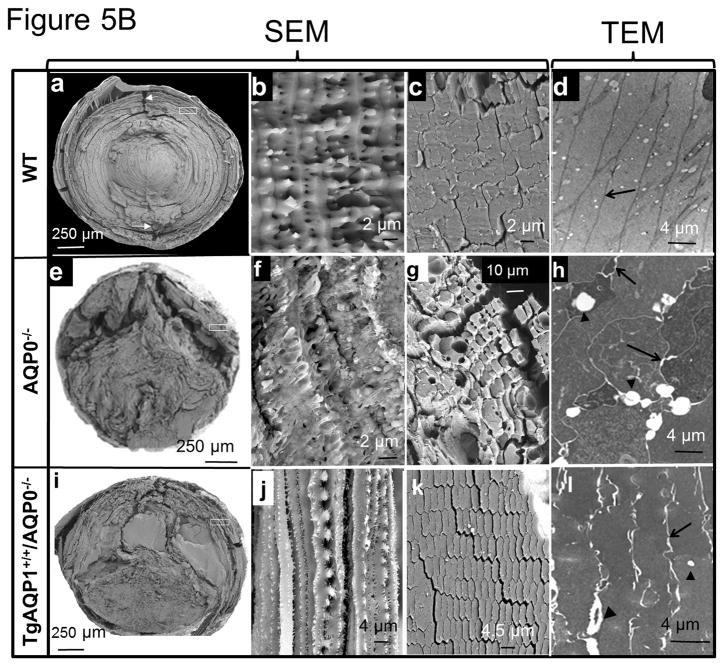
Electron microscopic analyses. A (a–i) Scanning Electron Microscopy of WT and TgAQP1+/+ lenses of 1-month old mice split along the polar axis (a, b, c, f, g and h) and equatorial axis (d and i). Normal anterior and posterior suture planes are shown by white arrows in a, and f. Outer cortex fiber cells of WT (b) and TgAQP1+/+ (g) have well defined lateral interdigitations with ball and socket along the length; inner cortex fiber cells of WT (c) and TgAQP1+/+ (h) have well defined tongue and grooves. (e,j) Transmission Electron Microscopic Analysis of WT (e), and TgAQP1+/+ (j) lenses of 1-month old mice sectioned along the polar axis showing tightly packed fiber cells. Arrows (a,f) indicate anterior and posterior sutures (a) B. Scanning electron microscopic analysis of WT, AQP0−/− and TgAQP1+/+/AQP0−/− lenses of 1-month old mice split along the polar axis (a,b,e,f,i and j) and equatorial axis (c,g and k). Normal anterior and posterior suture planes are shown by white arrows in the WT (a). Boxed areas in a, e, and i are magnified and shown as b, f and j, respectively; (b) well defined ball and socket interdigitations, (f) severely degenerated interdigitations and (j) better interdigitations compared to (f); (c) WT showing compactly arranged fiber cells; (g,k) showing loss of compactness of the fiber cells in AQP0−/− (g) and TgAQP1+/+/AQP0−/− (k) lenses compared to the WT (c). Transmission Electron Microscopic Analysis of WT (d), AQP0−/− (h) and TgAQP1+/+/AQP0−/− (l) lenses of 1-month old mice sectioned along the polar axis. Black arrows - extracellular space; Arrowhead - vacuoles.
A comparison of the ultrastructure of WT, AQP0−/−, and TgAQP1+/+/AQP0−/− lenses is shown in Figure 5B. WT lens (a) showed crescent-shaped cells and typical ‘Y’ sutures (white arrows); in AQP0−/− (e) and TgAQP1+/+/AQP0−/− (i) lenses, ‘Y’ sutures were absent. In AQP0−/− lens, fiber cell width varied considerably, probably due to localized osmotic alteration leading to fiber cell swelling at different regions (Fig. 5B, f–h). In TgAQP1+/+/AQP0−/− lenses, the crescent shape of fiber cells was observed under low magnification (Fig. 5B, i). The shape and width were not as uniform as observed in WT (Fig. 5B, compare panels (b) and (j)). Fiber cells in the WT were tightly packed (Fig. 5B, c). Conversely, in the AQP0−/− (g) and TgAQP1+/+/AQP0−/− (k) lenses, fiber cells were loosely packed, indicating lack of cell-to-cell adhesion, which in turn implies a structural role for AQP0 in holding the fiber cells in close proximity to each other. In the AQP0−/− (f), lens fiber cells showed poorly developed interdigitations compared to the WT (b); in the TgAQP1+/+/AQP0−/− (j) lens, the interdigitations were less pronounced than in WT but better than in AQP0−/− (f) lens. In the WT, inner cortex fiber cells contained well-defined tongue and groove interdigitations (Fig. 5A, c); in comparison, the AQP0−/− lenses contained highly degenerated fiber cells, and TgAQP1+/+/AQP0−/− lenses had slightly improved fibers with no defined membrane morphology (images not shown). Transmission electron microscopic (TEM) images of WT lens sections exhibited tightly packed fiber cells with very narrow extracellular spaces (black arrow, Fig. 5B, d) ranging between 0.5 to 0.7 nm. In AQP0−/− (Fig. 5B, h), these spaces ranged between 0.8 to 2.0 nm, and vacuole-like extracellular spaces (black arrowheads) were even wider ranging from 1.4 to 1.8 nm; TEM images of TgAQP1+/+/AQP0−/− fiber cells (Fig. 5B, l) showed extracellular spaces ranging between 0.8 to 1.15 nm (black arrows) and vacuole-like spaces ranging from 1.3 to 1.8 nm (black arrowheads). Even though TgAQP1+/+/AQP0−/− (Fig. 5B, l) lens showed comparatively fewer extracellular spaces and vacuoles than AQP0−/− (Fig. 5B, h) lens, the fiber cell arrangement was less compact implying AQP0 is necessary for providing fiber cell structural integrity. This attribute may be a consequence of AQP0 mediation of cell-to-cell adhesion. In other words, fiber cell AQP1 transgene was able to replace the role/s of AQP0 only partially even after compensating the lens membrane water permeability in the AQP0 knockout mice.
Even though transgene AQP1 expression improved the shape, structure and outer cortical fiber cell transparency, it was still ineffective in reducing the large extracellular spaces to facilitate contact between adjacent fibers and in aiding the formation of ‘Y’ sutures. These structural features ensure contact between opposite fibers. Such compactness is associated with less fiber cell death and cataractogenesis in the WT lens. Transgenic AQP1 was unable to improve the fiber cell architecture in the TgAQP1+/+/AQP0−/− mouse lenses to the extent seen in the WT lenses, suggesting that AQP1 was able to replace the function/(s) of AQP0 only partially, even though it created 2.6 times higher membrane water permeability [36] and improved the morphology of the fiber cells in the outer cortex. These results suggest that while AQP0 has an analogous function as a water channel similar to AQP1, it may have a unique structural adhesion function that AQP1 cannot provide [35,44]. Several investigators have speculated that AQP0 might function as a cell-to-cell adhesion protein [31,34,45–48]. Our findings support this suggestion, since AQP0−/− and TgAQP1+/+/AQP0−/− lens fiber cells showed less compact arrangement compared to the WT. Similar to our study, the unique and analogous functions of mouse connexin43 (Cx43) had been deduced using genetically engineered mice. Knocking out Cx43 was lethal, as the pups died shortly after birth due to cardiac malformation [49]. The postnatal lethality of Cx43-deficient mice could be prevented by knocking in Cx40, 32 or 26 indicating that these four connexins share analogous (common) function/s [50,51]. However, compared to the wild type, the knock-in mice showed morphological and functional differences, reflecting the possibility of Cx43-associated unique function(s) that cannot be replaced by any of the tested connexins. These studies and our data demonstrate that while respective members of a multigene family of proteins have analogous function/s that can be replaced by another member of the same multigene family, some members have unique, non-redundant functions.
AQP0, the most abundant membrane protein in the lens, undergoes post-translational modifications during aging of the fiber cells. In the inner cortical and nuclear regions of the lens, cleavage of the N- and C-termini of AQP0 occurs as a post-translational modification. The cleaved forms are retained throughout life, as these regions of the avascular ocular lens have no protein turnover. In humans, cleavage of AQP0 occurs in normal lenses throughout life [52–54] and happens at an accelerated rate in cataractous lenses [20,55–58]. While the possibility of a cell-to-cell adhesion function for AQP0 is being corroborated, other questions emerge, such as, whether intact and cleaved forms perform both water channel and cell-to-cell adhesion functions or whether they have specific functions. Truncation of lens proteins has been regarded as part of normal aging process with no functional bearing on the physiology of the lens [17,59,60]. However, researchers have differing views on the role(s) of intact and cleaved forms of AQP0. Structural studies using electron diffraction microscopy indicate that intact AQP0 functions as an open water channel but not as an adhesion protein. C-terminal-cleaved AQP0 functions as an adhesion protein but is closed as a water pore [34,61]. Data gathered by two other structural studies [33,48] contradict the hypothesis that only intact AQP0 can function as a water channel [34]. In a WT lens, while the cleaved form of AQP0 is confined to the inner cortical and nuclear regions, its outer cortex with intact AQP0 has a highly ordered arrangement of the fiber cells with firm adherence to each other. If cell-to-cell adhesion is provided by adhesion proteins like cadherin whereas intact AQP0 only provides water permeability function, then cataractogenesis in the AQP0 knockout lens should have been eliminated by the transgenic AQP1.
We have shown previously that mutation [19,20] or deletion [7,36] of AQP0 in mouse lens leads to loss of fiber cell membrane water permeability. In vitro and ex-vivo studies have revealed that accumulation of mutated AQP0 in the cultured cells and mutant mouse lenses causes cytotoxicity, and leads to necrosis [18,62]. Lack of cell-to-cell adhesion due to the functional impairment of AQP0 could be another reason for the development of autosomal dominant lens cataract in humans and mice with mutated AQP0.
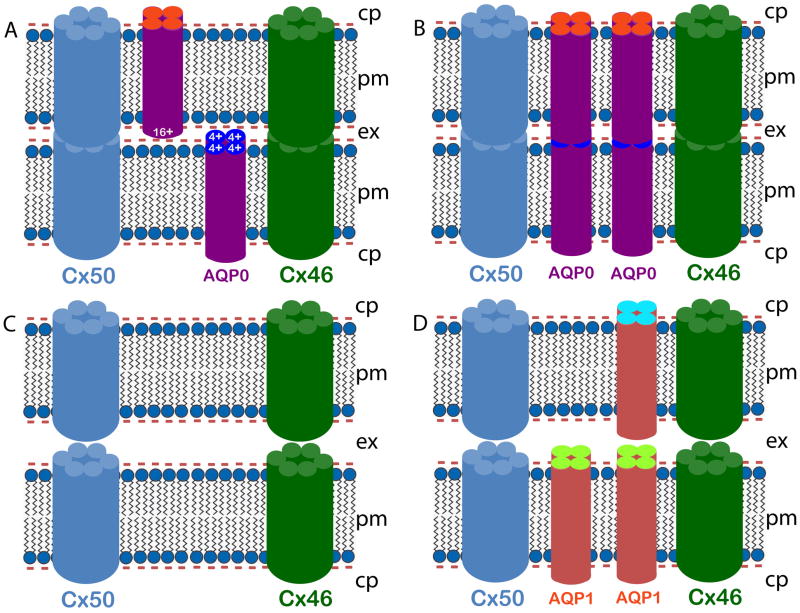
Using adhesion-deficient L-cells, we have confirmed the in vitro cell-to-cell adhesion property of intact WT AQP0 [35]. Recently, Liu et al. [63], demonstrated that chicken AQP0 promotes cell-to-cell adhesion, while loss of AQP0 leads to reduction in connexin 50-induced cell-to-cell coupling. Their study concluded that the loss of gap junctional coupling could possibly be due to the loss of AQP0-induced cell-to-cell adhesion. From the electron micrographs of the present study, it is evident that in the WT, presence of AQP0 (Fig. 5Bc,d) facilitates reduction in fiber cell extracellular space in contrast to AQP0 knockout (Fig. 5B, g,h) and TgAQP1+/+/AQP0−/− (Fig. 5B, k,l) lenses in which these spaces are dilated. Michea et al. [46,47] have shown that AQP0 adheres to negatively charged lipid vesicles, indicating the electrostatic nature of the adhesion. Based on X-ray crystallographic studies, Harries et al. [33] suggested that adhesion could be the result of ionic interaction between the positive charges of one AQP0 tetramer and the negative charges in the lipid membrane, as suggested also by Michea et al. [47]. Electron diffraction microscopy by Gonen et al., [61,34] indicated a possible interaction of the extracellular loops 1 and 2 of one AQP0 of a monomer of a tetramer with the matching loops of another monomer of the opposing tetramer; a total of eight such interactions between two opposing tetramers could narrow the extracellular space and bring the membranes closer together. Figure 6 shows schematic models illustrating how AQP0 could be mediating fiber cell-to-fiber cell adhesion that also results in increased cell-to-cell communication. Figure 6A represents the positively charged extracellular domain of AQP0 interacting with a negatively charged lipid membrane to reduce extracellular space. Figure 6B illustrates the possible interaction of AQP0 extracellular domain(s) from two opposing fiber cell plasma membranes to draw the membranes closer together. In either case, the proximity of membranes allows the hemichannels of gap junction channels, such as those in connexin 46 (Cx46) and 50, to come together to form channels, thereby facilitating intercellular communication. In AQP0−/− and TgAQP1+/+/AQP0−/− mouse lenses, lack of AQP0 exposes the negatively charged membranes to face each other which results in repulsion causing wider extracellular spaces (Fig. 6C) between fibers, eventually perturbing cell-to-cell communication. Unlike AQP0, AQP1 does not possess a net positive charge at the extracellular domains. This could also have contributed to the widening of the spaces between membranes of adjacent fiber cells in the TgAQP1+/+/AQP0−/− mouse lenses (Fig. 6D).
Fig. 6.
Schematic models illustrating AQP0 mediated fiber cell-to-fiber cell adhesion and cell-to-cell communication. Lens fiber cells express AQP0 in the membrane (~ 45%). AQP0 forms functional water channels as an analogous function. It also facilitates reduction in fiber cell extracellular space in the WT lenses in contrast to AQP0 knockout and TgAQP1+/+/AQP0−/− lenses, as shown by the EM studies (Fig. 5B), for reducing light scattering, and for increasing lens transparency and fiber cell communication through gap junction channels. AQP0 aids in pulling the fiber cell plasma membranes of opposing cells closer by functioning as an adhesion protein which is a unique role to maintain a streamlined architecture. Four models are proposed here, based on the available literature and our ultrastructural studies. Two possible working models for wild type mouse lens (A, B) show narrow extracellular space (see also Fig. 5B, c, d). A) Positively charged extracellular domain of AQP0 interacts with negatively charged lipid membrane to draw the membranes closer, and B) AQP0 extracellular domains of opposing fiber cell plasma membranes interact with each other to reduce the extracellular space. Two possible working models (C, D) show wide extracellular space. C) Wide extracellular space in the AQP0−/− (as seen in Fig. 5B, g, h) due to negatively charged fiber cell membranes opposing each other. D) Wide extracellular space between the TgAQP1+/+/AQP0−/− mouse lens fiber cells (see also Fig. 5B, k, l) due to the absence of a net +ve charge in the extracellular domains of AQP1, and/or due to the repulsion caused by negatively charged membranes facing each other. cp — cytoplasm, pm — plasma membrane, ex — extracellular space.
Taking into account, the need for AQP0 to elicit cell-to-cell adhesion and prevent fiber cell disorganization described here, and the inferences from other related studies [35,36,42,46,63], it is becoming evident that AQP0 has an essential in vivo structural role as an adhesion molecule.
Acknowledgments
We thank Dr. Peter Agre, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, for providing human AQP1 cDNA; Dr. Ana B. Chepelinsky, NIH, for αA-crystallin promoter; Dr. Alan Shiels, Washington University, St. Louis, MO, for AQP0 knockout mouse, as well as for critically reading the manuscript; Dr. Sangly P. Srinivas, School of Optometry, Indiana University, Bloomington, IN, for critically going through the manuscript.
Funding
This work was supported by NIH NEI grants R01: EY20506 (to K. Varadaraj), and EY06391 (to R. T. Mathias), and by a grant from Alcon Research Ltd., (# 39733: to K. Varadaraj).
Abbreviations
- AQP0
Aquaporin 0
- AQP1
Aquaporin 1
- TgAQP1
wild type mouse expressing transgene AQP1 in the lens fiber cells
- TgAQP1+/+/AQP0−/−
AQP0 knockout mouse expressing transgene AQP1 in the lens fiber cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszakgon JR. Lens structure in MIP-deficient mice. Anat Rec A Discov Mol Cell Evol Biol. 2001;273:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- 2.Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Han J, Schey KL. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. J Proteome Res. 2008;7:2696–2702. doi: 10.1021/pr700737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 5.Chepelinsky AB. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb Exp Pharmacol. 2009;190:265–297. doi: 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Jiang J, Zhu Y, Li J, Jin C, Shentu X, Yao K. A novel mutation in the major intrinsic protein (MIP) associated with autosomal dominant congenital cataracts in a Chinese family. Mol Vis. 2010;16:534–539. [PMC free article] [PubMed] [Google Scholar]
- 7.Shiels A, Bassnett S, Varadaraj K, Mathias RT, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- 8.Swamy-Mruthinti S, Shaw SM, Zhao HR, Green K, Abraham EC. Evidence of a glycemic threshold for the development of cataracts in diabetic rats. Curr Eye Res. 1999;18:423–429. doi: 10.1076/ceyr.18.6.423.5271. [DOI] [PubMed] [Google Scholar]
- 9.Swamy-Mruthinti S. Glycation decreases calmodulin binding to lens transmembrane protein, MIP. Biochim Biophys Acta. 2001;1536:64–72. doi: 10.1016/s0925-4439(01)00031-x. [DOI] [PubMed] [Google Scholar]
- 10.Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional chip water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- 11.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4309. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 12.Kushmerick C, Rice SJ, Baldo GJ, Haspel HC, Mathias RT. Ion, water and neutral solute transport in Xenopus oocytes expressing frog lens MIP. Exp Eye Res. 1995;61:351–362. doi: 10.1016/s0014-4835(05)80129-0. [DOI] [PubMed] [Google Scholar]
- 13.Mulders SM, Preston GM, Deen PMT, Guggino WB, Vanos CH, Agre P. Water channel properties of major intrinsic protein of lens. J Biol Chem. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- 14.Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- 15.Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- 16.Németh-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- 17.Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1–243) observed in the aging human lens. Invest Ophthalmol Vis Sci. 2003;44:4820–4828. doi: 10.1167/iovs.02-1317. [DOI] [PubMed] [Google Scholar]
- 18.Varadaraj K, Kumari S, Patil R, Wax MB, Mathias RT. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008;87:9–21. doi: 10.1016/j.exer.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- 20.Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- 21.Németh-Cahalan KL, Kalman K, Froger A, Hall JE. Zinc modulation of water permeability reveals that aquaporin 0 functions as a cooperative tetramer. J Gen Physiol. 2007;130:457–464. doi: 10.1085/jgp.200709826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Németh-Cahalan KL, Kalman K, Hall JE. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol. 2004;123:573–580. doi: 10.1085/jgp.200308990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalman K, Németh-Cahalan KL, Froger A, Hall JE. AQP0-LTR of the CatFr mouse alters water permeability and calcium regulation of wild type AQP0. Biochim Biophys Acta. 2006;1758:1094–1099. doi: 10.1016/j.bbamem.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Kalman K, Németh-Cahalan KL, Froger A, Hall JE. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. J Biol Chem. 2008;283:21278–21283. doi: 10.1074/jbc.M801740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichow SL, Gonen T. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure. 2008;16:1389–1398. doi: 10.1016/j.str.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, Schey KL. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry. 2008;47:339–347. doi: 10.1021/bi701980t. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald PG, Bok D, Horwitz J. The distribution of the main intrinsic membrane polypeptide in ocular lens. Curr Eye Res. 1985;4:1203–1218. doi: 10.3109/02713688509003365. [DOI] [PubMed] [Google Scholar]
- 28.Dunia I, Manenti S, Rousselet A, Benedetti EL. Electron microscopic observations of reconstituted proteoliposomes with the purified major intrinsic membrane protein of eye lens fibers. J Cell Biol. 1987;105:1679–1689. doi: 10.1083/jcb.105.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costello MJ, McIntosh TJ, Robertson JD. Distribution of GJs and square array junctions in the mammalian lens. Invest Ophthalmol Vis Sci. 1989;30:975–989. [PubMed] [Google Scholar]
- 30.Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein-composition of lens fiber junctions. J Cell Biol. 1989;108:2255–2275. doi: 10.1083/jcb.108.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fotiadis D, Hasler L, Muller DJ, Stahlberg H, Kistler J, Engel A. Surface tongue-and-groove contours on lens MIP facilitate cell-to-cell adherence. J Mol Biol. 2000;300:779–789. doi: 10.1006/jmbi.2000.3920. [DOI] [PubMed] [Google Scholar]
- 32.Grey AC, Li L, Jacobs MD, Schey KL, Donaldson PJ. Differentiation-dependent modification and subcellular distribution of aquaporin-0 suggests multiple functional roles in the rat lens. Differentiation. 2009;77:70–83. doi: 10.1016/j.diff.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc Natl Acad Sci USA. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonen T, Cheng YF, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQPO crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumari SS, Varadaraj K. Intact AQP0 Performs Cell-to-Cell Adhesion. Biochem Biophys Res Commun. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varadaraj K, Kumari SS, Mathias RT. Transgenic Expression of AQP1 in the Fiber Cells of AQP0 Knockout Mouse: Effects on Lens Transparency. Exp Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47:4931–4934. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- 38.Varadaraj K, Kumari S, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Dev Dyn. 2007;236:1319–1328. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiels A, King JM, Mackay DS, Bassnett S. Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Invest Ophthalmol Vis Sci. 2007;48:500–508. doi: 10.1167/iovs.06-0947. [DOI] [PubMed] [Google Scholar]
- 40.Kuszak JR, Macsai MS, Rae JL. Stereo scanning electron microscopy of the crystalline lens. Scan Electron Microsc. 1983;III:1415–1426. [PubMed] [Google Scholar]
- 41.Taylor VI, Al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–1410. [PubMed] [Google Scholar]
- 42.Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- 43.Duke-Elder S, Wybar KC. The refractive media. In: Duke-Elder S, editor. Systems of ophthalmology. II. St. Louis: C.V. Mosby Co; 1961. pp. 309–324. [Google Scholar]
- 44.Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi AY. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 45.Costello MJ, McIntosh TJ, Robertson JD. Distribution of gap junctions and square array junctions in the mammalian lens. Invest Ophthalmol Vis Sci. 1989;30:975–989. [PubMed] [Google Scholar]
- 46.Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669. doi: 10.1021/bi00190a021. [DOI] [PubMed] [Google Scholar]
- 47.Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995;61:293–301. doi: 10.1016/s0014-4835(05)80124-1. [DOI] [PubMed] [Google Scholar]
- 48.Palanivelu DV, Kozono DE, Engel A, Suda K, Lustig A, Agre P, Schirmer T. Co-axial association of recombinant eye lens aquaporin-0 observed in loosely packed 3D crystals. J Mol Biol. 2006;355:605–611. doi: 10.1016/j.jmb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 50.Plum A, Hallas G, Magin T, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M, Meda P, Traub O, Willecke K. Unique and shared functions of different connexins in mice. Curr Biol. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- 51.Winterhager E, Pielensticker N, Freyer J, Ghanem A, Schrickel JW, Kim JS, Behr R, Grümmer R, Maass K, Urschel S, Lewalter T, Tiemann K, Simoni M, Willecke K. Replacement of connexin 43 by connexin 26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev Biol. 2007;7:26. doi: 10.1186/1471-213X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy D, Spector A, Farnsworth PN. Human lens membrane: comparison of major intrinsic polypeptides from young and old lenses isolated by a new methodology. Exp Eye Res. 1979;28:353–358. doi: 10.1016/0014-4835(79)90097-6. [DOI] [PubMed] [Google Scholar]
- 53.Horwitz J, Robertson NP, Wong MM, Zigler JS, Kinoshita JH. Some properties of lens plasma membrane polypeptides isolated from normal human lenses. Exp Eye Res. 1979;28:359–365. doi: 10.1016/0014-4835(79)90098-8. [DOI] [PubMed] [Google Scholar]
- 54.Takemoto L, Takehana M, Horwitz J. Covalent changes in MIP26K during aging of the human lens membrane. Invest Ophthalmol Vis Sci. 1986a;27:443–446. [PubMed] [Google Scholar]
- 55.Takemoto L, Takehana M, Horwitz J. Antisera to synthetic peptides of MIP26K as probes of membrane changes during human cataractogenesis. Exp Eye Res. 1986b;42:497–501. doi: 10.1016/0014-4835(86)90009-6. [DOI] [PubMed] [Google Scholar]
- 56.Takemoto L, Smith J, Kodama T. Major intrinsic polypeptide (MIP26K) of the lens membrane: covalent change in an internal sequence during human senile cataractogenesis. Biochem Biophys Res Commun. 1987;142:761–766. doi: 10.1016/0006-291x(87)91479-3. [DOI] [PubMed] [Google Scholar]
- 57.Takemoto L, Takehana M. Covalent change of major intrinsic polypeptide (MIP26K) of lens membrane during human senile cataractogenesis. Biochem Biophys Res Commun. 1986c;135:965–971. doi: 10.1016/0006-291x(86)91022-3. [DOI] [PubMed] [Google Scholar]
- 58.Schey KL, Little M, Fowler JG, Crouch RK. Characterization of human lens major intrinsic protein structure. Invest Ophthalmol Vis Sci. 2000;41:175–182. [PubMed] [Google Scholar]
- 59.Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 2004;43:9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- 60.Korlimbinis A, Berry Y, Thibault D, Schey KL, Truscott RJ. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 2009;88:966–973. doi: 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;429:193–197. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- 62.Varadaraj K, Kumari SS, Okamura T, Kasai N, Shiels A, Patil R, Wax MB, Mathias RT. Congenital cataract and microopthalmia result from cytotoxicity and necrosis in AQP0 mutant mouse, CatTohm. Invest Ophthalmol Vis Sci. 2008;49:E-5859. [Google Scholar]
- 63.Liu J, Xu J, Gu S, Nicholson BJ, Jiang JX. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J Cell Sci. 2011;124:198–206. doi: 10.1242/jcs.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]