Abstract
The pathophysiological roles of the angiotensin II type 2 receptor (AT2) in cardiac hypertrophy remain unclear. By the targeted deletion of mouse AT2 we were able to prevent the left ventricular hypertrophy resulting from pressure overload, while cardiac contractile functions remained normal. This implies that AT2 is a mediator of cardiac hypertrophy in response to increased blood pressure. The effects of AT2 deletion were independent of activation of embryonic genes for cardiac hypertrophy. However, p70S6k, one of the key factors in cardiac hypertrophy, was markedly and specifically reduced in the ventricles of Agtr2–/Y mice. We propose that p70S6k plays a major role in AT2-mediated ventricular hypertrophy.
This article may have been published online in advance of the print edition. The date of publication is available from the JCI website, http://www.jci.org. J. Clin. Invest. 105:R25–R29 (2000).
Introduction
Cardiac hypertrophy is an adaptive response of the heart to several forms of cardiac disease. Although sustained hypertrophy is an initial compensatory mechanism to preserve cardiac function, it is also a major risk factor for congestive heart failure and sudden death.
Angiotensin II (Ang II) plays an important role in the development of cardiac hypertrophy (1). It is supplied by the circulating renin-angiotensinogen system and also is generated locally (2). Treatment with angiotensin-converting enzyme inhibitors or Ang II–receptor antagonists induces marked but incomplete regression and prevention of the cardiac hypertrophy (3).
Of two major subtypes of Ang II receptors, type 1 (AT1) and type 2 (AT2), only the latter appears to increase under pathological conditions including cardiac hypertrophy (4). However, its function remains controversial. Recent studies reported that pressure overload–induced left ventricular hypertrophy (LVH) still occurs in mice lacking the AT1A gene (Agtr1A–/–) (5, 6). In vitro studies on AT2 in cultured cells have been difficult because its functions are often poorly reproducible. In the present in vivo studies, AT2 gene-targeted mice (Agtr2–/Y) were used to delineate the role of AT2 in cardiac hypertrophy. The well-established ability of Ang II to induce cardiac hypertrophy prompted us to investigate the hypothesis that pressure overload–induced LVH would be prevented in the Agtr2–/Y mice.
Methods
Agtr2–/Y mice.
Ten-week-old male Agtr2–/Y (n = 9) and wild-type (n = 7) mice weighing 22–24 g were used. The Agtr2–/Y mice were produced as described previously (7), by using E 14-1 embryonic stem cells from male 129 Ola mice and blastocysts derived from the C57BL/6 strain (The Jackson Laboratory, Bar Harbor, Maine, USA), backcrossed to C57BL/6 genetic background by mating C57BL/6 males to heterozygous AT2 females. The Agtr2–/Y mice from the 8th generation of the backcross to C57BL/6 mice were used. The wild-type mice were generated as littermates by the same mating procedure. Experimental protocols were approved by Vanderbilt Animal Care Committee.
Pressure overload by aortic constriction.
Pressure overload was produced by a modification of the published banding method of the abdominal aorta (8). As controls, sham-operated mice were produced in both the Agtr2–/Y and wild-type groups, using a procedure that was identical except for the aortic banding above the left renal artery.
Blood pressure measurement.
To ascertain the pressure gradient of aortic constriction, the left carotid and femoral arteries were cannulated with stretched PE 10 intramedic polyethylene tubing (Clay Adams, division of Becton Dickinson and Co., Parsippany, New Jersey, USA) after characterization of cardiac properties. Blood pressure was measured under anesthesia with ketamine (50 mg/kg) and xylazine (20 mg/kg).
Echocardiography.
Transthoracic echocardiography was performed with a Sonos 5500 with an S12 transducer (12 MHz) (Hewlett-Packard, Andover, Massachusetts, USA). Mice were weighed, anesthetized with ketamine (50 mg/kg) and xylazine (20 mg/kg), and settled in the left decubitus or supine position (9). Good two-dimensional views of the left ventricle were obtained for guided M-mode measurements of the intraventricular septum (IVS), left ventricular end-diastolic diameter (LVDd), left ventricular posterior wall (LVPW), and left ventricular end-systolic diameter (LVDs). Percent fractional shortening (%FS) was calculated by the formula: [(LVDd – LVDs)/LVDd] × 100. Mean of three measurements was determined for each mouse. The echocardiographic left ventricular mass (LVM) was calculated using the specific gravity of myocardium (1.055 mg/mm3) and the cube formula: LVM = 1.055 × [(IVS + LVPW + LVDd)3 – (LVDd)3] (ref. 10). After the echocardiographic and pressure gradient study, mice were euthanized. Major blood vessels, both atria, and the right ventricle were removed from the heart. Then the left ventricle was dissected and weighed. This value was used as the autopsied left ventricular weight.
Histology.
Histological analysis was performed on aortic-banded Agtr2–/Y and wild-type mice as well as sham-operated Agtr2–/Y and wild-type mice. Briefly, hearts were isolated after perfusion with 40 mM KCl, fixed in 4% paraformaldehyde with PBS, dehydrated in graded ethanol solution, and transferred to xylene and then into paraffin. The paraffin-embedded hearts were sectioned at 4 μm and subsequently were stained with hematoxylin, eosin, and Van Gieson solution. Left ventricular myocyte cross-sectional area (CSA) was measured on sections of mid–free wall of the left ventricle. Suitable cross sections were defined as having nearly circular capillary profiles and circular to oval myocyte cross sections. The outer borders of the myocytes were traced and myocyte areas were calculated with an NIH image system (NIH, Research Service Branch). Approximately 100 cells were counted per sample and the average was used for analysis.
Western blot analysis.
Western blot analysis was performed for ventricular extracts of aortic-banded Agtr2–/Y and wild-type mice and sham-operated Agtr2–/Y and wild-type mice as previously reported (11). Rabbit polyclonal phosphospecific antibodies to p70S6k, extracellular signal–regulated kinase (ERK), p38 mitogen-activated protein kinase (p38 MAPK), and Jun NH2-terminal kinase (JNK) (New England Biolabs Inc., Beverly, Massachusetts, USA) and rabbit polyclonal antibodies to p70S6k (New England Biolabs Inc.), collagen type I (Calbiochem-Novabiochem Corp., La Jolla, California, USA), and calcineurin (Santa Cruz Biotechnology Inc., Beverly, Massachusetts, USA) were used. ECL (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) was used for detection of immunoreactive bands. The outer borders of the bands were traced and the areas and densities were determined with the NIH image system.
Northern blot analysis.
Northern blot analysis of Nkx 2.5, GATA4, myocyte enhancer factor 2C (MEF2C), β–myosin heavy chain (β-MHC), atrial natriuretic peptide (ANP), and GAPDH were performed as previously reported (12).
RNase protection assay and calcineurin activity.
Mouse AT1 RNase protection assay was performed on aortic-banded Agtr2–/Y and wild-type mice as previously reported (13). The following primers for PCR were used:
sense 5′-GGTGGGAATATTTGGAAACAG-3′_______and
antisense 5′-AAGAAGAAAAGCACAATCGCC-3′.
Calcineurin enzyme activity was determined as previously reported (14).
Statistical analysis.
For all statistical tests, multiple comparisons were performed by two-way ANOVA and Fisher’s exact probability test. A P value less than 0.05 was considered significant.
Results
Effect of the pressure overload on systolic pressure.
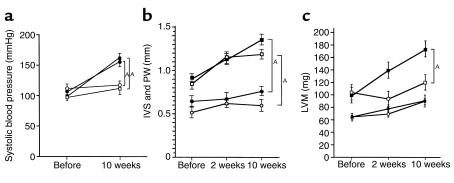
Changes in the left carotid artery systolic pressure after aortic constriction were determined by the direct method. Ten weeks after abdominal aortic constriction, left carotid artery systolic pressure increased significantly as compared with sham-operated mice. The magnitude of increase was similar in aortic-banded Agtr2–/Y (56 ± 6 mmHg) and wild-type mice (62 ± 7 mmHg) (Figure 1a).
Figure 1.
(a) Systolic blood pressure in Agtr2–/Y and wild-type mice. Filled circles: aortic-banded Agtr2–/Y mice; filled squares: aortic-banded wild-type mice; open circles: sham-operated Agtr2–/Y mice; open squares: sham-operated wild-type mice. AP < 0.05. (b) IVS and LVPW in aortic-banded Agtr2–/Y and wild-type mice. Filled circles: IVS in aortic-banded Agtr2–/Y mice; filled squares: IVS in aortic-banded wild-type mice; open circles: LVPW in aortic-banded Agtr2–/Y mice; open squares: LVPW in aortic-banded wild-type mice. AP < 0.05. (c) LVM of Agtr2–/Y and wild-type mice. Filled circles: aortic-banded Agtr2–/Y mice; open circles: sham-operated Agtr2–/Y mice; filled squares: aortic-banded wild-type mice; open squares: sham-operated wild-type mice. AP < 0.05.
Determination of effects of the pressure overload on left ventricular dimension and function by echocardiography.
In the native state, IVSs and LVPWs of the Agtr2–/Y mice were thinner than those of the wild-type mice. Surprisingly, in the Agtr2–/Y mice aortic constriction with concomitant elevation in systolic blood pressure induced little or no thickening of the ventricular walls, whereas marked LVH was seen in aortic-banded wild-type mice (Figure 1b). LVM in the Agtr2–/Y mice remained at almost the same levels as in sham-operated animals, whereas in the wild-type, a marked difference was seen between the sham and aortic-constricted groups (Figures 1c and 2a). In contrast with IVS, LVPW, and LVM, we did not see significantly different CSAs between the Agtr2–/Y and wild-type before bonding. CSAs in aortic-banded Agtr2–/Y mice increased slightly as compared with those in sham-operated Agtr2–/Y mice. However, they were considerably less than those in aortic-banded wild-type mice (Figure 2b). The correlation of autopsied left ventricular weight and LVM was excellent, with correlation constants of 0.70 and 0.85, respectively (data not shown).
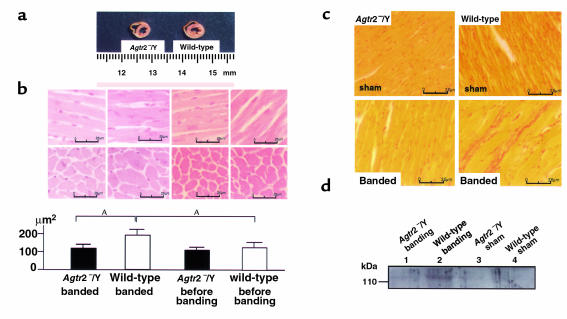
Figure 2.
Pathological analysis in the Agtr2–/Y and wild-type mouse heart. (a) Cross section of midportion of left ventricle in aortic-banded Agtr2–/Y and wild-type mice. (b) Left ventricular CSA in aortic-banded Agtr2–/Y and wild-type mice, and in Agtr2–/Y and wild-type mice before banding. AP < 0.05. (c and d) Collagen type I in aortic-banded Agtr2–/Y and wild-type mouse hearts and in sham-operated Agtr2–/Y and wild-type mouse hearts. Lane 1: aortic-banded Agtr2–/Y; lane 2: aortic-banded wild-type; lane 3: sham-operated Agtr2–/Y; lane 4: sham-operated wild-type mouse hearts. Data are representative of three independent experiments with nearly identical results.
Left ventricular functions of Agtr2–/Y and wild-type mice.
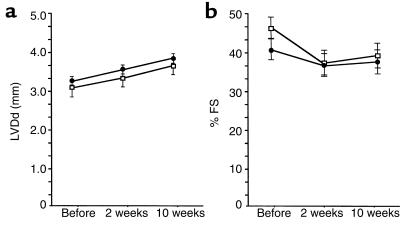
To determine whether the unique morphological features of the left ventricles and the absence of hypertrophic response to aortic constriction in Agtr2–/Y mice are related to any functional abnormality, we determined LVDd and %FS. Despite thinner left ventricular wall thickness and a markedly reduced hypertrophic response to pressure overload, these indices were comparable between the Agtr2–/Y and wild-type mice (Figure 3, a and b). AT1 expression examined by RNase protection assay was also comparable between the Agtr2–/Y and wild-type mice, and it remained comparable between the two groups 10 weeks after aortic constriction (data not shown). Thus, the features of the hearts of the Agtr2–/Y mice are (a) normal ventricular function, (b) thinner ventricular wall thickness, and, most interestingly, (c) a markedly suppressed hypertrophic response to pressure overload.
Figure 3.
Heart function in aortic-banded Agtr2–/Y and wild-type mice. (a) LVDd in aortic-banded Agtr2–/Y (filled circles) and wild-type (open squares) mice. (b) %FS in aortic-banded Agtr2–/Y (filled circles) and wild-type (open squares) mice.
Reduced collagen type I in Agtr2–/Y mice.
Left ventricular and interstitial collagen type I was markedly reduced in aortic-banded Agtr2–/Y mice compared with aortic-banded wild-type mice (Figure 2, c and d).
Markedly reduced p70S6k in Agtr2–/Y mice.
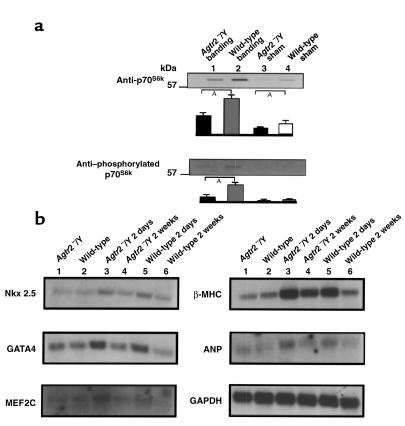
To determine the signaling mechanisms underlying the resistance of the left ventricle of the Agtr2–/Y mouse to growth stimulation by pressure overload, we compared the expression of various compounds involved in ventricular growth stimulation between the Agtr2–/Y and wild-type mice. p70S6k is one of the key factors in cardiac hypertrophy (15). We found that phosphorylated p70S6k and total p70S6k were markedly reduced in the Agtr2–/Y mice compared with the wild-type mice. Although aortic constriction increased both, their levels were still markedly below those of similarly treated wild-type mice (Figure 4a). These observations suggest that the markedly attenuated hypertrophic response of the Agtr2–/Y mouse may be due, in large part, to suppression of p70S6k expression and activation in mice lacking the AT2 receptor.
Figure 4.
(a) Phosphorylated p70S6k and total p70S6k in the Agtr2–/Y and wild-type mouse hearts. Lane 1: aortic-banded Agtr2–/Y; lane 2: aortic-banded wild-type; lane 3: sham-operated Agtr2–/Y; lane 4: sham-operated wild-type. AP < 0.05. Data are representative of three independent experiments with nearly identical results. (b) Nkx 2.5, GATA4, MEF2C, β-MHC, ANP, and GAPDH in aortic-banded Agtr2–/Y and wild-type mouse hearts. Lane 1: Agtr2–/Y before surgery; lane 2: wild-type before surgery; lane 3: Agtr2–/Y 2 days after surgery; lane 4: Agtr2–/Y 2 weeks after surgery; lane 5: wild-type 2 days after surgery; lane 6: wild-type 2 weeks after surgery. Data are representative of three independent experiments with nearly identical results.
On the other hand, no difference was observed between the Agtr2–/Y and wild-type mice in the following substances, which are considered to stimulate hypertrophy: phosphorylated ERK, p38 MAPK, JNK, calcineurin and calcineurin enzyme activity. Aortic banding did increase these factors slightly but to similar extents in the Agtr2–/Y and wild-type mice (data not shown). No difference was detected in mRNA of the following well-known markers of ventricular hypertrophy: Nkx 2.5, GATA4, MEF2C, β-MHC, and ANP. In the native state, there was no significant difference between the groups in any of these markers. Two days after surgery, they were increased equally in both groups and reduced to base lines at 2 weeks after surgery (Figure 4b). Based on these results, we submit that attenuation of the signal pathway linking AT2 to p70S6k activation may be involved in the resistance to pressure overload–induced LVH in Agtr2–/Y mice.
Discussion
The present studies demonstrate that pressure overload failed to induce LVH in the Agtr2–/Y mice, whereas substantial LVH occurred in similarly treated wild-type mice. We also found that p70S6k was markedly and specifically reduced whereas other hypertrophy-stimulating genes were not reduced. The AT2 receptor appears to play a pivotal role in regulating pressure overload–induced LVH. Although many LVH-inducing genes and proteins have been described, by gain-of-function studies (16–18), only a few have been shown to be responsible for cardiac hypertrophy by loss-of-function studies (19). Particularly, we are not aware of any previous reports on the prevention of pressure overload–induced cardiac hypertrophy by deletion of the AT2 receptor. The present results indicate a role for the expressed AT2 receptor in the development of LVH in response to pressure overload.
Several recent reports show that the AT2 receptor functions counteract those of the AT1 receptor (20). In the present studies, we utilized the Agtr2–/Y mice to delineate the mechanism of hypertrophy suppression associated with the chronic effect of the absence of the AT2 receptor. In their native states, the Agtr2–/Y mice had thinner ventricular walls than those of the wild-type, as shown in Figure 1, b and c. However, their cardiac function was normal. After chronic aortic constriction, the cardiac function remained normal and cardiac enlargement was not seen in the Agtr2–/Y mice. This indicates that the Agtr2–/Y mice are not a model of dilated cardiomyopathy.
The ability to maintain normal cardiac function under pressure overload without evoking LVH is remarkable and seems to imply a primary pathological role of the LVH itself, rather than a secondary effect to compensate the pressure overload (21). It is surprising that the left ventricular myocardium was able to maintain normal fiber length and contractility with no evidence of left ventricular dilation and failure. The markedly reduced collagen type I in aortic-banded Agtr2–/Y mice may explain at least partly the normal contractile function (22, 23).
Since cardiac hypertrophy is dependent mainly on protein synthesis, and the S6 kinases, particularly p70S6k, play a pivotal regulatory role, the present observations suggest that the marked resistance to hypertrophy in the Agtr2–/Y mice implicates suppression of p70S6k. The marked reduction in the myofilament diameter in the Agtr2–/Y mice can also be explained by the reduced p70S6k (15). Thus, the present findings suggest the possibility that induction of LVH requires the expression of AT2, and the regulation of LVH by AT2 may use a pathway distinct from that of AT1, even though both may converge to activate p70S6k (24). These observations suggest that long-term pressure overload effect via AT2 regulates the expression of p70S6k, which may regulate ventricular myocyte protein synthesis.
Several MAPKs, particularly ERKs 1 and 2, are considered to be key factors regulating cell growth (25). However, no difference was seen in the phosphorylated forms of any of the three MAPKs between the Agtr2–/Y and wild-type mice. Since expression of ANP in aortic-banded Agtr2–/Y mice, a sensitive marker for ventricular response to pressure overload (26), is not different from that of aortic-banded wild-type mice, transcriptional mechanisms related to ANP gene expression may not be involved in the AT2-related hypertrophy. Recently, mechanisms involving calcineurin and nuclear factor of activated T cells–3 (NFAT-3), and/or activation of MEF2C by Ca2+-calmodulin–activated protein kinases II and IV, have been proposed for cardiac hypertrophy (27). Again, these factors do not appear to be involved in the resistance to LVH in Agtr2–/Y mice. In the Agtr2–/Y mice, all of the hypertrophy-inducing factors discussed above were comparable to those of the wild-type mice.
The almost complete abrogation of hypertrophic response in the Agtr2–/Y mouse heart may indicate that the presence of AT2 is a major factor in the cardiac hypertrophy. This hypothesis finds support in the observation of Harada et al. (5) and Hamawaki et al. (6) that aortic constriction can still induce LVH in Agtr1A–/– mice and that AT1 is only partially responsible for LVH. This latter conclusion is in contradiction to the current consensus that AT1 and AT2 have effects in antagonistic to growth regulation. However, the present in vivo studies suggest that AT2 and p70S6k can be determinants in regulating LVH, and they support the paradoxical phenotypic observation that AT2 is essential for LVH rather than for its inhibition in the heart. However, it is not clear how these mechanisms are related to several reported AT2 functions (28, 29).
In summary, we have found that in Agtr2–/Y mice, the left ventricle maintains normal contractile function but hypertrophic response to pressure overload is completely lost. The expression of ventricle-specific genes and marked suppression of p70S6k, which regulates the DNA synthesis in Agtr2–/Y mice, may provide an explanation for their loss of LVH response. Since a wide variety of other growth-regulating components are expressed normally, the present results indicate specific roles of p70S6k regulation in the Agtr2–/Y mouse heart and suggest that it is a major factor in mechanisms of cardiac hypertrophy.
Acknowledgments
This study was supported in part by NIH grants HL-58205 and DK-20593. We thank Mona Nemer for probes for Nkx2.5, GATA4, and MEF2C; Ronald Forczek and Peggy Petty for technical assistance with echocardiography; Erwin Landon for reading the manuscript; and Tina Stack for secretarial assistance.
References
- 1.Baker KM, Aceto JF. Angiotensin II stimulation of protein synthesis and cell growth in chick heart cells. Am J Physiol. 1990;259:H610–H618. doi: 10.1152/ajpheart.1990.259.2.H610. [DOI] [PubMed] [Google Scholar]
- 2.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer JM, Pfeffer MA, Mirsky I, Braunwald E. Regression of left ventricular hypertrophy and prevention of left ventricular dysfunction by captopril in the spontaneously hypertensive rat. Proc Natl Acad Sci USA. 1982;79:3310–3314. doi: 10.1073/pnas.79.10.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez JJ, et al. Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am J Physiol. 1994;267:H844–H852. doi: 10.1152/ajpheart.1994.267.2.H844. [DOI] [PubMed] [Google Scholar]
- 5.Harada K, et al. Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation. 1998;97:1952–1959. doi: 10.1161/01.cir.97.19.1952. [DOI] [PubMed] [Google Scholar]
- 6.Hamawaki M, et al. Pressure-overload hypertrophy is unabated in mice devoid of AT1A receptors. Am J Physiol. 1998;274:H868–H873. doi: 10.1152/ajpheart.1998.274.3.H868. [DOI] [PubMed] [Google Scholar]
- 7.Ichiki T, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 8.Komuro I, Kurabayashi M, Takaku F, Yazaki Y. Expression of cellular oncogenes in the myocardium during the developmental stage and pressure-overloaded hypertrophy of the rat heart. Circ Res. 1988;62:1075–1079. doi: 10.1161/01.res.62.6.1075. [DOI] [PubMed] [Google Scholar]
- 9.Fentzke RC, et al. Evaluation of ventricular and arterial hemodynamics in anesthetized closed-chest mice. J Am Soc Echocardiogr. 1997;10:915–925. doi: 10.1016/s0894-7317(97)80008-9. [DOI] [PubMed] [Google Scholar]
- 10.Manning WJ, Wei JY, Katz SE, Litwin SE, Douglas PS. In vivo assessment of LV mass in mice using high-frequency cardiac ultrasound: necropsy validation. Am J Physiol. 1994;266:H1672–H1675. doi: 10.1152/ajpheart.1994.266.4.H1672. [DOI] [PubMed] [Google Scholar]
- 11.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockman HA, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg PA, Melton DA. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- 14.Matsui H, Pallen CJ, Adachi AM, Wang JH, Lam PH. Demonstration of different metal ion-induced calcineurin conformations using a monoclonal antibody. J Biol Chem. 1985;260:4174–4179. [PubMed] [Google Scholar]
- 15.Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 16.Thorburn J, Frost JA, Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glennon PE, et al. Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 18.Kudoh S, et al. Angiotensin II stimulates c-Jun NH2-terminal kinase in cultured cardiac myocytes of neonatal rats. Circ Res. 1997;80:139–146. doi: 10.1161/01.res.80.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Hirota H, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 21.Bartunek J, et al. Chronic N(G)-nitro-L-arginine methyl ester-induced hypertension: novel molecular adaptation to systolic load in absence of hypertrophy. Circulation. 2000;101:423–429. doi: 10.1161/01.cir.101.4.423. [DOI] [PubMed] [Google Scholar]
- 22.Levine HJ. Compliance of the left ventricle. Circulation. 1972;46:423–426. doi: 10.1161/01.cir.46.3.423. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest. 1991;88:1141–1146. doi: 10.1172/JCI115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schluter KD, Piper HM. Regulation of growth in the adult cardiomyocytes. FASEB J. 1999;13(Suppl.):S17–S22. doi: 10.1096/fasebj.13.9001.s17. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T, et al. Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. J Biol Chem. 1993;268:12069–12076. [PubMed] [Google Scholar]
- 26.Arai H, et al. Augmented expression of atrial natriuretic polypeptide gene in ventricles of spontaneously hypertensive rats (SHR) and SHR-stroke prone. Circ Res. 1988;62:926–930. doi: 10.1161/01.res.62.5.926. [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lokuta AJ, Cooper C, Gaa ST, Wang HE, Rogers TB. Angiotensin II stimulates the release of phospholipid-derived second messengers through multiple receptor subtypes in heart cells. J Biol Chem. 1994;269:4832–4838. [PubMed] [Google Scholar]
- 29.Martens JR, Wang D, Sumners C, Posner P, Gelband CH. Angiotensin II type 2 receptor-mediated regulation of rat neuronal K+ channels. Circ Res. 1996;79:302–309. doi: 10.1161/01.res.79.2.302. [DOI] [PubMed] [Google Scholar]