Summary
Invariant natural killer T-cells (‘iNKT’) are the best-known CD1d-restricted T-cells, with recently-defined roles in controlling adaptive immunity. CD1d-restricted T-cells can rapidly produce large amounts of Th1 and/or Th2//Treg/Th17-type cytokines, thereby regulating immunity. iNKT can stimulate potent anti-tumor immune responses via production of Th1 cytokines, direct cytotoxicity, and activation of effectors. However, Th2//Treg-type iNKT can inhibit anti-tumor activity. Furthermore, iNKT are decreased and/or reversibly functionally impaired in many advanced cancers. In some cases, CD1d-restricted T-cell cancer defects can be traced to CD1d+ tumor interactions, since hematopoietic, prostate, and some other tumors can express CD1d. Ligand and IL-12 can reverse iNKT defects and therapeutic opportunities exist in correcting such defects alone and in combination. Early stage clinical trials have shown potential for reconstitution of iNKT IFN-gamma responses and evidence of activity in a subset of patients, with rational new approaches to capitalize on this progress ongoing, as will be discussed here.
Keywords: cytokines, tumor immunity, CD1, CD1d-reactive T cells, iNKT, NKT
1.1. CD1d-restricted T Cell Populations-I: Invariant natural killer T cells
Natural killer T cells (NKT) are a population of innate-like T cells with unique activation properties and effector functions related to NK cells. The best characterized population of NKT cells, termed invariant NKT (iNKT), was initially identified by a restricted T cell receptor repertoire. iNKT express a canonical, invariant T cell antigen receptor comprised of Vα14 and Jα18 in mice and rats and Vα24-Jα18 in humans and non-human primates, with preferred (although not essential or invariable) Vβs, in both cases. Unlike classical T cells, which recognize peptides presented by highly polymorphic MHC molecules, iNKT cells recognize (glyco-)lipids via MHC-like, non-polymorphic CD1d molecules (1–10). The basis of iNKT regulatory function is their rapid secretion of multiple cytokines and chemokines accompanied by CD1dspecific cytotoxicity following TCR triggering (1–7). iNKT rapidly secrete large amounts of different cytokines after activation and thereby regulate immune responses. These products include both regulatory factors (e.g. IL-4, IL-10, IL-13) as well as pro-inflammatory agents such as IL-2, IL-17, TNFa, CCL3 (MIP1a), and IFNγ, reflecting their capacity to suppress or stimulate immune responses (1–7). iNKT were shown to contribute to immune surveillance in early stage tumors and in chemically-induced cancers (3- 5;11–13). The originally-defined prototypic high-affinity iNKT glycolipid ligand, alpha-galactosylceramide (αGalCer) was identified in a screen for anti-cancer agents. αGalCer and iNKT have subsequently been shown to have anti-cancer activity in animal models and anti-tumor potential in patients (3-5;11,12), as well as to have anti-pathogenic activity (14–18). Although exogenous CD1d ligands similar to αGalCer have been identified from pathogenic as well as non-pathogenic microorganisms (14,16,19), the identity of physiological endogenous ligands that can also mediate CD1d-dependent T cell activation remains supposed, but so far identification has been limited to ligands for subsets (14,16,19–22). Furthermore, unike T cells, iNKT can also be activated by cytokine combinations (21).
The main CD1d-expressing cell types have been identified as dendritic cells (DCs), macrophages, B and murine T cells (1–4;16,23–27). To date, physiological functions of CD1d in anti-tumor activity (11–13), tolerance induction (5) and host defense (14–18) have been best established in the case of DCs. The interaction between iNKT and APC (antigen presenting cells: monocytes, macrophages, DCs) appears to be of central importance in regulating immune responses. Monocytes or DCs loaded with αGalCer can activate iNKT in vitro and in vivo, with subsequent iNKT stimulation of DC maturation (1–10;19–22;28–31). The interactions between iNKT and DCs appear to share many features with those between conventional CD4+ T cells and DCs, although priming is not required and there are other key differences. DCs pulsed with a specific Ag (in this case αGalCer) stimulate iNKT through TCR ligation and this can be enhanced by B7 (CD80/86) ligation of CD28 on the iNKT (20–22; 28–31). iNKT activation and production of IFNγ are markedly enhanced by DCs producing IL-12, with increased expression of IL-12 receptor on activated iNKT (1–7; 28–31). CD40L expression by activated iNKT can in turn activate DCs through ligation of CD40, with iNKT IFNγ further stimulating DC IL-12 (1–7; 28–31). These interactions provide a mechanism by which iNKT markedly amplify IL-12 production by DCs, and are consistent with the requirement by iNKT, in some anti-tumor responses, for low-dose exogenous and/or physiological endogenous IL-12 (1–7;11–13; 28–31). In several systems, iNKT are the primary responders to low dose IL-12 rather than NK cells (11–13;32–36). Finally, it should be noted that the profile of chemokine receptors expressed by peripheral blood iNKT indicates that they primarily traffic to peripheral tissues, consistent with their biological function being to interact with immature DC in tissues and stimulate their maturation (37,38).
1.2. CD1d-restricted T Cell Populations-II: relation of iNKT to other CD1d-reactive T cells
Human and rodent iNKT have many common features and closely resemble one another in activity and in general properties. However, iNKT frequencies are lower in humans than in mice (39). This is true both in the periphery and in the organs including liver. In fact, human liver is dominated by non-invariant CD1d-restricted T cells (40–42), many of which do not even express NK markers (41), although “tip-of-the iceberg” iNKT behave similarly (43). Furthermore, both human and murine bone marrow tend to be dominated by the “non-invariant” (‘Type 2’) CD1d-restricted NKT cells (1–7;44).
Human iNKT proportions decline with age (45,46), whereas in mice they rise (47), possibly due to declines in other lymphocyte populations. This, together with minimal surface expression of CD1d by healthy human hepatocytes (23,41) unlilke mouse (24,26), may explain why αGalcer is lethal in older mice, causing a Con-A-like acute hepatitis (48), but has minimal effects in most adult humans. Indeed, cytokine responses to αGalcer administration are only detected in patients with higher levels of iNKT (49). Furthermore, men generally have less iNKT than women, and levels are also typically lower in those with chronic diseases of many types, including multiple auto-immune diseases and cancers 39,45,46). Therefore, despite overall conservation to the level of species cross-reactivity of iNKT with CD1d (50), there are important CD1d-restricted T cell species differences.
Finally, while all the CD1d-restricted T cells described above are αβ T cells, there is no reason in principle that γδ T cells could not also be CD1d-restricted, as have been described for other CD1 molecules (16). Indeed, such cells have been described and are not always protective. CD1d is up-regulated in murine model coxsackie virus infection and its recognition specifically by Vγ4+ T cells is associated with the autoimmune viral myocarditis sequelae of otherwise successful anti-picornaviral responses (51).
2.1. Principle of CD1d-restricted T cell anti-tumor activities
CD1d-restricted T cell populations physiologic role in tumor immunosurveillance is mediated at least partly through APC maturation and IL-12 induction and via both NK and CD8+ T cells (11–13;21,27– 30;39). In addition, immunity against many tumor models is observed with therapeutic activation of iNKT by selective agonist α-galactosylceramide (αGalCer) presented by CD1d+ APC (11–13). Sequential production of IFNγ, initiated by iNKT and subsequently produced by NK cells, is pivotal for the antimetastatic activity of αGalCer and other agents affecting CD1d-restricted T cells, such as IL-12 and cytokine combinations such as IL-12 and IL-18 (11–13;21). Direct CD1d-restricted T cell cytotoxicity may contribute via classic granule-mediated as well as TNF family mechanisms, since some tumors express CD1d (see below). Despite normal IL-4 production and activation marker up-regulation, iNKT in tumor bearing mice have defective IFNγ responses (11–13;52–54). These are reminiscent of cancer patient iNKT defects in activation by αGalCer or IL-12 (55–57). Indeed, the presence of Th1-type IFNγ-producing CD1d-restricted T cell populations is a positive prognostic indicator in a number of cancers described (see below).
2.2. CD1d-restricted T Cell Populations in Cancer Patients and CD1d Expression in Cancer
Quantitative defects in the iNKT pool are found in various types of cancer including melanoma, colon, lung, breast, head and neck squamous cell carcinoma (HNSCC), prostate cancer, myelodysplastic syndromes and progressive multiple myeloma (13;45,46;55–58). However, these differences are not absolute, there is overlap between groups, and these defects are not unique to cancers, being associated with many inflammatory conditions (59).
Importantly, these statistically significant quantitative defects are frequently accompanied in certain advanced cancers by profound but reversible defects in the capacity of iNKT to both proliferate (55–57,60) and produce IFNγ in vitro and ex vivo (55–57). Interestingly, both the proliferative defect and the Th1 response block are reversible in vitro, and unlike committed memory T cells, iNKT defects can therefore potentially be corrected in patients. IL-2 reverses the iNKT proliferative defect (55,60), similarly to how it well-known overcome some forms of T cell anergy. Similarly, IL-12 can reverse the block in IFNγ production in response to αGalCer in vitro (55), reflecting the in vivo block we subsequently identified in a tumor model (54). Importantly, conventional T cell responses from the same patients were normal, indicating that these defects are iNKT-specific (55–57,60). Not all cancers exhibit iNKT defects and relative iNKT cell Th2 biases are not found in all (especially early) stages or cancers and other clear exceptions have been reported (58,61).
Certain tumor types are known to express CD1d and thus can be directly recognized by iNKT (42,61–66). These include some of the very tumors where selective functional defects of iNKT have been identified: hematopoietic malignancies, prostate cancer, and certain but not all neurological tumors (54–57;61). The functional consequences of CD1d on tumor cells are not well understood, but increasing evidence suggests this may impact iNKT. For example, early and intermediate stage myeloma cells express CD1d, which can be targeted for killing, but this is lost on advanced myeloma and most myeloma cell lines (67–69). Other mostly solid human and model tumors have generally been believed to be CD1d-negative (B16 melanoma cell lines represent a good example of this), reflecting limited CD1d expression outside normal hematopoietic cell types (23–27). However, there is evidence for at least some CD1d expression on various solid tumor cell lines (70–73). Furthermore, very low levels of CD1d are sufficient for presentation to iNKT, just as for MHC-peptide complexes and other T cells. The presence of CD1d on some tumors makes them sensitive to iNKT cytotoxicity (61,63,74,75) as well as potentially direct mechanisms of apoptosis/cell suicide, as seen in CD1d+ myelomas upon CD1d cross-linking (69). Therefore, CD1d expression may be a common demoninator in advanced cancers with failure of Th1 iNKT driven by tumor acting as non-professional APCs. Of course, other factors may contribute, including CD1d expression by stroma and infiltrating immune cells in various tumors and stages, as well as in response to some therapies. Indeed, another mechanism of targeting tumors by iNKT can involve them killing tumor-associated macrophage-type cells (76).
Most human cancer studies utilize blood iNKT and therefore investigate systemic effects. However, some studies have been performed using human iNKT associated with tumors. Results show that tissue and tumor-infiltrating lymphocyte (TIL) iNKT and other CD1d-reactive ‘NKT’-type cells are distinct and can be enriched relative to in matched blood (42,77–79). In liver cancer, iNKT cytokine responses can be Th1-biased (42,77), like such cells in healthy liver and other liver diseases (40–42), and unlike the equivalent Th0 cells in mouse (1–7). Interestingly, TIL can be enriched for CD4+ iNKT, which at least from healthy donors, produce higher levels of regulatory/Th2 cytokines ex vivo (1–7;36;78). Human iNKT have shown the ability to kill tumor cells ex vivo. In many cancer patients, these cells are depleted from tissues, and in some cases replaced by Type 2 NKT (42,77).
There is also the remarkable finding that iNKT and other CD1d-reactive peripheral blood T cell populations producing IFNγ in vitro are strongly and selectively associated with improved prognosis in patients with glioma, colon cancer HNSCC, and hematological cancers (55,58,80,81). This latter specific feature of CD1d-restricted T cell Th1 responses could be of diagnostic value.
2.3. Role of iNKT in Prostate Tumor Immunity: an example of the relation of human and model studies
We first described reversible numerical and functional iNKT defects in patients with advanced prostate cancer (55). Similarly, a functional iNKT defect was found in TRAMP mice stimulated with glycolipid αGalCer in vivo (Figure 1; 54). Furthermore, iNKT deficiency exacerbates sensitivity to the TRAMP prostate cancer model (53). iNKT can be found in established TRAMP tumors in the TIL compartment. We characterized the interaction of tumor cells with iNKT cells from TRAMP mice ex vivo compared to iNKT cells from healthy mice. Systemic iNKT in both normal and TRAMP mice constitutively express low levels of CD69. However, high levels of CD69 as well as IL-12RB1 are expressed by TIL iNKT, suggesting iNKT are hyper-activated within tumors (54).
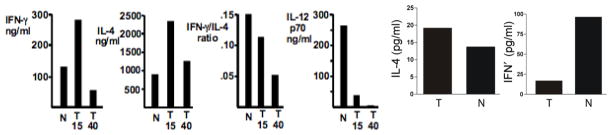
Fig. 1. αGalCer stimulated NKT-dependent cytokine production is decreased in prostate cancer model in vivo and in vitro.
Left: 15 or 40 wk. old mice with prostate tumors (T) or normal controls (N) were stimulated with αGalCer in vivo and serum cytokines analyzed 90 min. later. Right: Splenocytes of 40 weeks old tumor-bearing mice or normal controls were stimulated in vitro with 100 pg/ml αGalCer and culture supernatants tested for IL-4 and IFNγ 24 hr. later. Cytokine levels of unstimulated mice splenocytes were below detection limit.
TRAMP tumor cell lines, human CaP lines and primary prostate epithelium as well as primary TRAMP tumors express CD1d (54). Moreover, CD1d on the TRAMP-C2 cell line is functional. TRAMPC2 pulsed with αGalCer stimulate IL-2 secretion by iNKT hybridomas (54). Therefore, we tested whether tumor cells could aberrantly activate iNKT. TRAMP tumor cells induce expression of activation markers CD25, PD-1 and IL-12RB1 on primary iNKT (54). TRAMP tumor cells modestly activate primary iNKT ex vivo even without exogenous ligand. αGalCer+ TRAMP tumor cells induce iNKT IL-4, but not IFNγ (54). IL-4 production occurs independently of co-stimulatory molecules presented by CD1d+ DC (30), whereas iNKT IFNγ is enhanced by IL-12 produced by DC (28-30). However, despite iNKT up-regulation of IL-12R, IL-12 alone was not sufficient to stimulate their IFNγ production. Only both IL-12 and the high-affinity ligand αGalCer together could induce IFNγ production by iNKT (54), thus showing that tumor cells can reversibly inhibit iNKT. This defect can be overcome by provision of strong TCR signals (such as provided by the high affinity ligand αGalCer) in combination with Th1 adjuvant IL-12.
The mechanism(s) by which iNKT in the presence of tumors acquire a Th2 like profile is not fully understood, but appears to be related to a specific defect in signaling to IFNγ production (54). As mentioned, DCs play a fundamental role in controlling iNKT effector functions, and iNKT can control DC maturation. This positive feedback loop provides a further potential in vivo contributing mechanism to help explain the observed qualitative iNKT defects. As discussed above, some other tumors express CD1d (62–73) as well as human and mouse prostate epithelial cells (54). Therefore, one may speculate that CD1d+tumors present endogenous glycolipids to iNKT, hence there is some cytokine production even in the absence of αGalCer (54), as has been shown in other systems (82,83), leading to iNKT activation that is distinct from normal DC-induced activation. Finally, provision of exogenous high affinity ligand-loaded tumor cells has been shown to break tumor tolerance in some systems even in the absence of IL-12 (84,85).
2.4. Further Examples of NKT-related therapy
2.4.1. CD1d mAb bypasses iNKT defects in models
CD1d mAb have widely been used to block CD1d-reactive T cell activity in vivo (1–7). However, an unexpected effect of this approach has been identified. Direct CD1d mAb administration induces a potent Th1 and type 1 interferon response both in vitro but also in vivo, through maturation of dendritic type and other CD1d+ antigen presenting cells, such as monocytes (86,87). As mentioned above, CD1d cross-linking of myeloma cell CD1d leads to apoptosis, which has been suggested as another element of a multi-pronged attack in CD1d+ tumors (69).
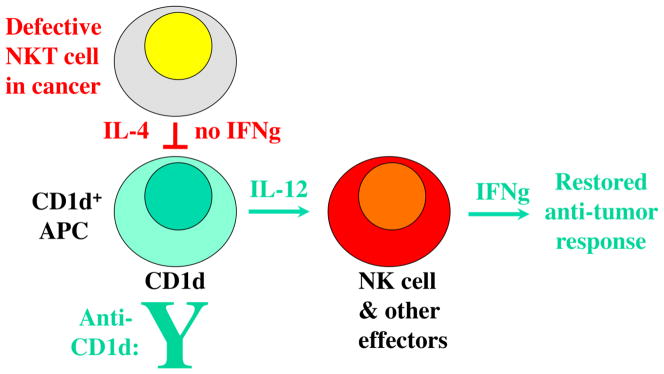
As described for several tumor cell line models, anti-CD1d mAb can also delay or actually prevent tumor growth (88). In models, this approach is synergistic with other approaches, both conventional and immuno-therapeutic (89). Therefore, this may be a future alternative means to bypass defects in NKT cells of cancer patients (Figure 2).
Fig. 2. Bypassing iNKT cell defects with CD1d cross-linking.
iNKT defects in cancer shown in red. CD1d mAb can stimulate CD1d+ APC maturation and IL-12 production (86,87). This can lead to activation of downstream Th1-type effectors such as NK cells, resulting in anti-tumor (green) as well as anti-viral effects (87-89). Blocking of Type 2 non- invariant CD1d-reactive T cells with Th2 bias may also contribute to net gain in anti-tumor activity. FInally, a direct pro-apoptotic effect of CD1d mAb on CD1d+ tumor cells has also been described (69), potentially augmenting this effect.
2.4.2. Further NKT-related therapeutic Approaches
Several further direct approaches to exploit the anti-tumor activity of CD1d-restricted T cell populations have been described. For example, direct transfer of iNKT cells is therapeutically active against a range of model tumors (11–13;34).
Unlike most classical T cells, IL-12 and IL-18 combined will also activate NKT independently of the TCR (21) and this combination has been shown to enhance anti-tumor activity (11,90). NKT activity is also synergistic with NK cells in mice treated with IL-12 and IL-18 (11,21,90).
Finally in this regard, iNKT have also been shown to have powerful adjuvant-like activity for T and B cell immune responses to model antigens, a range of pathogens, and against tumors (91-98). In particular, as also mentioned above, αGalCer presented on CD1d+ tumor cells or carried to DC on some CD1d-negative tumors (84,85), or presented by dendritic cells and other APC, augments anti-tumor activity in numerous models (96-99). Therefore, NKT have the potential synergise with other immuno- and conventional therapies. This is discussed further below for models and in the context of clinical trials.
2.5. Optimizing Cancer Vaccines through iNKT cells
While some potentially tumor reactive T cell clones may be lost (by central or peripheral mechanisms) in tumor-bearing individuals, other clones appear to remain naïve or tolerized and can potentially be activated by tumor vaccines. Multiple tumor vaccines have been shown to require iNKT presence for optimal activity, including both GM-CSF-transduced (‘GVax’) (100) and some types of CpG based vaccines (101–105). It is likely that other vaccine types are purely (but still usefully) synergistic with NKT-related therapies. Such synergy has been described, as above, for CD1d mAbs with other immunotherapies in various models (89). The efficacy of GM-CSF tumor vaccine is largely impaired in iNKT cell deficient as well as CD1d KO mice (100), which supports a critical role for iNKT in the requisite maturation of DCs for effective antigen presentation in this system. However, as in direct anti-tumor responses (11,12), CD1d-restricted T cells are not protective for all cancer vaccines, with CD1/noninvariant CD1d-restricted T cells apparently having deleterious effect on anti-tumor immunity (106).
3.1. iNKT-related clinical trials
Cumulatively, in vivo results from tumor models (Figure 3) and in vitro patient results, along with results in various other diseases have led to a range of current and planned clinical trials exploiting the NKT system in different ways (107). Initial phase clinical trials attempting to induce antitumor immunity through activation of iNKT have revolved around the ligand, αGalCer. Further details are provided below, as described in detail in other reviews in this issue of Clin. Immunol. (108–113).
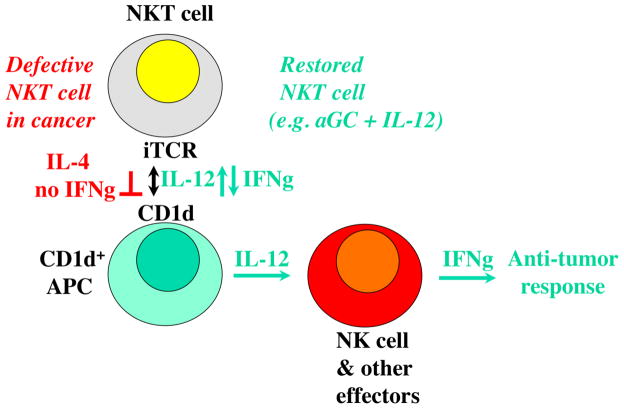
Fig. 3. Simplified model for iNKT involvement in anti-tumor responses.
iNKT cell defects in cancer shown in red. iNKT defects can be corrected (green). NKT cells can contribute to anti-tumor responses through several mechanisms, but the dominant protective pathway described to date depends on mutual stimulation of iNKT cells interacting with CD1d+ APC, such as some myeloid dendritic cells in humans, most APC in rodents.
In the first iNKT trial, in which patients with solid tumors received intravenous αGalCer, signs of immune activation only occurred in the fraction of patients with relatively normal iNKT numbers (49,109). Despite overall relatively specific effects, αGalCer has been reported to have an off-iNKT target effect of inducing type-1 IFN (114), which could also affect anti-tumor activity in vivo.
As murine experiments indicated that anti-tumor acitivity of αGalCer is enhanced by loading onto DC (11,12;99), similar approaches have been evaluated in clinical trials for solid and hematological malignancies. αGalCer-pulsed monocyte-derived ‘DC’ produced more potent immune activity, including inflammatory tumor responses, tumor necrosis and decreases in tumor markers in some patients, as well as expansion of iNKT up to a few months and an increase in adaptive T cell immunity (11-13;112,113,115-117). Local targeting of αGalCer-pulsed APC has been shown to be well-tolerated in HNSCC patients (118).
iNKT adoptive transfer has also been effective in mouse tumor models (11–13;34,119). Based on these observations and those that the size of the IFNγ-producing NKT-type cell pool also appears to correlate with survival (15, 55,58,80,81), a study evaluated feasibility of adoptive transfer of autologous iNKT-enriched populations in cancer patients, and reported that the treatment was well-tolerated and could be accompanied by clinical responses (120). Combining iNKT-enriched product with αGalCer-pulsed APC has been associated with evidence of some clinical responses as well as increased cytokine responses in HNSCC (121). As purity of initial enriched iNKT products has been variable and mostly modest, future studies aim to use more homogeneous populations of iNKT to allow evaluation of clinical responses.
Current immunotherapeutic approaches in cancer may also be expected to be substantially more potent when combined synergistically with iNKT restoration/activation. For examples, iNKT can activate other T cells and limitations on the latter are being relieved in current trials involving anti-CTLA4 and anti PD-1 mAbs. It is likely that direct positive iNKT activation will be synergistic with approaches to inihibit conventional T cell inhibitory pathways.
Further trials have had impacts on various NKT-type populations. In pioneering model studies by several groups, bone marrow NKT-type cells (which unlike mouse liver, but like human bone marrow are dominated by non-invariant NKT; 4,20,44;122-125), were shown to contribute to graft-versus-tumor (GvT) activity, but also be capable of suppressing graft-versus-host disease (GvHD) (123–125). This potential double benefit was exploited in several regimens in which the non-invariant (‘Type 2’) NKT were enriched and activated in vivo. NKT in general are resistant cells, which can also recover faster than other T cells from potent stimulations and/or insults, such as pharmacological doses of IL-12, anti-CD3 mAb, steroids, etc. (1–7;21,22,90). Dr. S. Strober’s group found that a clinical protocol-derived treatment with total lymphoid organ irradiation and anti-thymocyte globulin (TLI + ATG) led to rapid restoration of NKT populations ahead of conventional T cells (123–125). These types of treatment could also enhance bone marrow transplant (BMT) efficacy in multiple models (123–126). However, a note of caution was raised by the finding that iNKT DC activation and IL-12 production downstream of αGalCer could exacerbate GvHD (127). Therefore, a clinical non-invariant NKT enhancing protocol combining TLI + ATG with BMT was tested and found to have a strong protective effect against acute GvHD (128,129). In parallel, non-myeloablative BMT and kidney transplantation without immunosuppression in patients with renal failure due to myeloma led to transient mixed chimerism and tumor control without GvHD or kidney rejection (130,131). Donor leukocyte infusions (DLI) can enhance BMT anti-tumor effects and most recently recipient leukocyte infusions (RLI) have been shown to have similar activity. In particular, iNKT were the protective population in models of the RLI approach (131).
Based on the large amounts of model data, others trials, and our preclinical data summarized above, a phase I clinical trial of autologous iNKT in patients with advanced cancer was initiated (PI. Dr. S. Balk; DF/HCC #06-432), and has now completed 8 melanoma patient treatments. The approach is feasible and well-tolerated, with only local/grade 1-2 toxicity. The trial is conducted through the Dana Farber/Harvard Cancer Center, in collaboration with Drs. S. Hodi and G. Dranoff, who have extensive expertise in the conduct of melanoma clinical trials. The methods for iNKT purification and expansion are similar to those we have published previously (55,133), with modifications for clinical grade use. Dr. J. Ritz (DFCI) supervises the iNKT cell culture. Patients undergo leukapheresis yielding ~1010 PBMC. Residual iNKT (~ 0.01% iNKT, ~ 106 cells) are purified from the leukapheresis product using GLP-grade biotinylated mouse anti-human mAb (6B11) against the human invariant Vα24Jα18 TCR chain (55,133) and GMP grade antibiotin micro-beads for purification on a magnetic column, as described (133). This procedure yields populations highly enriched in iNKT (~ 50% pure), with little or no losses. The bulk of purified iNKT are then expanded in vitro with CD3 mAb and IL-2 (133) for therapeutic use of up to > 100 million total iNKT/infusion at 3 bi-weekly infusions. The first 3 patients received iNKT alone. Since there was no grade 3 or greater toxicity, subsequent patients additionally receive GM-CSF subcutaneously for 10 days with second and third iNKT administrations, to mobilize and activate dendritic cells. Patients are restaged as appropriate 3 – 4 weeks after final iNKT infusion and followed thereafter. Complete formal clinical and immunological results will be reported at conclusion of the trial. In summary, these data confirm feasibility of such a clinical trial, show isolation and expansion of iNKT from patients with advanced melanoma, and infusion safety.
4.1. Conclusions
In conclusion, although iNKT cells and other CD1d-reactive T cells appear to frequently suffer attrition in advanced cancers and some other diseases, this can be reversible and mechanistic insights reveal rational approaches to restore NKT cell physiological protective activities (Figure 3) for cancer (as above), as well as for other therapeutic settings such as sickle cell disease (134). At least some ‘Type 2’ non-invariant CD1d-reactive T cells and even iNKT cells can inhibit anti-tumor responses, including those of other iNKT in tumor models (135-137), so CD1d-reactive T cell-related therapeutic approaches need to avoid augmenting undesirable responses alongside protective ones. Although highly specific for iNKT, based on lack of activity in Jα18 KO mice (lacking only the TCR J region used by iNKT and a small number of other T cells; 1–7), there is a report of direct type-1 IFN inducing activity of αGalCer on human liver cells (114), which could contribute either positively or negatively where αGalCer is used therapeutically. A spectrum of αGalCer analogues are now in preclinical development (e.g. 111,138), some of which will be deployed in phase 1 trials imminently. Although, as expected, toxicity has been minimal, the therapeutic potential of NKT-based approaches has not yet been fully realised in early clincal trials. However, a number of recent immunological and clinical observations suggest both that progress is being made and that various means to improve on NKT-related therapies will become available in the near future (108–113). Indeed, a wide-ranging series of approaches involving (or even bypassing) NKT cell populations are at various stages of late-stage preclinical development.
Table.
Examples of Published and Ongoing iNKT-Targeted Clinical Trials
| Published iNKT Trial | Indication | Ref. |
|---|---|---|
| αGalcer i.v. | Advanced Cancer | 49, 109 |
| αGalcer-loaded monocyte-derived DC. | Advanced Cancer | 115 |
| αGalcer-loaded monocyte-derived matured DC. | Multiple Myeloma | 116, 113 |
| αGalcer-loaded monocyte-derived APC. | Non-Small Cell Lung Cancer | 117, 112 |
| αGalcer-loaded monocyte-derived DC. | Head & Neck Squamous Cell Cancer | 118, 112 |
| αGalcer-expanded iNKT-enriched T cells. | Non-Small Cell Lung Cancer | 120, 112 |
| αGalcer-loaded APC & expanded iNKT. | Head & Neck Squamous Cell Cancer | 121, 112 |
|
| ||
| Ongoing Clinical Trial | Indication Status | (04/2011)/Ref. |
|
| ||
| Anti-iNKTCR mAb purified & expanded iNKT. | Stage IV Melanoma | ongoing; n = 8 |
| iNKT-expressed adenosine receptor agonist. | Sickle Cell Disease | ongoing; 134 |
| αGalcer-loaded matured DC + lenalidomide. | Multiple Myeloma | ongoing; 113 |
Highlights.
There are 2 major CD1d-restricted T cell populations
Both CD1d-restricted T cell subsets can contribute to anti-tumor immunity
Both CD1d-restricted T cell subsets can “lose” against progressive tumors
Both CD1d-restricted T cell subsets defects can be reversed
Improved CD1d-restricted T cell therapies are nearing clinical trials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. AnnuRevImmunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi M, Tashiro T, Dashtsoodol N, Hongo N, Watarai H. The specialized iNKT cell system recognizes glycolipid antigens and bridges the innate and acquired immune systems with potential applications for cancer therapy. Int Immunol. 2010 Jan;22(1):1–6. doi: 10.1093/intimm/dxp104. Epub 2009 Oct 25. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, Kronenberg M. Going both ways: immune regulation via NKT cells. J Clin Inv. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Vliet HJ, Molling JW, von Blomberg BM, Nishi N, Kolgen W, van den Eertwegh AJ, Pinedo HM, Giaccone G, Scheper RJ. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol. 2004 Jul;112(1):8–23. doi: 10.1016/j.clim.2004.03.003. Review. [DOI] [PubMed] [Google Scholar]
- 6.Nowak M, Stein-Streilein J. Invariant NKT cells and tolerance. IntRevImmunol. 2007;26:95–119. doi: 10.1080/08830180601070195. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss- Army knife’ of the immune system. CurrOpinImmunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh VV, Wilson MT, Van Kaer L. iNKT-cell responses to glycolipids. CritRevImmunol. 2005;25:183–213. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, Silk JD, Cerundolo V. Recent advances in processing and presentation of CD1 bound lipid antigens. Curr Opin Immunol. 2010 Feb;22(1):81–8. doi: 10.1016/j.coi.2009.12.008. Epub 2010 Jan 14. [DOI] [PubMed] [Google Scholar]
- 10.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010 Apr;22(2):68–78. doi: 10.1016/j.smim.2009.10.003. Epub 2009 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 12.Berzofsky JA, Terabe M. The contrasting roles of NKT cells in tumor immunity. Curr Mol Med. 2009 Aug;9(6):667–72. doi: 10.2174/156652409788970706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhodapkar MV. Harnessing human CD1d restricted T cells for tumor immunity: progress and challenges. Front Biosci. 2009 Jan 1;14:796–807. doi: 10.2741/3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009 Aug;21(4):391–6. doi: 10.1016/j.coi.2009.07.002. Epub 2009 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diana J, Lehuen A. NKT cells: friend or foe during viral infections? Eur J Immunol. 2009 Dec;39(12):3283–91. doi: 10.1002/eji.200939800. [DOI] [PubMed] [Google Scholar]
- 16.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 17.Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;129:1628–42. doi: 10.1038/jid.2009.30. Epub Mar 5. [DOI] [PubMed] [Google Scholar]
- 18.Tessmer MS, Fatima A, Paget C, Trottein F, Brossay L. NKT cell immune responses to viral infection. Expert Opin Ther Targets. 2009 Feb;13(2):153–62. doi: 10.1517/14712590802653601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfrey DI, McConville MJ, Pellicci DG. Chewing the fat on natural killer T cell development. J Exp Med. 2006 Oct 2;203(10):2229–32. doi: 10.1084/jem.20061787. Epub 2006 Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009 Feb;218(2):246–50. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly EC, Wands JR, Brossay L. Cytokine dependent and independent iNKT cell activation. Cytokine. 2010 Sep;51(3):227–31. doi: 10.1016/j.cyto.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde S, Fox L, Wang X, Gumperz JE. Autoreactive natural killer T cells:promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology. 2010 Aug;130(4):471–83. doi: 10.1111/j.1365-2567.2010.03293.x. Epub 2010 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, et al. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 24.Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL, Kronenberg M. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997 Aug 1;159(3):1216–24. [PubMed] [Google Scholar]
- 25.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- 26.Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 27.Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, Patton KT, Blumberg RS, Porcelli S, Chott A, Balk SP. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)- 12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomura M, Yu WG, Ahn HJ, Yamashita M, Yang YF, Ono S, et al. A novel function of Valpha14 + CD4 + NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 30.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda K, Hayakawa Y, Atsuta M, Hong S, Van Kaer L, Kobayashi K, Ito M, Yagita H, Okumura K. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000 Jun;12(6):909–14. doi: 10.1093/intimm/12.6.909. [DOI] [PubMed] [Google Scholar]
- 33.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000 Sep 1;165(5):2665–70. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 34.Shin T, Nakayama T, Akutsu Y, Motohashi S, Shibata Y, Harada M, Kamada N, Shimizu C, Shimizu E, Saito T, Ochiai T, Taniguchi M. Inhibition of tumor metastasis by adoptive transfer of IL-12-activated Valpha14 NKT cells. Int JCancer. 2001 Feb 15;91(4):523–8. doi: 10.1002/1097-0215(20010215)91:4<523::aid-ijc1087>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003 Feb 1;170(3):1197–201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 36.Gumperz JE. CD1d-restricted “NKT” cells and myeloid IL-12 production: an immunological crossroads leading to promotion or suppression of effective anti-tumor immune responses ? J Leukoc Biol. 2004 Aug;76(2):307–13. doi: 10.1189/jlb.0104038. Epub 2004 May 3. [DOI] [PubMed] [Google Scholar]
- 37.Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516–519. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 38.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 39.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011 Feb;11(2):131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 40.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d- reactive T cells in hepatitis C virus infected liver. J Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 41.Durante-Mangoni, Wang R, Shaulov A, He Q, Nasser, Afdhal N, Koziel MJ, Exley M. Hepatic CD1d Expression in Hepatitis C Virus Infection and Recognition by Resident Proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–66. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 42.Kenna T, O’Brien M, Hogan AE, Exley MA, Porcelli SA, Hegarty JE, O’Farrelly C, Doherty DG. CD1 expression and CD1-restricted T cell activity in normal and tumour-bearing human liver. Cancer Immunol Immunother. 2007 Apr;56(4):563–72. doi: 10.1007/s00262-006-0215-x. Epub 2006 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, Nuti S, Colombo M, Callea F, Porcelli SA, Panina-Bordignon P, Abrignani S, Casorati G, Dellabona P. Production of pro-fibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–25. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- 44.Exley M, Tahir S, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk S. Cutting edge: A major fraction of human bone marrow lymphocytes are Th2-Like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 45.Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Vα24+Vβ11+ NKT cells by age, malignancy and conventional anticancer therapies. Br J Cancer. 2004;91:1880–6. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Peripheral blood IFN-γ-secreting Vα24+Vβ11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 47.Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175:3102–9. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- 48.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver NKT cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 50.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998a;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber S, Sartini D, Exley M. Role of CD1d in coxsackie virus B3-induced myocarditis. J Immunol. 2003;170:3147. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 52.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand alpha-galactosylceramide in cancer-bearing state mediated by CD11b+Gr-1+ cells producing nitric oxide. Cancer Res. 2006 Dec 1;66(23):11441–6. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 53.Bellone M, Ceccon M, Grioni M, Jachetti E, Calcinotto A, Napolitano A, Freschi M, Casorati G, Dellabona P. iNKT cells control mouse spontaneous carcinoma independently of tumor-specific cytotoxic T cells. PLoS One. 2010 Jan 13;5(1):e8646. doi: 10.1371/journal.pone.0008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowak M, Arredouani MS, Tun-Kyi A, Schmidt-Wolf I, Sanda MG, Balk SP, Exley MA. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with alpha-galactosylceramide. PLoS One. 2010 Jun 25;5(6):e11311. doi: 10.1371/journal.pone.0011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFNgamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 56.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–76. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003 Aug;122(4):617–22. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 58.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007 Mar 1;25(7):862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 59.van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, Polman CH, Rustemeyer T, Lips P, van den Eertwegh AJ, Giaccone G, Scheper RJ, Pinedo HM. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001 Aug;100(2):144–8. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 60.Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002 Jun 15;168(12):6494–9. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 61.Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, Steinman RM. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004 May 10;109(6):893–9. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 62.Zheng Z, Venkatapathy S, Rao G, Harrington CA. Expression profiling of B cell chronic lymphocytic leukemia suggests deficient CD1-mediated immunity, polarized cytokine response, altered adhesion and increased intracellular protein transport and processing of leukemic cells. Leukemia. 2002 Dec;16(12):2429–37. doi: 10.1038/sj.leu.2402711. [DOI] [PubMed] [Google Scholar]
- 63.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003 Jun;17(6):1068–77. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 64.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, Groshen S, Wilson SB, Seeger RC. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004 May 3;199(9):1213–21. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fais F, Tenca C, Cimino G, Coletti V, Zanardi S, Bagnara D, Saverino D, Zarcone D, De Rossi G, Ciccone E, Grossi CE. CD1d expression on B-precursor acute lymphoblastic leukemia subsets with poor prognosis. Leukemia. 2005 Apr;19(4):551–6. doi: 10.1038/sj.leu.2403671. [DOI] [PubMed] [Google Scholar]
- 66.Nebozhyn M, Loboda A, Kari L, Rook AH, Vonderheid EC, Lessin S, Berger C, Edelson R, Nichols C, Yousef M, Gudipati L, Shang M, Showe MK, Showe LC. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006 Apr 15;107(8):3189–96. doi: 10.1182/blood-2005-07-2813. Epub 2006 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, Jagannath S, Dhodapkar MV. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006 Jul 15;108(2):618–21. doi: 10.1182/blood-2005-10-4184. Epub 2006 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song W, van der Vliet HJ, Tai YT, Prabhala R, Wang R, Podar K, Catley L, Shammas MA, Anderson KC, Balk SP, Exley MA, Munshi NC. Generation of antitumor invariant natural killer T cell lines in multiple myeloma and promotion of their functions via lenalidomide: a strategy for immunotherapy. Clin Cancer Res. 2008 Nov 1;14(21):6955–62. doi: 10.1158/1078-0432.CCR-07-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spanoudakis E, Hu M, Naresh K, Terpos E, Melo V, Reid A, Kotsianidis I, Abdalla S, Rahemtulla A, Karadimitris A. Regulation of multiple myeloma survival and progression by CD1d. Blood. 2009 Mar 12;113(11):2498–507. doi: 10.1182/blood-2008-06-161281. Epub 2008 Dec 3. [DOI] [PubMed] [Google Scholar]
- 70.Jenkinson HJ, Wainwright SD, Simpson KL, Perry AC, Fotiadou P, Holmes CH. Expression of CD1D mRNA transcripts in human choriocarcinoma cell lines and placentally derived trophoblast cells. Immunology. 1999 Apr;96(4):649–55. doi: 10.1046/j.1365-2567.1999.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fiedler T, Walter W, Reichert TE, Maeurer MJ. Regulation of CD1d expression by murine tumor cells: escape from immunosurveillance or alternate target molecules? Int J Cancer. 2002 Mar 20;98(3):389–97. doi: 10.1002/ijc.10141. [DOI] [PubMed] [Google Scholar]
- 72.Konishi J, Yamazaki K, Yokouchi H, Shinagawa N, Iwabuchi K, Nishimura M. The characteristics of human NKT cells in lung cancer--CD1d independent cytotoxicity against lung cancer cells by NKT cells and decreased human NKT cell response in lung cancer patients. Hum Immunol. 2004 Nov;65(11):1377–88. doi: 10.1016/j.humimm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Cabrita M, Pereira CF, Rodrigues P, Cardoso EM, Arosa FA. Altered expression of CD1d molecules and lipid accumulation in the human hepatoma cell line HepG2 after iron loading. FEBS J. 2005 Jan;272(1):152–65. doi: 10.1111/j.1432-1033.2004.04387.x. [DOI] [PubMed] [Google Scholar]
- 74.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999 Oct 15;59(20):5102–5. [PubMed] [Google Scholar]
- 75.Motohashi S, Kobayashi S, Ito T, Magara KK, Mikuni O, Kamada N, Iizasa T, Nakayama T, Fujisawa T, Taniguchi M. Preserved IFN-alpha (gamma) production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002 Nov 10;102(2):159–65. doi: 10.1002/ijc.10678. Erratum in: Int J Cancer. 2003 May 10;104(6):799. [DOI] [PubMed] [Google Scholar]
- 76.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009 Jun;119(6):1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O’Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003 Aug 15;171(4):1775–9. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 78.Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im JS, Alves PM, Martinet O, Halkic N, Cerottini JC, Romero P, Porcelli SA, Macdonald HR, Speiser DE. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009 Apr 15;182(8):5140–51. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 79.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009 Jul;39(7):1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 80.de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, Paganoni AM, Maccario R, Di Cesare-Merlone A, Zecca M, Locatelli F, Dellabona P, Casorati G. Invariant NKT Cell Reconstitution in Pediatric Leukemia Patients Given HLA-Haploidentical Stem Cell Transplantation Defines Distinct CD4+ and CD4- Subset Dynamics and Correlates with Remission State. J Immunol. 2011 Feb 25; doi: 10.4049/jimmunol.1003748. [Epub] [DOI] [PubMed] [Google Scholar]
- 81.Shaulov ASC, Yue R, Wang RM, Joyce SP, Balk HT, Kim DE, Avigan L, Uhl R, Sackstein Exley MA. Peripheral blood progenitor cell product contains Th1-biased non-invariant CD1dreactive natural killer T cells: implications for post-transplant survival. Exp Hematol. 2008;36:464–72. doi: 10.1016/j.exphem.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003 Jul 7;198(1):173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. nflammation-associated lysophospholipids as ligands for CD1drestricted T cells in human cancer. Blood. 2008 Aug 15;112(4):1308–16. doi: 10.1182/blood-2008-04-149831. Epub 2008 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, et al. Innate NKT cells confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–16. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007 Mar 1;178(5):2853–61. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 86.Yue SC, Shaulov A, Wang R, Balk SP, Exley MA. CD1d ligation on human monocytes directly signals rapid NF-kappaB activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yue SC, Wang R, Shaulov A, Balk SP, Exley MA. Direct CD1d-mediated stimulation of APC IL-12 production & protective immune response to virus infection in vivo. J Immunol. 2009;184:268–76. doi: 10.4049/jimmunol.0800924. e-Pub. Nov. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teng MW, Yue S, Sharkey J, Exley MA, Smyth MJ. CD1d activation and blockade: a new antitumor strategy. J Immunol. 2009;182:3366-71. doi: 10.4049/jimmunol.0802964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teng MW, Sharkey J, McLaughlin NM, Exley MA, Smyth MJ. CD1d-based combination therapy eradicates established tumors in mice. J Immunol. 2009;183:1911–20. doi: 10.4049/jimmunol.0900796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baxevanis CN, Gritzapis AD, Papamichail M. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol. 2003 Sep 15;171(6):2953–9. doi: 10.4049/jimmunol.171.6.2953. [DOI] [PubMed] [Google Scholar]
- 91.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 93.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003 Apr 21;197(8):1051–7. doi: 10.1084/jem.20021616. Epub 2003 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006 Sep;119(1):116–25. doi: 10.1111/j.1365-2567.2006.02413.x. Epub 2006 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):3984–9. doi: 10.1073/pnas.0700191104. Epub 2007 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007 Dec;220:183–98. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 97.Lang ML. How do natural killer T cells help B cells? Expert Rev Vaccines. 2009 Aug;8(8):1109–21. doi: 10.1586/erv.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009 Jan;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 99.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol. 1999;163:2387–91. [PubMed] [Google Scholar]
- 100.Gillessen S, Naumov YN, Nieuwenhuis EE, Exley MA, Lee FS, Mach N, Luster AD, Blumberg RS, Taniguchi M, Balk SP, Strominger JL, Dranoff G, Wilson SB. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci U S A. 2003;100(15):8874–9. doi: 10.1073/pnas.1033098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hafner M, Zawatzky R, Hirtreiter C, Buurman WA, Echtenacher B, Hehlgans T, Monnel DN. Antimetastatic effect of CpG DNA mediated by type I IFN. Cancer Res. 2001 Jul 15;61(14):5523–8. [PubMed] [Google Scholar]
- 102.Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 2004 Dec 1;64(23):8754–60. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- 103.Wakita D, Chamoto K, Zhang Y, Narita Y, Noguchi D, Ohnishi H, Iguchi T, Sakai T, Ikeda H, Nishimura T. An indispensable role of type-1 IFNs for inducing CTL-mediated complete eradication of established tumor tissue by CpG-liposome co-encapsulated with model tumor antigen. Int Immunol. 2006 Mar;18(3):425–34. doi: 10.1093/intimm/dxh381. Epub 2006 Jan 13. [DOI] [PubMed] [Google Scholar]
- 104.Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, Krieg AM, Landay AL, Wilson SB. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol. 2006 Jul 15;177(2):1028–39. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 105.Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009 Feb 15;182(4):1846–53. doi: 10.4049/jimmunol.0802492. [DOI] [PubMed] [Google Scholar]
- 106.Sfondrini L, Besusso D, Zoia MT, Rodolfo M, Invernizzi AM, Taniguchi M, Nakayama T, Colombo MP, Monard S, Balsari A. Absence of the CD1 molecule up-regulates antitumor activity induced by CpG oligodeoxynucleotides in mice. J Immunol. 2002 Jul 1;169(1):151–8. doi: 10.4049/jimmunol.169.1.151. [DOI] [PubMed] [Google Scholar]
- 107.van der Vliet HJ, Koon HB, Atkins MB, Balk SP, Exley MA. Exploiting regulatory T cell populations for the immunotherapy of cancer. J ImmunoTherapy. 2007;30:591–5. doi: 10.1097/CJI.0b013e31805ca058. [DOI] [PubMed] [Google Scholar]
- 108.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1dnegative tumors in humans. Clin Immunol. 2010 Nov 20; doi: 10.1016/j.clim.2010.10.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ, Verheul HM, de Gruijl TD, van der Vliet HJ. Clinical experience with α-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2010 Dec 17; doi: 10.1016/j.clim.2010.11.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 110.Dellabona P, Casorati G, de Lalla C, Montagna D, Locatelli F. On the use of donor-derived iNKT cells for adoptive immunotherapy to prevent leukemia recurrence in pediatric recipients of HLA haploidentical HSCT for hematological malignancies. Clin Immunol. 2010 Dec 23; doi: 10.1016/j.clim.2010.11.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Padte NN, Li X, Tsuji M, Vasan S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin Immunol. 2010 Dec 24; doi: 10.1016/j.clim.2010.11.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Motohashi S, Okamoto Y, Yoshino I, Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. 2011 Jan 27; doi: 10.1016/j.clim.2011.01.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Dhodapkar MV, Richter J. Harnessing natural killer T (NKT) cells in human myeloma: progress and challenges. Clin Immunol. 2011 Mar; doi: 10.1016/j.clim.2010.12.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehta AS, Gu B, Conyers B, Ouzounov S, Wang L, Moriarty RM, Dwek RA, Block TM. alpha-Galactosylceramide and novel synthetic glycolipids directly induce the innate host defense pathway and have direct activity against hepatitis B and C viruses. Antimicrob Agents Chemother. 2004 Jun;48(6):2085–90. doi: 10.1128/AAC.48.6.2085-2090.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 116.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosylceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, Hanaoka H, Shimizu N, Suzuki M, Yoshino I, Taniguchi M, Fujisawa T, Nakayama T. A phase I-II study of alpha-galactosylceramide- pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009 Feb 15;182(4):2492–501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 118.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008 Mar;57(3):337–45. doi: 10.1007/s00262-007-0373-5. Epub 2007 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005 Nov 7;202(9):1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006 Oct 15;12:6079–86. doi: 10.1158/1078-0432.CCR-06-0114. Epub 2006 Oct 6. [DOI] [PubMed] [Google Scholar]
- 121.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, Taniguchi M, Nakayama T, Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009 Jun;100(6):1092–8. doi: 10.1111/j.1349-7006.2009.01135.x. Epub 2009 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sykes M, Hoyles KA, Romick ML, Sachs DH. In vitro and in vivo analysis of bone marrow-derived CD3+, CD4-, CD8-, NK1.1+ cell lines. Cell Immunol. 1990 Sep;129(2):478–93. doi: 10.1016/0008-8749(90)90222-d. [DOI] [PubMed] [Google Scholar]
- 123.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003 Jun;9(6):355–63. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 124.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007 May 15;178(10):6242–51. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kohrt HE, Pillai AB, Lowsky R, Strober S. NKT cells, Treg, and their interactions in bone marrow transplantation. Eur J Immunol. 2010 Jul;40(7):1862–9. doi: 10.1002/eji.201040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morris ES, MacDonald KP, Rowe V, Banovic T, Kuns RD, Don AL, Bofinger HM, Burman AC, Olver SD, Kienzle N, Porcelli SA, Pellicci DG, Godfrey DI, Smyth MJ, Hill GR. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. J Clin Invest. 2005 Nov;115(11):3093–103. doi: 10.1172/JCI25249. Epub 2005 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morris ES, MacDonald KP, Kuns RD, Morris HM, Banovic T, Don AL, Rowe V, Wilson YA, Raffelt NC, Engwerda CR, Burman AC, Markey KA, Godfrey DI, Smyth MJ, Hill GR. Induction of natural killer T cell-dependent alloreactivity by administration of granulocyte colony-stimulating factor after bone marrow transplantation. Nat Med. 2009 Apr;15(4):436–41. doi: 10.1038/nm.1948. Epub 2009 Mar 29. [DOI] [PubMed] [Google Scholar]
- 128.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, Laport GG, Stockerl-Goldstein KE, Johnston LJ, Hoppe RT, Bloch DA, Blume KG, Negrin RS, Strober S. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005 Sep 29;353(13):1321–31. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 129.Kohrt HE, Turnbull BB, Heydari K, Shizuru JA, Laport GG, Miklos DB, Johnston LJ, Arai S, Weng WK, Hoppe RT, Lavori PW, Blume KG, Negrin RS, Strober S, Lowsky R. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009 Jul 30;114(5):1099–109. doi: 10.1182/blood-2009-03-211441. Epub 2009 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2008;358:353. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, Ballen K, Delmonico F, Saidman S, Sachs DH, Cosimi AB. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011 Mar 27;91(6):672–6. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saito TI, Li HW, Sykes M. Invariant NKT cells are required for antitumor responses induced by host-versus-graft responses. J Immunol. 2010 Aug 15;185(4):2099–105. doi: 10.4049/jimmunol.0901985. Epub 2010 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Exley MA, Hou R, Shaulov A, Tonti E, Dellabona P, Casorati G, Akbari O, Akman HO, Greenfield EA, Gumperz JE, Boyson JE, Balk SP, Wilson SB. Selective activation, expansion, and monitoring of human iNKT cells with a mAb specific for the TCR α-chain CDR3 loop. Eur J immunol. 2008;20:1756–6. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Field J, Nathan D, Linden J. Targeting iNKT cells for the treatment of sickle cell disease. Clin Immunol. 2011 Mar; doi: 10.1016/j.clim.2011.03.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005 Dec 19;202(12):1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–45. doi: 10.1182/blood-2007-05-092866. Epub 2008 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang W, Li H, Mayhew E, Mellon J, Chen PW, Niederkorn JY. NKT cell exacerbation of liver metastases arising from melanomas transplanted into either the eyes or spleens of mice. Invest Ophthalmol Vis Sci. 2011 Feb 17; doi: 10.1167/iovs.10-7067. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hogan A, O’Reilly V, Dunnea M, Derec R, Zenga S, O’Briena C, Amua S, Fallona P, Exley M, O’Farrelly C, Zhuc X, Doherty D. Activation of human invariant natural killer T cells with a thioglycoside analogue of α-galactosylceramide. Clin Immunol. 2011 April 12; doi: 10.1016/j.clim.2011.03.016. ePub. [DOI] [PubMed] [Google Scholar]