Abstract
Our laboratory has previously demonstrated that viral administration of glial cell line-derived neurotrophic factor (AdGDNF), one week prior to a controlled cortical impact (CCI) over the forelimb sensorimotor cortex of the rat (FL-SMC) is neuroprotective, but does not significantly enhance recovery of sensorimotor function. One possible explanation for this discrepancy is that although protected, neurons may not have been functional due to enduring metabolic deficiencies. Additionally, metabolic events following TBI may interfere with expression of therapeutic proteins administered to the injured brain via gene therapy. The current study focused on enhancing the metabolic function of the brain by increasing cerebral blood flow (CBF) with L-arginine in conjunction with administration of AdGDNF immediately following CCI. An adenoviral vector harboring human GDNF was injected unilaterally into FL-SMC of the rat immediately following a unilateral CCI over the FL-SMC. Within 30 min of the CCI and AdGDNF injections, some animals were injected with L-arginine (i.v.). Tests of forelimb function and asymmetry were administered for 4 weeks post-injury. Animals were sacrificed and contusion size and GDNF protein expression measured. This study demonstrated that rats treated with AdGDNF and L-arginine post-CCI had a significantly smaller contusion than injured rats who did not receive any treatment, or injured rats treated with either AdGDNF or L-arginine alone. Nevertheless, no amelioration of behavioral deficits was seen. These findings suggest that AdGDNF alone following a CCI was not therapeutic and although combining it with L-arginine decreased contusion size, it did not enhance behavioral recovery.
Keywords: traumatic brain injury, growth factors, behavioral assessments, forelimb sensorimotor cortex, controlled cortical impact, cerebral blood flow
1. INTRODUCTION
Traumatic brain injury (TBI) is a complex neurological event that results in the disruption of numerous cellular and physiological processes. In addition to activation of cell death through necrosis and apoptosis, TBI also affects brain metabolism, ionic fluxes, growth factors, neurotransmitter levels, and inflammation (for a review see (Margulies and Hicks, 2009)). The diversity of cellular sequelae and the lack of a neuroprotective drug for TBI point to the need for studies that examine combination therapies in rodent models of TBI. Studies have begun to explore combining cell-based, pharmacological, and rehabilitative therapies in animal models of TBI (Atif et al., 2009; Chen et al., 2008; Griesbach et al., 2008; Mahmood et al., 2008; Xiong et al., 2009). The majority of these studies demonstrate a positive synergistic effect of the combined therapies on both cellular and behavioral outcomes. In the current study, the therapeutic potential of combining neurotrophic factor gene therapy with enhancement of brain metabolism post-injury is examined in the controlled cortical impact (CCI) rodent model of TBI.
Previous work in our laboratory has demonstrated that injection of an adenoviral vector expressing glial cell line-derived neurotrophic factor (AdGDNF) one week prior to a CCI in the adult male rat is neuroprotective, decreasing cortical volume and cell loss by 50% (Minnich et al., 2010). GDNF is a member of the transforming growth factor-β superfamily, and has been shown to protect dopaminergic, motor, hippocampal, and sensorimotor neurons (Giehl et al., 1998; Li et al., 1995; Lin et al., 1993). Choi-Lundberg et al (1997) were the first to demonstrate the clinical potential of adenoviral-delivered GDNF in a rodent model of Parkinson’s disease. Since then, virally-mediated delivery of GDNF has been shown to be a potent neuroprotective factor in animal models of Parkinson’s disease (Bohn, 2000; Connor et al., 1999; Grondin, 2003; Kirik, 2004; Kozlowski et al., 2000), spinal cord injury (Tai, 2003), and stroke (Wang, 2002). In addition to providing neuroprotection, AdGDNF also enhanced behavioral function in these animal models. When AdGDNF was injected prior to CCI it too provided neuroprotection but it did not result in a significant amelioration of behavioral deficits (Minnich et al., 2010). One possible explanation for this discrepancy may have been due to GDNF protein expression not being maintained during the duration of the study, possibly due to metabolic deficiencies in the traumatically injured brain. Virally-mediated expression of trophic factors relies on a cell’s ability to produce protein. Following CCI, protein synthesis is compromised (Lee, 1999), potentially decreasing virally-mediated protein expression. A number of factors play a role in TBI-induced decrease in protein synthesis, one of which is a decrease in cerebral blood flow. It has been well documented that a profound decrease in cerebral blood flow (CBF) occurs immediately after a TBI (for review see (Golding et al., 1999)). Specifically, following a CCI, CBF is decreased focally in the area of the contusion (Bryan et al., 1995; Cherian et al., 1994) and can still be depressed out to one year post-CCI around the contusion cavity (Kochanek et al., 2002). This reduction in CBF may compromise substrate delivery producing a metabolic crisis resulting in decreased protein synthesis and eventually cell death (Hovda, 1996; Lee, 1999). Cerebral blood flow also plays a role in the distribution of viral particles in the parenchyma. Rats with decreased cerebral blood flow showed a significant decrease in the distribution of viral particles within the brain (Hadaczek et al., 2006). Given the role of CBF in both neurodegeneration following TBI and in the delivery of viral vectors, the current study chose to combine AdGDNF (previously used in the CCI model in our laboratory) with a drug long known to enhance CBF, l-arginine (l-arg). The timing of the administration of both AdGDNF and L-arg was within the first hour post-injury. This is different from the previous study that injected AdGDNF one week prior to CCI (Minnich et al., 2010). This change was made so that the administration of both of these agents would be examined in a more clinically relevant time frame.
The amino acid l-arg has been shown to significantly increase CBF following CCI in a dose-dependent way (Cherian et al., 2003; Cherian and Robertson, 2003). In addition to increasing blood flow, l-arg has been shown to significantly decrease contusion volume (Cherian et al., 2003) but its effects on behavioral function have not been examined. This study will focus on enhancing the metabolic function of the brain with l-arg in conjunction with administration of AdGDNF, post contusion, in the CCI rat model of TBI. Together, the expression of a trophic factor in combination with enhanced brain metabolism may result in a smaller contusion and improved behavioral recovery.
2. RESULTS
2.1 Contusion Size
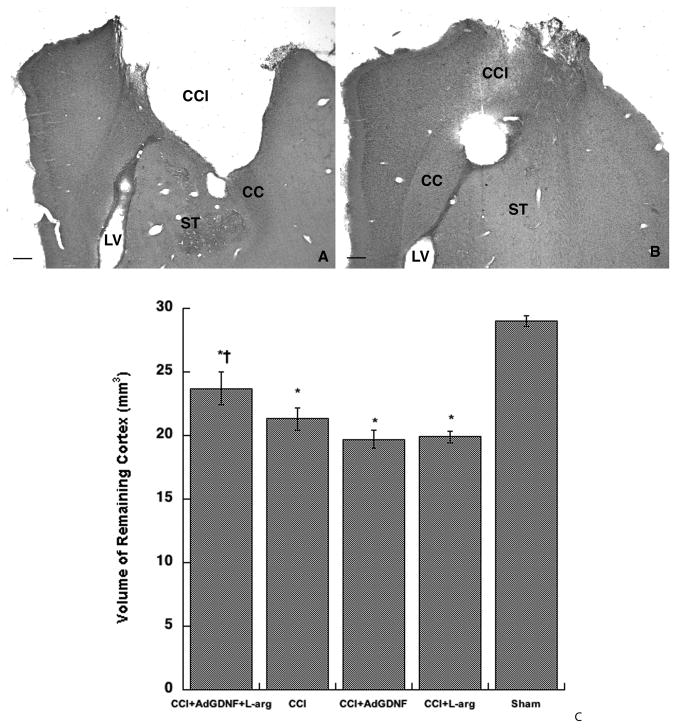
There were no significant differences in the volume of remaining cortex between Sham and Sham+AdGDNF+L-arg groups (p=0.40) and between control CCI groups (i.e. CCI Only, CCI+AdGFP, CCI+Vehicle; p=0.10) as predicted by previous studies (Minnich et al., 2010); therefore, to simplify the presentation of the data, the Sham groups and CCI Control groups were pooled and labeled “Sham” and “CCI”, respectively. Administering both AdGDNF and L-arginine post injury significantly decreased the size of the contusion by approximately 32–44% (See Figure 1). One-way ANOVA indicated a significant difference in volume of remaining cortex between groups (F(4,43)=31.26; p< 0.0001; Figure 1). Post-hoc analysis further revealed that rats treated with the combination of AdGDNF and L-arginine post-CCI had significantly smaller contusions (i.e. a larger volume of remaining cortex) than rats that received no treatment post-CCI (32% smaller), rats treated with L-arginine only post-CCI (44% smaller), or rats treated with AdGDNF only post-CCI (44% smaller; all comparisons = p<0.05). Interestingly, administering AdGDNF alone post-CCI did not have a therapeutic effect. We did not expect that the dose of l-arginine would affect contusion size significantly due to previous findings (Cherian et al., 2003).
Figure 1.
Combining AdGDNF and L-arginine Significantly Reduces Contusion Size. A) Coronal section of an animal that received a CCI, but received no treatment post injury. B) Animal received a CCI and was treated with AdGDNF and L-arginine immediately post-injury resulting in a significantly smaller contusion. CCI=Contusion cavity; CC = Corpus Callosum; ST=Striatum; LV= Lateral Ventricle; Scale bar (A&B) = 250μm C) Volume of Remaining Cortex - Animals treated with AdGDNF and L-arginine post-CCI had significantly smaller contusions (greater amount of remaining cortex) than animals in the CCI group, which did not receive any treatment; as well as injured animals treated with AdGDNF or L-arginine alone (*p<0.05 significantly different from sham; †significantly different from CCI, CCI+L-arg, CCI+AdGDNF p<0.05)
2.2 Foot Fault Test
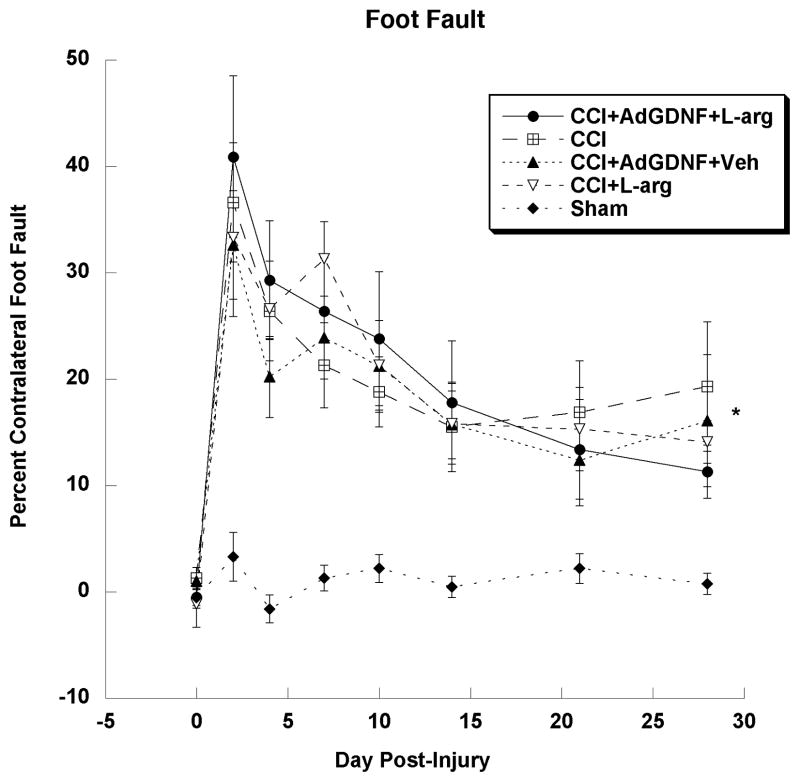
The Foot Fault Test was performed to examine deficits in forelimb motor coordination following an injury to the FL-SMC and to examine behavioral recovery over time. A one-way repeated-measure ANOVA of Sham groups indicated that L-arginine and AdGDNF had no effect on motor coordination in uninjured rats (p=0.76). Therefore, the groups Sham and Sham+AdGDNF+L-arginine were pooled and labeled “Sham”. A one-factor repeated-measure ANOVA of CCI Only, CCI+Vehicle and CCI+AdGFP+Vehicle groups indicated that AdGFP or vehicle had no affect on motor coordination in injured rats (p=0.72). Therefore, the groups CCI Only, CCI+Vehicle and CCI+AdGFP+Vehicle were pooled and labeled “CCI”. Once the groups were pooled, a one-factor repeated- measures ANOVA of all treatment groups indicated a significant effect of Group (F(4,43)=30.792; p<0.0001); as well as a significant effect of Days post-injury (F(7,301)=29.024; p<0.0001), and a significant group by day interaction (F(28,301)=2.36; p<0.002). A Tukey-Kramer post-hoc analysis of the Group effect showed a significant difference between the Sham group and all CCI groups (p<0.05) indicating that all injured groups showed deficits in forelimb coordination compared to uninjured rats (See Figure 2). However, there were no significant differences between any of the injured (CCI) groups. All CCI animals had deficits that began to recover over time, but did not reach Sham levels. Although combining AdGDNF and L-arginine post-injury resulted in a smaller contusion, it did not affect deficits in or recovery of motor coordination.
Figure 2.
Foot Fault Test. All CCI groups had significantly larger deficits in motor coordination than the Sham group (*p<0.05), yet there were no significant differences between the CCI groups. These deficits began to recover over time but did not reach uninjured Sham levels by day 28.
2.3 Limb Use
Observations of forelimb use for weight-bearing movements during exploration of a Plexiglas cage were made in order to examine forelimb preferences and asymmetries following a unilateral contusion to the FL-SMC. Forelimb use is observed during rearing, exploration of the wall and landing. Each of these behaviors is combined to obtain a “Total Ipsilateral Forelimb Preference” score. Typically, unilateral injuries to the FL-SMC result in the preferential use of the ipsilateral (non-impaired) limb (Kozlowski et al., 1996; Minnich et al., 2010). Limb use preferences during exploration of the wall alone (Wall Ipsilateral Forelimb Preference) and all behaviors combined (Total Ipsilateral Forelimb Preference) have been shown in previous studies, to be the most sensitive to effects of contusion on limb use as well as the most sensitive to therapeutic manipulations (Kozlowski et al., 1996; Kozlowski et al., 2000). Therefore, these two measures were analyzed individually. As with the Foot Fault Test, Sham and Sham+AdGDNF+L-arginine groups were pooled and labeled “Sham” as there were no significant affects of AdGDNF and L-arginine on limb use in uninjured rats (Total- p=0.72, Wall- p=0.82). Also, a one-factor repeated-measures ANOVA found AdGFP or Vehicle had no significant effect on limb use in an injured rat (Total = p=0.19, Wall = p=0.37), therefore, the CCI Only, CCI+Vehicle, and CCI+AdGFP+Vehicle group were pooled and labeled “CCI”.
2.3.1 Total Ipsilateral Forelimb Preference
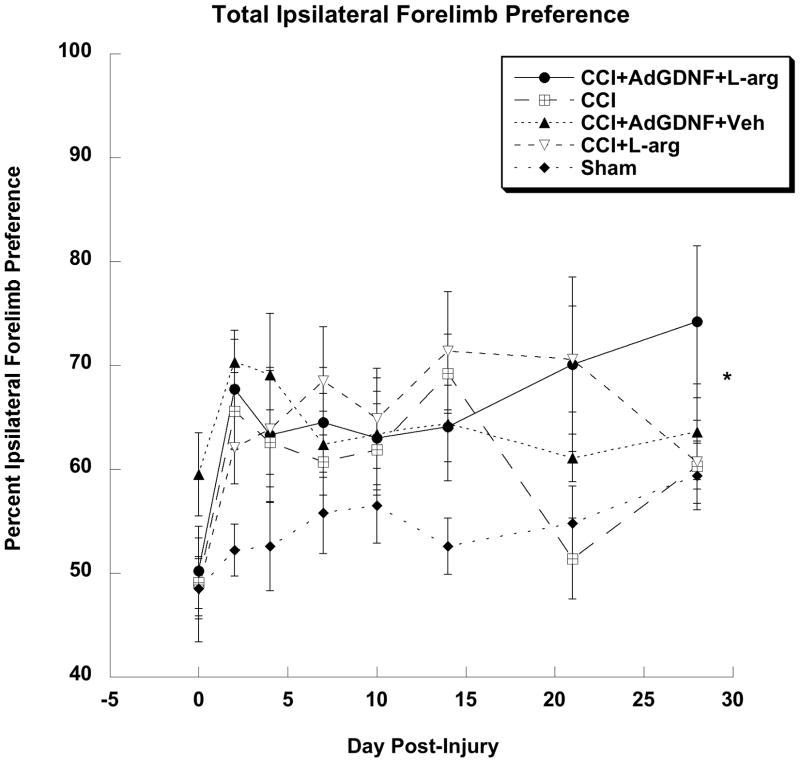
When examining total ipsilateral forelimb preference of all treatment groups, a one-factor repeated-measure ANOVA indicated a significant difference between treatment groups (F(4,43)=4.648; p<0.005); as well as a significant effect of days post-injury (F(7,301)=6.378; p<0.0001), however, no significant group by day interaction was found. A Tukey-Kramer post-hoc analysis of the Group effect showed injured rats treated with AdGDNF, L-arginine, or both preferred the ipsilateral forelimb when compared to shams (p<0.05; Figure 3). However, there were no significant differences between all of the injured rats, which indicate that AdGDNF, L-arginine or the combination of the two did not lessen preferences for the uninjured forelimb.
Figure 3.
Total Ipsilateral Forelimb Preference. Injured animals treated with AdGDNF, L-arginine, or both preferred the ipsilateral forelimb when compared to Shams (*p<0.05). There were no significant differences between the CCI groups.
2.3.2. Wall Ipsilateral Forelimb Preference
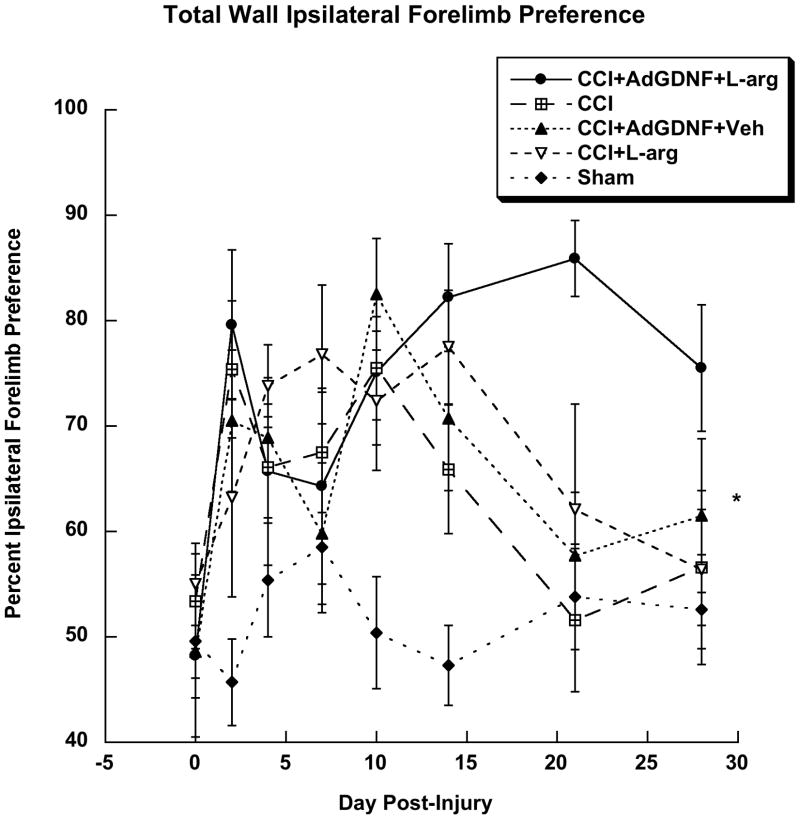
A one-factor repeated-measure ANOVA of percent ipsilateral forelimb preference along the wall indicated a significant difference between treatment groups (F(4,43)=8.108; p<0.0001); as well as a significant effect of Day post-injury (F(7,301)=3.517; p<0.001), and a significant group by day interaction (F(28,301)=1.556; p<0.04). A Tukey-Kramer post-hoc analysis of groups found injured rats that received no treatment, injured rats treated with AdGDNF, L-arginine, or both preferred the ipsilateral forelimb when compared to shams (p<0.05), but no significant difference between the injured groups was found (Figure 4). All injured animals showed asymmetries in bilateral forelimb use that began to recover over time.
Figure 4.
Wall Ipsilateral Forelimb Preference. Injured animals that received no treatment, treated with AdGDNF, L-arginine, or both preferred the ipsilateral forelimb when compared to Shams (*p<0.05). There were no significant differences between CCI groups.
2.4 Expression of Virally-Mediated Proteins – GDNF and GFP
2.4.1 GDNF Expression
2.4.1.1 Qualitative Analysis of Immunohistochemical Staining
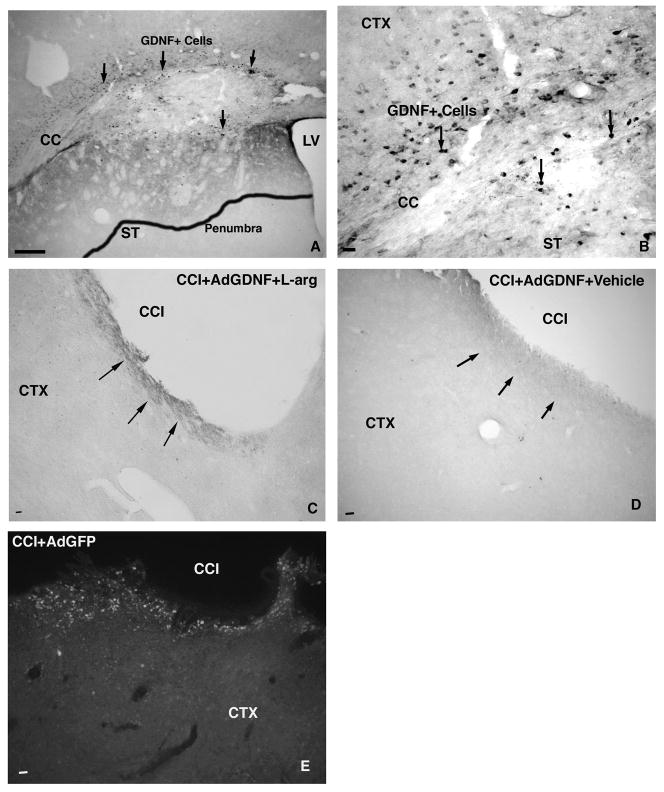
GDNF protein expression was measured qualitatively by observation of GDNF stained tissue using a Leica microscope. Prior to the onset of the current study, two rats, which did not receive an injury, were injected with AdGDNF and sacrificed seven days post injection to ensure that the vector resulted in protein expression (Figure 5A&B). In these rats, a large number of cells (20–24) positively expressed GDNF in the FL-SMC. Surrounding these positive cells was an area of diffuse GDNF staining of the parenchyma, termed the “penumbra”, which is thought to correspond to an area of secreted GDNF protein.
Figure 5.
GDNF expression –GDNF protein expression is robust in Sham animals one week post-injection, producing a penumbra of staining (outlined area in A) and significant numbers of GDNF+ Cells (B). Following injury, GDNF expression decreases significantly, with slightly more expression seen in the animals treated with AdGDNF+L-Arginine (C) versus AdGDNF alone (D). Following injury, GFP+ Cells are still present surrounding the contusion cavity (E). Scale bar - A,C, D, &E = 250μm; B=25 μm. Arrows point to GDNF penumbra and or GDNF+cells. LV=Lateral Ventricle; CC = Corpus Callosum; ST=Striatum; CTX =Cortex; CCI=Contusion cavity
No rats in the current study that were sacrificed 28 days post injection showed this type of GDNF protein expression. GDNF expression decreased in the cortex over time in both injured and uninjured animals. In Sham+AdGDNF rats, GDNF stained cells were seen in and around the injection site, with most sections averaging 7–9 positive GDNF cells. Only one of the five Sham rats exhibited a penumbra. Injured rats treated with both AdGDNF and l-arg averaged 5–6 GDNF positive cells per section around the contusion site. Only three of the seven rats exhibited a penumbra. Injured rats treated with only AdGDNF averaged 2–3 GDNF positive cells per section in and around the contusion. Two of the seven rats did have a very faint penumbra; however, these penumbras were not as dark as those in the other injected rats. Animals that received AdGDNF+l-arg were slightly more likely to exhibit a penumbra and generally expressed a few more cells than the AdGDNF+Vehicle groups. However, with such low GDNF protein expression in all animals, the slight qualitative differences between these groups are most likely not significant.
2.4.1.2. Quantitative Analysis of Tissue Protein Using ELISA
Combining AdGDNF and l-arg resulted in a much smaller contusion size in this study. To examine whether this was due to an enhanced expression of GDNF in the first week post injury, a separate group of rats was studied. The effects of l-arg on virally-mediated GDNF expression at one week post injury was assessed quantitatively to ascertain whether l-arg could perhaps enhance GDNF expression, and whether this might potentially be the mechanism by which neuroprotection was occurring. At one week post-injection, L-arginine did not significantly increase GDNF protein expression (GDNF+L-arg = 3.32±0.67 ng/ml vs. GDNF = 3.7±1.32 ng/ml; (F(1,8)=5.31; p=.76)). Our previous study indicates the level of GDNF in cortical tissue in uninjured animals seven days post-surgery is approximately 0.09–0.12 ng/ml (Minnich et al., 2010). Therefore, although AdGDNF did enhance expression of GDNF above uninjured levels, l-arg did not further increase GDNF protein levels. Therefore, the effects of AdGDNF+L-arg are not due to increased GDNF protein expression at one-week post-CCI.
2.4.2 GFP Expression
GFP protein expression was measured qualitatively using a Leica microscope. All injured rats injected with AdGFP showed robust to moderate expression of GFP protein in and around the contusion site (Figure 5E). Robust expression consisted of dozens of positive cells, while moderate expression consisted of five to ten positive GFP cells. As GFP is not an endogenous protein, it is not secreted, and therefore a penumbra is not expected and was not observed. All rats injected with AdGFP successfully expressed the protein.
3. Discussion
In the quest to find a new therapeutic approach for traumatic brain injury, the goal of this study was to combine a drug which would enhance metabolism by increasing cerebral blood flow (by means of femoral injections of L-arginine) with neurotrophic factor gene therapy (AdGDNF) administered post-CCI and examine their effects on contusion size, behavioral measures of forelimb function, and viral-mediated protein expression. The results of this study demonstrated that AdGDNF combined with L-arginine post injury decreased contusion size although it did not ameliorate behavioral deficits.
Rats treated with AdGDNF and L-arginine post-CCI had a significantly smaller contusion than injured rats who did not receive any treatment, injured rats treated with AdGDNF alone, or injured rats treated with L-arginine alone. Our lab has previously demonstrated that when AdGDNF was injected prior to a CCI, significantly smaller contusions were found when compared to control rats (Minnich et al., 2010). AdGDNF has also been shown to be neuroprotective in animal models of stroke, Parkinson’s disease, cold injury, and spinal cord injury (Choi-Lundberg et al., 1998; Connor et al., 1999; Hermann, 2001; Kozlowski et al., 2000; Tai, 2003; Wang, 2002) when injected before or after the induction of degeneration. However, in the present study, injured rats treated with AdGDNF alone did not benefit from its neuroprotective properties. Rats in this group had significantly larger contusions than injured rats treated with AdGDNF and L-arginine, and contusions similar in size to all other injured groups. In our previous study utilizing AdGDNF in the CCI model, AdGDNF was injected one week prior to the injury. In the present study, AdGDNF was injected within the first 30 min post-CCI. Therefore, it was injected directly into traumatically injured cortex, into the area surrounding the core of a contusion, an environment that is undergoing numerous pathophysiological processes (Margulies and Hicks, 2009). This difference in timing of administration may explain its lack of effect. The environment of an acutely injured cortex may not be suitable for virally-mediated trophic factor expression. The stress of having to undergo metabolic processes necessary to translate, secrete, and utilize the virally-mediated GDNF protein may cause already endangered cells to be challenged past their limit potentially resulting in cell death. Ip et al (2003) demonstrated that a secondary cell death was caused by further stimulating metabolically challenged neurons 1 day after a lateral fluid percussion injury. This study found that if neurons are stimulated to complete energy dependent processes post TBI, such as secreting or utilizing a protein, the additional energy demands will result in their death (Ip et al., 2003). This may explain why injured rats treated with AdGDNF alone post-injury had slightly larger contusions than injured rats without treatment. Alternatively, host immune responses targeted against adenovirus may also play a role in diminishing vector expression along with increased cell death (Thomas et al., 2001). Lastly, the CCI surgery requires both a craniotomy as well as the induction of the brain injury using the impactor. It is known that a craniotomy itself can induce pathological consequences (Cole et al., 2011) potentially exacerbating the effects of the CCI. Therefore, further studies need to be conducted to examine the possible neuroprotective qualities of AdGDNF alone following CCI that include (but are not limited to) injecting the vector into subcortical locations, at different concentrations, and with Sham controls that contain craniotomies.
In the current study, rats treated with only L-arginine post-CCI had significantly larger contusions when compared to rats treated with both L-arginine and AdGDNF and a contusion similar in size to all other injured groups. Previous work has demonstrated L-arginine’s intrinsic capabilities to cross the blood brain barrier, reduce cerebral hypoperfusion, increase cerebral blood flow, all without detrimentally increasing intracranial pressure (Cherian et al., 2003; Cherian and Robertson, 2003; He et al., 1995). Studies have also supported L-arginine’s ability to reduce contusion size post-CCI in the rat and mouse model. When 300 mg/kg of L-arginine was injected i.p. or infused femorally post injury, contusion volume was significantly reduced (Cherian et al., 2003). The present study used L-arginine at a lower dose - 150 mg/kg. A lower dose was chosen in this study because it was previously shown to increase CBF but not significantly reduce contusion volume when compared to untreated rats (although this dose did decrease contusion size to some extent in Cherian et al. 2003, it was not statistically significant due to variability). When choosing combination pharmacological therapies, it is important to choose a dose that has previous shown to effect your therapeutic measure to some extent, but one that does not produce a maximal ceiling effect which could not be overcome by the combination therapy. Therefore, it is not surprising that L-arginine alone at the 150mg/kg dose did not significantly decrease contusion size in the present study.
Taken together, the results suggest that the effect of AdGDNF and L-arginine combined may not necessarily be due to an additive effect of two independently neuroprotective therapies. It was initially hypothesized that increasing CBF with L-arginine would enhance the metabolic environment of the cortex, potentially enhancing the distribution of and expression of virally-mediated GDNF protein. Our study demonstrates that this was not the case. GDNF expression at one week post-injection was not affected by L-arginine. Nevertheless, L-arginine may have prolonged GDNF expression; however, this was not examined in the study quantitatively, although a qualitative examination of GDNF immunohistochemistry suggests this as a possibility. Alternatively, combining AdGDNF with l-arginine may have produced a synergistic inhibition of apoptosis. AdGDNF is known to exert its neuroprotective effects by reducing apoptosis in dopaminergic neurons (Clarkson et al., 1997) and in rat models of ischemia (Kitagawa, 1999; Tsai, 2000), and cold injury (Hermann, 2001). The mechanism by which GDNF inhibits apoptosis include the inhibition of caspase 3 activation (Zhao et al., 2004) activation of Akt via the RET signalling pathway (Akt increases survival promoting proteins such as Bcl-2 and Bcl-xl) (Jin et al., 2002) and decreasing NMDA receptor activity (Nicole, 2001) all of which have been shown to be involved in cell death following TBI (Raghupathi, 2004). L-arginine on the other hand, affects apoptosis through nitric oxide pathways. Nitric oxide (NO) is synthesized from L-arginine by neuronal nitric oxide synthase (NOS) enzymes (Choi et al., 2002). NO has been shown to be both pro-and anti-apoptotic. NO in high quantities has been shown to induce apoptosis whereas more physiological concentrations are antiapoptotic (for review see (Guix et al., 2005)). Its antiapoptotic mechanisms range from inhibition of caspase 3, 1, & 9, inhibition of cytochrome c release, and activation of Akt and the maintainence of Bcl-2 levels (Guix et al., 2005). Although we do not know the direct mechanisms by which the combination of AdGDNF and l-arginine decreased contusion size following CCI, their combined effect may be due to the fact that they both inhibit apoptosis via a variety of different pathways. Combined, their effects may have produced a significant antiapoptotic effect, limiting the size of the contusion formed by the CCI. Future studies, utilizing a time course study of early time points post injury (hours to 2 days) could elucidate whether combining AdGDNF and L-arginine can enhance the inhibition of anti-apoptotic proteins, perhaps those that involve caspases 3, 1, 9 and Akt. Delineation of this mechanism cannot be done in the current study due to its late post-injury time point.
While injured rats treated with AdGDNF and L-arginine had a smaller contusion, this was not accompanied by enhancement of behavioral recovery. A previous study in this lab, in which AdGDNF was injected prior to a CCI also demonstrated a decreased contusion size but not a significant enhancement of overall behavioral recovery, albeit in some behaviors (motor coordination) there was a significant enhancement of function. (Minnich et al., 2010). Numerous studies have also found neural protection in the absence of behavioral enhancement: following GDNF administration in the 6-OHDA lesion model of Parkinson’s disease (Connor et al., 1999; Rosenblad, 2000), fibroblast growth factor delivery to the Schwann cell implanted transected spinal cord (Meijs, 2004) and calbindin D28K gene transfer after an excitotoxic insult (Phillips, 2001). Following TBI, mice overexpressing the anti-apoptotic protein Bcl-2 have significantly smaller contusions and increased hippocampal survival but no changes in motor or cognitive function (Tehranian, 2006). The lack of a correlation between behavioral recovery and cellular neuroprotection may be due to: 1) neurons that were protected but are still in the process of neurodegeneration 2) protected neurons that do not maintain physiological function and 3) protected neuronal cell bodies in the absence of spared neuronal connections to subcortical targets. It is most likely that protected neurons did not maintain physiological function and/or did not maintain functional connections to subcortical targets. In uninjured rodents, motor skill learning was significantly reduced when protein synthesis was repressed by an injection of the protein synthesis inhibitor (anisomycin) into the cortex (Luft et al., 2004). All of this occurred without the presence of cell death. Perhaps depression of protein synthesis, without cell loss, may still be occurring in injured rats treated with AdGDNF and L-arginine, negatively affecting motor skills in the present study. Lastly, it is possible that cells protected by AdGDNF+L-arginine were functional but that they were no longer connected to their subcortical targets. Following a CCI, there is not just cell loss and the formation of a contusion cavity, but also a significant amount of axonal injury (Hall et al., 2008) which leads to retrograde degeneration and loss of cell bodies in striatal and thalamic areas extending also to the contralateral hemisphere (Chen et al., 2003; Hall et al., 2008). It is very possible in the current study that although cell bodies were protected, their functional connections to subcortical targets were not. Neuroprotection of cell bodies but not connections has been previously demonstrated in the 6-OHDA model (Connor et al., 1999; Rosenblad, 2000) and in axotomized rat retinal ganglion cells (Weise, 2000). In the 6-OHDA model, AdGDNF injected into the striatum or substantia nigra resulted in neuroprotection, however preservation of the connections between SN & Striatum by AdGDNF injection into the striatum resulted in enhanced behavioral function (Connor et al., 1999). This emphasizes that when examining the therapeutic potential of an agent, cellular and behavioral neuroprotection are both important. In future studies, examination of axonal injury and functional connections in addition to cell loss will also be important when considering a potential therapeutic manipulation or combination therapy.
Lastly, inadequate GDNF protein expression may have attributed to the lack of behavioral improvement in this study. Both injured and sham rats treated with AdGDNF had decreased expression thirty days post injection (Figure 5). In pilot studies, uninjured rats showed robust GDNF protein expression at one week post-injection and expression at 2-3 weeks post-injection (albeit to a much lesser extent (Minnich et al., 2010)). Using AdGDNF, protein expression was seen in the striatum and substantia nigra of a Parkinsonian rat model from three to seven weeks post injection (Choi-Lundberg et al., 1997; Connor et al., 1999; Connor et al., 2001; Kozlowski et al., 2000) and in the MPTP monkey out to 2 months post-injection (Bohn et al., 1999). This is the first study to inject AdGDNF into the cortex following a CCI. GDNF protein expression was most likely inadequate due to the fact that it was being injected into a metabolically challenged environment of a CCI. Others have introduced viral vectors into the metabolically challenged environment of a stroke (Abe and Hayashi, 1997; Abe et al., 1997; Abe, 1998; Tsai, 2000; Zhang, 2002), however they only examined successful vector expression for hours or days post injury and not out to the time point examined in this study. In the current study, it was the hope that L-arginine would enhance GDNF expression out to 28 days post injury. Although GDNF protein expression seemed slightly increased in L-arginine treated rats, there was still a significant decrease in expression in the injured cortex over time. These findings suggest that perhaps gene therapy should be targeted not to the cortical contusion area but perhaps to a subcortical target like the striatum where GDNF might be picked up and retrogradely transported to the injured cortex. This would allow the vector to work in a more stable environment, perhaps helping to maintain cortical connectivity and eventually ameliorating behavioral deficits.
3.1 Conclusions
This is the first study to deliver AdGDNF following a CCI, to combine AdGDNF with L-arginine as a therapeutic option for TBI, and to examine behavioral results of injured rats treated with L-arginine. Although this study successfully decreased contusion size when treating injured rats with both AdGDNF and L-arginine post-CCI, it did not enhance behavioral recovery. This lack of behavioral recovery may be due to a lack of functional neurons remaining in the protected cortex or to the lack of maintenance of functional connections with subcortical targets. Future studies should focus on targeting subcortical structures for gene therapy before further combination treatment strategies are attempted.
4. EXPERIMENTAL PROCEDURE
4.1 Animals and Treatment Groups
Male Fischer-344 rats (Charles River Laboratories; 225–300g) housed within the DePaul University Animal Facility in clear plastic cages, were kept on a 12:12h light and dark cycle, with food and water available ad-libitum. The NIH Guide for the Care and Use of Laboratory Animals and Institutional Guidelines were strictly adhered to during all procedures. Rats were anesthetized with Equithesin (149 mg/100g chloral hydrate, 31mg/100g sodium pentobarbital i.p.) during all surgical procedures and during euthanasia. Rats were randomly assigned into treatment and control groups: CCI+AdGDNF+L-arginine (n=7), CCI+AdGDNF+Vehicle (n=7), CCI+AdGFP+Vehicle (n=7), CCI+L-arginine (n=8), CCI Only (n=4) CCI+Vehicle (n-=4), Sham+AdGDNF+L-arginine (n=5), Sham Only (n=6), Sham+AdGDNF+Vehicle (n=4). The Sham+ AdGDNF+Vehicle animals (n=4) were added later in the study and were not included in the behavioral analysis. They were only included in the qualitative analysis of GDNF expression, as a comparison for the CCI+AdGDNF+Vehicle group. Additionally, our pilot work (unpublished data) showed that contusion size and behavioral deficits in injured animals were not effected by a femoral injection of saline, therefore an n=4 was used in the CCI and CCI+Vehicle groups to reduce the number of animals in the study, with the intention of pooling the groups once statistical analysis confirmed no group differences.
4.2 Controlled Cortical Impact (CCI)
To inflict a cortical injury, rats were anesthetized, shaved, and placed in a Kopf stereotaxic apparatus (Kopf, Tujunga, CA). A CCI was delivered unilaterally to the forelimb sensorimotor cortex (FL-SMC), through a 4 mm diameter craniotomy at 0.5 mm anterior and 4.0 mm lateral to bregma. The impact was delivered by a controlled cortical impact device, which consisted of a small bore and double acting pneumatic piston. The 3 mm impactor tip was placed at the end of the piston and centered over the craniotomy at an 18° angle. The piston entered the craniotomy at a velocity of 3.0 m/s, to a depth 2.5 mm below the cortical surface for 250 ms (Sutton et al., 1993). Following the CCI, some rats received a vector injection while in others, the wound was sutured, and covered with topical anesthetics and antibiotics. The rats were then placed in a warm home cage for recovery. A sham CCI involved anesthetizing the rat, exposing the skull, and suturing the wound. The body temperature of the rats was maintained at 37C with a heating pad during surgery and during recovery until the rat was ambulatory.
4.3 Vector Injections
Immediately following CCI or Sham surgery, viral vectors were injected into the cortex just medial and lateral to the injury or in corresponding areas in uninjured cortex of the Sham group. First generation adenoviral vectors harboring either human prepro-GDNF (AdGDNF) or green fluorescent protein (AdGFP) were used. The vectors had E1a and E3 deletions, a Rous Sarcoma Virus promotor, and were prepared as previously described (Choi-Lundberg, 1997). Two injections of AdGDNF or AdGFP (2×107 plaque forming units (pfu) total in two-2 μl injections using a 10 μl Hamilton syringe fitted with a 30 gauge flat needle) were administered to the cortex medial and lateral (+0.5 A/P, −2.3 and 4.7 M/L, −1.7 D/V) to the injury within 30 min post-CCI at a flow rate of 0.5μl/min with a Stoelting microinjector (Stoelting, Wood Dale, IL). After the vector injection, the wound was sutured and covered with topical analgesics and antibiotics. The rats were kept at 37C with a heating pad during surgery and recovery until ambulatory.
4.4 L-arginine Injection
In some rats, L-arginine or vehicle was injected into the femoral vein (150 mg/kg in sterile 0.9% saline) within 30 min post-CCI and post vector injections. A dose of 150 mg/kg was chosen due to previous studies demonstrating that this dose increased blood flow but did not result in a significant decrease in contusion size (Cherian et al., 2003; Cherian and Robertson, 2003; He et al., 1995). The left femoral vein was exposed by shaving the anesthetized rat’s hindlimb and thoroughly swabbing the area with alcohol. Through a small incision and with the aid of a dissecting microscope, L-arginine or vehicle (sterile 0.9% saline) was injected into the left femoral vein with a 27.5 gauge needle. The hindlimb incision was then sutured, and topical analgesics and antibiotics were applied.
4.5 Measures of Forelimb Function
Behavioral measures of forelimb function were administered on day 0 (pre-injury and injections) to achieve a baseline, then on days 2, 4, 7, 10,14, 21, and 28 post-injury.
4.5.1 Foot Fault Test
Forelimb coordination was examined using the Foot Fault Test. Rats with a unilateral injury to the FL-SMC exhibit deficits in forelimb coordination contralateral to the injury (Hernandez and Schallert, 1988; Minnich et al., 2010). The rat was placed on a grid made of test tube racks covering an area of 33.02 × 25.40 × 7.62 cm and with openings of 2.54 cm for 4 min. During that time, the number of left and right forelimb “faults” were counted. In order to be considered a “fault” the rat’s forelimb had to fall completely through the 2.54 cm opening. The number of steps the rat took on the grid was also counted during this time. Percent contralateral forelimb faults score was calculated using the following formula: [Contra faults-Ipsi faults/steps]*100.
4.5.2. Limb Use
Limb Use observations were made to examine the symmetrical use of the forelimbs during weight bearing exploratory movements. Rats with unilateral lesions to the FL-SMC typically develop a preference for the forelimb ipsilateral to the injury for exploration and prefer not to use the affected, contralateral forelimb (Kozlowski et al., 1996; Minnich et al., 2010). Limb Use was performed in a 22.86 × 37.46 × 24.13 cm Plexiglas platform. The rat was placed in the platform and videotaped for 5 minutes. The videotape was then observed in slow-motion and incidences of right and left forelimb use for weight-bearing exploratory behaviors were recorded. Data was collected for the following behaviors: rears, in which both forelimbs leave the ground and the rat is at least half way up to a standing position (with and without touching the wall), exploring the wall in either horizontal or vertical movements, and lands onto the floor of the platform following exploration. Ipsilateral preference was first calculated by adding the vertical and horizontal wall behaviors with the ipsilateral limb to obtain wall preference; while a total ipsilateral preference was obtained by adding each individual behavior with the ipsilateral limb(rears, rears before a wall, vertical wall, horizontal wall, lands, and total behavior). A percent Ipsilateral forelimb preference was obtained by taking the sums and dividing by contralateral and ipsilateral limb use (i.e. total limb use) for that behavior, and multiplying by 100 to achieve percent ipsilateral forelimb preference ([I/I+C]*100). This percent ipsilateral forelimb preference was calculated and analyzed for Wall behaviors and Total behaviors separately.
Behavioral observations were completed in all groups except for the Sham+AdGDNF+Vehicle group. This group was added later to study the differences in virally-mediated GDNF protein expression. Previous work in our lab has demonstrated that AdGDNF does not affect the behavior of uninjured rats on these two behavioral tests (Minnich et al., 2010).
4.6 Histology
Twenty-eight days post-injections and CCI the rats were sacrificed by cardiac perfusion with 0.9% saline followed by 4% paraformaldehyde in phosphate buffered saline (PBS). Once the brains were post-fixed at 4°C, and cryoprotected in 30% sucrose in PBS, they were sliced serially into 40 μm coronal sections in sets of 6, spaced 240 μm apart.
4.6.1. Contusion Size Analysis
Measurements of contusion size were obtained by measuring the volume of remaining cortex in H&E stained sections (Minnich et al., 2010). Measurements were conducted in eight sections between 2.7mm anterior to bregma until 2.7mm posterior to bregma. These sections were analyzed using NeuroLucida Software (MicroBrightfield, Williston VT) and a Leica Microscope. For each section, a contour was drawn around the remaining cortex of one hemisphere of the brain using a low power objective and an area obtained. For Shams, a random hemisphere was chosen. For injected Shams and injured rats, the injured or injected hemisphere was chosen. The area of remaining cortex for each section was obtained, added, and multiplied by the distance between successive sections in the set (240μm) to obtain total cortical volume mm3.
4.6.2. GDNF Immunohistochemistry
In order to detect the presence of the virally-induced GDNF protein in the brain tissue of AdGDNF injected rats, GDNF immunohistochemistry (polyclonal rabbit anti-human GDNF (R&D Systems, Minneapolis, MN) 1:250 in 1% NGS, 1% BSA, and 0.5% TX-100 in PBS) was performed as previously described (Minnich et al., 2010). Sections without primary antibody were also processed to verify specificity of staining. Verification of GDNF protein expression was achieved qualitatively using light microscopy.
4.6.3. GFP Expression
GFP expression was measured qualitatively by mounting one set of sections onto slides and coverslipping with FluorSave (Calbiochem, LaJolla, CA). GFP fluorescence was examined in these sections using a Leica fluorescent microscope with a FITC filter.
4.7 Quantitation of Virally-mediated GDNF Protein Expression
To quantitatively examine the effects of L-arginine on the expression of virally-mediated GDNF protein, a separate group of rats was given a CCI, AdGDNF injection and L-arginine as above (CCI+AdGDNF+L-arginine, n=5) and compared to another group of rats given a CCI+AdGDNF but no-L-arginine (n=5). One week post-injury the rats were sacrificed by CO2 inhalation. The cortical area around the injury was quickly dissected, weighed and snap-frozen in liquid nitrogen. Control tissue consisting of the contralateral homotopic cortex and cerebellum from each rat was also dissected, weighed and snap frozen. All tissue was stored at−80C. Protein isolation and GDNF Elisa was performed as described previously (Kozlowski et al., 2001; Minnich et al., 2010). The amount of GDNF protein (ng/ml) of tissue was calculated.
4.8 Statistical Analysis
A one-way ANOVA and Fisher’s protected LSD post-hoc tests were performed on contusion volume and GDNF ELISA data. A repeated measures, one-way ANOVA and Tukey-Kramer post-hoc tests were performed on behavioral data. For contusion volume and behavioral data, planned comparisons were first conducted to demonstrate that there were no differences in Sham groups and no differences in the control groups which received CCI, specifically (CCI+AdGFP, CCI Only and CCI+Vehicle). When no significant differences were found, the groups were pooled and labeled “Sham” or “CCI” respectively. Data are presented as mean ± SEM. Virally-mediated GFP and GDNF expression was examined qualitatively.
Highlights.
This study examines the therapeutic effects of AdGDNF and L-arginine post traumatic brain injury.
AdGDNF and L-arginine were injected into cortex immediately post Controlled cortical impact in the rat.
Contusion size was decreased by the combination but not be each treatment alone.
Behavioral recovery was not affected.
Combining AdGDNF and L-arginine may be beneficial post-injury, but injection site should be re-examined.
Acknowledgments
The authors would like to thank the University of Iowa Gene Transfer Vector Core, supported in part by the NIH and the Roy J. Carver Foundation for viral vector preparations. This study was supported by: NIH R-15 NS42583-01 (D.A.K.), DePaul University Liberal Arts & Sciences Research Grant and University Research Council Grants (D.A.K.)
Abbreviations
- AdGDNF
adenoviral vector harboring glial cell line-derived neurotrophic factor
- AdGFP
adenoviral vector harboring green fluorescent protein
- CCI
Controlled Cortical Impact
- FL-SMC
Forelimb-sensorimotor cortex
- GDNF
Glial Cell Line Derived Neurotrophic Factor
- l-arg
L-arginine
- TBI
Traumatic Brain Injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4.10 References
- Abe K, Hayashi T. Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transcient MCA occlusion. Brain Res. 1997;776:230–234. doi: 10.1016/s0006-8993(97)01041-x. [DOI] [PubMed] [Google Scholar]
- Abe K, Setoguchi Y, Hayashi T, Itoyama Y. In vivo adenovirus-mediated gene transfer and the expression in ischemic and reperfused rat brain. Brain Res. 1997;763:191–201. doi: 10.1016/s0006-8993(97)00389-2. [DOI] [PubMed] [Google Scholar]
- Abe K, Kitagawa H, Setoguchi Y. Temporal profile of adenovirus-mediated E.. coli lacZ gene expression in normal and post-ischemic gerbil hippocampus and ventricle. Neurol Res. 1998;20:689–696. doi: 10.1080/01616412.1998.11740585. [DOI] [PubMed] [Google Scholar]
- Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;9-10:328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MC, Choi-Lundberg DL, Davidson BL, Leranth C, Kozlowski DA, Smith JC, O’Banion MK, Redmond DE. Adenoviral-mediated transgene expression in non-human primate brain. Human Gene Therapy. 1999;10:1175–1184. doi: 10.1089/10430349950018166. [DOI] [PubMed] [Google Scholar]
- Bohn MCC, Kozlowski BDA, Mohajeri MH. Gene transfer for neuroprotection in animal models of Parkinson’s disease and amyotrophic lateral sclerosis. Novartis Foundation Symposium. 2000;231:70–89. doi: 10.1002/0470870834.ch5. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Jr, Cherian L, Robertson C. Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth Analg. 1995;80:687–95. doi: 10.1097/00000539-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87–102. doi: 10.1016/s0014-4886(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Beziaud T, Plotkine M, Marchand-Leroux C. Combination therapy with fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, and simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, on experimental traumatic brain injury. J Pharmacol Exp Ther. 2008;326:966–74. doi: 10.1124/jpet.108.140368. [DOI] [PubMed] [Google Scholar]
- Cherian L, Robertson CS, Contant CF, Jr, Bryan RM., Jr Lateral cortical impact injury in rats: cerebrovascular effects of varying depth of cortical deformation and impact velocity. J Neurotrauma. 1994;11:573–85. doi: 10.1089/neu.1994.11.573. [DOI] [PubMed] [Google Scholar]
- Cherian L, Chacko G, Goodman C, Robertson CS. Neuroprotective effects of L-arginine administration after cortical impact injury in rats: dose response and time window. J Pharmacol Exp Ther. 2003;304:617–23. doi: 10.1124/jpet.102.043430. [DOI] [PubMed] [Google Scholar]
- Cherian L, Robertson CS. L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J Neurotrauma. 2003;20:77–85. doi: 10.1089/08977150360517209. [DOI] [PubMed] [Google Scholar]
- Choi BM, Pae HO, Jang SI, Kim YM, Chung HT. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J Biochem Mol Biol. 2002;35:116–26. doi: 10.5483/bmbrep.2002.35.1.116. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang Y-N, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:338–341. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang Y-N, Chiang YL, Qian J, Bardwaj L, Bohn MC. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Zawada MW, Freed CR. GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell & Tissue Res. 1997;289:207–210. doi: 10.1007/s004410050867. [DOI] [PubMed] [Google Scholar]
- Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard Hb, O’Neill T, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Craniotomy: True sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28(3):359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, Schallert T, Tillerson JL, Davidson BL, Bohn MC. Differential effects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged parkinsonian rat. Gene Therapy. 1999;6:1936–1951. doi: 10.1038/sj.gt.3301033. [DOI] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, Unnerstall JR, Elsworth JD, Tillerson JL, Schallert T, Bohn MC. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp Neurol. 2001;169:83–95. doi: 10.1006/exnr.2001.7638. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Schutte A, Mestres P, Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J Neurosci. 1998;18:7351–7360. doi: 10.1523/JNEUROSCI.18-18-07351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM., Jr The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens. 1999;21:299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–40. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, Zhang A, Ai Y, Gash DM, Gerhardt GA. Intracranial delivery of proteins and peptides as a therapy for neurodegenerative diseases. Progress in Drug Res. 2003;61:101–123. doi: 10.1007/978-3-0348-8049-7_4. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–52. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K. The Perivascular Pump Driven by Arterial Pulsation is a Powerful Mechanism for the Distribution of Therapeutic Molecules within the Brain. Mol Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–47. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- He Z, Ibayashi S, Nagao T, Fujii K, Sadoshima S, Fujishima M. L-arginine ameliorates cerebral blood flow and metabolism and decreases infarct volume in rats with cerebral ischemia. Brain Res. 1995;699:208–13. doi: 10.1016/0006-8993(95)00907-8. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M. Adenovirus-mediated glial cell line-derived neurotrophic factor (GDNF) expression protects against subsequent cortical cold injury in rats. Neurobio of Disease. 2001;8:964–973. doi: 10.1006/nbdi.2001.0448. [DOI] [PubMed] [Google Scholar]
- Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- Hovda DA. Metabolic Dysfunction. In: Narayan RK, Wilberger JE, Povlishock JT, editors. Neurotrauma. McGraw-Hill; New York: 1996. pp. 1459–1478. [Google Scholar]
- Ip EY, Zanier ER, Moore AH, Lee SM, Hovda DA. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23:900–10. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Jin G, Omori N, Li F, Sato K, Nagano I, Manabe Y, Shoji M, Abe K. Activation of cell-survival signal Akt by GDNF in normal rat brain. Brain Res. 2002;958:429–33. doi: 10.1016/s0006-8993(02)03521-7. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson’s disease. Nature Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Sasaki C, Sakai K, Mori A, Mitsumoto Y, Mori T, Fukuchi Y, Setoguchi Y, Abe K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents ischemic brain injury after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1999;19:1336–1344. doi: 10.1097/00004647-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hendrich KS, Dixon CE, Schiding JK, Williams DS, Ho C. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J Neurotrauma. 2002;19:1029–37. doi: 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–86. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski DA, Connor B, Tillerson JL, Schallert T, Bohn MC. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp Neurol. 2000;166:1–15. doi: 10.1006/exnr.2000.7463. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, Bremer E, Redmond DE, Jr, George D, Larson B, Bohn MC. Quantitative analysis of transgene protein, mRNA, and vector DNA following injection of an adenoviral vector harboring glial cell line-derived neurotrophic factor into the primate caudate nucleus. Mol Ther. 2001;3:256–61. doi: 10.1006/mthe.2000.0256. [DOI] [PubMed] [Google Scholar]
- Lee SM, Wong MD, Samii A, Hovda DA. Evidence for energy failure following irreversible traumatic brain injury. Annals NY Acad Sci. 1999;893:337–340. doi: 10.1111/j.1749-6632.1999.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Li LX, Wu WT, Lin L-FH, Lei M, Oppenheim RW, Houenou LJ. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. PNAS. 1995;92:9771–9775. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24:6515–20. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Goussev A, Lu D, Qu C, Xiong Y, Kazmi H, Chopp M. Long-lasting benefits after treatment of traumatic brain injury (TBI) in rats with combination therapy of marrow stromal cells (MSCs) and simvastatin. J Neurotrauma. 2008;25:1441–7. doi: 10.1089/neu.2007.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–39. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijs MFL, Timmers L, Pearse DD, Tresco PA, Bates ML, Joosten EAJ, Bunge MB, Oudega M. Basic fibroblast growth factor promotes neuronal survival but not behavioral recovery in the transected and schwann cell implanted rat thoracic spinal cord. J Neurotrauma. 2004;21:1415–1430. doi: 10.1089/neu.2004.21.1415. [DOI] [PubMed] [Google Scholar]
- Minnich JE, Mann SL, Stock M, Stolzenbach KA, Mortell BM, Soderstrom KE, Bohn MC, Kozlowski DA. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects cortical neurons from dying following a traumatic brain injury. Restor Neurol Neurosci. 2010;28:293–309. doi: 10.3233/RNN-2010-0528. [DOI] [PubMed] [Google Scholar]
- Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, Buisson A. Neuroprotection mediated by glial cell line-derived neurotrophic factor: Involvement of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neuroscience. 2001;21:3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Monje ML, Giuli LC, Meier TJ, Yenari MA, Kunis D, Sapolsky RM. Gene therapy effectiveness differs for neuronal survival and behavioral performance. Gene Therapy. 2001;8:579–585. doi: 10.1038/sj.gt.3301397. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Bjorklund A. Sequential administration of GDNF into the substantia nigra and striatum promotes dopamine neuron survival and axonal sprouting but not striatal reinnervation or functional recovery in the partial 6-OHDA lesion model. Exp Neurol. 2000;161:503–516. doi: 10.1006/exnr.1999.7296. [DOI] [PubMed] [Google Scholar]
- Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: vascular disruption and temporal development of cortical necrosis. J Neurotrauma. 1993;10:135–149. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- Tai MH, Cheng H, Wu JP, Liu YL, Lin Pr, Kuo JS, Tseng CJ, Tzeng SF. Gene transfer of glial cell line-derived neurotrophic factor promotes functional recovery following spinal cord contusion. Exp Neurol. 2003;183:508–515. doi: 10.1016/s0014-4886(03)00130-4. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Rose ME, Vagni V, Griffith RP, Shasha W, Maits S, Zhang X, Clark RSB, Dixon CE, Kochanek PM, Bernard O, Graham SH. Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 2006;1101:126–135. doi: 10.1016/j.brainres.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Tsai T-H, Chen SL, Chiang YH, Lin SZ, Ma HJ, Kuo SW, Tsao YP. Recombinant adeno-associated virus vector expressing glial cell line-derived neurotrophic factor reduces ischemia-induced damage. Exp Neurol. 2000;166:266–275. doi: 10.1006/exnr.2000.7505. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Morales M, Chiang YH, Hoffer J. Protective effects of glial cell line-derived neurotrophic factor in ischemic brain injury. Annals NY Acad Sci. 2002;962:423–437. doi: 10.1111/j.1749-6632.2002.tb04086.x. [DOI] [PubMed] [Google Scholar]
- Weise J, Isenmann S, Klocker N, Kugler S, Hirsch S, Gravel C, Mathias Bahr. Adenovirus-mediated expression of ciliary n eurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobio Disease. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Qu C, Mahmood A, Liu Z, Ning R, Li Y, Kaplan DL, Schallert T, Chopp M. Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. Brain Res. 2009;1263:183–91. doi: 10.1016/j.brainres.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WR, Sato K, Isai M, Nagano I, Manabe Y, Abe K. Therapeutic time window of adenovirus-mediated GDNF gene transfer after transient middle cerebral artery occlusion in rat. Brain Res. 2002;947:140–145. doi: 10.1016/s0006-8993(02)02923-2. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol. 2004;190:356–72. doi: 10.1016/j.expneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]