Abstract
Background.
Despite widespread use, there are no data on initiation of thyroid hormone use in older people. We report the prevalence of thyroid hormone use and predictors of thyroid hormone initiation in a population of older men and women.
Methods.
Thyroid hormone medication data were collected annually from 1989 to 2006 in community-dwelling individuals aged 65 years and older enrolled in the Cardiovascular Health Study (N = 5,888). Associations of age, sex, race, body mass index, education, and coronary heart disease with initiation were evaluated using discrete-time survival analysis.
Results.
In 1989–1990, 8.9% (95% confidence interval 8.1%–9.7%) of participants were taking a thyroid hormone preparation, increasing to 20.0% (95% confidence interval 8.2%–21.8%) over 16 years. The average initiation rate was 1% per year. The initiation rate was nonlinear with age, and those aged 85 years and older initiated thyroid hormone more than twice as frequently as those aged 65–69 years (hazard ratio = 2.34; 95% confidence interval 1.43–3.85). White women were more likely to initiate thyroid hormone than any other race and sex group. Higher body mass index was independently associated with higher risk for initiation (p = .002) as was greater education (p = .02) and prevalent coronary heart disease (p = .03).
Conclusions.
Thyroid hormone use is common in older people. The indications and benefits of thyroid hormone use in older individuals with the highest rate of thyroid hormone initiation—the oldest old, overweight and obese individuals, and those with coronary heart disease—should be investigated.
Keywords: Thyroid hormone, Levothyroxine, Elderly population
HYPOTHYROIDISM is more common in the elderly population, and the classic signs and symptoms found in younger people are often not apparent (1), making this an attractive demographic for biochemical screening. In addition, screening identifies subclinical hypothyroidism, which is found in up to 15% of older people (2). Management of subclinical hypothyroidism is controversial, and expert panels have published guidelines for (3) and against (4) routine treatment of subclinical hypothyroidism. An increase in the use of routine thyroid-stimulating hormone (TSH) screening and recognition of overt and subclinical hypothyroidism would be anticipated to lead to rising use of thyroid hormone supplementation in the elderly population. To date, there have been no studies to determine if an increase in thyroid hormone use over time has occurred. We conducted an analysis of individuals taking thyroid hormone preparations who were enrolled in the Cardiovascular Health Study (CHS), a population-based study of community-dwelling individuals aged 65 years and older. We sought to describe trends in the prevalence of thyroid hormone use and predictors of thyroid hormone initiation in a population of elderly men and women.
METHODS
These analyses are based on data from the CHS (5). The CHS is a population-based, longitudinal study in 5,888 adults 65 years and older. Enrollment of an original cohort of 5,201 adults at four U.S. sites occurred between May 1989 and June 1990, and an additional cohort of 687, predominantly African Americans, was enrolled in 1992–1993. The institutional review boards of all four sites and of the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
Thyroid hormone medication use was assessed annually from Study Year 1 (1989–1990) through Study Year 10 (1998–1999) via medication bottle examination during annual study visits and from Study Years 11 to 17 (1999–2000 to 2005–2006) by annual surveillance phone calls. Body mass index (BMI) was calculated as kg/m2 using objective measures. Diabetes mellitus was classified using the American Diabetes Association criteria (6). Coronary heart disease (CHD) was present if one of the following was reported and confirmed by adjudication: myocardial infarction, angina pectoris, or history of angioplasty or bypass surgery (7). Stroke diagnosis was also confirmed by adjudication.
Statistical Analysis
Study participants’ baseline characteristics were summarized by study cohort, and participants taking a thyroid hormone preparation at baseline were compared with those who were not using a t-test or chi-square test as appropriate. The percentage taking thyroid hormone medication was calculated by year, overall and stratified by sex and race. Annual initiation rates were calculated after exclusion of 508 participants taking thyroid hormone at baseline. Time to initiation was defined as the number of years after baseline that thyroid medication use was first reported. Discrete-time survival analysis was used to evaluate associations of baseline age, sex, race, education, income, smoking, BMI, weight gain, self-reported health, fatigue, diabetes, hypertension, prevalent CHD, stroke, and difficulty in activities of daily living with initiation of use. Only statistically significant (p < .05) variables were retained in the final model. Age and BMI were modeled continuously, and results shown on both continuous and categorical scales, with p values for all variables derived from the continuous model. Participants were censored at the time of their last visit. All analyses were performed using STATA version 9 (Stata Corp., College Station, TX).
RESULTS
The mean age was 72.8 years (range 65–100 years), 58% were women and 84% white. At baseline, thyroid hormone users were more likely to be women, Caucasian, and high school graduates than nonusers were (Table 1). They were less likely to self-report good or excellent health, which was statistically significant in the original cohort, and more likely to have CHD only in the minority cohort.
Table 1.
Characteristics of Cohort by Thyroid Medication Status at Baseline
| Characteristic | Thyroid Medication |
|||
| Original Cohort |
New Cohort |
|||
| No (n = 4737) | Yes (n = 464) | No (n = 643) | Yes (n = 44) | |
| Age, mean (SD) | 72.8 (5.6) | 72.9 (5.4) | 73.0 (5.8) | 73.1 (5.5) |
| Male, n (%) | 2,149 (45.4) | 90 (19.4)‡ | 249 (38.7) | 7 (15.9)† |
| Caucasian, n (%) | 4,476 (94.5) | 449 (96.8)* | 0 | 0 |
| High school graduate, n (%) | 3,393 (71.8) | 356 (77.1)* | 359 (56.0) | 31 (72.1)* |
| Income ≥$25,000, n (%) | 1,946 (41.1) | 194 (41.8) | 137 (21.3) | 10 (22.7) |
| Current smoker, n (%) | 551 (11.6) | 50 (10.8) | 95 (14.8) | 4 (9.3) |
| BMI, kg/m2, mean (SD) | 26.4 (4.5) | 26.7 (4.9) | 28.7 (5.6) | 29.4 (6.4) |
| Good or excellent health, n (%) | 3,643 (77.0) | 336 (72.4)* | 379 (59.2) | 22 (50.0) |
| Diabetes mellitus, n (%) | 714 (15.2) | 74 (16.1) | 153 (25.0) | 12 (27.9) |
| Hypertension, n (%) | 2,687 (56.8) | 255 (55.0) | 479 (74.5) | 36 (81.8) |
| Coronary heart disease, n (%) | 930 (19.6) | 92 (19.8) | 118 (18.4) | 14 (31.8)* |
| Stroke, n (%) | 181 (3.8) | 18 (3.9) | 47 (7.3) | 3 (6.8) |
| Any ADL difficulty, n (%) | 332 (7.0) | 42 (9.1) | 93 (14.5) | 9 (20.5) |
Notes: ADL = activities of daily living; BMI = body mass index.
p < .05; †p < .01; ‡p < .001 for comparison by medication use within cohort.
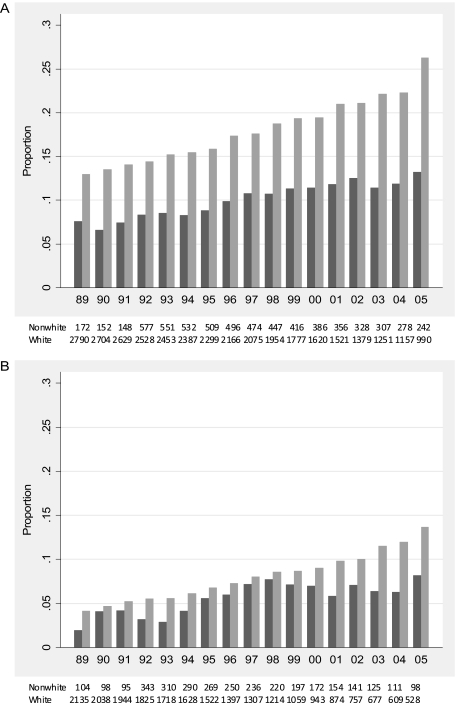
In 1989–1990, 8.9% (95% confidence interval [CI] 8.1%–9.7%) of participants were taking a thyroid hormone preparation, increasing to 20.0% (95% CI 18.2%–21.8%) over 16 years, by 2005–2006. More thyroid hormone use was seen in women than in men, with a greater proportion of users at each year between 1989 and 1990 and 2005 and 2006 (Figure 1), and a greater proportion of users in whites than in nonwhites. At initiation of the study, 12.9% (95% CI 11.7%–14.2%) of white women were taking thyroid hormone, increasing to 26.3% (95% CI 23.5%–29.0%) by 2005–2006. In nonwhite women, 7.6% (95% CI 3.6%–11.5%) were taking thyroid hormone at enrollment, increasing to 13.2% (95% CI 8.9%–17.5%) by 2005–2006. Despite the lower proportion of thyroid hormone use across all years, white and nonwhite men also demonstrated a trend of increasing thyroid hormone use over calendar time. Only 4.1% (95% CI 3.3%–5.0%) of white men and 1.9% (95% CI 0.01%–4.1%) of nonwhite men were taking a thyroid hormone preparation at baseline, increasing to 13.6% (95% CI 10.7%–16.6%) and 8.2% (95% CI 2.7%–13.6%), respectively, by the end of follow-up.
Figure 1.
Proportion of participants taking thyroid hormone medication by calendar year: (A) white and nonwhite women and (B) white and nonwhite men. Black bars indicate nonwhites, gray bars whites.
After excluding the 508 thyroid medication users at baseline, there was a rate of initiation of 0.6%–1.4% per year among nonusers, with a total of 498 people initiating thyroid hormone after baseline. The average rate of initiation was 1% per year and without a consistent linear trend with time. The average age at thyroid hormone initiation was 79.7 ± 6.4 years. However, the association with age was not linear, with a higher crude incidence rate, at 1.50% per year, in those aged 85 years and older at baseline than in the younger age groups, in which the crude incidence rate varied from 0.80% to 0.88% per year. In multivariable analyses (Table 2), those aged 85 years and older at baseline were more than twice as likely to initiate thyroid hormone (hazard ratio 2.34; 95% CI 1.43–3.85) than those aged 65–69 years. White women were more likely to initiate thyroid hormone than any other race and sex group (overall p value <.001), with no significant difference in rates of thyroid hormone initiation among white men, nonwhite women, and nonwhite men. Thyroid hormone initiation was higher in those whose BMI was above 25 kg/m2 than below, achieving statistical significance for the overweight (hazard ratio 1.26; 95% CI 1.03–1.54) and extremely obese (hazard ratio 2.72; 95% CI 1.45–5.10) groups. Initiation of thyroid hormone was also independently associated with more years of education (p = .022) and CHD at baseline (p = .028).
Table 2.
Hazard Ratios (95% confidence intervals [CI]) From Discrete-Time Survival Analysis of Initiation of Thyroid Medication Use
| Characteristic | n | Hazard Ratio | 95% CI | p Value* |
| Age at baseline, y | — | 1.02 | 1.00–1.04 | .019 |
| 65—69 | 1,825 | 1.00 | Reference | — |
| 70—74 | 1,664 | 1.05 | 0.85–1.30 | — |
| 75—79 | 1,051 | 1.05 | 0.81–1.36 | — |
| 80—84 | 493 | 1.26 | 0.88–1.82 | — |
| 85+ | 188 | 2.34 | 1.43–3.85 | — |
| Sex/race group | — | — | — | <.001 |
| White women | 2,385 | 1.00 | Reference | — |
| White men | 1,972 | 0.61 | 0.50–0.75 | — |
| Nonwhite women | 535 | 0.42 | 0.28–0.63 | — |
| Nonwhite men | 329 | 0.52 | 0.32–0.85 | — |
| BMI, kg/m2 | — | 1.03 | 1.01–1.05 | .002 |
| Normal (<25.0) | 2,005 | 1.00 | Reference | — |
| Overweight (25.0–29.9) | 2,170 | 1.26 | 1.03–1.54 | — |
| Obese (30.0–39.9) | 959 | 1.25 | 0.96–1.62 | — |
| Extremely obese (>40.0) | 68 | 2.72 | 1.45–5.10 | — |
| Education, y | — | 1.03 | 1.00, 1.07 | .022 |
| CHD at baseline | 988 | 1.31 | 1.04, 1.65 | .028 |
Notes: BMI = body mass index; CHD = coronary heart disease.
The p values shown are derived from a model in which each variable was modeled continuously. Results for age and BMI using categorical scales are also displayed.
CONCLUSIONS
We found a high proportion of thyroid hormone users in our cohort of community-dwelling individuals aged 65 years and older, particularly among white women. Our study is the first to follow patients over an extended time frame, demonstrating a steady trend in thyroid hormone initiation. We also provide new demographic information about who initiates thyroid hormone, with age 85 years or older, being a white woman, more years of education, high BMI, and prevalent CHD independently predicting thyroid hormone initiation over our 16-year time frame of study.
Our study demonstrates a similar prevalence of thyroid hormone use by sex and race to that reported in the 70- to 79-year-olds enrolled in the Health, Aging, and Body Composition Study in 1997–1999 and to a white, community-dwelling population of women aged 65 years or older enrolled in the Study of Osteoporotic Fractures in 1986–1988, during each of these time frames (8,9). Hashimoto’s thyroiditis is more common in women than in men, and thus, our finding of greater thyroid hormone use in women is not surprising and parallels this sex difference in indication for prescription of thyroid hormone. The differences we found in thyroid hormone use by sex and race correspond to reported findings of demographic differences in TSH distribution (10–13) and suggest that bias in screening practices by sex and race likely play a minor role. We also found a higher thyroid hormone initiation rate with increasing educational level and with CHD, which could suggest higher screening rates in more educated individuals and in those with cardiovascular disease.
We found that a BMI above normal is also associated with increased initiation of thyroid hormone preparations in this elderly cohort. A higher prevalence of subclinical hypothyroidism has been shown in obesity (14), and it is likely that individuals with concerns about their weight were more likely to have thyroid function testing performed and, in turn, to be prescribed thyroid hormone replacement. Interestingly, weight loss after bariatric surgery has been shown to reverse subclinical hypothyroidism in obese younger individuals (15). These data suggest that obesity depletes thyroid reserves, resulting in subclinical hypothyroidism, rather than the converse effect of mild thyroid dysfunction inducing weight gain.
Our most intriguing finding is a higher rate of thyroid hormone initiation among those aged 85 years or older that is independent of sex or race. Although it is possible that there is an increase in overt hypothyroidism in this age-group, the more likely explanation is prescription of thyroid hormone for treatment of subclinical hypothyroidism, which is present in 14.5% of the population of men and women aged 80 years and older (10). However, the benefits of thyroid hormone supplementation of subclinical hypothyroidism are unclear in this age-group. Data from the Leiden 85+ study show lower mortality in 85-year-old men and women with elevated TSH levels compared with their euthyroid counterparts and no difference in functional status (16). Offspring of nonagenarians tend to have higher TSH levels than their partners do, also suggesting a favorable effect of slower thyroid metabolism on longevity (17). A small study of nonagenarians showed no association between TSH level and mortality, although only 4% had elevated TSH levels (18). Furthermore, we have previously found in CHS that overreplacement with thyroid hormone is common in older people (19), and in Study of Osteoporotic Fractures, thyroid hormone use was independently associated with greater declines in lower extremity performance (20). Furthermore, we and others have previously found that overreplacement with thyroid hormone is common in older people (21,22), and others have shown adverse cardiac, skeletal, and cognitive effects in older people with low TSH levels (23–25). In aggregate, these data suggest the need to define a target TSH for thyroid hormone initiation, risk–benefit ratio of thyroid hormone replacement, and therapeutic goals that are specific to the 85 years and older age-group. This need is only highlighted by the fact that those aged 85 years and older are the fastest growing demographic group (26), and a group in whom polypharmacy is a serious problem (27,28).
A major strength of this study is the use of a large, population-based cohort of older men and women to examine trends in thyroid hormone replacement over 16 years. Thyroid function testing was not performed in the CHS main study, and when it was performed using banked samples, results were never released to participants or their physicians. Thus, participation in CHS should not have influenced the prescribing patterns of participant’s physicians. Limitations of our study include the lack of information on thyroid function testing prior to thyroid hormone initiation or the prescriber’s indication for thyroid hormone prescription, and use of baseline covariates in the models. We were also unable to provide data after the 2005–2006 participant phone call.
Implications
Levothyroxine sodium was the fourth most commonly dispensed medication in the United States in 2008 (29). Mild TSH elevations increase in prevalence with increasing age, particularly in those aged 70 years or older (30). The management of subclinical hypothyroidism in the elderly population is controversial, with observational studies largely showing no harm in those with TSH levels lower than 10 mU/L (31,32) and no data from randomized clinical trials with clinical outcomes. Our data support the need to further investigate the threshold TSH level for thyroid hormone initiation and benefits of thyroid hormone use in the elderly population, particularly in the oldest old (aged 85 years and older), overweight and obese individuals, and those with CHD, who have the highest rates of thyroid hormone initiation.
FUNDING
This work was supported by the National Institute on Aging at the National Institutes of Health (R01-AG032317). The Cardiovascular Health Study was supported by contract numbers N01-HC-80007, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and grant number U01 HL-080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01-AG-015928, R01-AG-020098, R01-AG-023629, and R01-AG-027058 from the National Institute on Aging; R01 HL-075366 from the National Heart, Lung, and Blood Institute; and P30-AG-024827 from the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Doucet J, Trivalle C, Chassagne P, et al. Does age play a role in clinical presentation of hypothyroidism? J Am Geriatr Soc. 1994;42:984–986. doi: 10.1111/j.1532-5415.1994.tb06592.x. [DOI] [PubMed] [Google Scholar]
- 2.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–585. doi: 10.1210/jc.2004-1231. [DOI] [PubMed] [Google Scholar]
- 4.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 6.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya AM, Harris F, Volpato S, Perez-Stable EJ, Harris T, Bauer DC. Association between thyroid dysfunction and total cholesterol level in an older biracial population: the health, aging and body composition study. Arch Intern Med. 2002;162:773–779. doi: 10.1001/archinte.162.7.773. [DOI] [PubMed] [Google Scholar]
- 9.Bauer DC, Rodondi N, Stone KL, Hillier TA. Thyroid hormone use, hyperthyroidism and mortality in older women. Am J Med. 2007;120:343–349. doi: 10.1016/j.amjmed.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 11.Boucai L, Surks MI. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 2009;70:788–793. doi: 10.1111/j.1365-2265.2008.03390.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab. 2008;93:1224–1230. doi: 10.1210/jc.2006-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagchi N, Brown TR, Parish RF. Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med. 1990;150:785–787. [PubMed] [Google Scholar]
- 14.Asvold BO, Bjoro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023–5027. doi: 10.1210/jc.2009-1180. [DOI] [PubMed] [Google Scholar]
- 15.Chikunguwo S, Brethauer S, Nirujogi V, et al. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis. 2007;3:631–635. doi: 10.1016/j.soard.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Gussekloo J, van EE, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 17.Rozing MP, Westendorp RG, de Craen AJ, et al. Low serum free triiodothyronine levels mark familial longevity: the Leiden Longevity Study. J Gerontol A Biol Sci Med Sci. 2010;65:365–368. doi: 10.1093/gerona/glp200. [DOI] [PubMed] [Google Scholar]
- 18.Formiga F, Ferrer A. Thyrotropin serum values and 3-year mortality in nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:1250–1251. doi: 10.1093/gerona/glq076. [DOI] [PubMed] [Google Scholar]
- 19.Simonsick EM, Newman AB, Ferrucci L, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169:2011–2017. doi: 10.1001/archinternmed.2009.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest KY, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: a 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61:1194–1200. doi: 10.1093/gerona/61.11.1194. [DOI] [PubMed] [Google Scholar]
- 21.Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94:1342–1345. doi: 10.1210/jc.2008-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diez JJ. Hypothyroidism in patients older than 55 years: an analysis of the etiology and assessment of the effectiveness of therapy. J Gerontol A Biol Sci Med Sci. 2002;57:M315–M320. doi: 10.1093/gerona/57.5.m315. [DOI] [PubMed] [Google Scholar]
- 23.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 24.Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ceresini G, Lauretani F, Maggio M, et al. Thyroid function abnormalities and cognitive impairment in elderly people: results of the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57:89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Census Bureau. U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin. 2004. U.S. Census Bureau Web site. http://www.census.gov/ipc/www/usinterimproj. Accessed March 18, 2004. [Google Scholar]
- 27.Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170–175. doi: 10.1093/gerona/61.2.170. [DOI] [PubMed] [Google Scholar]
- 28.Bernabei R, Caputi A, Di CL, et al. Need for redesigning pharmacologic research in older individuals. A position statement of the Geriatric Working Group of the Agenzia Italiana del Farmaco (AIFA) J Gerontol A Biol Sci Med Sci. 2011;66:66–67. doi: 10.1093/gerona/glq179. [DOI] [PubMed] [Google Scholar]
- 29.IMS Health. IMS National Sales Perspectives. 2009. www.imshealth.com. Accessed August 10, 2010. [Google Scholar]
- 30.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 31.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health Study. J Am Coll Cardiol. 2008;52:1152–1159. doi: 10.1016/j.jacc.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]