Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that are critical for induction of adaptive immunity and tolerance. Traditionally DCs have been divided into two discrete subtypes, which comprise conventional and non-conventional DCs. They are distributed across various organs in the body and comprise a heterogeneous population, which has been shown to display differences in terms of surface marker expression, function and origins. Recent studies have shed new light on the process of DC differentiation and distribution of DC subtypes in various organs. Although monocytes, macrophages and DCs share a common macrophage–DC progenitor, a common DC progenitor population has been identified that exclusively gives rise to DCs and not monocytes or macrophages. In this review, we discuss the recent advances in our understanding of DC differentiation and subtypes and provide a comprehensive overview of various DC subtypes with emphasis on their function and origins. Furthermore, in light of recent developments in the field of DC biology, we classify DCs based on the precursor populations from which the various DC subsets originate. We classify DCs derived from common DC progenitor and pre-DC populations as conventional DCs, which includes both migratory and lymphoid-resident DC subsets and classify monocyte-derived DCs and plasmacytoid DCs as non-conventional DCs.
Keywords: dendritic cells, lymphoid dendritic cells, monocyte-derived dendritic cells, T cells, tolerance
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells and are essential mediators of immunity and tolerance. They were first discovered in 1973 as a novel cell population in mouse spleen that was clearly distinct from macrophages.1–5 Similar to other leucocytes, DCs are derived from haematopoietic stem cells. Although, the pathways leading to generation of DCs were not completely understood, recent findings have shed light on DC ontogeny.6–8 During haematopoiesis, haematopoietic stem cells give rise to common myeloid progenitors (CMP) and common lymphoid progenitors (CLP), with monocytes, macrophages, megakaryocytes, granulocytes and erythrocytes originating from CMP and T cells, B cells and natural killer cells, originating from CLP.9,10 Studies have documented that injection of purified CLP as well as CMP into irradiated mice results in their differentiation into DCs.11 Recently, it has also been shown that human multi-lymphoid progenitors can give rise to all lymphoid cell types along with monocytes, macrophages and DCs.12 Although DCs are traditionally thought to be of myeloid origin, these studies indicate that even lymphoid progenitors can give rise to DCs. However, in a recent study by Schlenner et al.,13 fate mapping strategy was used to identify that under steady-state conditions most of the DCs are derived from the myeloid lineage.
Dendritic cells are comprised of a heterogeneous population of cells with DCs in various organs possessing unique sets of cell surface markers. Moreover, different routes of DC differentiation from precursors add another layer of complexity to the heterogeneity of DC populations. It has become evident that many distinct DC subtypes exist, each with a particular location and a specialized function in the immune system.14 In this review we discuss the recent advances in our understanding of DC differentiation and DC subtypes with an emphasis on classification of various murine DC subsets as conventional or non-conventional DCs based on their ontogeny.
Differentiation/origin of dendritic cells
Over the last decade, studies have established the potential of monocytes to differentiate into DCs. Monocytes are circulating leucocytes that were classically known as precursors to macrophages. Mouse monocytes have been classified into two subsets: Ly6Chigh monocytes, which are CX3CR1low, CCR2+, CD62L+, and CCR5−, and Ly6Clow monocytes, which are CX3CR1high, CCR2−, CD62L−, and CCR5+.15 Previous studies indicated that it is particularly during inflammation that DCs arise from monocytes. However, recent findings challenge the notion and instead indicate that even during steady-state conditions DCs can arise from monocytes. Ly6Chigh monocytes have been shown to give rise to CD103− DCs in the intestinal lamina propria under steady-state conditions.16,17 The Ly6Clow monocytes are thought to play a tolerogenic role and recent evidence indicates that they can be differentiated into DCs in vitro upon culturing with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).18 Moreover, in vivo studies indicate that injection of apoptotic thymocytes results in their uptake by Ly6Clow monocytes, which subsequently migrate to the spleen and differentiate into immunosuppressive DCs.18,19 It is important to note that adoptive transfer of purified monocytes under steady-state conditions to mice has failed to reconstitute the entire DC repertoire, whereas upon induction of inflammation using complete Freund's adjuvant monocytes have been able to differentiate into certain DC subsets.20 Therefore, monocytes cannot be regarded as the absolute precursors to conventional DCs but probably differentiate into specialized DC subsets under specific conditions.
The common precursor to macrophages, monocytes and DCs is the macrophage-DC progenitor (MDP) which is classified as Lin− CX3CR1+ CD11b− CD115+cKit+ CD135+.6 The MDP is derived from CMP and only gives rise to monocytes, macrophages and DCs.6 The MDP probably differentiates into a DC-restricted progenitor, called the common DC progenitor (CDP), which exclusively gives rise to DCs but not monocytes or macrophages.7 Although both MDP and CDP reside exclusively in the bone marrow, a precursor DC population (pre-DCs), derived from CDP, has been identified in bone marrow, blood, spleen and lymph nodes, which comprise < 0·05% of the leucocytes in respective tissues.7,8 These pre-DCs have been shown to migrate to lymphoid tissues through the blood and undergo proliferation and differentiation into DCs.7 Therefore CMPs give rise to MDPs, which give rise to CDPs, which subsequently give rise to pre-DCs, which function as immediate precursors to DCs. Figure 1 provides a schematic for differentiation of DCs from precursors.
Figure 1.
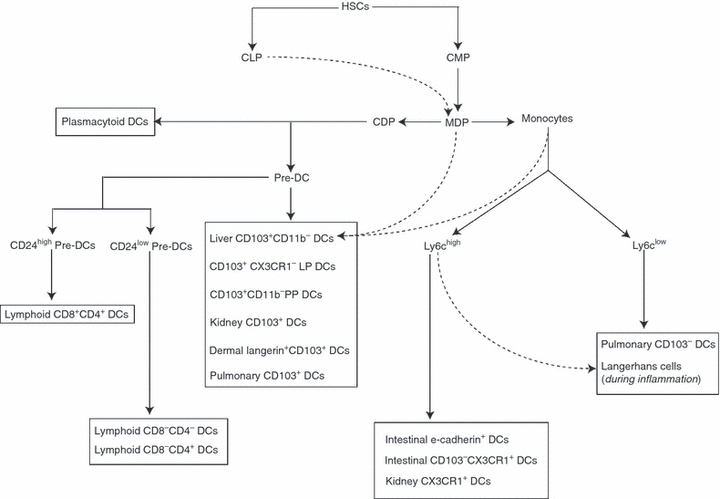
Differentiation of dendritic cells (DCs) from haematopoietic stem cells (HSC). The HSCs differentiate into common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs); CMPs subsequently differentiate into monocytes and pre-DCs in the bone marrow. Subsequently, monocytes and pre-DCs enter the blood and migrate to lymphoid organs and peripheral tissues, where they give rise to lymphoid DCs and tissue-resident DCs. In addition to CMPs, CLPs also have the potential to give rise to DCs, but their contribution is not well understood.
Although the myeloid origin of DCs has been established, the lymphoid origins of DCs from CLPs cannot be ignored. Recently, studies have identified that induction of Toll-like receptor 9 (TLR9) via CpG DNA on CLPs promotes the generation of DCs.21 It has also been shown that induction of TLR4 signalling via lipopolysaccharide treatment of CLPs promotes DC differentiation.22 Flt3, a receptor tyrosine kinase, is involved in haematopoiesis and although it is not needed for the generation of CDPs in bone marrow, it plays a role in DC development in peripheral tissue along with DC homeostasis and expansion.23 It is particularly important for development of plasmacytoid DCs along with CD8+ DCs and CD103+ DCs and functions by signalling through the mammalian target of rapamycin (or mTOR) pathway.24 Studies have indicated that adoptive transfer of CLPs followed by injection of Flt3L drives DC differentiation from CLPs, which indicates that CLPs do have the potential to differentiate into DCs but still does not address whether under steady-state conditions, CLPs act as precursors to DC populations.25 Therefore, it is likely that under certain conditions, certain subtypes of DCs can be derived from lymphoid progenitors as well. Table 1 provides an overview of the various DC populations and their precursors.
Table 1.
Dendritic cell subtypes and their precursors
| Dendritic cell subtypes | Origin |
|---|---|
| Lung | |
| CD103+ CD11chi CD11b− DCs | Pre-DCs |
| CD103− CD11chi CD11b+ DCs | Monocytes |
| Plasmacytoid DCs | Pre-DCs? |
| Intestinal tract | |
| LP CD103+ CX3CR1− CD11b+ DCs | Pre-DCs |
| LP CD103− CX3CR1+ CD11b+ DCs | Monocytes |
| PP CD103+ CX3CR1+ DCs | Pre-DCs |
| E-cadherin+ DCs | Monocytes |
| Liver | |
| CD103+ CD11b− DCs | Pre-DCs |
| CD103− CD11b+ DCs | Monocytes |
| Plasmacytoid DCs | Pre-DCs |
| Kidney | |
| CX3CR1+ CD11b+ DCs | Monocytes |
| CX3CR1+ CD11b− DCs | Monocytes or Pre-DCs? |
| CD103+ CX3CR1− DCs | Pre-DCs |
| Lymphoid DCs | |
| CD8+ CD4+ DCs | Pre-DCs |
| CD8− CD4− DCs | Pre-DCs |
| CD8− CD4+ DCs | Pre-DCs |
| Skin | |
| Langerhans cells | Yolk sac primitive macrophages, monocytes |
| Dermal langerin− CD103− DCs | Monocytes or Pre-DCs? |
| Dermal langerin+ CD103+ DCs | Pre-DCs |
DC, dendritic cell; LP, lamina propria; PP, Peyer's patches.
Dendritic cell subtypes
Dendritic cells were initially broadly classified into two groups, which include the steady-state conventional DCs and non-conventional DCs.26 Steady-state conventional DCs were regarded as having a DC form and function, whereas non-conventional DCs were DCs that were usually not seen in steady state but that arose in response to inflammatory stimuli. Non-conventional DCs initially included plasmacytoid DCs and monocyte-derived DCs.7,8,14,26 However, the identification of DC subsets that are monocyte-derived but arise in the absence of inflammation under steady-state conditions further complicates DC classification. As DCs have multiple routes of development, those which arise from pre-DCs with a classical DC function can be regarded as conventional DCs, whereas non-conventional DCs can include monocyte-derived DCs along with plasmacytoid DCs, which although are derived from CDP and not monocytes are distinct in their function. Figure 2 provides a classification of various DC subtypes as conventional or non-conventional DCs.
Figure 2.
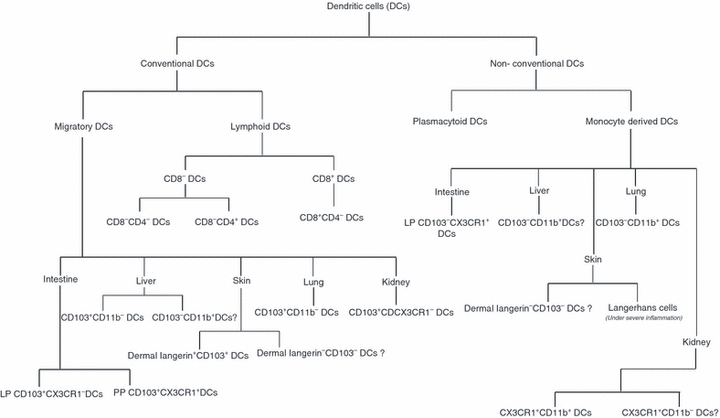
Classification of dendritic cell (DC) subsets as conventional and non-conventional DCs. Conventional DCs are derived from common DC progenitor and pre-DC populations and are further divided into migratory and lymphoid DCs. Non-conventional DCs include plasmacytoid DCs, which are derived from the pre-DC population along with monocyte-derived DC subsets, and are found in various peripheral organs.
Conventional steady-state DCs
Conventional DCs are comprised of DC subsets derived from CDP and pre-DCs and can be further divided into migratory and lymphoid DCs. Migratory DCs have the ability to migrate from peripheral tissues to lymphoid organs, whereas lymphoid DCs reside in the lymphoid organs and lack migratory function. Migratory DC subsets include DC subsets found in the skin, lung, intestinal tract, liver and kidneys. Lymphoid DCs are found in lymphoid organs such as lymph nodes, spleen and thymus and have been further divided depending on the varied expression of CD4 and CD8.
Migratory DCs
Migratory DCs are derived from CDPs and pre-DCs and reside in peripheral tissues such as skin, lung, intestinal tract, liver and kidney. Migratory DCs are characterized by their unique ability to acquire antigen and subsequently migrate to the draining lymph nodes, where interaction with T cells takes place.
Skin
Skin DCs include Langerhans cells (LCs) and dermal DCs, which participate in the immune response against pathogens that gain access to the epidermis and the dermis layers. These DCs have slow turnover with half-life of > 21 days for LCs and approximately 12 days for dermal DCs. Dermal DCs includes dermal langerin+ CD103+ DCs and langerin− CD103− DCs. CD103 corresponds to integrin αE, which is expressed on a subset of effector CD8+ T cells and CD4+ and CD8+ regulatory T (Treg) cells along with DC subsets.27 Dermal langerin+ DCs can be classified as conventional DCs, derived from bone marrow precursors with pre-DC precursor as the likely precursor for this dermal DC subset.28 However, the origins of dermal langerin− CD103− DCs are not well understood.
Dermal langerin+ CD103+ DCs are the most efficient subset in processing viral antigens to the MHC I pathway, probably via cross-presentation.29 Recently, this particular subset has been shown to play a key role in initiating T helper type 1 (Th1) and Th17 response during subcutaneous sensitization and has also been shown to cross-present antigens expressed by keratinocytes.30,31 Studies have also reported that dermal langerin+ DCs can play a role in mediating contact hypersensitivity with no evidence of tolerance induction.32–34 In addition to dermal langerin+ DCs, langerin− dermal DCs have also been shown to potentiate CD8+ T-cell responses, with dermal langerin− DCs comprising the major population of migrating DCs following intradermal injection of lentiviral vectors.35 However, in models of leishmania, it has been shown that dermal langerin− DCs play a role in priming the CD4+ T-cell response and dermal langerin+ DCs play a role in priming the CD8+ T-cell response.36 Overall, studies point to a role for both dermal DCs and dermal langerin+ DCs as initiators of the immune response.
Lung
Lung consists of three DC subpopulations which include CD103+ CD11chigh CD11b− DCs in the intraepithelial network and CD103− CD11chigh CD11b+ DCs in the lamina propria of conducting airways along with plasmactyoid DCs.37 Among the three subsets, the CD103+ CD11chigh CD11b− DC population is regarded as a conventional migratory DC population that is derived from the pre-DC precursor population.38 CD103+ DCs in the lung have the ability to migrate to the draining lymph nodes, produce IL-12 and are specialized in cross-presenting antigens to CD8+ T cells.39,40 Among the resident pulmonary DCs, it appears CD103+ DCs are the major initiators of the CD8+ T-cell response to poxvirus infection.41 However, it appears that pulmonary CD103+ DCs may comprise a heterogeneous population with CD8α+ and CD8α− DCs, with CD8α− CD103+ DCs being the major initiators of the CD8+ cytotoxic T-cell response.41,42
Intestinal tract
In the intestinal tract, DCs are found in Peyer's patches, lamina propria (LP) and mesenteric lymph nodes. Intestinal DCs are classified primarily on varied expression of CD103 and CX3CR1. The two main LP subsets are CD103+ CX3CR1− CD11b+ (CD103+ LP) DCs and CD103− CX3CR1+ CD11b+ DCs, with with the latter outnumbering CD103+ LP DCs by threefold to fourfold. The CD103+ LP DC is a conventional DC population derived from CDPs along with pre-DCs, under the control of Fltl3 and GM-CSFR ligands.16,17 CD103+ DCs are also found in Peyer's patches and are also a conventional DC subset derived from CDPs as well as pre-DCs under the control of Fltl3 but not GM-CSF signalling.16 Studies have documented CD103+ DCs as serving both a regulatory and an immunogenic role in the intestinal tract.
The CD103+ LP DCs express high levels of CCR7 and are substantially depleted in the mesenteric lymph nodes of CCR7−/− mice but not in the LP of CCR7−/− mice.16,43,44 This indicates that CD103+ DCs constitute the major population of migratory DCs. In various experimental models such as salmonella infection, CD103+ DCs have been shown to be the DC subset responsible for antigen transport to the mesenteric lymph nodes.16 CD103+ LP DCs have been shown to recognize pathogenic intestinal bacteria via TLR5 and secrete pro-inflammatory cytokines.45 Additionally, CD103+ DCs have also been shown to induce expression of gut homing receptor CCR9 on T cells and to drive induction of gut homing CD8+ T cells.44,46 The TLR5-mediated bacterial recognition by CD103+ DCs combined with their migratory ability makes CD103+ DCs the major DC subset responsible for initiating adaptive immune responses in the intestinal tract.
Contrary to their role in initiating immune responses, CD103+ DCs have also been shown to serve a regulatory function, because depletion of CD103+ DCs exacerbates colitis in mice.17 The CD103+ DCs have also been shown to drive Treg-cell differentiation under steady-state conditions through a mechanism dependent on transforming growth factor-β (TGF-β) and retinoic acid.47 The DC-specific β-catenin knockout mice display reduced numbers of Treg cells in the intestine with normal Treg-cell frequencies in the spleen, indicating a role of β-catenin signalling in intestinal DCs to promote tolerance.48β-Catenin signalling is essential in intestinal DCs to promote expression of anti-inflammatory mediators such as retinoic acid metabolizing enzymes, IL-10, TGF-β and suppression of pro-inflammatory cytokines, which cumulatively promotes tolerance induction.48 Furthermore, intestinal epithelial cells are also known to secrete factors such as thymic stromal lymphopoietin which can promote tolerance induction by CD103+ DCs under steady state conditions.49 In the absence of inflammation, E-cadherin from intestinal epithelial cells may interact with CD103 on CD103+ DCs to activate the β-catenin pathway promoting tolerance. However, during the presence of inflammatory stimuli CD103 and E-cadherin interaction may be affected, which could inactivate the β-catenin pathway and drive CD103+ DCs to an inflammatory phenotype to prime immune responses.
Liver
Hepatic DCs were initially identified as CD11c+ B220− DCs and CD11c+ B220+ plasmacytoid DCs.50 CD11c+ B220− hepatic DCs have been shown to play a role in peripheral tolerance under steady-state conditions and can reduce ischaemia/reperfusion-induced liver injury through secretion of IL-10.51 However, CD11c+ B220− DCs have been further divided into CD103+ CD11b− and CD103− CD11b+ subsets, with both expressing aldehyde dehydrogenase, an enzyme that controls retinoic acid production and has been associated with Treg-cell induction.52 Fltl3 is highly expressed in the CD103+ subset compared with the CD103− subset and Fltl3 knockout mice are substantially depleted of liver CD103+ DCs.38 Flt3 is involved in the generation of DCs from pre-DCs pointing to pre-DCs being the precursor to CD103+ DCs, which can therefore be classified as conventional DCs.38 In contrast to liver CD103+ DCs, CD103− hepatic DCs can originate from MDPs as well as from monocytes, indicating that CD103− DCs may include both pre-DC as well as monocyte-derived populations.8 In lieu of the recent findings, although CD103+ CD11b− hepatic DCs can be classified as conventional, CD103− CD11b+ hepatic DCs require further investigation to identify their origins.
Kidney
Subsets of DCs in kidney include the recently identified interstitial CX3CR1+ CD11b+ DCs, CX3CR1+ CD11b− DCs and CD103+ DCs.53 The CD103+ subset is largely a conventional DC subset, arising from the pre-DC precursor population.38 CD103+ DCs have been shown to play an important role in mediating tolerance to kidney allografts.54 Renal DCs have been shown to secrete IL-10 and their depletion has been associated with aggravated glomerular damage in a model of nephrotoxic nephritis.55 In contrast, renal DCs have recently been also shown to play a role in the progression of kidney disease.56 It is likely that CD103+ kidney DCs may play a tolerogenic role, with an immunological role attributed to other renal DC subsets. The discrepancy could largely be because of studies relying on CD11c as a marker for renal DCs. Further investigation into subsets of renal DCs along with expression of CD103 on CX3CR1+ CD11b+ and CX3CR1+ CD11b− DCs is further required to shed light on the role of various renal DC subsets in tolerance versus immunity.
Lymphoid DCs
Lymphoid DC subsets are found in lymphoid organs such as thymus, spleen and lymph nodes and include CD8+ CD4+ DCs, CD8− CD4− DCs and CD8− CD4+ DCs. Both CD8− CD4− and CD8− CD4+ share a higher degree of similarity than CD8+ CD4+ DCs and hence are collectively referred to as CD8− DCs. CD8− CD4− DCs and CD8− CD4+ DCs differ significantly in one functional aspect: whereas CD8− CD4− DC, like CD8+ DC, can make IL-12 p70 when appropriately stimulated, CD4+ DC appear unable to do so.57 Under steady-state conditions although CD8+ DCs can be observed in the T-cell areas of the lymphoid tissues, CD8− DCs are found within the marginal zones of lymphoid tissues and only migrate to the T-cell areas upon stimulation.58,59 The recently identified pre-DC precursors can give rise to both CD8− and CD8+ DCs.20 However, pre-DC precursors are further divided into two subsets based on CD24 expression and these include CD24high and CD24low pre-DC precursors. The CD24high pre-DC, which are DEC205− MHCII−, further differentiate into CD8− CD24+ DEC205+ MHCII+ cells which differentiate into CD8+ DCs without dividing.60 In contrast to CD24high pre-DCs, the CD24low population gives rise to CD8− DCs.59
Although, CD8+ DCs have been well characterized, our understanding of CD8− DCs is fairly limited. Among lymphoid DC subsets, CD8− CD4− DCs have been shown to secrete the highest amounts of interferon-γ (IFN-γ) and act as potent initiators of the cytotoxic T-cell response upon intravenous immunization with male antigen.61,62 In contrast to their role in driving T-cell responses, other studies have reported that CD8− CD4− DCs are poor stimulators of CD8+ T cells in vitro and instead prime regulatory Tr1 cells, which secrete IL-10 and suppress the immune response.63 The ability of CD8− CD4− DCs to induce tolerance by driving Tr1 differentiation is mediated via TGF-β1 secretion whereas the ability of CD8− CD4− DCs in driving immunity is dependent on TLR9 signalling. Stimulation of CD4− CD8− DCs via TLR9 signalling has been shown to convert tolerogenic CD8− CD4− DCs into immunogenic DCs, which could potentiate a T-cell response.64 This raises the likelihood that under steady-state conditions, CD8− CD4− DCs may play a role in tolerance induction but upon stimulation by TLR9 ligands, may revert to an immunogenic phenotype that can prime a cytotoxic T-cell response. CD8− CD4+ DCs have been shown to reduce the severity of experimental autoimmune encephalitis in murine models, first through secretion of IL-10 and through tolerizing effects on Th1 cells.65 Studies have shown that CD8− CD4+ DCs are unable to drive IFN-γ production in T cells and this ability is independent of IL-10 production.65
CD8+ DCs are found in the spleen, lymph nodes and thymus and their turnover ranges from only 3 days in the spleen to up to 10 days in the thymus.66 Moreover, though CD8+ DCs in the spleen and the lymph nodes probably derive from pre-DCs, the DNA of thymic CD8+ DCs contains IgH gene D–J arrangements as in T cells, raising the likelihood that thymic CD8+ DCs may have lymphoid origins.67 CD8+ DCs play a key role in viral immunity along with immune responses to intracellular pathogens, particularly in the spleen and the lymph nodes.66 CD8+ DCs are the most potent producers of IFN-α among lymphoid DCs, which plays a role in increasing cytotoxicity of natural killer and T cells and further contributes to viral immunity.62 Initially, CD8+ DCs were thought to express the complete CD8 molecules found on T cells. However, later it became apparent that T cells express the CD8αβ heterodimer, whereas CD8+ DCs express the CD8αα homodimer. Although CD8 is used as a marker to classify DCs, no studies to date have been able to show a functional and/or developmental significance of CD8 on DC surface. CD8+ DCs express CD36 and Clec9A, which are receptors that give CD8+ DCs the ability to readily phagocytose dead cells.68 However, the distinguishing feature of CD8+ DCs is their ability to cross-present exogenous antigens through an MHC class I pathway.69 Initially the ability of CD8+ DCs to phagocytose dead cells was thought to be responsible for their ability to cross-present. However, later studies identified that CD8+ DCs can even uptake soluble antigens and cross-present via the MHC I pathway, indicating an intrinsic difference in their antigen-processing machinery compared with other DC subsets.70 This makes CD8+ DCs potent inducers of the CD8+ T-cell response to exogenous antigens and CD8− DCs potent inducers of the CD4+ T-cell response.71 Stimulation of CD8+ DCs with a TLR ligand induces CD40 induction on CD8+ DCs, which drives the production of high levels of IL-12p70, which in combination with MHC class I antigen presentation drives an effector CD8+ T-cell response.72 Furthermore, CD8+ DCs have also been shown to secrete IFN-λ in response to polyinosinic : polycytidilic acid, which is a double-stranded RNA known to function as adjuvant.73
In addition to the induction of the immune response, CD8+ DCs have also been implicated in tolerance induction. DEC205 is an endocytic receptor highly expressed on CD8+ DCs that mediates the efficient processing and presentation of antigens on MHC class II products in vivo.74 Targeting of antigen to DEC205 by coupling with anti-DEC205 antibodies has been shown to induce CD8+ T-cell tolerance.75 This was mainly attributed to deletional tolerance and also to the induction of regulatory T cells.76 Furthermore, CD8+ DCs have been shown to induce peripheral self-tolerance by capturing self antigens and presenting to both naive CD4+ and CD8+ T cells via the cross-presentation pathway.77 It is likely that exposure to an antigen in the presence of TLR ligands, which drive CD40 induction, results in induction of the immune response, whereas in the absence of any inflammatory stimuli, the antigen is cross-presented and tolerance is achieved.
Non-conventional DCs
Non-conventional DCs include plasmacytoid DCs, which, although derived from CDP, are unique in their ability to secrete high amounts of IFN; this distinguishes them from conventional DCs, resulting in them being classified as non-conventional DCs. Additionally, there are several DC subsets derived from monocytes and not CDPs, which are also classified as non-conventional DCs.
Plasmacytoid DCs
Plasmacytoid DCs (pDCs) comprise a distinct DC subset found both in lymphoid and non-lymphoid organs and characterized by rapid production of type I interferons in response to viral infections. Differentiation of pDCs is dependent on Fltl3 and MDP and CDP give rise to pDCs.7,78 pDCs express TLR7 and TLR9, which recognize viral RNA and DNA and signal downstream via phosphatidyl inositol 3-kinase, which regulates interferon regulatory factor 7 (IRF7), a key transcriptional regulatory of IFN to drive type I interferon production.79 The TLR signalling and IFN production by pDCs is positively regulated by Ly49Q, which is expressed on all pDCs and binds to MHC I.80 Recognition of viral particles by TLRs leading to IFN production by pDCs does not require viral replication. Instead, TLR recognition of virus leads to IFN production, which positively feedbacks via interferon receptor to drive further IFN production by pDCs.81 In addition to serving as a source of IFN, pDCs have also been shown to be critical for differentiation of activated B cells to plasma cells via secretion of type I interferons and IL-6.82 Activated pDCs behave differently than conventional DCs in antigen presentation following stimulation via TLR9 ligands such as CpG DNA. In models of influenza infection, conventional DCs undergo maturation and present antigens in complex with MHC II, with a parallel down-regulation of MHC II synthesis. In contrast, although pDCs also undergo maturation and present antigens, MHC II synthesis is not down-regulated, giving pDCs the ability to continuously present endogenous viral antigens in activated state.83
Plasmacytoid DCs have been associated with maintenance of peripheral tolerance as well as in induction of the autoimmune response. Studies have shown that in models of experimental autoimmune encephalitis, pDCs migrate to the lymph nodes and interact with myelin-specific CD4+ T cells via MHC II and induce Treg-cell expansion, which dampens the autoimmune response.84 Moreover, pDCs have also been shown to up-regulate inducible T-cell costimulator ligand expression upon undergoing maturation, which drives induction of IL-10-producing Treg cells.85 In contrast to tolerance, pDCs have also been associated with autoimmune responses. Antimicrobial peptide L77 (CAMP) has been shown to bind to self DNA, which is then recognized by TLR9 in the endocytic compartment of pDCs and drives IFN production leading to an autoimmune response.86 Moreover, in the absence of conventional DCs, alloantigen-expressing pDCs have been shown to prime the T-cell response in models of graft-versus-host disease.87 The differential ability of pDCs to drive immunity versus tolerance is not completely understood and may be attributed to individual pDC subsets. The ability of pDCs to drive tolerance induction could be attributed to a CCR9-expressing subset of pDCs, which have been shown to be potent inducers of Treg cells and suppressors of antigen-specific immune responses.88
Plasmacytoid DCs in the liver and the lung
Although pDCs have been implicated to play a key role in hepatic immune responses, their role in the liver is not completely understood. Under steady-state conditions, hepatic DCs are poor stimulators of T-cell proliferation but have a pro-inflammatory cytokine profile.89 Hepatic pDCs, upon TLR9 ligation become potent inducers of natural killer cells, natural killer T cells and antigen-specific CD8+ T cells in vitro.89 During hepatitis C infection, hepatitis C virus-infected cells trigger a robust IFN response in pDCs, which plays a role in inhibiting infection.90 Furthermore, during chronic hepatitis C infection, depletion of pDCs is observed, which may contribute to viral persistence, indicating that hepatic pDCs play a key role in initiating the immune response against hepatitis C.91 However, hepatic pDCs may also play a role in tolerance. Hepatic pDCs express high levels of NOD2, a pattern recognition molecule, which binds to its ligand muramyl dipeptide.92 Treatment of pDCs with muramyl dipeptide results in the reduction of T-cell stimulatory capacity along with an increased expression of IRF4, which is inhibitory to the nuclear factor-κB pathway.92 Taken together, these studies indicate that though pDCs can drive hepatic inflammation, they may also possess a self-regulatory mechanism to control hepatic inflammation.
The role of pulmonary pDCs is controversial, with studies pointing towards their role in immunity as well as tolerance. Adenoviral delivery to mice induces maturation of both pDC and conventional DCs, with only conventional DCs migrating to the draining lymph nodes, raising the likelihood that pulmonary pDCs may play an indirect role in potentiating immune responses by modulating conventional DCs.93 In contrast to the role of pulmonary pDCs in the immune response, studies have shown that pulmonary pDCs can suppress the generation of effector T cells in asthma models.94 Moreover, in the model of dust mite-induced allergy, increased frequency of pDCs in the lung is associated with suppression of airway inflammation.95 Studies indicate that the programmed death-1/programmed death ligand 1 pathway is important for pDC-mediated suppression of airway inflammation and is independent of the pDC maturation status.96 Furthermore, pulmonary pDCs may also suppress conventional DC maturation as an increased ratio of pDC to conventional DCs in the lungs is associated with reduced inflammation along with a reduction in Th2-driving chemokines.97 Plasmacytoid DCs in the lung may affect the balance between Th1 and Th2 in the lung and shift towards a Th1 response, which may also result in amelioration of the Th2-associated inflammation observed in asthma models.
Monocyte-derived DCs
Monocytes are derived from CMPs and MDPs and can give rise to DCs under inflammatory as well as steady-state conditions. Monocyte-derived DCs are found in peripheral tissues such as the intestine, lung, skin and kidneys, and also have the ability to uptake antigen and subsequently migrate to draining lymph nodes.
Intestinal monocyte-derived DCs
E-cadherin+ DCs and CD103− CX3CR1+ intestinal DCs are monocytic in origin. Ly6hi monocytes home to sites of inflammation in the intestine and give rise to E-cadherin+ DCs, which have a pro-inflammatory gene expression profile.98 E-cadherin+ DCs secrete very high amounts of IL-23 and IL-12 upon stimulation and also exacerbate T-cell colitis in mice, pointing towards their role in intestinal inflammation.98 Another monocyte-derived DC population in the intestine is the CD103− CX3CR1+ DCs, which are derived from Ly6Chigh monocytes under the control of GM-CSF.16,17 CX3CR1+ DCs are capable of taking up bacteria via transepithelial dendrites from intestinal lumen, indicating that this DC subset may act as the first line of defence to mucosal pathogens.99 CD103− CX3CR1+ DCs are longer lived than conventional intestinal DCs and are poor stimulators of T-cell proliferation with poor migration to the draining lymph nodes.100 During the course of an infection, such as Salmonella, depletion of CX3CR1+ DCs only during the early course of infection attenuates Salmonella-induced colitis.101 These findings point towards the role of CX3CR1+ DCs in mediating the initial innate immune response against pathogens and not in initiating intestinal T-cell response, thereby playing a role in gut homeostasis.
Pulmonary monocyte-derived DCs
Pulmonary CD103− CD11chi CD11b+ DCs are monocyte derived and Ly6lo monocytes have been shown to give rise to this particular subset under steady-state conditions.102 However, in response to a pulmonary fungal infection, largely Ly6hi monocytes have been shown to give rise to this particular subset.103 In response to allergen challenge, monocytes are recruited from the blood to the lung where they undergo differentiation into CD103− CD11chi CD11b+ DCs. These DCs are the key producers of CCL17 and CCL22, which are critical for infiltration of Th2 cells and eosinophils into the airways to drive allergen-induced inflammation.104 In the house-dust-mite-induced airway allergy model, CD11b+ DCs uptake antigen, increase in numbers, undergo maturation and secrete cytokines that play a role in inducing a Th17 immune response.95 The increased ratio of CD11b+ DCs compared with pDCs corresponds to an increased pulmonary immune response, indicating that CD103− CD11chi CD11b+ DCs play a critical role in airway inflammation by regulating Th2 and Th17 immune responses.
Monocyte-derived DCs in the skin
Langerhans cells possess a unique cellular organelle called the Birbeck granule, with langerin (CD207), a C-type lectin, as its main component, which plays a role in antigen uptake.105 Among skin DCs, only epithelial resident LCs express E-cadherin, which mediates their attachment to neighbouring keratinocytes as well as langerin.106 In human LCs, glycolipids from pathogens such as Mycobacterium leprae are endocytosed via langerin and then loaded onto CD1a antigen without endosomal acidification, which can then subsequently present antigens to T cells.107 TGF-β1 is crucial to the development of LCs because mice lacking TGF-β1 lack LCs.108 Furthermore, it is the TGF-β1 derived from LCs that acts in an autocrine manner for development/survival of LCs.109 Receptor for colony-stimulating factor-1 (CSF-1) is also important for LC development because under steady-state conditions CSF-1 lacking mice lack LCs.110 Ly6Chi monocytes have been shown to give rise to LCs during inflammation, pointing towards a myeloid lineage of LCs.110 Along with the myeloid ancestry of LCs, studies have also shown that mouse lymphoid progenitors can give rise to LCs upon transfer, pointing towards a lymphoid ontogeny of LCs.111 In addition to the studies supporting myeloid/lymphoid ancestry of LCs, several studies point to a distinct route of LC development, whereby yolk sac primitive macrophages migrate to developing skin from E10 to E16.5 and populate the LC compartment.112 Inspite of the controversy surrounding development of LCs, LCs can be regarded as non-conventional DCs because of their origins from monocytes and/or macrophages.
Under steady-state conditions, LCs do not show MHC II expression of surface and most MHC II molecules are intracellular. Langerhans cells do express E-cadherin, which mediates their adherence to keratinocytes. However, upon encounter with an immunogen, MHC II is rapidly transported to the cell surface and expression of E-cadherin is down-regulated, which mediates LCs to detach from keratinocytes and migrate to the skin-draining lymph nodes.113 Activation-induced LCs also elongate their dendrites and penetrate the keratinocyte tight junctions to sample external antigens below the stratum corneum of the skin.114 The LCs carry the antigen to the draining lymph nodes, where CD8+ DCs take up the antigen and cross-present to CD4+ and CD8+ T cells.115,116 However, studies have challenged the immunogenic potential of LCs and have instead showed that depletion of LCs leads to an increase in ear swelling in contact hypersensitivity models, pointing towards a role of LCs in mediating immunological tolerance.32 It has been shown that disruption of E-cadherin under steady-state conditions leads to up-regulation of co-stimulatory molecules, MHC II and chemokine receptors on LCs, triggered via the β-catenin pathway.117 However, these LCs fail to release immunostimulatory cytokines and instead promote tolerance induction via generation of regulatory T cells. As β-catenin signalling in DCs has been association with a tolerogenic phenotype; it is likely that under steady-state conditions, the β-catenin pathway promotes LC-induced tolerance induction.48 However, signalling induced during the presence of inflammatory stimuli may override the β-catenin pathway and promote induction of the immune response instead of tolerance.
Monocyte-derived DCs in kidney
CX3CR1+ interstitial DCs, which comprise CD11b+ and CD11b− DCs and are found in the kidney, also express F4/80, a macrophage marker, indicating that this DC subset could be monocyte derived.53 CD11b+ (CX3CR1+ CD11b+) DCs in the kidney express the monocytic marker Gr1, indicating monocytic origin and they have been shown to increase in numbers in a mouse model of glomerular disease and are critical for the infiltration of T cells.56 In contrast to CD11b+ DCs, the function and the origins of CX3CR1+ CD11b− DCs are not well understood, although the presence of CX3CR1 and F4/80 indicate that CX3CR1+ CD11b− may also be monocytic in origin.
Concluding remarks
Although DCs comprise a heterogenous population, they are mostly derived from pre-DCs or monocytes. However, lymphoid progenitors can also give rise to DCs and the contribution of lymphoid progenitors to DC development is not completely understood. Further studies are needed to identify whether lymphoid-derived DCs arise normally during steady-state conditions or under the influence of inflammatory stimuli. Studies have tried to associate various DC subsets with an ability to drive tolerance verus immunity. It is likely that the local environment and the presence of extrinsic signals, which drive a DC subset to behave in either a tolerogenic or an inflammatory manner. Recent studies have shed light on such signals such as β-catenin pathway, but further studies are needed to better understand the signals that can drive DC from behaving tolerogenically to becoming immunostimulatory.
Acknowledgments
This work was supported in part by Operating Grants from the Canadian Institutes of Health Research, the Canadian Cystic Fibrosis Foundation (CCFF), and the Foundation Fighting Blindness-Canada to J.H. J.H. was a CCFF Scholar and recipient of the CCFF Zellers Senior Scientist Award, and held a Premier's Research Excellence Award of Ontario, Canada. R.K. is a recipient of the CCFF doctoral award.
Disclosures
The authors declare no financial or conflict of interest.
References
- 1.Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149:1. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Adams JC, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. IV. Identification and distribution in mouse spleen. J Exp Med. 1975;141:804. [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139:1431. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 7.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 9.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 10.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 11.Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 12.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 13.Schlenner SM, Madan V, Busch K, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Latchman Y, Elkon KB. Ly6Clow monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 21.Welner RS, Pelayo R, Nagai Y, et al. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waskow C, Liu K, Darrasse-Jeze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathaliyawala T, O'Gorman WE, Greter M, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 27.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 28.Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedoui S, Whitney PG, Waithman J, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 30.Henri S, Poulin LF, Tamoutounour S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207:953. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiki R, Kabashima K, Sugita K, Atarashi K, Shimauchi T, Tokura Y. IL-10-producing Langerhans cells and regulatory T cells are responsible for depressed contact hypersensitivity in grafted skin. J Invest Dermatol. 2009;129:705. doi: 10.1038/jid.2008.304. [DOI] [PubMed] [Google Scholar]
- 35.Furmanov K, Elnekave M, Lehmann D, Clausen BE, Kotton DN, Hovav AH. The role of skin-derived dendritic cells in CD8+ T cell priming following immunization with lentivectors. J Immunol. 2010;184:4889. doi: 10.4049/jimmunol.0903062. [DOI] [PubMed] [Google Scholar]
- 36.Brewig N, Kissenpfennig A, Malissen B, Veit A, Bickert T, Fleischer B, Mostbock S, Ritter U. Priming of CD8+ and CD4- T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J Immunol. 2009;182:774. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- 37.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 40.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp NM, Busick RY, Alexander-Miller MA. Functional divergence among CD103+ dendritic cell subpopulations following pulmonary poxvirus infection. J Virol. 2010;84:10191. doi: 10.1128/JVI.00892-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pascual DW, Wang X, Kochetkova I, Callis G, Riccardi C. The absence of lymphoid CD8+ dendritic cell maturation in L-selectin−/− respiratory compartment attenuates antiviral immunity. J Immunol. 2008;181:1345. doi: 10.4049/jimmunol.181.2.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang MH, Sougawa N, Tanaka T, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 44.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 46.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 50.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 51.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilliams M, Crozat K, Henri S, et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 53.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 54.Degauque N, Lair D, Dupont A, et al. Dominant tolerance to kidney allografts induced by anti-donor MHC class II antibodies: cooperation between T and non-T CD103+ cells. J Immunol. 2006;176:3915. doi: 10.4049/jimmunol.176.7.3915. [DOI] [PubMed] [Google Scholar]
- 55.Scholz J, Lukacs-Kornek V, Engel DR, et al. Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol. 2008;19:527. doi: 10.1681/ASN.2007060684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards AD, Chaussabel D, Tomlinson S, Schulz O, Sher A, Reis e Sousa C. Relationships among murine CD11chigh dendritic cell subsets as revealed by baseline gene expression patterns. J Immunol. 2003;171:47. doi: 10.4049/jimmunol.171.1.47. [DOI] [PubMed] [Google Scholar]
- 58.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 59.Sathe P, Shortman K. The steady-state development of splenic dendritic cells. Mucosal Immunol. 2008;1:425. doi: 10.1038/mi.2008.56. [DOI] [PubMed] [Google Scholar]
- 60.Bedoui S, Prato S, Mintern J, et al. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 61.McLellan AD, Kapp M, Eggert A, Linden C, Bommhardt U, Brocker EB, Kammerer U, Kampgen E. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 62.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Huang H, Yuan J, Sun D, Hou WS, Gordon J, Xiang J. CD4−8− dendritic cells prime CD4+ T regulatory 1 cells to suppress antitumor immunity. J Immunol. 2005;175:2931. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Munegowda MA, Yuan J, Wei Y, Xiang J. Optimal TLR9 signal converts tolerogenic CD4−8− DCs into immunogenic ones capable of stimulating antitumor immunity via activating CD4+ Th1/Th17 and NK cell responses. J Leukoc Biol. 2010;88:393. doi: 10.1189/jlb.0909633. [DOI] [PubMed] [Google Scholar]
- 65.Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 67.Corcoran L, Ferrero I, Vremec D, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 68.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnorrer P, Behrens GM, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 72.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 73.Lauterbach H, Bathke B, Gilles S, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–17. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46:1229. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 77.Belz GT, Behrens GM, Smith CM, et al. The CD8alpha+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Auffray C, Fogg DK, Narni-Mancinelli E, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tai LH, Goulet ML, Belanger S, et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting Edge: TLR-Dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-alpha production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 82.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 83.Young LJ, Wilson NS, Schnorrer P, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 84.Irla M, Kupfer N, Suter T, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito T, Yang M, Wang YH, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 87.Koyama M, Hashimoto D, Aoyama K, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 88.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, McKeating J, Adams DH. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47:338. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 92.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kushwah R, Cao H, Hu J. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. J Immunol. 2008;180:4098. doi: 10.4049/jimmunol.180.6.4098. [DOI] [PubMed] [Google Scholar]
- 94.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kool M, van Nimwegen M, Willart MA, et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183:1074. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 97.Kohl J, Baelder R, Lewkowich IP, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 100.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hapfelmeier S, Muller AJ, Stecher B, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J Immunol. 2008;180:3019. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 103.Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medoff BD, Seung E, Hong S, et al. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J Immunol. 2009;182:623. doi: 10.4049/jimmunol.182.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jakob T, Udey MC. Regulation of E-cadherin-mediated adhesion in Langerhans cell-like dendritic cells by inflammatory mediators that mobilize Langerhans cells in vivo. J Immunol. 1998;160:4067. [PubMed] [Google Scholar]
- 107.Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anjuere F, del Hoyo GM, Martin P, Ardavin C. Langerhans cells develop from a lymphoid-committed precursor. Blood. 2000;96:1633. [PubMed] [Google Scholar]
- 112.Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 113.Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 114.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 117.Jiang A, Bloom O, Ono S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


