Abstract
The immunological synapse forms as a result of the tight apposition of a T cell with an antigen-presenting cell (APC) and it is the site where the T-cell receptor (TCR) is triggered by its antigen ligand, the peptide–MHC complex present in the APC membrane. The immunological synapse was initially characterized in the T-cell membrane as three concentric rings of membrane receptors and their underlying cytoskeletal and signalling proteins. The inner circle, or central supramolecular activation cluster (cSMAC), concentrates most of the TCR and CD28, and it is surrounded by the peripheral SMAC that is formed by integrins. Finally, the most external ring or distal SMAC (dSMAC) is where proteins with large ectodomains are located, such as CD43 and CD45, far from the cSMAC. This arrangement was initially thought to be responsible for maintaining sustained TCR signalling, however, this typical concentric bull's-eye pattern is not found in the immunological synapses formed with the APCs of dendritic cells. Interestingly, TCR signalling has been detected in microclusters formed in the dSMAC area and it extinguishes as the TCRs reach the cSMAC. Hence, it appears that TCR signalling and full T-cell activation do not require the formation of the cSMAC and that this structure may rather play a role in TCR down-regulation, as well as participating in the polarized secretion of lytic granules. Here, we shall review the historical evolution of the role of the cSMAC in T-cell activation, finally discussing our most recent data indicating that the cSMAC serves to internalize exhausted TCRs by phagocytosis.
Keywords: central supramolecular activation cluster, down-regulation, immunological synapse, phagocytosis, T-cell receptor
Introduction
A synapse is a specialized structure that forms when the plasma membranes of two cells come into close apposition to transmit signals. Initially, this term brings to mind the long-lasting structures, sometimes life-long, formed between two neurons, or between neurons and other cell types such as muscle cells. However, cells of the immune system also form synapses that, although more transient than neural synapses, are nevertheless structured and essential for cell activation. T cells, B cells and natural killer cells form synapses that are referred to as immunological synapses (ISs). Of these, we will focus on the ISs formed between T cells and antigen-presenting cells (APCs), a structure that forms during the recognition of the peptide antigen–major histocompatibility complex (pMHC) ligand by the T-cell antigen receptor (TCR). The TCR and pMHC are both membrane bound so it is clear that the TCR will only be triggered by its ligand at the interface between T cells and APCs. The term ‘immunological synapse’ was first coined as a result of the seminal work of Kupfer and colleagues1 showing that a specialized structure forms at the T-cell : APC interface, consisting of two concentric rings of molecules. These rings are visible by confocal microscopy and they were named the central supramolecular activation cluster (cSMAC) and peripheral supramolecular activation cluster (pSMAC). Both the TCR and intracellular signalling molecules like protein kinase Cθ (PKCθ) and Lck have been detected in the cSMAC, whereas the integrin lymphocyte function-associated antigen-1 and talin, an actin cytoskeleton-bound protein, are integrated into the pSMAC ring that surrounds the TCR. Although both naive T cells and T-cell blast cells were found to form cSMAC and pSMAC structures with the typical bull's-eye pattern when contacting B cells, tumour cells and artificial planar bilayers, the formation of this structure is not universal.2,3 More significantly, T cells do not appear to form the bull's-eye pattern when contacting dendritic cells (DCs), which on the other hand are excellent professional APCs capable of strongly activating T cells. Instead, T cells establish what have been termed ‘multifocal ISs’ with DCs, because the TCR appears to cluster at multiple sites at the T-cell : DC interface.4 Therefore, although the initial hypothesis was that the bull's-eye organization of the cSMAC and pSMAC was crucial to provide a sustained TCR signal, acting as a ‘molecular machine’5 that provokes full activation of T cells, the variations on this theme indicate that this is not necessarily the case. Moreover, it has been possible to show that stimulation through the TCR occurs before the formation of the mature IS, again supporting the idea that the IS is not required for T-cell activation. On the basis of these data, the IS is now broadly considered to be any structure formed at the interface of functional T-cell : APC contacts, whereas the cSMAC and pSMAC are structures restricted to certain conditions, the function of which is less clear. In this review, we do not aim to exhaustively describe the components and events occurring at the IS, for this readers are referred to recent excellent reviews,6–10 rather we aim to analyse the physiological function of the structures formed at the IS in a somewhat critical manner.
The structural diversity of immunological synapses
Bull's-eye structures, i.e. a clear focal concentration of the TCR surrounded by a ring of integrins that forms within minutes after T-cell : APC contact, are frequently observed when CD4 T cells are stimulated with B-cell lymphoma tumour cell lines or with an artificial lipid bilayer system that anchors pMHC and intercellular adhesion molecule 1 (ICAM-1). This latter approach has permitted high-resolution light microscopy studies to follow the formation of the molecular aggregates that ultimately generate the cSMACs and pSMACs. The lipid bilayer system allows these processes to be visualized in detail, although the trade-off is that it is artificial.11 Cytotoxic T cells (CTLs) also form bull's-eye ISs with their target cells and this is believed to be important for the polarized delivery of cytolytic granules into the IS space. In this situation, the ring formed by the pSMAC might serve to prevent the diffusion of perforins and granzymes beyond the IS space that is defined by the cSMAC and the target cell plasma membrane.12 Strikingly, the propensity of differentiated effector CD4 T cells to form ISs with a bull's-eye pattern when they contact B-cell APCs or artificial lipid bilayers depends on whether they are T helper type 1 (Th1) or Th2 cells: the Th1 cells form well-defined cSMAC and pSMAC, whereas Th2 cells form multifocal ISs.13 The term multifocal refers to the formation of small accumulations of TCRs that do not contain ICAM-1, although they do contact pMHC on the APC and are spread across the T-cell : APC interface. Double-positive thymocytes seem to form multifocal ISs rather than a well-defined cSMAC and pSMAC 14 but more importantly, multifocal ISs are characteristic of the interactions of DCs with naive CD4 and CD8 T cells, and with activated CD4 T cells.4 Furthermore, the interaction between the T-cell and APC does not just seem to be a stable contact. In vivo imaging of lymph nodes suggests that T cells seem to interact with the APCs in either a stable or a rapid transient manner, forming ISs and immunological kinapses, respectively. The immunological kinapse is a brief contact that the T cell makes with different DCs during its migration in which small accumulations of TCR are produced. For example, CD4 cells and DCs can form multifocal ISs or kinapses, both producing calcium flux and a proliferative response. Hence, it would appear that the formation of the bull's-eye pattern with well-defined cSMACs and pSMACs is not an absolute requirement for full T-cell activation.
Going backwards from the cSMAC to microclusters and TCR nanoclusters
The initial observations that Lck and PKCθ co-localized at the cSMAC suggested that this structure was involved in transmitting TCR signals.1 However, this paradigm began to change when it was shown that these putative markers of TCR signalling co-localize with the pSMAC and not with the cSMAC.15 This observation suggested that although the TCR was more concentrated in the cSMAC, active TCR was mainly present in the external pSMAC ring. Meanwhile, it was demonstrated that T cells formed small TCR clusters within seconds of contacting coverslips coated with anti-CD3 antibodies, which were associated with the kinase zeta chain-associated (TCR) protein kinase 70 kDa (ZAP70) and with adaptors such as Grb2, linker for activation of T cells (LAT) and SH2 domain containing leukocyte protein of 76 kDa (SLP76).16 Subsequently, the use of recombinant pMHC and ICAM-1 molecules embedded into artificial planar lipid bilayers enabled cSMAC and pSMAC formation at the T-cell : planar bilayer interface to be followed in detail. Using this technique it was found that the TCR formed small aggregates, microclusters, at the periphery of the interface that then moved centripetally and finally converged in the cSMAC.17,18 These TCR microclusters were formed beyond the pSMAC ring, in the distal SMAC (dSMAC). The newly formed microclusters were associated with signalling molecules such as ZAP70, Lck, LAT and SLP76, but these associations were lost as the microclusters migrated towards the cSMAC. Therefore, it was proposed that TCR signalling is initiated and sustained in peripheral microclusters, and that the accumulated TCR in the cSMAC was not necessarily signalling competent. Hence, the cSMAC appeared to be a dumping ground for exhausted TCR rather than a TCR signalling site.
Nevertheless, the relationship between peripheral TCR microclusters and signalling activity, and between cSMAC and inactivity, does not seem as clear when strong and weak agonist pMHCs are compared. Whereas stimulation with a strong agonist peptide antigen results in a cSMAC deprived of tyrosine phosphorylated proteins, such proteins are detected in the cSMAC following stimulation with a weak agonist.19 However, strong agonists are also known to induce the formation of cSMACs more efficiently than weak agonists.1,5 Therefore, while most TCR signalling probably takes place in microclusters, the cSMAC may also participate depending on the strength of the signal. Alternatively, cSMAC formation may simply be a consequence and not a cause of strong TCR signalling.
The TCR microclusters are associated with the CD4 and CD8 co-receptors, the co-stimulatory CD28 receptor, and internally with PKCθ, adaptors, and the Lck and ZAP70 tyrosine kinases. Treating T cells with PP2, the Src tyrosine kinase inhibitor that also inhibits the TCR priming tyrosine kinases Lck and Fyn, prevents ZAP70 recruitment but not the formation of TCR microclusters, suggesting that microclusters are the site where TCR signalling initiates.17 This raises the question as to how TCR microclusters are formed? Are they the result of the lateral aggregation of TCRs promoted by pMHC binding or is a TCR signal required that is independent of Src kinase activity?
Our own work showed that the TCR is organized into pre-existing oligomers in resting T cells.20 These TCR oligomers contain between two and 20 TCRs in a linear arrangement that can be detected under the electron microscope, but not using standard optical microscopy because they are smaller than the wavelength of visible light (< 200 nm). As a result of their nanometer size, we named these pre-existing TCR oligomers as TCR nanoclusters.21 The organization of TCRs into nanoclusters suggests that TCR signalling might be initiated in these structures by mechanisms that do not necessarily involve TCR clustering because the TCR is already pre-clustered. Hence, conformational changes might be responsible for initiating TCR signalling. Interestingly, using ligands of different valences that are separated by spacers of variable length, we concluded that both TCR cross-linking (as established previously22,23) and conformational changes were required for full T-cell activation.21,24 Whether this cross-linking of different TCRs occurs between TCRs within a single nanocluster or between TCRs of different nanoclusters, thereby bringing together several nanoclusters, is something that still remains unclear. Nevertheless, experiments using single molecule detection by high-speed photoactivated localization microscopy (hsPALM) indicate that in resting T cells, the TCR forms nanoclusters in protein-rich areas called ‘protein islands’, and that these nanoclusters grow in size after pMHC engagement.25 Interestingly, the LAT adaptor was also found in nanoclusters independent of the TCR nanoclusters, although the TCR and LAT nanoclusters coalesced in a lateral manner upon activation with pMHC, without intermixing.26 Therefore, our working hypothesis is that TCR signalling is initiated in pre-existing nanoclusters and these grow until they become visible by confocal microscopy, as well as associating with adaptors and other signalling molecules to generate the microclusters that are detected as signalling assemblies in the periphery of the IS.
The role of the cSMAC: activation or deactivation?
In terms of signalling, the initial idea of the cSMAC as a structure necessary to provide a sustained TCR signal has progressively changed to one in which the cSMAC fulfils a dual role in sustaining and terminating the TCR signal, depending on the strength of TCR ligation by pMHC. Strong agonist stimulation was found to produce cSMAC formation without detectable tyrosine phosphorylation, whereas stimulation with weak agonists results in cSMACs with active tyrosine kinase phosphorylation.19,27 This difference may indicate that the cSMAC is important for the signalling induced by weak but not strong agonists. Alternatively, cSMAC formation may simply be a consequence of the intensity of TCR signalling, serving to internalize and down-regulate the TCR from the ISs. Accordingly, weak agonists would form cSMACs in which tyrosine phosphorylation persists because the TCR is not internalized. This hypothesis is also consistent with the finding that CD28 and PKCθ co-localize strongly in cSMACs, although they remain segregated from most of the TCR that accumulates in the centre.28 These results indicate that there is still signalling activity in the periphery of the cSMAC but that the TCR at the centre has been designated for down-regulation. The idea of the cSMAC serving a termination role is favoured by the finding that Cbl-b, a ubiquitin ligase, and a strong ubiquitin signal both concentrate in the cSMAC.29,30 The multivesicular body marker lysobisphosphatidic acid (LBPA) is also found in the cSMAC, further suggesting that this structure is associated with internalization and degradation.
Irrespective of whether the cSMAC participates in sustained TCR signalling, the bull's-eye structure could be important for CTL killing activity. The CTLs establish ISs in which the cSMAC is divided into two zones: one with a high concentration of TCR and the other serving as a space into which cytolytic granules deliver their content.31 The two concentric pSMAC and cSMAC rings might be important for CTL function because the integrin-rich pSMAC could serve as a sealing ring to prevent the diffusion of perforins and granzymes beyond the cSMAC. Hence, the activity of these proteins would be concentrated on the target membrane, and the actin-free cSMAC would facilitate granule fusion and secretion through the CTL's membrane. The polarization of lytic granule release into the IS space is made possible by the polarization of the microtubule cytoskeleton: lytic granules are transported along microtubules in a plus-end direction, that is moving away from the centrosome. TCR-triggering promotes the translocation of the centrosome to a position just beneath the cSMAC, which aids the polarization of lytic granule secretion by CTLs and of Golgi stacks, which appear quite close to the IS membrane.32 This Golgi polarization towards the IS means that CD4 T cells secrete the cytokines interleukins 2, 4, and 5 and interferon-γ towards the synaptic space defined by the cSMAC.33,34 Furthermore, the receptor for interferon-γ is also translocated to the IS in a polarized manner, suggesting that it may influence the TCR signalling at this site that promotes T-cell differentiation to the Th1 stage.35 The cell polarization caused by the formation of the IS is also thought to be decisive for asymmetric cell division, provoking differentiation towards effector and memory T cells.36
Accordingly the IS appears to polarize T cells in a manner that might be determinant for their effector functions, such as target cell killing, targeted cytokine secretion, T-cell differentiation and asymmetric T-cell division. Furthermore, the cSMAC may exert an important structural function by becoming the site for polarized secretion of lytic granules and cytokines because of it being an actin-free membrane site.
Actin polymerization at the immunological synapse
The TCR signalling promotes the rearrangement of the actin cytoskeleton at the IS.16 Conversely, the actin cytoskeleton is required for both the formation of TCR microclusters at the periphery of the IS and their centripetal movement towards the cSMAC.17,30,37 Interestingly, microtubules do not play an important role in the formation of TCR microclusters, whereas they are determinant, together with the actin cytoskeleton, for the centripetal migration of microclusters.8,17,38 Indeed, inhibition of myosin IIa (both pharmacologically and by RNAi) does not affect the formation of TCR microclusters but rather, it prevents their centripetal migration towards the cSMAC.39 Hence, this actin motor might be involved in ‘pulling’ the TCR microclusters to the cSMAC and in the retraction of the peripheral actin ring. Surprisingly, proximal TCR signalling is prevented by myosin IIa inhibition, including the phosphorylation of ZAP70 and LAT, suggesting that actin contraction is necessary for the integration of the TCR signalling complex.40 Hence, it would be interesting to determine if myosin IIa intervenes in the aggregation of the pre-existing TCR and LAT nanoclusters detected by hsPALM.41
The cSMAC as a phagocytic structure
T cells acquire plasma membrane fragments and MHC molecules from APCs by a process widely known as trogocytosis, which in the case of T cells is dependent on IS formation.42 Trogocytosis is not a form of clathrin-mediated endocytosis. Rather, we recently showed that the small TC21 and RhoG GTPases are required for the acquisition of APC membrane fragments and MHC, and that the TCR is not internalized from the cSMAC in the absence of these GTPases or in the presence of dominant negative or constitutively active mutants.43 Interestingly, the cSMAC appears to be completely devoid of clathrin pits when examined by electron microscopy, and knockdown of the clathrin heavy chain does not affect TCR internalization from the cSMAC by the TC21- and RhoG-dependent pathway. RhoG mediates phagocytosis of apoptotic bodies by macrophages and caveolin-dependent endocytosis, whereas phagocytosis is characterized by the absolute dependence on actin cytoskeleton remodelling and phosphatidylinositol 3-kinase (PI3K) activity. We previously found that TC21 directly binds to the non-phosphorylated immunoreceptor tyrosine-based activation motifs of the TCR, and it regulates tonic TCR signalling by recruiting and activating the p110δ catalytic subunit of PI3K. Moreover, we found that T cells can phagocytose large inert particles by a TCR-triggered TC21- and RhoG-dependent mechanism. Accordingly, we proposed that the cSMAC primarily serves to down-regulate the TCR by phagocytosis.43 Hence, the trogocytosis of MHC and membrane fragments from the APC would be the result of frustrated phagocytosis. When a substantial number of TCRs accumulate in the cSMAC, a TC21-mediated signal is produced that activates PI3K and the formation of an invagination, which serves to ‘swallow’ the cSMAC and all the accumulated TCR (Fig. 1). It has been considered that TCR signalling is equivalent to tyrosine phosphorylation of the TCR itself and of its downstream effectors. However, it remains to be determined whether what is an apparently inert cSMAC, in terms of signalling, is indeed a site of TC21-dependent PI3K activity in the absence of detectable tyrosine phosphorylation. In this regard, the accumulation of PI3K products in the cSMAC has been detected under conditions of strong agonist stimulation, when tyrosine phosphorylation is not evident.19
Figure 1.
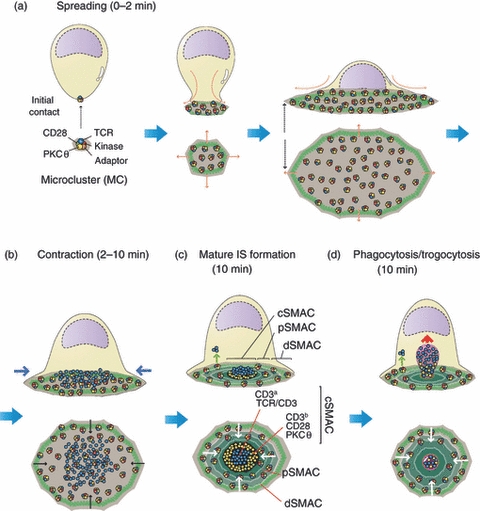
T-cell receptor (TCR) internalization from the immunological synapse (IS). The cartoon depicts the formation of a classical IS between a T cell and either an antigen-presenting cell (APC) or a supported planar bilayer, which are not shown for simplicity. In resting T cells, the TCR is forming pre-existing nanometer-scale oligomers that are called TCR nanoclusters. A few seconds after recognition of the antigen peptide major histocompatibility complex (pMHC) on the APC membrane, the TCR starts forming larger oligomers that are visible in the light microscope and are called microclusters (MC). In these MC the TCR is associated with co-stimulatory receptors (CD28) to tyrosine kinases (Lck and ZAP70), serine kinases [protein kinase C (PKCθ)] and adaptor molecules (LAT, SLP76). Signals transmitted by TCR MC promote the polymerization of the actin cytoskeleton in the form of an expanding ring that spreads the T cell's membrane on the pMHC-loaded APC's membrane. This spreading of the T cell's membrane allows the interaction of more TCRs with new pMHC ligands. From this point of maximal expansion (a), the IS starts to contract resulting from a contraction of the peripheral actin ring (b). At this point, a centripetal movement of TCR MC is observed in which kinases and adaptors are being released from the TCR MC resulting in the accumulation on the centre of the IS of TCR MC not apparently associated with signalling proteins. New signalling MC are continuously formed at the periphery of the IS, which continuously migrate towards the centre of the IS. This process leads to the formation of the classical bull's-eye pattern of a mature IS (c) in which the TCR concentrates in an internal ring essentially devoid of signalling proteins called the central supramolecular activation cluster (cSMAC). The periphery of the cSMAC is still decorated with CD28 and PKCθ. The cSMAC is surrounded by a ring of integrins (peripheral SMAC; pSMAC), which is also surrounded by a more distal ring (the dSMAC) of membrane proteins like CD43 and CD45 with large ectodomains. The expanding and contracting actin rings are indicated by green circles. The cSMAC is devoid of polymerized actin. (d) The TCR can be endocytosed by a clathrin-dependent mechanism (green arrow) from the pSMAC and other areas external to the cSMAC and is also internalized from the cSMAC by a clathrin-independent phagocytic mechanism (red arrow) that is accompanied by the trogocytosis of APC membrane fragments containing pMHC. This figure has been inspired by Yokosuka et al.10
These data suggest that the cSMAC primarily serves a structural role that is critical for the polarized secretion of lytic granules, and for the phagocytosis/trogocytosis of the TCR and APC membrane fragments. This trogocytosis at the cSMAC would desensitize T cells by serving as a site for the disposal and phagocytosis of previously engaged TCRs. The accumulation of ubiquitinylated proteins and multivesicular body (MVB) markers at this site would be consistent with this interpretation.
Acknowledgments
We wish to thank Mark Sefton for his critical reading of the manuscript. This work was supported by grants SAF2006-01391 and SAF2010-14912 from CICYT. Institutional support from the Fundación Ramón Areces to the CBMSO is also acknowledged.
Disclosures
The authors have no financial disclosures.
References
- 1.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 2.Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–7. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Thauland TJ, Parker DC. Diversity in immunological synapse structure. Immunology. 2010;131:466–72. doi: 10.1111/j.1365-2567.2010.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, Trautmann A. Multifocal structure of the T cell–dendritic cell synapse. Eur J Immunol. 2005;35:1741–53. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 5.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation [see comments] Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 6.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. Epub 2010 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasserre R, Alcover A. Cytoskeletal cross-talk in the control of T cell antigen receptor signaling. FEBS Lett. 2010;584:4845–50. doi: 10.1016/j.febslet.2010.09.001. Epub 2010 Sep 7. [DOI] [PubMed] [Google Scholar]
- 9.Padhan K, Varma R. Immunological synapse: a multi-protein signalling cellular apparatus for controlling gene expression. Immunology. 2010;129:322–8. doi: 10.1111/j.1365-2567.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 11.Wulfing C, Tskvitaria-Fuller I, Burroughs N, Sjaastad MD, Klem J, Schatzle JD. Interface accumulation of receptor/ligand couples in lymphocyte activation: methods, mechanisms, and significance. Immunol Rev. 2002;189:64–83. doi: 10.1034/j.1600-065x.2002.18907.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–13. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, Parker DC. Th1 and Th2 cells form morphologically distinct immunological synapses. J Immunol. 2008;181:393–9. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4+ CD8+ thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–48. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–42. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–29. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 17.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. Epub 2005 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. Epub 2005 Nov 6. [DOI] [PubMed] [Google Scholar]
- 19.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–22. doi: 10.1016/j.immuni.2008.06.014. Epub 2008 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcon B. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. Epub 2005 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schamel WW, Risueno RM, Minguet S, Ortiz AR, Alarcon B. A conformation- and avidity-based proofreading mechanism for the TCR-CD3 complex. Trends Immunol. 2006;27:176–82. doi: 10.1016/j.it.2006.02.005. Epub 2006 Mar 9. [DOI] [PubMed] [Google Scholar]
- 22.Boniface JJ, Rabinowitz JD, Wulfing C, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–66. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 23.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–50. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 24.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. Epub 2006 Dec 21. [DOI] [PubMed] [Google Scholar]
- 25.Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci U S A. 2006;103:18992–7. doi: 10.1073/pnas.0609009103. Epub 2006 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–6. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KH, Dinner AR, Tu C, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. Epub 2003 Sep 25. [DOI] [PubMed] [Google Scholar]
- 28.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. Epub 2008 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–40. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 32.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–5. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 33.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci U S A. 1991;88:775–9. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poo WJ, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–80. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado RA, Irvine DJ, Schreiber R, Glimcher LH. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 2004;431:527–32. doi: 10.1038/nature02916. Epub 2004 Sep 22. [DOI] [PubMed] [Google Scholar]
- 36.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. Epub 2007 Mar 1. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–21. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–75. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–9. doi: 10.1038/ni.1723. Epub 2009 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–9. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2009;11:90–6. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261–9. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-Martin N, Fernandez-Arenas E, Cemerski S, et al. TCR internalization from the immunological synapse mediated by TC21- and RhoG-dependent phagocytosis. Immunity. 2011 doi: 10.1016/j.immuni.2011.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


