Abstract
The intravascular administration of iodine-based contrast media remains a common cause of acute kidney injury and a leading cause of iatrogenic renal disease. Past research has elucidated the principal risk factors for contrast-induced acute kidney injury (CIAKI) and helped to establish the efficacy of various interventions for the prevention of this condition. The importance of preventing CIAKI has been underscored by a growing number of studies demonstrating strong associations of CIAKI with serious, adverse short and long-term outcomes. However, it remains unclear whether these associations are causal. This is important as considerable healthcare resources are used to prevent CIAKI. If CIAKI is a marker, but not a mediator, of serious, adverse downstream outcomes, more judicious and selective utilization of preventive care may be appropriate. Moreover, with an increasing number of studies reporting the under-utilization of coronary angiography in patients with acute coronary syndrome and underlying CKD, presumably due in part out of a fear of CIAKI, a clear understanding of whether this condition directly results in adverse downstream outcomes is essential. Careful inspection of past studies that investigated the association of CIAKI with adverse short and long-term events sheds light on their strengths and weaknesses and provides insight into how future research may be better able to characterize the short and long-term implications of this iatrogenic condition.
Introduction
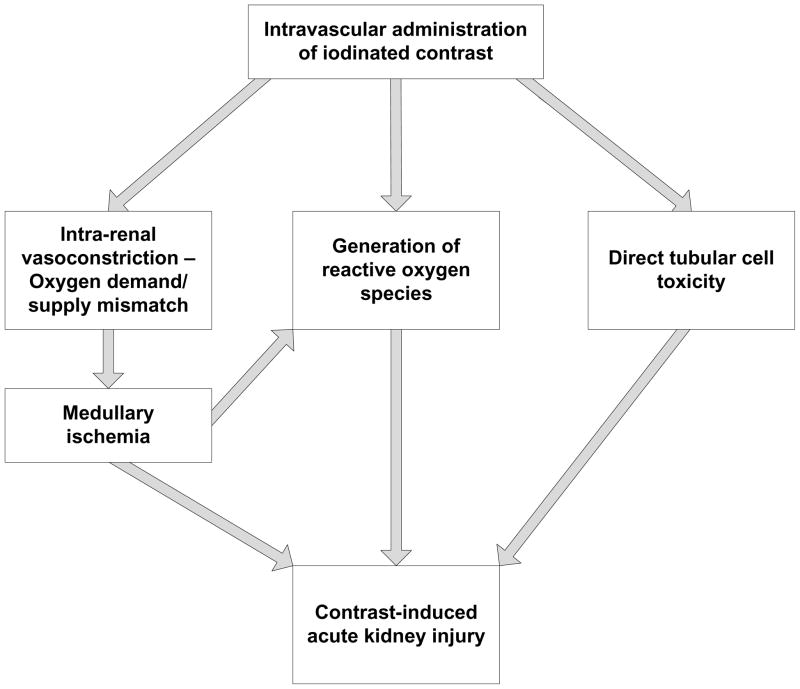
Contrast-induced acute kidney injury (CIAKI) is defined as a sudden decline in kidney function following the intravascular administration of iodinated contrast media for diagnostic imaging.1–3 While the threshold level of kidney injury used to define CIAKI varies across studies, the most commonly employed definition has been an increase in the serum creatinine concentration (SCr) of at least 0.5 mg/dL and/or 25% within 3–4 days of contrast exposure.4–6 Precise estimates of the incidence of CIAKI following angiography vary considerably based on patient characteristics, procedural factors, and the threshold change in SCr utilized.4, 7 A recent prospective observational analysis found that CIAKI occurred in 8.5% of clinically stable patients with eGFR < 60 ml/min/1.73 m2 undergoing non-urgent coronary angiography and 13.2% of clinically stable patients with eGFR < 60 ml/min/1.73 m2 undergoing non-urgent, non-coronary angiography.8 As many as 33% of very high-risk patients develop this condition following contrast-enhanced procedures.9 Pathophysiological processes thought to contribute to the development of CIAKI include renal vasoconstriction leading to medullary ischemia, direct tubular cytotoxicity of contrast, and the generation of reactive oxygen species which contribute to cell damage (Figure 1). Past research has broadened our understanding of the risk factors for CIAKI and elucidated the efficacy of preventive interventions for this condition; work that has made it possible for providers to easily identify patients who are at high risk for CIAKI and implement preventive care. Nonetheless, due to limitations in sample size and study design, most clinical trials have not been able to demonstrate that interventions that reduce the incidence of CIAKI also prevent the adverse downstream events thought to be direct sequelae of this condition. Whether this relates to a lack of statistical power to examine hard, patient-centered outcomes or to the absence of a causal relationship between CIAKI and adverse downstream events is not clear. The current review briefly discusses the risk factors and preventive interventions for CIAKI and critically examines the data linking this condition with serious, adverse short and long-term patient-centered outcomes.
Figure 1.
Pathophysiology of contrast-induced acute kidney injury
Risk Factors for CIAKI
Research over the past three decades has elucidated the principal patient- and procedure-related risk factors for the development of CIAKI (Table 1). Underlying kidney dysfunction is recognized as the most important risk factor, with increasing levels of renal impairment associated with escalating levels of risk.10 The presence of diabetes mellitus substantially amplifies the risk for CIAKI in patients with concomitant renal disease;10–13 however, diabetes in the setting of intact kidney function does not appear to be a significant risk factor.12 Patients with intravascular volume depletion are also susceptible to renal injury from iodinated contrast, as are patients with advanced heart failure.10 In both clinical states, decreased effective circulating volume and reduced renal perfusion potentiate renal vasoconstriction following the administration of intravascular contrast. The risk of CIAKI also increases with larger volumes of administered contrast.14, 15 It is also believed that the risk for CIAKI is greater following intra-arterial contrast administration than with intravenous administration. Recognition of these major risk factors has helped providers identify which patients are most likely to develop CIAKI and has informed research efforts to assess the efficacy of preventive interventions for this iatrogenic condition.
Table 1.
Principal risk factors for CIAKI
|
amplifies risk in the setting of renal insufficiency
Preventive Interventions for CIAKI
Renal injury resulting from iodinated contrast is potentially preventable. Procedures that utilize intravascular contrast are frequently scheduled in advance and thus provide sufficient time to implement prophylactic care and patients at increased risk for CIAKI are easily identifiable by the presence of known clinical risk factors. Past efforts to find effective preventive strategies for CIAKI have focused on four principal approaches: 1) use of less nephrotoxic contrast agents; 2) provision of pre-emptive renal replacement therapy to remove contrast from the circulation prior to its filtration at the glomerulus; 3) expansion of the intravascular space and enhanced diuresis with IV fluids; and 4) utilization of pharmacologic agents to counteract the nephrotoxic effects of contrast media. These data have recently been reviewed and are briefly summarized below.16
Starting in the 1980s, the so called “low-osmolal” contrast agents (with an osmolality of 500 to 700 mOsm/kg) began to supplant the considerably more nephrotoxic high-osmolal agents (osmolality of >1400 mOsm/kg), resulting in a decreased incidence of CIAKI.12, 17 Over the past decade, several trials have compared the renal effects of an iso-osmolal to the low-osmolal contrast media.18–20 While these studies have yielded conflicting data, it is clear that the incidence of CIAKI remains substantial in high-risk patients despite the use of these less nephrotoxic agents.21–24
Renal replacement therapies for the prevention of CIAKI have been largely ineffective, and in some instances, prophylactic hemodialysis has been associated with harm.25–27 The interpretation of studies of hemofiltration for the prevention of CIAKI has been confounded by the use of change in SCr, a variable that is directly impacted by the intervention, as the primary study endpoint.28, 29 As a result, use of renal replacement therapy to prevent CIAKI is not currently recommended.30
Trials of pharmacologic agents, including furosemide, dopamine, fenoldopam, calcium channel blockers, and mannitol have failed to demonstrate benefit and in some cases have documented an increased risk of CIAKI.9, 31–35 Studies on the benefit of natriuretic peptides, aminophylline, theophylline, statins, and ascorbic acid have yielded mixed results, yet the paucity of data on these interventions and potential safety concerns with natriuretic peptides, aminophylline, and theophylline has led experts to recommend against their routine use.36
Based on a more complete understanding of the pathophysiology of CIAKI, recent research has focused on the role of IV fluids and N-acetylcysteine. Over the past half-decade, clinical trials have compared the effectiveness of IV sodium bicarbonate (bicarbonate) with IV sodium chloride (saline). While several trials demonstrated bicarbonate to be more effective than saline for the prevention of CIAKI, other trials reported no difference between these two IV fluids.37–46 Clinical trials investigating the efficacy of NAC have also been inconsistent in their results.47–71. Multiple meta-analyses attempting to reconcile the conflicting studies on these interventions have themselves, been inconclusive.72–98 Consequently, there remains clinical quipoise regarding the superiority of bicarbonate (compared to saline) and role of NAC for the prevention of CIAKI.
At the present time, the mainstay of preventive care for CIAKI involves the discontinuation of nephrotoxic medications (e.g., non-steroidal anti-inflammatory agents) prior to contrast administration, the use of LOCM or IOCM in the lowest possible dose, peri-procedural administration of IV isotonic bicarbonate or saline, and the provision of NAC prior to and following the contrast-enhanced procedure.
Clinical implications of CIAKI
The importance of preventing CIAKI has been supported by a large and growing body of research demonstrating an association of CIAKI with serious, adverse short and long-term outcomes. A careful review of past studies that reported these associations provides a clear understanding of the data supporting a link between CIAKI and adverse downstream events, as well as an appreciation for the weaknesses and shortcomings of these data.
Short-term implications of CIAKI
Short-term mortality associated with CIAKI
A series of retrospective studies have demonstrated an association between CIAKI (defined by small relative and/or absolute changes in SCr) and increased short-term mortality (Table 2).11, 99–103 Levy et al. reported that the incidence of in-hospital death among 183 hospitalized patients who developed CIAKI (defined by an increase in SCr of ≥25% to at least 2.0 mg/dL) was 34% compared to 7% in 174 matched controls without CIAKI (unadjusted OR: 6.5, p<0.001).99 After adjusting for underlying severity of illness, CIAKI remained a strong predictor of in-hospital death (OR = 5.5, p<0.001). A subsequent study by McCullough et al. of 1,826 patients who had undergone percutaneous coronary intervention found an incidence of in-hospital mortality of 7.1% among patients who developed CIAKI (defined by an increase in SCr of >25%) compared to 1.1% in those without this change in SCr (p< 0.0001).11 In patients who developed CIAKI that required renal replacement therapy, in-hospital mortality was 35.7%. Similarly, in a retrospective study by Rihal et al. that examined outcomes in 7,586 patients who underwent coronary angiography with percutaneous intervention, patients who developed CIAKI had a markedly higher incidence of in-hospital mortality (22% v. 1.4%, p<0.0001).103 In multivariate analyses, CIAKI had a strong independent association with in-hospital mortality (OR=10.8, p<0.0001). In a series of over 20,000 patients who underwent percutaneous coronary intervention, Bartholomew found that CIAKI, defined by more robust changes in SCr (≥1.0 mg/dL), was associated with a marked increase in in-hospital mortality (OR = 22, 95% CI 16–31). From et al. performed a case-matched cohort study of patients who underwent contrast-enhanced procedures and found that CIAKI, defined by an increase in SCr of ≥25% or ≥0.5 mg/dL, was associated with an increase risk for 30-day mortality after adjustment for a series of potential confounding variables (OR 3.37, 95% CI 2.58–4.41). 101 Shema et al. recently reported the findings of an analysis of over 1,100 hospitalized patients who underwent contrast-enhanced radiographic procedures. The investigators demonstrated that CIAKI was independently associated with a nearly 10-fold increase in in-hospital mortality (OR = 9.8, 95% CI: 4.4–22.0). 104 Finally, in a retrospective analysis of over 27,000 patients who underwent coronary angiography, we reported that even a small post-procedure increase in SCr of greater than 0.25 mg/dL but no higher than 0.5 mg/dL was independently associated with increased in-hospital mortality (OR=1.83, 95% CI:1.35–2.49).102
Table 2.
Association of CIAKI with short-term mortality
| Study authors | Number of patients | Definition of CIAKI | Adjusted ORa | 95% CI |
|---|---|---|---|---|
| Bartholomew et al.100 | 20,479 | ↑ SCr ≥ 1.0 mg/dL | 22 | 16–31 |
| From et al.101 | 3,236 | ↑ SCr ≥ 25% or ≥ 0.5 mg/dL | 3.4 | 2.6–4.4 |
| Levy et al.99 | 357 | ↑ SCr ≥ 25% to ≥ 2.0 mg/dL | 5.5 | 2.9–13.2 |
| McCullough et al.11 | 1,826 | ↑ SCr > 25% | 6.6 | 3.3–12.9 |
| Rihal et al.103 | 7,586 | ↑ SCr > 0.5 mg/dL | 10.8 | 6.9–17.0 |
| Shema et al. 104 | 1,111 | ↑ SCr ≥ 0.5 mg/dL | 9.8 | 4.4–22.0 |
| Weisbord et al.102 | 27,608 | ↑ SCr 0.25–0.5 mg/dL | 1.8 | 1.4–2.5 |
OR denotes odds ratio for death
While consistent in demonstrating a robust relationship between small changes in SCr and short-term mortality, these seven studies were retrospective and hence, susceptible to ascertainment bias and to potential problems with missing data.11, 99–103 Nonetheless, prospective observational studies and clinical trials report similar findings.39, 49, 105 Gruberg and colleagues conducted a prospective observational study of 439 patients with CKD undergoing percutaneous coronary intervention and found that in-hospital mortality was considerably more common among patients who developed CIAKI (14.9% v. 4.9%, p=0.001).105 In a clinical trial, Marenzi et al. also found that patients who developed CIAKI had a significantly increased incidence of in-hospital mortality compared to patients without this decline in renal function (26% v. 1.4%, p<0.001).49 Finally, in a clinical trial of patients undergoing coronary angiography, Maioli et al. demonstrated that in-hospital mortality among patients who developed CIAKI was markedly higher than among patients who did not develop this post-procedure complication (11.1% v. 0.2%, p=0.001).39 Thus, data from observational studies and clinical trials are consistent with the findings of retrospective analyses demonstrating an association of small post-angiography decrements in renal function with short-term mortality.
Prolonged hospitalization with CIAKI
A series of observational studies and clinical trials also document an association of CIAKI with a prolongation in hospitalization.46, 100, 102, 106–108 In our group's recent analysis of over 27,000 patients who underwent coronary angiography, a rise in SCr of 0.25 – 0.5 mg/dL was associated with a prolongation in hospital length of stay after adjusting for underlying severity of illness.102 Progressively larger increases in SCr were associated with even longer lengths of stay. Bartholomew and colleagues found that patients who developed CIAKI after PCI were 15-times more likely to have their hospitalization prolonged more than four days.100 In the study by Shema et al., patients who developed CIAKI had a marked increase in hospital length of stay compared to patients without this renal complication (24 v. 13 days, p<0.001). Adolph and colleagues found that patients with a post-angiography increase in SCr of ≥25% or ≥0.5 mg/dL remained in the hospital a mean of two days longer than patients without such an increase in SCr in a clinical trial comparing IV fluids for the prevention of CIAKI.46 While the difference in the length of stay between patients who did and did not develop CIAKI in these study varies due to differences in patient populations, these studies are consistent in demonstrating that the development of CIAKI is associated with a prolongation in hospitalization.
Increased hospital-related costs with CIAKI
The extended length of hospital stay associated with the development of CIAKI is associated with increased healthcare expenditures.107, 108 An analysis of 598 diabetics with CKD undergoing coronary angiography found that CIAKI, defined by a rise in SCr of ≥50%, was independently associated with a 2-fold increase in hospital costs.107, 108 A study by Subramanian et al. that used a decision analytic model reported that CIAKI resulted in an average increase in hospital-related costs of more than $10,000 per episode.106 Based on estimates of the number of angiograms performed across the United States, there may be 110,000 cases of angiography-related CIAKI yearly nationwide, with a cumulative cost of greater than $1.1 billion.106, 109 This estimate would increase considerably if the costs of CIAKI following other imaging procedures such as computed tomography scans are considered.
Long-term implications of CIAKI
Long-term mortality associated with CIAKI
In addition to short-term complications, CIAKI defined by small increases in SCr has also been linked with long-term mortality (Table 3). 103, 110–114 Solomon et al. demonstrated that CIAKI following angiography (defined by an increase in SCr of ≥0.3 mg/dL) was associated with a greater than 3-fold increased risk of major adverse outcomes (death, stroke, myocardial infarction, end-stage renal disease requiring renal replacement therapy) at 1-year of follow up.113 In an analysis by Harjai et al. of 985 patients who underwent PCI, CIAKI was independently associated with increased mortality at 24 months of follow-up (HR = 2.6; 95% CI: 1.5–4.4).111 Brown et al. examined long-term survival among 7,856 patients who underwent percutaneous coronary intervention.114 Patients with either transient or persistent deterioration in renal function following angiography had a 2–3 fold increase in long-term mortality. Goldenberg and colleagues reported that among 78 patients who underwent coronary angiography, the development of CIAKI that fully recovered within 7 days of the procedure was associated with a significant increase in 5-year mortality (HR=2.66, 95% CI 1.72-4.46).110 The previously described study by Rihal et al. also demonstrated that the 5-year mortality rate among patients who underwent coronary angiography and survived to hospital discharge was significantly higher in those who had experienced CIAKI (44.6% v. 14.5%).103 Similarly, a prospective cohort study by Roghi et al. of 2,860 patients who underwent percutaneous coronary intervention demonstrated a borderline independent association of CIAKI with increased 2-year mortality (HR 1.83; 95% CI 0.98-3.44). 112 Finally, James and colleagues found a hazard ratio for death of 2.0 (95% CI: 1.69–2.36) over the ensuing 3 years in patients sustaining a 50% to 100% increase in SCr and a hazard of death of 3.72 (95% CI: 2.92–4.76) in patients sustaining an acute increase in SCr of >100% following coronary angiography.115 Thus, collectively these studies indicate that small decrements in renal function following contrast-enhanced procedures, even if transient, are associated with increased long-term mortality.
Table 3.
Association of CIAKI with long-term mortality
| Study authors | Number of Patients | Definition of CIAKI | Follow-up (months) | Adjusted HR | 95% CI |
|---|---|---|---|---|---|
| Brown et al.114 | 7856 | ↑SCr ≥ 0.5 mg/dL | 90 | 3.1 | 2.4–4.0 |
| Goldenberg et al.110 | 78 | ↑SCr ≥ 0.5 mg/dL or ≥ 25% | 60 | 2.7 | 1.7–4.5 |
| Harjai et al.111 | 985 | ↑SCr ≥ 0.5 mg/dL | 24 | 2.6 | 1.5–4.4 |
| Rihal et al.103 | 7075 | ↑ SCr > 0.5 mg/dL | 6 | a | a |
| Roghi et al.112 | 2860 | ↑SCr ≥ 0.5 mg/dL | 24 | 1.8 | 1.0–3.4 |
| Solomon et al.113 | 294 | ↑SCr ≥ 0.3 mg/dL | 12 | 3.2 b | 1.1–8.7 |
HR denotes hazard ratio for death
HR not reported: 6-month mortality of 9.8% with CIAKI v. 2.3% without CIAKI (p<0.0001)
reflects incident rate ratio of death, CVA, AMI, ESRD requiring renal replacement therapy
Progression of CKD following CIAKI
Past studies have also documented an association of CIAKI with more rapid progression of underlying CKD.39, 110, 115, 116 Goldenberg and colleagues examined downstream outcomes among 78 patients with CKD and found that patients who manifested a transient post-procedure rise in SCr of ≥25% or ≥0.5 mg/dL following coronary angiography experienced a larger decrement in eGFR two years following the procedure as compared to patients without these small post-angiography increases in SCr (ΔeGFR = – 20 ± 11 ml/min.1.73 m2 v. – 6 ± 16 ml/min/1.73 m2, p=0.02).110 In a study by Maioli et al., patients who developed CIAKI had a 0.2 mg/dL higher mean SCr at one month post-angiography compared to patients who had not developed CIAKI (p=0.001).39 Finally, James et al. recently reported that patients who developed an increase in SCr of ≥0.3 mg/dL or 50–99% within 7 days following coronary angiography experienced a greater rate of loss of kidney function on long-term follow-up compared to patients who had not experienced this change in SCr following angiography (loss of eGFR 0.8 ml/min/1.73m2/yr v. 0.2 ml/min/1.73m2/yr) (Figure 2).116 For patients who experienced an even larger post-angiography increase in SCr (≥100%), the long-term rate of loss of eGFR was even more pronounced (2.8 ml/min/1.73m2/yr).116 Additional analyses from the same group have also demonstrated an increased risk for development of end-stage renal disease (ESRD) during three years of follow-up in patients who sustain CIAKI following coronary angiography, with greatest risk in patients with more severe kidney injury (hazard ratio of 4.15 [95% CI: 2.32–7.42] in patients with a 50% to 100% increase in SCr and 11.74 [95% CI: 6.38–21.59] in patients with a >100% increase in SCr).115
Figure 2.
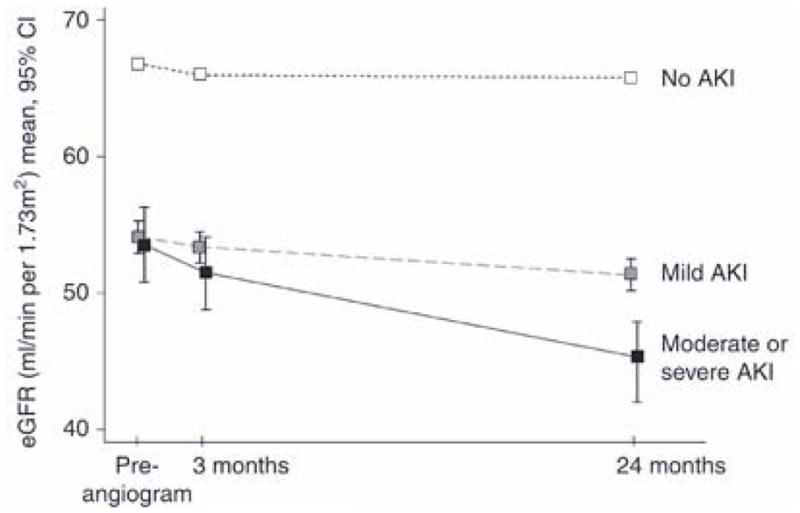
Kidney function following coronary angiography among patients with post–angiography eGFR < 90 ml/min/1.73m2, according to acute kidney injury status*
*Reprinted by permission from Macmillan Publishers Ltd: Kidney International ; James MT et al. Acute kidney injury following coronary angiography is associated with a long–term decline in kidney function.78;803–809,2010
Increased long-term costs with CIAKI
As part of the aforementioned economic analysis by Subramanian and colleagues, the long-term costs associated with an episode of CIAKI were examined. Considering incremental expenditures related to hospital prolongation, the need for dialysis, and downstream complications, the investigators estimated that CIAKI was associated with an increase in 1-year costs of more than $11,800.106
Interpretation of the data linking CIAKI with adverse clinical outcomes
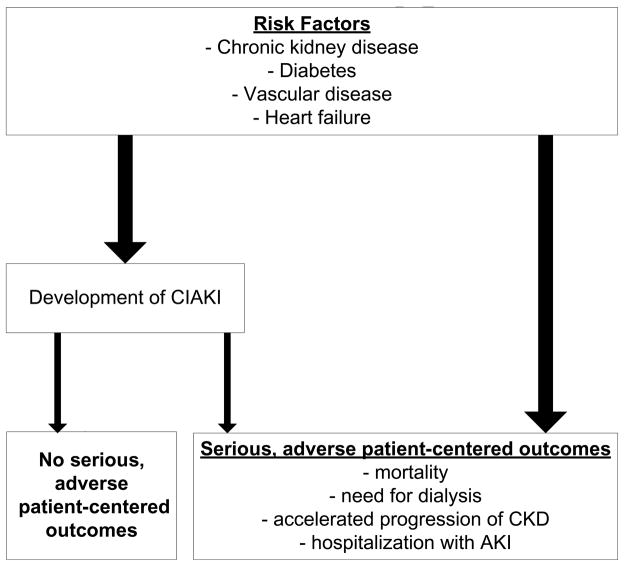
Collectively, these studies demonstrate robust associations of CIAKI with clinically significant short and long-term adverse outcomes and health resource utilization. However, whether these data demonstrating that CIAKI is associated with serious adverse short and long-term outcomes justify the avoidance of indicated contrast-enhanced procedures in patients with CKD is an essential question. Although the strength of the association between CIAKI and the adverse outcomes is strong, it must be recognized that association does not imply causality. The majority of patients who undergo angiography have significant underlying comorbid illnesses including CKD, vascular disease, heart failure, and diabetes. These conditions increase the risk for CIAKI. However, it is clear that not all episodes of CIAKI lead to adverse outcomes (Figure 3). Moreover, while serious, downstream events following angiography may occur as a direct consequence of CIAKI, they may also develop independent of this intermediate event, as many of the clinical conditions that predispose patients to the development of CIAKI (e.g., CKD, diabetes mellitus, heart failure) are also independently associated with mortality and other adverse outcomes (Figure 3). Due to their inherent limitations and biases, observational studies are not able to determine whether CIAKI is a mediator of serious downstream events or simply serves as a marker of patients at particularly high-risk for these outcomes. Demonstration of a causal relationship will require prospective studies that show that interventions that prevent CIAKI also decrease the longer term consequences. Unfortunately, none of the trials conducted to date has had sufficient statistical power to address this question.
Figure 3.
Potential pathways to adverse patient-centered outcomes following contrast-enhanced imaging procedures
Considering the limitations of the studies to date, concern for the clinical consequences of CIAKI seem perfectly justified, but should not preclude the routine performance of clinically indicated and potentially life-saving procedures. Nonetheless, it appears from a growing number of studies that provider concern about the clinical implications of CIAKI may contribute to sub-optimal clinical care. Chertow and colleagues conducted an analysis of more than 55,000 patients to assess whether patients with CKD presenting with acute MI underwent coronary angiography at a rate comparable to patients with intact kidney function.117 While the provision of coronary angiography was associated with a significant reduction in mortality (OR = 0.62, 95% CI: 0.54–0.70), those with CKD deemed to be appropriate candidates for this procedure were less than half as likely to undergo angiography compared to patients without CKD (OR=0.47, 95% CI: 0.40–0.52). Although the authors did not systematically examine the reasons for under-utilization of angiography in individuals with CKD, they posited that concern for the development of CIAKI may explain this finding. Subsequent studies have also documented under-utilization of coronary angiography in patients with CKD. Han et al. examined processes of care delivered to over 45,000 patients presenting with non-ST segment elevation acute coronary syndromes and reported that after adjustment for potential confounders, patients with moderate to severe CKD were considerably less likely to undergo percutaneous coronary intervention that patients without CKD (OR 0.67, 95% CI 0.62–0.71).118 These authors also speculated that concern for CIAKI may underlie this observation. In another analysis of over 13,000 patients with non-ST segment elevation acute coronary syndrome, Goldenberg et al. reported that patients with CKD who underwent coronary angiography experienced a 36% lower risk of inhospital mortality as compared to patients with CKD who did not undergo this procedure.119 However, compared to patients without CKD, patients with CKD were considerably less likely to undergo coronary angiography (49.9% v. 67.8%, p<0.001). Thus, three observational studies demonstrate less frequent performance of coronary angiography in patients with acute coronary syndrome who have underlying CKD. Provider concern for adverse outcomes related to the development of CIAKI may, at least in part, motivate decisions on the performance of angiography in patients with CIAKI.
It is also important to note that nearly all past clinical trials investigating the efficacy of interventions for the prevention of CIAKI have used small perturbations in SCr as the primary study endpoint. Use of such a surrogate biochemical endpoint is based upon past studies that demonstrated an association of CIAKI with serious, adverse short and long-term outcomes. However, recognizing that these epidemiological studies were unable to determine the causal nature of these associations, the practice of using small changes in SCr as a surrogate endpoint in clinical trials is potentially problematic. Use of small changes in SCr rather than hard, patient-centered events as the primary endpoint in past clinical trials has justified the enrollment of smaller numbers of study participants because CIAKI occurs with considerable greater frequency than serious, adverse downstream outcomes. However, this has rendered nearly all past trials underpowered to determine the impact of preventive interventions on the hard outcomes that are of greatest importance to patients. Therefore, future trials on the prevention of CIAKI should be designed and powered to investigate the impact of clinical interventions on serious, adverse events. Trials that establish an intervention to be effective for the prevention of hard, patient-centered outcomes will subsequently be able to determine whether the benefit of the intervention is mediated through a decrease in the development of CIAKI.
CIAKI and renal angiography
The preponderance of data on the incidence, prevention and outcomes of CIAKI emanates from studies of patients undergoing coronary angiography with or without percutaneous intervention. Considerably less is known about the precise incidence of CIAKI following renal angiography. Based on the mechanisms of renal injury from iodinated contrast media, which includes vasoconstriction in the renal medulla, it seems highly plausible that the risk of renal injury would be greater with the direct injection of contrast into the renal arteries. However, direct comparisons of the incidence of CIAKI following renal angiography compared to coronary angiography are lacking. Data on the incidence of AKI following renal angioplasty are provided by the ASTRAL trial, a randomized trial comparing revascularization and medical therapy for the treatment of renal artery stenosis in patients with chronic kidney disease.120 In this trial, AKI occurred in 25 of 383 (7%) patients who underwent renal angioplasty. While this appears to be a lower incidence of CIAKI than many past studies involving coronary angiography, the assessment and definition of AKI were not specified and the volume of contrast administered was not described. A small study by Lufft el al. compared the incidence of CIAKI, defined by an increase in SCr of >25% or 0.5 mg/dL, between patients undergoing renal angiography without angioplasty and with angioplasty.121 CIAKI occurred in 25% of patients who underwent renal angiography with angioplasty and in 6.9% of patients who underwent renal angiography without angioplasty. Notwithstanding the results of these and other small studies, future research on the incidence and implications of CIAKI following renal angiography with or without intervention is needed.
Conclusions
The small decrements in renal function that define CIAKI occur commonly following angiography and other contrast-enhanced imaging procedures. While a series of retrospective analyses, prospective observational studies, and clinical trials demonstrate that CIAKI is associated with serious adverse short and long-term events, evidence that CIAKI is a mediator rather than a marker of patients at particularly high risk for adverse outcomes is lacking. The importance of elucidating the nature of this relationship is underscored by the growing number of studies demonstrating the underperformance of angiography in patients with CKD who seemingly have clear indications for these procedures. Future research on CIAKI should focus not merely on the prevention of small increases in SCr, but on the efficacy of interventions for the prevention of hard outcomes that matter most to patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven D. Weisbord, Staff Physician, Renal Section and Core Investigator, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System and Assistant Professor of Medicine, Renal-Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Paul M. Palevsky, Chief, Renal Section, VA Pittsburgh Healthcare System and and Professor of Medicine, Renal-Electrolyte Division, Department of Medicine, University of Pittsburgh, School of Medicine Pittsburgh, PA.
References
- 1.Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol. 1994;5:125–37. doi: 10.1681/ASN.V52125. [DOI] [PubMed] [Google Scholar]
- 2.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material–induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–9. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 3.Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Contrast media-associated nephrotoxicity. Curr Opin Nephrol Hypertens. 1996;5:127–33. doi: 10.1097/00041552-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Reddan D, Laville M, Garovic VD. Contrast-induced nephropathy and its prevention: What do we really know from evidence-based findings? J Nephrol. 2009;22:333–51. [PubMed] [Google Scholar]
- 5.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–28. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Mehran R, Nikolsky E. Contrast–induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006:S11–5. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98:5K–13K. doi: 10.1016/j.amjcard.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Mor MK, Resnick AL, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168:1325–32. doi: 10.1001/archinte.168.12.1325. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. Jama. 2003;290:2284–91. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Adam A, Becker CR, et al. Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006;98:27K–36K. doi: 10.1016/j.amjcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 11.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 12.Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47:254–61. doi: 10.1038/ki.1995.32. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259–65. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- 14.Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86:649–52. doi: 10.1016/0002-9343(89)90437-3. [DOI] [PubMed] [Google Scholar]
- 15.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–20. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 16.Weisbord SD, Palevsky PM. Strategies for the prevention of contrast–induced acute kidney injury. Curr Opin Nephrol Hypertens. doi: 10.1097/MNH.0b013e32833d42e3. [DOI] [PubMed] [Google Scholar]
- 17.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–8. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 18.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–9. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 19.Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189–96. doi: 10.1161/CIRCULATIONAHA.106.671644. [DOI] [PubMed] [Google Scholar]
- 20.McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006;48:692–9. doi: 10.1016/j.jacc.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 21.Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. Jacc. 2009;2:645–54. doi: 10.1016/j.jcin.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006;48:924–30. doi: 10.1016/j.jacc.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Juergens CP, Winter JP, Nguyen-Do P, et al. Nephrotoxic effects of iodixanol and iopromide in patients with abnormal renal function receiving N-acetylcysteine and hydration before coronary angiography and intervention: a randomized trial. Internal medicine journal. 2009;39:25–31. doi: 10.1111/j.1445-5994.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 24.Laskey W, Aspelin P, Davidson C, et al. Nephrotoxicity of iodixanol versus iopamidol in patients with chronic kidney disease and diabetes mellitus undergoing coronary angiographic procedures. Am Heart J. 2009;158:822–8. e3. doi: 10.1016/j.ahj.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48:361–71. doi: 10.1053/j.ajkd.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Reinecke H, Fobker M, Wellmann J, et al. A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol. 2007;96:130–9. doi: 10.1007/s00392-007-0473-4. [DOI] [PubMed] [Google Scholar]
- 27.Lehnert T, Keller E, Gondolf K, Schaffner T, Pavenstadt H, Schollmeyer P. Effect of haemodialysis after contrast medium administration in patients with renal insufficiency. Nephrol Dial Transplant. 1998;13:358–62. doi: 10.1093/oxfordjournals.ndt.a027830. [DOI] [PubMed] [Google Scholar]
- 28.Marenzi G, Lauri G, Campodonico J, et al. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119:155–62. doi: 10.1016/j.amjmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Marenzi G, Marana I, Lauri G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349:1333–40. doi: 10.1056/NEJMoa023204. [DOI] [PubMed] [Google Scholar]
- 30.Stacul F, Adam A, Becker CR, et al. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98:59K–77K. doi: 10.1016/j.amjcard.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Cacoub P, Deray G, Baumelou A, Jacobs C. No evidence for protective effects of nifedipine against radiocontrast-induced acute renal failure. Clin Nephrol. 1988;29:215–6. [PubMed] [Google Scholar]
- 32.Kellum JA. The use of diuretics and dopamine in acute renal failure: a systematic review of the evidence. Crit Care (Lond) 1997;1:53–9. doi: 10.1186/cc103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury Z, Schlicht JR, Como J, et al. The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15:59–65. [PubMed] [Google Scholar]
- 34.Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. [see comments.] New England Journal of Medicine. 1994;331:1416–20. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein JM, Heyman S, Brezis M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron. 1992;62:413–5. doi: 10.1159/000187090. [DOI] [PubMed] [Google Scholar]
- 36.Shammas NW, Kapalis MJ, Harris M, McKinney D, Coyne EP. Aminophylline does not protect against radiocontrast nephropathy in patients undergoing percutaneous angiographic procedures. J Invasive Cardiol. 2001;13:738–40. [PubMed] [Google Scholar]
- 37.Brar SS, Shen AY, Jorgensen MB, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. Jama. 2008;300:1038–46. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 38.Briguori C, Airoldi F, D'Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–7. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 39.Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100:781–6. doi: 10.1016/j.amjcard.2007.03.098. [DOI] [PubMed] [Google Scholar]
- 41.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. Jama. 2004;291:2328–34. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 42.Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled trial. Am Heart J. 2007;154:539–44. doi: 10.1016/j.ahj.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Pakfetrat M, Nikoo MH, Malekmakan L, et al. A comparison of sodium bicarbonate infusion versus normal saline infusion and its combination with oral acetazolamide for prevention of contrast-induced nephropathy: a randomized, double–blind trial. International urology and nephrology. 2009;41:629–34. doi: 10.1007/s11255-008-9520-y. [DOI] [PubMed] [Google Scholar]
- 44.Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007;49:1283–8. doi: 10.1016/j.jacc.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 45.Vasheghani-Farahani A, Sadigh G, Kassaian SE, et al. Sodium bicarbonate plus isotonic saline versus saline for prevention of contrast-induced nephropathy in patients undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:610–8. doi: 10.1053/j.ajkd.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Adolph E, Holdt-Lehmann B, Chatterjee T, et al. Renal Insufficiency Following Radiocontrast Exposure Trial (REINFORCE): a randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coronary artery disease. 2008;19:413–9. doi: 10.1097/MCA.0b013e3283021ac6. [DOI] [PubMed] [Google Scholar]
- 47.Baker CS, Baker LR. Prevention of contrast nephropathy after cardiac catheterisation. Heart (British Cardiac Society) 2001:361–2. doi: 10.1136/heart.85.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298–303. doi: 10.1016/s0735-1097(02)01958-7. [DOI] [PubMed] [Google Scholar]
- 49.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 50.Coyle LC, Rodriguez A, Jeschke RE, Simon-Lee A, Abbott KC, Taylor AJ. Acetylcysteine In Diabetes (AID): a randomized study of acetylcysteine for the prevention of contrast nephropathy in diabetics. Am Heart J. 2006;151:1032, e9–12. doi: 10.1016/j.ahj.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Kay J, Chow WH, Chan TM, et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. Jama. 2003;289:553–8. doi: 10.1001/jama.289.5.553. [DOI] [PubMed] [Google Scholar]
- 52.Gomes VO, Poli de Figueredo CE, Caramori P, et al. N-acetylcysteine does not prevent contrast induced nephropathy after cardiac catheterisation with an ionic low osmolality contrast medium: a multicentre clinical trial. Heart (British Cardiac Society) 2005;91:774–8. doi: 10.1136/hrt.2004.039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung JW, Szeto CC, Chan WW, et al. Effect of N-acetylcysteine for prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: a randomized trial. Am J Kidney Dis. 2004;43:801–8. doi: 10.1053/j.ajkd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Durham JD, Caputo C, Dokko J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62:2202–7. doi: 10.1046/j.1523-1755.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 55.Allaqaband S, Tumuluri R, Malik AM, et al. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv. 2002;57:279–83. doi: 10.1002/ccd.10323. [DOI] [PubMed] [Google Scholar]
- 56.Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol. 2002;40:1383–8. doi: 10.1016/s0735-1097(02)02308-2. [DOI] [PubMed] [Google Scholar]
- 57.Sandhu C, Belli AM, Oliveira DB. The role of N-acetylcysteine in the prevention of contrast–induced nephrotoxicity. Cardiovascular and interventional radiology. 2006;29:344–7. doi: 10.1007/s00270-005-0127-8. [DOI] [PubMed] [Google Scholar]
- 58.Rashid ST, Salman M, Myint F, et al. Prevention of contrast–induced nephropathy in vascular patients undergoing angiography: a randomized controlled trial of intravenous N–acetylcysteine. J Vasc Surg. 2004;40:1136–41. doi: 10.1016/j.jvs.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 59.Oldemeyer JB, Biddle WP, Wurdeman RL, Mooss AN, Cichowski E, Hilleman DE. Acetylcysteine in the prevention of contrast–induced nephropathy after coronary angiography. Am Heart J. 2003;146:E23. doi: 10.1016/S0002-8703(03)00511-8. [DOI] [PubMed] [Google Scholar]
- 60.Ochoa A, Pellizzon G, Addala S, et al. Abbreviated dosing of N–acetylcysteine prevents contrast–induced nephropathy after elective and urgent coronary angiography and intervention. J Interv Cardiol. 2004;17:159–65. doi: 10.1111/j.1540-8183.2004.09880.x. [DOI] [PubMed] [Google Scholar]
- 61.MacNeill BD, Harding SA, Bazari H, et al. Prophylaxis of contrast-induced nephropathy in patients undergoing coronary angiography. Catheter Cardiovasc Interv. 2003;60:458–61. doi: 10.1002/ccd.10684. [DOI] [PubMed] [Google Scholar]
- 62.Kefer JM, Hanet CE, Boitte S, Wilmotte L, De Kock M. Acetylcysteine, coronary procedure and prevention of contrast-induced worsening of renal function: which benefit for which patient? Acta cardiologica. 2003;58:555–60. doi: 10.2143/AC.58.6.2005321. [DOI] [PubMed] [Google Scholar]
- 63.Goldenberg I, Shechter M, Matetzky S, et al. Oral acetylcysteine as an adjunct to saline hydration for the prevention of contrast-induced nephropathy following coronary angiography. A randomized controlled trial and review of the current literature. European heart journal. 2004;25:212–8. doi: 10.1016/j.ehj.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803–7. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 65.Diaz-Sandoval LJ, Kosowsky BD, Losordo DW. Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial) Am J Cardiol. 2002;89:356–8. doi: 10.1016/s0002-9149(01)02243-3. [DOI] [PubMed] [Google Scholar]
- 66.Azmus AD, Gottschall C, Manica A, et al. Effectiveness of acetylcysteine in prevention of contrast nephropathy. J Invasive Cardiol. 2005;17:80–4. [PubMed] [Google Scholar]
- 67.Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J. 2004;148:422–9. doi: 10.1016/j.ahj.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 68.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 69.Balderramo DC, Verdu MB, Ramacciotti CF, et al. Renoprotective effect of high periprocedural doses of oral N-acetylcysteine in patients scheduled to undergo a same-day angiography. Revista de la Facultad de Ciencias Medicas (Cordoba, Argentina) 2004;61:13–9. [PubMed] [Google Scholar]
- 70.Carbonell N, Blasco M, Sanjuan R, et al. Intravenous N-acetylcysteine for preventing contrast-induced nephropathy: a randomised trial. Int J Cardiol. 2007;115:57–62. doi: 10.1016/j.ijcard.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 71.Amini M, Salarifar M, Amirbaigloo A, Masoudkabir F, Esfahani F. N–acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials. 2009;10:45. doi: 10.1186/1745-6215-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brar SS, Hiremath S, Dangas G, Mehran R, Brar SK, Leon MB. Sodium bicarbonate for the prevention of contrast induced-acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1584–92. doi: 10.2215/CJN.03120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho KM, Morgan DJ. Use of isotonic sodium bicarbonate to prevent radiocontrast nephropathy in patients with mild pre-existing renal impairment: a meta-analysis. Anaesthesia and intensive care. 2008;36:646–53. doi: 10.1177/0310057X0803600503. [DOI] [PubMed] [Google Scholar]
- 74.Hogan SE, L'Allier P, Chetcuti S, et al. Current role of sodium bicarbonate-based preprocedural hydration for the prevention of contrast-induced acute kidney injury: a meta-analysis. Am Heart J. 2008;156:414–21. doi: 10.1016/j.ahj.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 75.Hoste EA, De Waele JJ, Gevaert SA, Uchino S, Kellum JA. Sodium bicarbonate for prevention of contrast-induced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp389. [DOI] [PubMed] [Google Scholar]
- 76.Joannidis M, Schmid M, Wiedermann CJ. Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a meta-analysis. Wiener klinische Wochenschrift. 2008;120:742–8. doi: 10.1007/s00508-008-1117-z. [DOI] [PubMed] [Google Scholar]
- 77.Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009;7:23. doi: 10.1186/1741-7015-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:617–27. doi: 10.1053/j.ajkd.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 79.Kanbay M, Covic A, Coca SG, Turgut F, Akcay A, Parikh CR. Sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of 17 randomized trials. International urology and nephrology. 2009;41:617–27. doi: 10.1007/s11255-009-9569-2. [DOI] [PubMed] [Google Scholar]
- 80.Zoungas S, Ninomiya T, Huxley R, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med. 2009;151:631–8. doi: 10.7326/0003-4819-151-9-200911030-00008. [DOI] [PubMed] [Google Scholar]
- 81.Brown JR, Block CA, Malenka DJ, O'Connor GT, Schoolwerth AC, Thompson CA. Sodium bicarbonate plus N-acetylcysteine prophylaxis: a meta-analysis. Jacc. 2009;2:1116–24. doi: 10.1016/j.jcin.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alonso A, Lau J, Jaber BL, Weintraub A, Sarnak MJ. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1–9. doi: 10.1053/j.ajkd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Bagshaw SM, Ghali WA. Acetylcysteine for prevention of contrast-induced nephropathy after intravascular angiography: a systematic review and meta-analysis. BMC Med. 2004;2:38. doi: 10.1186/1741-7015-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 85.Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv. 2005;64:471–9. doi: 10.1002/ccd.20342. [DOI] [PubMed] [Google Scholar]
- 86.Faddy SC. Significant statistical heterogeneity in a meta-analysis of the usefulness of acetylcysteine for prevention of contrast nephropathy. Am J Cardiol. 2004;94:414. doi: 10.1016/j.amjcard.2004.02.079. [DOI] [PubMed] [Google Scholar]
- 87.Gawenda M, Moller A, Wassmer G, Brunkwall J. Prophylaxis of contrast-induced nephropathy with N-acetylcysteine. Zentralblatt fur Chirurgie. 2007;132:227–31. doi: 10.1055/s-2007-960756. [DOI] [PubMed] [Google Scholar]
- 88.Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32. doi: 10.1186/1741-7015-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guru V, Fremes SE. The role of N-acetylcysteine in preventing radiographic contrast-induced nephropathy. Clin Nephrol. 2004;62:77–83. doi: 10.5414/cnp62077. [DOI] [PubMed] [Google Scholar]
- 90.Isenbarger DW, Kent SM, O'Malley PG. Meta-analysis of randomized clinical trials on the usefulness of acetylcysteine for prevention of contrast nephropathy. Am J Cardiol. 2003;92:1454–8. doi: 10.1016/j.amjcard.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 91.Kshirsagar AV, Poole C, Mottl A, et al. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15:761–9. doi: 10.1097/01.asn.0000116241.47678.49. [DOI] [PubMed] [Google Scholar]
- 92.Liu R, Nair D, Ix J, Moore DH, Bent S. N-acetylcysteine for the prevention of contrast-induced nephropathy. A systematic review and meta-analysis. J Gen Intern Med. 2005;20:193–200. doi: 10.1111/j.1525-1497.2005.30323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Misra D, Leibowitz K, Gowda RM, Shapiro M, Khan IA. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: a meta-analysis. Clinical cardiology. 2004;27:607–10. doi: 10.1002/clc.4960271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nallamothu BK, Shojania KG, Saint S, et al. Is acetylcysteine effective in preventing contrast-related nephropathy? A meta-analysis. Am J Med. 2004;117:938–47. doi: 10.1016/j.amjmed.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 95.Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004;65:1366–74. doi: 10.1111/j.1523-1755.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 96.Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122:874, e9–15. doi: 10.1016/j.amjmed.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 97.Vaitkus PT, Brar C. N-acetylcysteine in the prevention of contrast-induced nephropathy: publication bias perpetuated by meta-analyses. Am Heart J. 2007;153:275–80. doi: 10.1016/j.ahj.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Zagler A, Azadpour M, Mercado C, Hennekens CH. N-acetylcysteine and contrast-induced nephropathy: a meta-analysis of 13 randomized trials. Am Heart J. 2006;151:140–5. doi: 10.1016/j.ahj.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 99.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. Jama. 1996;275:1489–94. [PubMed] [Google Scholar]
- 100.Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–9. doi: 10.1016/j.amjcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 101.From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095–100. doi: 10.4065/83.10.1095. [DOI] [PubMed] [Google Scholar]
- 102.Weisbord SD, Chen H, Stone RA, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17:2871–7. doi: 10.1681/ASN.2006030301. [DOI] [PubMed] [Google Scholar]
- 103.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 104.Shema L, Ore L, Geron R, Kristal B. Contrast-induced nephropathy among Israeli hospitalized patients: incidence, risk factors, length of stay and mortality. Isr Med Assoc J. 2009;11:460–4. [PubMed] [Google Scholar]
- 105.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–8. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 106.Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast-induced nephropathy: implications for prevention strategies. Journal of medical economics. 2007;10:119–34. doi: 10.3111/200710119134. [DOI] [PubMed] [Google Scholar]
- 107.Weisbord SDBF, Saul MI, Chang CH, Palevsky PM. The high costs of acute renal failure following the administration of intravenous contrast media in hospitalized patients. Journal of the American Society of Nephrology. 2002;13:447A. [Google Scholar]
- 108.Weisbord SDBF, Saul MI, Palevsky PM. Provider use of preventive strategies for radiocontrast nephropathy in high risk patients. Nephron-Clinical Practice. 2003;96:c56–c62. doi: 10.1159/000076400. [DOI] [PubMed] [Google Scholar]
- 109.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 110.Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. American journal of nephrology. 2009;29:136–44. doi: 10.1159/000151772. [DOI] [PubMed] [Google Scholar]
- 111.Harjai KJ, Raizada A, Shenoy C, et al. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101:812–9. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 112.Roghi A, Savonitto S, Cavallini C, et al. Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. Journal of cardiovascular medicine (Hagerstown, Md) 2008;9:375–81. doi: 10.2459/JCM.0b013e3282eee979. [DOI] [PubMed] [Google Scholar]
- 113.Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–9. doi: 10.2215/CJN.00550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown JR, Malenka DJ, DeVries JT, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–54. doi: 10.1002/ccd.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–16. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 116.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–9. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 117.Chertow GM, Normand SL, McNeil BJ. "Renalism": inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–8. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 118.Han JH, Chandra A, Mulgund J, et al. Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med. 2006;119:248–54. doi: 10.1016/j.amjmed.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 119.Goldenberg I, Subirana I, Boyko V, et al. Relation between renal function and outcomes in patients with non-ST-segment elevation acute coronary syndrome: real-world data from the European Public Health Outcome Research and Indicators Collection Project. Arch Intern Med. 170:888–95. doi: 10.1001/archinternmed.2010.95. [DOI] [PubMed] [Google Scholar]
- 120.Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 121.Lufft V, Hoogestraat-Lufft L, Fels LM, et al. Contrast media nephropathy: intravenous CT angiography versus intraarterial digital subtraction angiography in renal artery stenosis: a prospective randomized trial. Am J Kidney Dis. 2002;40:236–42. doi: 10.1053/ajkd.2002.34501. [DOI] [PubMed] [Google Scholar]