Abstract
Objectives
The lack of durability in resin–dentine bonds led to the use of chlorhexidine as MMP-inhibitor to prevent the degradation of hybrid layers. Biomimetic remineralisation is a concept-proven approach in preventing the degradation of resin–dentine bonds. The purpose of this study is to examine the integrity of aged resin–dentine interfaces created with a nanofiller-containing etch-and-rinse adhesive after the application of these two approaches.
Methods
The more established MMP-inhibition approach was examined using a parallel in vivo and in vitro ageing design to facilitate comparison with the biomimetic remineralisation approach using an in vitro ageing design. Specimens bonded without chlorhexidine exhibited extensive degradation of the hybrid layer after 12 months of in vivo ageing.
Results
Dissolution of nanofillers could be seen within a water-rich zone within the adhesive layer. Although specimens bonded with chlorhexidine exhibited intact hybrid layers, water-rich regions remained in those hybrid layers and degradation of nanofillers occurred within the adhesive layer. Specimens subjected to in vitro biomimetic remineralisation followed by in vitro ageing demonstrated intrafibrillar collagen remineralisation within hybrid layers and deposition of mineral nanocrystals in nanovoids within the adhesive.
Conclusions
The impact was realized by understanding the lack of an inherent mechanism to remove water from resin–dentine interfaces as the critical barrier to progress in bonding with the etch-and-rinse technique. The experimental biomimetic remineralisation strategy offers a creative solution for incorporating a progressive hydration mechanism to achieve this goal, which warrants its translation into a clinically applicable technique.
Keywords: Ageing, Biomimetic remineralisation, Chlorhexidine, Degradation, Hybrid layer, Matrix metalloproteinase
1. Introduction
Resin–dentine bonds are less durable than resin–enamel bonds.1 The lack of durability in the adhesive joint between resin composites and dentine is one reason that accounts for the short lifetimes of tooth-coloured fillings.2 Replacing deceptive dental fillings costs about five billion dollars in the US alone.3 Bonding to dentine relies on the formation of a hybrid layer to couple adhesives/resin composites to the underlying mineralised dentine.4 In vivo human data accrued over the last few years indicates that dentine hybrid layers created by etch-and-rinse adhesives are susceptible to degradation.5–7 Like all mineralised connective tissues, dentine contains endogenous matrix metalloproteinases (MMPs).8–11 When apatite minerals are dissolved by acid etchants used in dentine bonding, some of the MMPs remain bound to dentine12 and are activated by the acidic resin components incorporated in etch-and-rinse adhesives.13 These activated host-derived MMPs are responsible for the degradation of water-rich resin-sparse collagen matrices within hybrid layers.14 Degradation of the collagen also results in the loss of static mechanical properties of the collagen matrix15 and probably the dynamic mechanical properties of the hybrid layers.
Recent studies demonstrated that degradation of hybrid layers may be prevented by application of chlorhexidine as a MMP-inhibitor to acid-etched dentine before the use of etch-and-rinse adhesives.5,6,16,17 Another potential method for reducing collagen degradation within hybrid layers is to increase the extent of cross-linking of the collagen fibrils prior to adhesive application.18 However, these existing intervention strategies fail to address the critical barrier to progress in dentine bonding—that water entrapped within the intrafibrillar compartments of collagen fibrils cannot be removed after the collagen is embedded by polymerised resins.19,20 A water-filled collagen matrix remains a weak matrix irrespective of how well its integrity is preserved. Water also serves as a functional medium for MMPs when the catalytic sites of these enzymes are not molecularly immobilised by polymerised adhesive resins to block their activities.21 Conversely, progressive dehydration of collagen fibrils by intrafibrillar apatite occurs naturally in hard tissue mineralisation22 and protects the organic matrix from degradation over a much longer time frame than what may be achieved by MMP-inhibitors or collagen cross-linking agents.23 By remineralising the water-rich naked collagen fibrils within the hybrid layer, a more definitive intervention of proteolytic degradation may be achieved through molecular immobilisation of MMPs in a manner that is analogous to what occurs during mineralisation of hard tissues.24,25 This protective mechanism must be recapitulated for long-term survival of resin–dentine bonds.
Biomimetic remineralisation of resin–dentine bonds26 is an in vitro technique that incorporates analogues of dentine matrix proteins for sequestration of amorphous calcium phosphate (ACP) derived from a remineralisation medium into nanophases.27 This particle-mediated self-assembly approach28,29 produces polyanion stabilised nanoprecursors that are small and fluidic enough to infiltrate the internal water compartments of collagen fibrils. Additional analogues that mimic the functional motifs of matrix phosphoproteins bind to collagen and act as templates for guiding the nucleation and growth of apatite within the collagen fibrils.27 As apatites are deposited in the internal water compartments of the collagen fibrils,30 compression of the collagen fibrils31 caused by intrafibrillar remineralisation displaces water and progressively dehydrates the fibrils.32,33 Thus, biomimetic remineralisation of resin–dentine bonds incorporates a progressive dehydration mechanism that recapitulates the collagen protective function of intrafibrillar apatites in natural mineralised tissues and is a potential means in preserving the interfacial integrity of resin–dentine bonds during ageing in an aqueous environment.34
The mechanisms involved in using MMP-inhibition and interfacial remineralisation as strategies for preserving the integrity of resin–dentine interfaces are considerably different. Thus, the objective of the present study was to examine the integrity of aged resin–dentine interfaces created with a nanofiller-containing etch-and-rinse adhesive after the application of these two approaches. The MMP-inhibition approach represents the current standard in the prevention of bond degradation. Thus, it was examined using a parallel in vivo and in vitro ageing design to facilitate comparison with the other less established approach. As the biomimetic remineralisation approach is in its exploratory phase of development, it could not be used clinically and was evaluated using an in vitro ageing design to determine if there are merits that warrant translation of this approach into a clinically applicable technique. The null hypothesis tested was that there are no differences in the manner in which resin–dentine interfaces are preserved following in vivo chlorhexidine application and in vitro biomimetic remineralisation.
2. Materials and methods
2.1. In vivo bonding and in vivo ageing
The clinical subjects in the present study were patients between the age of 12 and 17 (median age 14 years) who undertook orthodontic treatment in the Department of Orthodontics, Benémerita Universidad Autónoma de Puebla, Mexico. The criteria for recruitment were: (a) informed consent from the patients and parents for restorative treatment and the teeth retrieved for research; (b) each subject with a contralateral pair of non-carious, unrestored premolars that permitted conservative Class I cavity preparations to be performed with intact enamel cavosurface margins; (c) both premolars scheduled for extraction as part of the orthodontic treatment plan. An optional criterion was that both restored premolars from a clinical subject had to be subjected to 12 months of intraoral function without compromising the orthodontic treatment results. Those subjects fulfilling all four criteria were scheduled for the in vivo ageing part of the study. Those subjects who fulfilled the first three criteria but could not fulfil the fourth criterion had their premolar pairs restored and extracted immediately for the in vitro ageing or biomimetic remineralisation parts of the study. The aforementioned protocol was reviewed and approved by Human Assurance Committee of the Benémerita Universidad Autónoma de Puebla, Mexico. Eight subjects met all four criteria, resulting in 8 tooth-pairs for in vivo ageing of the restorations. Ten subjects could only fulfil the first three criteria and restorations prepared from those 10 tooth-pairs were used for in vitro ageing or biomimetic remineralisation.
For each of the eight subjects designated for the in vivo ageing part of the study, one member of the tooth-pair was randomly designated as the chlorhexidine group and the other as the non-chlorhexidine group. Each tooth received a 3 mm deep and 2.5 mm wide occlusal cavity preparation under local anaesthesia and rubber dam isolation. The cavity preparation in each control tooth was etched with phosphoric acid for 15 s. For the chlorhexidine group, a 2% chlorhexidine digluconate aqueous solution (Cavity Cleanser, Bisco Inc., Schaumburg, IL, USA) was applied to the acid-etched dentine for 30 s using a cotton pellet to permit binding of the chlorhexidine to the collagen matrix. The etched dentine was gently blot-dried so that the dentine surface remained visibly moist prior to placement of the adhesive. For the non-chlorhexidine group, deionised water was applied to the acid-etched dentine for 30 s using a cotton pellet. For both groups, a two-step nanofiller-containing adhesive (Prime&Bond XP, Dentsply Caulk, Milford, DE, USA) was applied to the acid-etched dentine using a moist bonding technique, gently air-dried to remove the adhesive solvent and light-cured for 20 s using a quartz-tungsten-halogen light-curing unit with an output intensity > 400 mW/cm2. A micro-filled composite (Epic-TMPT, Parkell Inc., Farmington, NY, USA) was placed in 2-mm thick increments and light-cured for 40 s per increment. All sixteen restorations from the eight clinical subjects were subjected to a 12-month period of clinical function prior to extraction and further laboratory processing.
After extraction under local anaesthesia, the root of each tooth was removed at the cementoenamel junction with a high-speed bur with water coolant to facilitate fixative penetration. The crown segments were placed in Karnovsky’s fixative for 4 h, stored in sodium cadcodylate buffer, and shipped to the Medical College of Georgia for transmission electron microscopy (TEM).
For each of the ten subjects designated for the in vitro ageing or biomimetic remineralisation part of the study, one member of the tooth-pair was also randomly designated as the chlorhexidine group and the other as the non-chlorhexidine group. These teeth were bonded and restored using exactly the same protocol employed for the corresponding in vivo ageing groups. These teeth were extracted within 10 min after completion of the restorative procedures. The teeth were stored in 0.9% NaCl containing 0.02% sodium azide to prevent bacteria growth and shipped to the Medical College of Georgia. The premolars of two subjects (i.e. four teeth) were processed immediately for TEM and served as the baseline control for comparison with the 12-month in vivo and in vitro specimens. The other 8 tooth-pairs were used for continuation of the in vitro part of the experiment until the end of the 12-month period before they were fixed in Karnovsky’s fixative and processed for TEM.
2.2. In vivo bonding and in vitro ageing/remineralisation
The 8 immediately extracted premolars from the chlorhexidine group were aged in simulated body fluid (SBF) for 12 months. Each tooth had its root removed at the cementoenamel junction using an Isomet saw (Buehler Ltd, Lake Bluff, IL, USA) under water-cooling. The crown was further sectioned occlusogingivally to create a 1-mm thick central tooth slab for the experiment. The SBF was prepared by dissolving 136.8 mM NaCl, 4.2 mM NaHCO3, 3.0 mM KCl, 1.0 mM K2HPO4·3H2O, 1.5 mM MgCl2·6H2O, 2.5 mM CaCl2 and 0.5 mM Na2SO4 in deionised water35 and adding 3.08 mM sodium azide to prevent bacterial growth. The SBF was buffered to pH 7.4 with 0.1 M Tris Base and 0.1 M HCl and filtered through a 0.22 μm Millipore filter. Each tooth slab from the chlorhexidine group was placed in a glass scintillation vial containing 15 mL of SBF and incubated at 37 °C, with the SBF changed every month for 12 months.
Each contralateral tooth from the non-chlorhexidine group was also sectioned in the same manner to produce a 1-mm thick slab for biomimetic remineralisation. The calcium and hydroxyl ion-releasing source was derived from set white Portland cement (Lehigh Cement Company, Allentown, PA, USA). The latter mixed with deionised water (water-to-powder ratio 0.35:1) and allowed to set in silicone moulds for one week before use. A remineralisation medium was prepared, consisting of SBF with 100–5000 μg/mL of polyacrylic acid and polyvinylphosphonic acid (Sigma–Aldrich, St. Louis, MO, USA) as the respective biomimetic sequestration analogue and templating analogue of dentine matrix proteins.27 The remineralisation medium was also buffered to pH 7.4.
Each tooth slab from the non-chlorhexidine group was placed over a set Portland cement block (ca. 1 g) inside a glass scintillation vial. The latter was filled with 15 mL of remineralisation medium and incubated at 37 °C. The medium was changed monthly, with its pH monitored weekly so that it was above 9.25 to facilitate formation of apatite instead of octacalcium phosphate.36 Biomimetic remineralisation was performed with monthly replacement of the remineralisation medium for 4 months. Thereafter, the Portland cement block and remineralisation medium were replaced with SBF for continuous in vitro ageing, with monthly replacement of the SBF for the rest of the ageing period. As polyvinylphosphonic acid, the templating analogue in the remineralisation medium is an effective MMP-inhibitor,37 no additional MMP-inhibitor was employed to prevent collagen degradation during the remineralisation period.
2.3. Transmission electron microscopy
For the baseline control groups and chlorhexidine and non-chlorhexidine groups that had undergone in vivo ageing, a 1-mm thick central slab was prepared from each tooth as previously described. Half of the tooth slabs in the respective group were completely demineralised in a formic acid/sodium formate buffer for two weeks, with the end point of demineralisation determined using digital radiography. These demineralised specimens were used to examine the status of collagen degradation within hybrid layers. The specimens were fixed in Karnovsky’s fixative and post-fixed in 1% osmium tetroxide. They were then dehydrated in an ascending ethanol series (50–100%), immersed in propylene oxide as the transitional medium and embedded in epoxy resin. Ninety nanometre thick sections were doubled-stained with 2% uranyl acetate and Reynold’s lead citrate and examined with a JEM-1230 TEM (JEOL, Tokyo, Japan) at 110 kV.
The rest of the tooth slabs in each respective group were not demineralised and were used to examine potential water-filled regions within the resin–dentine interfaces using a silver tracer technique.38 The tooth slabs were immersed in a 50 wt% aqueous ammoniacal AgNO3 tracer solution for 48 h. They were then rinsed with deionised water for 30 min, placed in a photodeveloping solution for 8 h under fluorescent light to reduce the diamine silver ions ([Ag(NH3)2]+) into metallic silver grains within the water-filled, resin-sparse regions of the hybrid layer. Thereafter, the silver-impregnated slabs were processed for TEM in the manner previously described. Non-demineralised, 90–110 nm thick sections were prepared and examined without further staining at 110 kV.
Specimens that had been subjected to 12 months of in vitro ageing or biomimetic remineralisation were not demineralised or silver-impregnated to prevent the loss of minerals or misinterpretation of silver deposits as remineralisation. Non-demineralised, 90–110 nm thick sections were prepared and examined without further staining at 110 kV.
3. Results
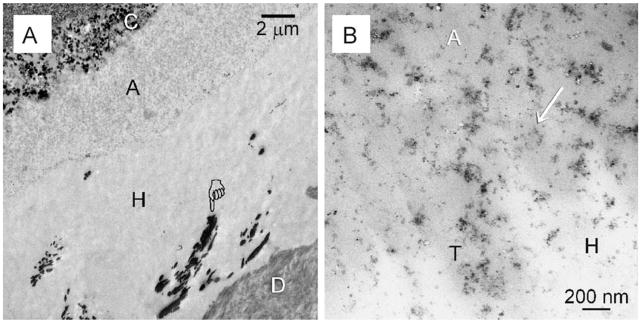
Baseline control specimens extracted immediately after bonding demonstrated intact hybrid layers with randomly distributed water-rich, resin-sparse regions. The latter were identified by the silver tracer in non-demineralised sections (Fig. 1A). Similar features were seen irrespective of whether the acid-etched dentine was pre-treated with chlorhexidine as MMP-inhibitor. Nanofiller clusters could be identified within the entire adhesive layer (Fig. 1B).
Fig. 1.
TEM images taken from unstained non-demineralised sections taken from the baseline control groups that were extracted immediately without ageing. This specimen was bonded using 2% chlorhexidine as a MMP inhibitor. The specimen was immersed in a silver nitrate tracer prior to TEM processing. (A) Silver-impregnated water-rich, resin-sparse regions (pointer) could be identified within the hybrid layer (H). C: composite; A: filled adhesive; D: dentine. Similar features were observed in the baseline control of premolars that were bonded without the use of chlorhexidine (not shown). (B) High magnification of (A). Nanofiller clusters (arrow) from the adhesive (A) could be seen in close proximity with the surface of the hybrid layer (H). T: dentinal tubule.
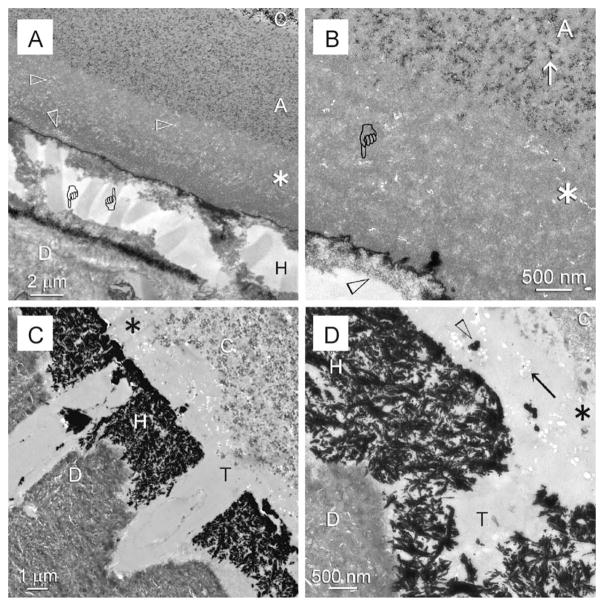
Specimens bonded without the use of chlorhexidine exhibited extensive degradation of the hybrid layer (Fig. 2A) in demineralised sections after 12 months of in vivo ageing. The degraded collagen fibrils within the hybrid layer were either completely destroyed or appeared as unravelled microfibrillar strands that were devoid of cross-banding patterns. Dissolution of the nanofillers could also be seen within a 3–5 μm thick zone of the adhesive layer that was located directly above the hybrid layer (Fig. 2B). Such a phenomenon occurred regardless of the thickness of the adhesive. That is, resin–dentine interfaces with adhesive layers thinner than 3–5 μm exhibited dissolution of nanofillers within the entire adhesive layer. Conversely, interfaces with adhesive layers thicker than 3–5 μm exhibited two zones within the adhesive layer, a nanofiller-depleted zone adjacent to the hybrid layer and a normal nanofiller-containing zone on top of the nanofiller-depleted zone. Examination of the silver-impregnated non-demineralised sections revealed extensive water-filled spaces within the degraded hybrid layer (Fig. 2C). The presence of silver-filled water channels that extended from the surface of the degraded hybrid layer into the adhesive was further indicative of a water-rich zone within the superficial part of the adhesive. Hydrolysis of the silica nanofillers resulted in the appearance of voids within the adhesive, which were partially impregnated with silver deposits (Fig. 2D).
Fig. 2.
TEM of specimens from the non-chlorhexidine group that were retrieved after 12 months of intraoral function. C: composite; A: filled adhesive; D: dentine. (A) Stained demineralised specimen showing that a degraded hybrid layer (H) with remnant denatured collagen (pointers) attached to the adhesive and underlying dentine. A void-bearing (open arrowheads), nanofiller-depleted zone was apparent in the basal part of the adhesive (asterisk) that was adjacent to the hybrid layer. (B) High magnification of (A). Nanofiller clusters (arrow) could be seen within the composite side but were absent from the hybrid layer side (asterisk) of the adhesive (A), where nanovoids (pointer) were observed in lieu of the nanofillers. Collagen within the remnant hybrid layer was grossly denatured (open arrowhead). (C) Unstained, non-demineralised, silver-impregnated specimen showing extensive silver deposits within the space occupied by the hybrid layer (H). As the adhesive layer was thin, the entire layer was devoid of nanofillers (asterisk) T: dentinal tubule. (D) High magnification of (C) showing clusters of nanovoids (arrow) within the nanofiller-depleted adhesive layer (asterisk). Some of these nanovoids were impregnated with silver deposits (open arrowhead). T: dentinal tubule.
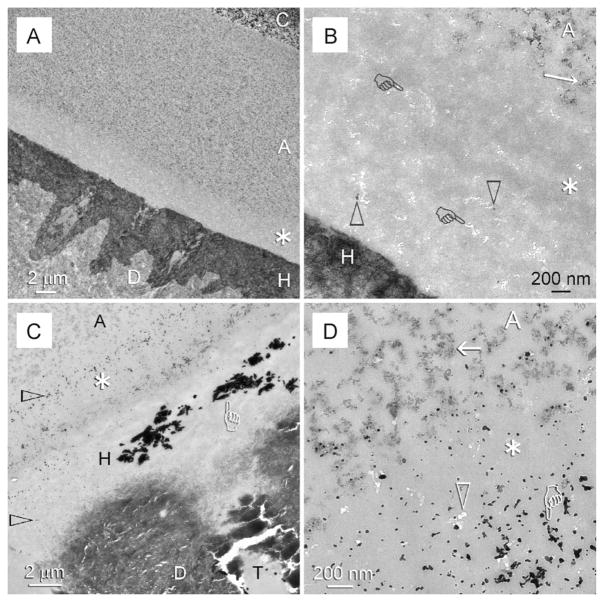
Stained demineralised sections prepared from specimens bonded with the adjunctive use of chlorhexidine as MMP-inhibitor demonstrated an intact hybrid layer after 12 months of in vivo ageing (Fig. 3A). However, the use of chlorhexidine did not eliminate water from the resin–dentine interface and hence did not prevent hydrolysis of the nanofillers from the 3–5 μm thick layer of adhesive that juxtaposed the water-rich surface of the hybrid layer (Fig. 3B). In silver-impregnated non-demineralised sections, silver-filled water-rich regions similar to those identified from the baseline control specimens could be observed within the hybrid layers (Fig. 3C). In addition, silver deposits were abundant in the nanovoids created by hydrolysis of nanofillers within the water-rich part of the adhesive (Fig. 3D).
Fig. 3.
TEM of specimens from the chlorhexidine group that were retrieved after 12 months of intraoral function. C: composite; A: filled adhesive; D: dentine. (A) Stained demineralised specimen with an intact hybrid layer (H). Although chlorhexidine prevented degradation of the collagen component of the hybrid layer, water entrapment within the resin–dentine interface resulted in a nanofiller-depleted zone (asterisk) within the basal portion of the adhesive. (B) High magnification of the nanofiller-depleted zone in (A). Nanofillers (open arrowheads) were almost completely dissolved, leaving behind clusters of nanovoids (pointers) within the adhesive resin matrix. By contract, nanofiller clusters (arrow) were readily observed within the top part of the adhesive (A). (C) An unstained, non-demineralised specimen from the same group showing silver-filled, water-rich regions (pointer) within the intact hybrid layer (H). Additional silver grains (open arrowheads) could be seen within the hybrid layer and the nanofiller-depleted zone of the adhesive (asterisk). T: dentinal tubule. (D) High magnification of (C) showing silver deposits (pointer) in nanovoids (open arrowhead) of the nanofiller-depleted zone (asterisk) of the adhesive (A). Intact nanofiller clusters (arrow) could be seen within the adhesive that was further away from the hybrid layer and dentinal tubules, where they were less susceptible to water hydrolysis during in vivo ageing.
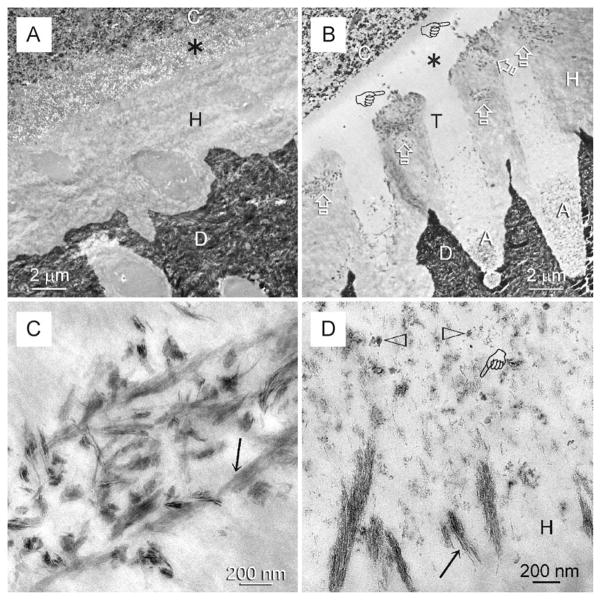
Specimens bonded in vivo with chlorhexidine also demonstrated intact hybrid layers after 12 months of in vitro ageing in SBF. Dissolution of nanofillers within the superficial water-rich part of the adhesive (Fig. 4A) was also similar to what was observed in the in vivo-aged specimens. Specimens bonded in vivo without chlorhexidine exhibited remineralisation zones within and along the surface of the hybrid layers after 4 months of in vitro biomimetic remineralisation followed by 8 months of in vitro ageing in SBF (Fig. 4B). Within the remineralised regions of the hybrid layer, collagen fibrils with intrafibrillar remineralisation were identified (Fig. 4C), corresponding with those water-rich, resin-sparse regions of the hybrid layers present in the silver-impregnated baseline and in vivo-aged specimens. Although the adhesive layer on top of the hybrid layer and resin tags around the dentinal tubular orifices were devoid of nanofillers, no voids could be seen in these regions (Fig. 4B) due to filling of those voids with nanocrystals that were similar to those present in the remineralised collagen fibrils (Fig. 4D).
Fig. 4.
TEM of unstained, non-demineralised specimens bonded in vivo with the same adhesive and extracted for in vitro ageing or biomimetic remineralisation. C: composite; A: filled adhesive; D: dentine. (A) A representative example of a specimen that was bonded in vivo with the use of chlorhexidine as an MMP-inhibitor and subjected to 12 months of in vitro ageing after extraction. Although the hybrid layer was intact, extensive dissolution of the nanofillers (asterisk) occurred within the thin adhesive layer. (B) A representative example of a contralateral specimen that was bonded in vivo without chlorhexidine and after extraction, subjected to 4 months of in vitro biomimetic remineralisation followed by 8 months of in vitro ageing in SBF. Remineralisation was evident (open arrows) within the hybrid layer and the water channels (pointers) that extended from the hybrid layer surface into the adhesive. Although the thin adhesive layer between the composite and the hybrid layer and within the dentinal tubule orifices (T) was devoid of nanofillers, no voids could be seen within those regions (asterisk). Conversely, nanofillers could be identified from that part of the adhesive (A) located beneath the tubular orifices. (C) High magnification of the remineralised part of the hybrid layer in (B) showing intrafibrillar remineralisation of the collagen fibrils (arrow). This suggests that the intrafibrillar compartments of those fibrils were occupied initially by water instead of adhesive resin. (D) High magnification of the surface of the hybrid layer (H) in (B). Nanofillers were sparse (open arrowheads) within the overlying adhesive and nanovoids generated by the dissolution of nanofillers were filled with nanocrystals (pointer) similar to those found within the remineralised collagen fibrils along the surface of the hybrid layer (arrow).
4. Discussion
The ultrastructural results in the present study demonstrated that both the use of chlorhexidine for MMP-inhibition and biomimetic remineralisation for filling water-filled spaces could effectively preserve the integrity of the hybrid layer over time. Prevention of degradation of the hybrid layer with chlorhexidine was in accordance with the results of previous in vivo studies.5,6 However, nanofillers in teeth bonded with chlorhexidine were almost completely dissolved after in vivo and in vitro ageing, leaving behind clusters of nanovoids within the basal portion of the adhesive layer. For the biomimetic remineralisation approach, intrafibrillar remineralisation of the collagen fibrils was evident within the hybrid layer after in vitro ageing. Although dissolution of the nanofillers also occurred within the adhesive layer and within the resin that occupied the tubular orifices, no voids could be seen within those nanofillers-depleted zones as they were filled by mineral nanocrystals. In terms of preventing the degradation of the hybrid layer, both approaches yielded similar results. In terms of preserving the integrity of the resin–dentine interfaces, the use of chlorhexidine as MMP-inhibitor did not replace the apatites that were initially removed from dentine but could not be completely replaced by adhesive resin. Thus, water-filled spaces within the hybrid layer that were initially present at the baseline were still present after in vivo ageing. The use of chlorhexidine as MMP-inhibitor also did not replace the nanofillers that were depleted from the adhesive after in vivo and in vitro ageing. From these perspectives, the postageing manifestations of the resin–dentine interfaces are different with the use of the two approaches. Thus, the null hypothesis that there are no differences in the status in which resin–dentine interfaces are preserved following in vivo chlorhexidine application and in vitro biomimetic remineralisation has to be rejected.
Contemporary dentine bonding technologies have achieved as much as they can in replacing minerals depleted from the extrafibrillar compartments of collagen matrices with resins. In terms of the results achieved compared with previous adhesive generations, the progress has insofar been phenomenal. However, the critical barrier to progress in contemporary dentine bonding is that both etch-and-rinse and self-etching approaches in establishing retention in dentine are hampered by their inability to completely replace free and loosely bound water from various internal water compartments39 of the collagen fibrils. Until recently, it is not known whether dentine adhesives are capable of infiltrating the intrafibrillar spaces of collagen fibrils to create a continuum with those resins that occupy the extrafibrillar spaces. This question has been raised for more than ten years4 but is generally perceived to be a philosophical issue with limited clinical relevance. Indeed, over the past decade, the term “nanoleakage”40 has taken on a life of its own in attempts to compare the status of resin infiltration within dentine or predict bonding success.41 Considering the fact that practically all of those studies consistently showed “nanoleakage” irrespective of whether hybrid layers were created with etch-and-rinse or self-etching adhesives, it is surprising that manufacturers were not alerted to the implicit message emancipating from those studies.
Resin–dentine bonding with etch-and-rinse adhesives is a crude form of biomimicry that depletes the mineral phase from dentine for the sake of “micromechanical retention”. The loss of intrafibrillar apatite from collagen without its appropriate replacement causes deterioration in the mechanical properties of mineralised tissues.42 Phase transition of the apatite-depleted collagen from insoluble collagen to more biodegradable gelatine represents the point-of-no-return in the survival of archaeological hard tissues.43 Collagen–gelatine transition also has a major adverse impact on the physical properties of bone.44 This irreversible transition is prevented by the presence of minerals45,46 and the close packing of collagen molecules in collagen fibrils.47 Both of these protective mechanisms are disrupted31 by techniques associated with contemporary dentine bonding. Progressive replacement of water by intrafibrillar apatite is a natural process in osteogenesis and dentineogenesis that preserves the integrity of collagen and other non-collagenous proteins. When protected by apatite, collagen and non-collagenous proteins in bone may remain intact for up to 50k years.48 This natural mode of protection highlights the critical barrier to progress in contemporary dentine adhesive technologies—they are incapable of completely replacing water from the intrafibrillar compartments of the collagen fibrils.
The experimental “ethanol wet bonding” technique involves progressive replacement of interfibrillar and intrafibrillar non-structural water by ethanol.49 Despite its philosophical nature, this bonding technique is able to produce durable resin–dentine bonds after 12 months19 and 18 months of in vitro ageing.50 Another study showed that when ethanol wet bonding was meticulously followed, there was no intrafibrillar remineralisation of collagen fibrils within hybrid layers19 using the same biomimetic remineralisation strategy employed in the present study. This is because resin has already replaced all non-structural water from the intrafibrillar water compartments and there is no room for the deposition of apatite. On the contrary, the observation of intrafibrillar remineralisation in collagen fibrils within hybrid layers in the present study provided indirect evidence that dentine adhesive applied to water-saturated demineralised dentine was incapable of infiltrating the intrafibrillar compartments of the collagen fibrils.
The observation that adhesive nanofillers partially disappeared in teeth bonded with/without chlorhexidine application implies that such a phenomenon is governed by a universal degradation mechanism which is unrelated to the MMP-inhibiting activity of chlorhexidine. The use of a silica nanofiller-containing adhesive in the present study provided a unique opportunity for discerning a phenomenon that is usually difficult to be recognised in morphologic work without resorting to chemoanalytical techniques—water sorption-induced hydrolysis of adhesive components. Hydrolytic degradation of pyrogenic silica fillers occurs in resin composites in spite of silane treatments.51 It is important to note that degradation of the silanised silica nano-particles in the present study was limited to the region immediately above the hybrid layer. The results suggest that this region is a water-rich zone. The adhesive nanofillers are pyrogenic hydrophilic silica nanoparticles that have been functionalised with a comparatively hydrophobic silane coupling agent.52 As these silica nanoparticles possess a very large specific surface area (380 m2/g),52 it is possible that silane coverage of the silica surface is incomplete and that residual silanol groups are present on the silica surface53 which are susceptible to hydrolysis54,55 after water sorption. The Si–O–Si bond that forms between the silane coupling agent and the mineral filler is also vulnerable to hydrolysis.56
It must be stressed that nanofiller degradation occurred irrespective of whether the resin-interfaces were subjected by MMP-inhibition or biomimetic remineralisation; the only difference between the two protocols being the manner in which the nanovoids created by the loss of nanofillers were subsequently filled (i.e. water versus mineral nanocrystals). Biomimetic remineralisation is not a process that prevents water sorption and hydrolysis of adhesive components. As specimens that were subjected to biomimetic remineralisation were bonded with the same hydrophilic adhesive, it is reasonable to perceive that water sorption or hydrolysis occurred to a similar extent when remineralisation was conducted in an aqueous medium. These processes are associated with the inherent chemistry of the adhesive and not related to the approach employed to prevent degradation of the resin–dentine interfaces. The potential consequence of adhesive nanofiller degradation on the durability of resin–dentine bonds is currently unknown. The nanovoid clusters that remain after the dissolution of nanofillers may be interconnecting and act as water channels for hydrolysis/leaching of hydrophilic resin components from the resin–dentine interface. Nanovoids were not present after the remineralised specimens were subjected to further in vitro ageing. This is probably because water sorption in hydrophilic resins occurs rapidly57 and most of the water sorption occurs during the first month.58 Filling of nanovoids by mineral nanocrystals fortifies the concept of progressive intrafibrillar water displacement within collagen fibrils, in resin–dentine interfaces that had been subjected to the biomimetic remineralisation scheme.34
The experimental biomimetic remineralisation strategy offers a solution for incorporating a “self-healing mechanism” by mimicking nature to enhance performance, provided that different components of the strategy can be successfully incorporated into the acid-etched dentine prior to bonding. The use of a proactive “self-healing” strategy for rejuvenating aged polymer composites is not without precedence and is a highly desirable goal in contemporary biomaterials research.59,60 One of the main challenges in biomimetics is that it demands creative solutions. Nature’s store of ideas is valuable only if it can be translated into usable technology, particularly in terms of processing methods. In the translation of the proof-of-concept biomimetic remineralisation approach into a clinically applicable technique, the authors anticipate the use of collagen size exclusion principles and the controlled release technology for delivering biomimetic analogues to the acid-etched dentine prior to the application of the adhesive. The remineralisation component may be delivered in the form of polyanionstabilised amorphous calcium phosphate nanophases and silicic acid to the acid-etched dentine prior to dentine bonding.61 Such a strategy ensures that the remineralising components are readily available at the base of the hybrid layers instead of relying on ionic diffusion through a polymerised adhesive layer. Investigations on these procedures are in order, to facilitate translation of the remineralisation approach from merely a creative solution into a pragmatic clinically applicable technique.
5. Conclusion
In the grand scheme of things, prevention of the degradation of denuded, water-filled collagen with the use of MMP-inhibitors or MMP-inhibitor-conjugated resin monomers represents the state-of-the-art for extending the longevity of resin–dentine bonds. Such an approach was developed during the era when the critical barrier to progress in dentine bonding was not completely understood. Inhibition of collagen degradation without removing the underlying cause of degradation (i.e. water) is analogous to the role played by palliative medicine in extending life expectancy. Palliative medicine is extremely valuable when the cause of a disease is unknown or when a definitive treatment method is unavailable. Preventing hybrid layer degradation with the use of MMP-inhibitors or MMP-inhibitor-conjugated resin monomers will continue to be the predominant method for extending the longevity of resin–dentine bonds until a more proactive solution becomes clinically available. Likewise, the use of this strategy is desirable to prevent degradation of naked collagen before biomimetic remineralisation can progressively dehydrate the collagen matrix and replace the water with intrafibrillar apatites, and extrafibrillar apatites where applicable. Chlorhexidine is not the only MMP-inhibitor that is useful for dentine bonding, as PVPA, one of the biomimetic analogues employed in the experimental remineralisation approach, also possesses anti-MMP potential.37
The results of the present study highlight the critical barrier to progress in contemporary dentine bonding, at least, with the use of etch-and-rinse adhesives. Whilst the observation of nanofiller degradation may be viewed upon as a phenomenological event exhibited by one particular adhesive, it points to an already known feature in dentine bonding—that a water-rich zone exists along the resin–dentine interface62 that is precipitated by the increased hydrophilicity of contemporary dentine adhesives.63
Acknowledgments
The in vivo part of this study was funded by the Benémerita Universidad Autónoma de Puebla, Mexico. The in vitro part of the study was supported by the National Institute of Dental and Craniofacial Research. Specifically, the funds were used to support transmission electron microscopy (R01 DE015306-06; PI. David H. Pashley) and biomimetic remineralisation of resin–dentine bonds (R21 DE019213-01; PI. Franklin R. Tay). We thank Michelle Barnes for her secretarial support and Thomas Bryan for his technical support.
References
- 1.Loguercio AD, Moura SK, Pellizzaro A, Del-Bianco K, Patslaff RT, Grande RHM, et al. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Operative Dentistry. 2008;33:79–88. doi: 10.2341/07-42. [DOI] [PubMed] [Google Scholar]
- 2.Abt E. The risk of failure is higher for composites than for amalgam restorations. The Journal of Evidence-Based Dental Practice. 2008;8:83–4. doi: 10.1016/j.jebdp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Projects 2–95. International Dental Journal. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Chicago: Quintessence Publishing Co., Ltd; 1998. pp. 1–17. [Google Scholar]
- 5.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 6.Carrilho MRO, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 7.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Operative Dentistry. 2009;34:379–83. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 8.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 9.Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Archives of Oral Biology. 2008;53:109–16. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos J, Carrilho M, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, et al. Determination of matrix metalloproteinases in human radicular dentin. Journal of Endodontics. 2009;35:686–9. doi: 10.1016/j.joen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Toledano M, Nieto-Aguilar R, Osorio R, Campos A, Osorio E, Tay FR, et al. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. Journal of Dentistry. 2010;38:635–40. doi: 10.1016/j.jdent.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, et al. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. Journal of Biomedical Materials Research Part A. 2009;88:697–703. doi: 10.1002/jbm.a.31920. [DOI] [PubMed] [Google Scholar]
- 13.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 15.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dental Materials. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YB, Li Y, Yao K, Liang GB. Effect of concentration of chlorhexidine on bonding durability of dentine and resin. Zhonghua Kou Qiang Yi Xue Za Zhi. 2010;45:94–7. [PubMed] [Google Scholar]
- 18.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2009;91:419–24. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadek FT, Castellan CS, Braga RR, Mai S, Tjäderhane L, Pashley DH, et al. One-year stability of resin–dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dental Materials. 2010;26:380–6. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim JR, Gu L, Breschi L, Tjäderhane L, Choi KK, Pashley DH, et al. Implication of ethanol wet bonding in hybrid layer remineralization. Journal of Dental Research. 2010 doi: 10.1177/0022034510363380. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutivet A, Schembri C, Kovensky J, Haupt K. Molecularly imprinted microgels as enzyme inhibitors. Journal of the American Chemical Society. 2009;131:14699–702. doi: 10.1021/ja901600e. [DOI] [PubMed] [Google Scholar]
- 22.Magne D, Weiss P, Bouler JM, Laboux O, Daculsi G. Study of the maturation of the organic (type I collagen) and mineral (nonstoichiometric apatite) constituents of a calcified tissue (dentin) as a function of location: a Fourier transform infrared microspectroscopic investigation. Journal of Bone and Mineral Research. 2001;16:750–7. doi: 10.1359/jbmr.2001.16.4.750. [DOI] [PubMed] [Google Scholar]
- 23.Collins MJ, Nielsen-Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, et al. The survival of organic matter in bone: a review. Archaeometry. 2002;44:383–94. [Google Scholar]
- 24.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. Journal of Dental Research. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Carpiaux W, Fan D, Fan Y, Lakshminarayanan R, Moradian-Oldak J. Apatite reduces amelogenin proteolysis by MMP-20 and KLK4 in vitro. Journal of Dental Research. 2010;89:344–8. doi: 10.1177/0022034509360660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay FR, Pashley DH. Biomimetic remineralization of resin-bonded acid-etched dentin. Journal of Dental Research. 2009;88:719–24. doi: 10.1177/0022034509341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–37. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Meldrum FC, Cölfen H. Controlling mineral morphologies and structures in biological and synthetic systems. Chemical Reviews. 2008;108:4332–432. doi: 10.1021/cr8002856. [DOI] [PubMed] [Google Scholar]
- 29.Pouget EM, Bomans PH, Goos JA, Frederik PM, de With G, Sommerdijk NA. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science. 2009;323:1455–8. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 30.Fullerton GD, Nes E, Amurao M, Rahal A, Krasnosselskaia L, Cameron I. An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biology International Reports. 2006;30:66–73. doi: 10.1016/j.cellbi.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Bonar LC, Lees S, Mook HA. Neutron diffraction studies of collagen in fully mineralized bone. Journal of Molecular Biology. 1985;181:265–70. doi: 10.1016/0022-2836(85)90090-7. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water content measured by proton–deuteron exchange NMR predicts bone mineral density and mechanical properties. Journal of Bone and Mineral Research. 2004;19:289–96. doi: 10.1359/JBMR.0301227. [DOI] [PubMed] [Google Scholar]
- 33.Chesnick IE, Mason JT, Giuseppetti AA, Eidelman N, Potter K. Magnetic resonance microscopy of collagen mineralization. Biophysical Journal. 2008;95:2017–26. doi: 10.1529/biophysj.107.120923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, et al. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices—implications in the aging of resin–dentin bonds. Acta Biomaterialia. 2010 doi: 10.1016/j.actbio.2010.03.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. Journal of Biomedical Materials Research. 1990;24:721–34. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 36.Meyer JL, Eanes ED. A thermodynamic analysis of the secondary transition in the spontaneous precipitation of calcium phosphate. Calcified Tissue Research. 1978;25:209–16. doi: 10.1007/BF02010771. [DOI] [PubMed] [Google Scholar]
- 37.Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphonic acid on functional MMP activities in human demineralized dentin. Acta Biomaterialia. 2010 doi: 10.1016/j.actbio.2010.05.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. Journal of Dental Research. 2002;81:472–6. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 39.Cameron IL, Short NJ, Fullerton GD. Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type 1 collagen. Cell Biology International Reports. 2007;31:531–9. doi: 10.1016/j.cellbi.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Pioch T, Staehle HJ, Duschner H, García-Godoy F. Nanoleakage at the composite-dentin interface: a review. American Journal of Dentistry. 2001;14:252–8. [PubMed] [Google Scholar]
- 41.Tagami J, Nikaido T, Nakajima M, Shimada Y. Relationship between bond strength tests and other in vitro phenomena. Dental Materials. 2010;26:e94–9. doi: 10.1016/j.dental.2009.11.156. [DOI] [PubMed] [Google Scholar]
- 42.Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. Journal of Structural Biology. 2008;62:404–10. doi: 10.1016/j.jsb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Child AM. Towards an understanding of the decomposition of bone in the archaeological environment. Journal of Archaeological Science. 1995;22:165–74. [Google Scholar]
- 44.Wang X, Bank RA, TeKoppele JM, Hubbard GB, Athanasiou KA, Agrawal CM. Effect of collagen denaturation on the toughness of bone. Clinical Orthopaedics and Related Research. 2000;371:228–39. doi: 10.1097/00003086-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Kronick PL, Cooke P. Thermal stabilization of collagen fibers by calcification. Connective Tissue Research. 1996;33:275–82. doi: 10.3109/03008209609028885. [DOI] [PubMed] [Google Scholar]
- 46.Trebacz H, Wójtowicz K. Thermal stabilization of collagen molecules in bone tissue. International Journal of Biological Macromolecules. 2005;37:257–62. doi: 10.1016/j.ijbiomac.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophysical Journal. 1999;76:3243–52. doi: 10.1016/S0006-3495(99)77476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CI, Craig OE, Prigodich RV, Nielsen-Marsh CM, Jans MME, Vermeer C, et al. Diagensis and survival of osteocalcin in archaeological bone. Journal of Archaeological Science. 2005;32:105–13. [Google Scholar]
- 49.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. American Journal of Dentistry. 2007;20:7–20. [PubMed] [Google Scholar]
- 50.Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. Journal of Dental Research. 2010;89:1499–504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. Journal of Dental Research. 1984;6:1248–54. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 52.Pflug K. Dental materials having a nanoscale filler. 6,693,143. US patent. 2004
- 53.Donnet JB, Ridaoui H, Balard H, Barthel H, Gottschalk-Gaudig T. Evolution of the surface polar character of pyrogenic silicas, with their grafting ratios by dimethylchlorosilane, studied by microcalorimetry. Journal of Colloid and Interface Science. 2008;325:101–6. doi: 10.1016/j.jcis.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Ma Y, Foster AS, Nieminen RM. Reactions and clustering of water with silica surface. Journal of Chemical Physics. 2005;122:144709, 1–9. doi: 10.1063/1.1878652. [DOI] [PubMed] [Google Scholar]
- 55.Rimer JD, Trofymluk O, Navrotsky A, Lobo RF, Vlachos DG. Kinetic and thermodynamic studies of silica nanoparticle dissolution. Chemistry of Materials. 2007;19:4189–97. [Google Scholar]
- 56.Antonucci JM, Dickens SH, Fowler BO, Xu HHK. Chemistry of silanes: interfaces in dental polymers and composites. Journal of Research of the National Institute of Standards and Technology. 2005;110:541–58. doi: 10.6028/jres.110.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–59. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 58.Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, et al. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–96. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 59.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, et al. Autonomic healing of polymer composites. Nature. 2001;409:794–9. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 60.Trask RS, Williams HR, Bond IP. Self-healing polymer composites: mimicking nature to enhance performance. Bioinspiration & Biomimetics. 2007;2:1–9. doi: 10.1088/1748-3182/2/1/P01. [DOI] [PubMed] [Google Scholar]
- 61.Kim YK, Gu LS, Bryan TE, Kim JR, Chen L, Liu Y, et al. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials. 2010;31:6618–27. doi: 10.1016/j.biomaterials.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CK. Water treeing in simplified dentin adhesives—déjà vu? Operative Dentistry. 2005;30:561–79. [PubMed] [Google Scholar]
- 63.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? Journal of the Canadian Dental Association. 2003;69:726–31. [PubMed] [Google Scholar]