Abstract
Objective
We investigated how study type, mean patient age, and amount of contact with research staff affected response rates to medication and placebo in acute antidepressant trials for pediatric depression.
Method
Data were extracted from 9 open, 4 active comparator, and 18 placebo-controlled studies of antidepressants for children and adolescents with depressive disorders. A multilevel meta-analysis examined how study characteristics affected response rates to antidepressants and placebo.
Results
The primary finding was a main effect of study type across patient age and contact amount, such that the odds of medication response were greater in open studies vs. placebo-controlled (OR 1.87, 95% CI 1.17 – 2.99, p = 0.012) and comparator studies (OR 2.01, 95% CI 1.16 – 3.48, p = 0.015) but were not significantly different between comparator and placebo-controlled studies. No significant main effects of patient age or the amount of contact with research staff were found for the analyses of response rates to medication and placebo. Response to placebo in placebo-controlled trials did significantly increase with the amount of therapeutic contact in older patients (age*contact OR 1.08, 95% CI 1.01 – 1.15, p = 0.038).
Conclusions
Whereas patient expectancy strongly influences response rates to medication and placebo in depressed adults, it appears to be less important in the treatment of children and adolescents with depression. Attempts to limit placebo response and improve the efficiency of antidepressant trials for pediatric depression should focus on other causes of placebo response apart from expectancy.
Keywords: Major Depressive Disorder, expectancy, placebo effect, randomized controlled trial, antidepressant
INTRODUCTION
Depressive disorders are common and impairing conditions in childhood and adolescence. Major Depressive Disorder (MDD) alone affects 2% of children and 4–8% of adolescents in the general population, and an additional 5–10% of young people have subsyndromal symptoms.1 Individuals with depressive disorders are at elevated risk of other psychiatric disorders, with 40–90% suffering from comorbid anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), or substance abuse disorders.2 Complications of pediatric depression include adverse effects on psychological development, elevated risk of suicide attempts and completion, and poor work, academic, and social functioning.3
The efficacy of pharmacologic treatment in pediatric depressive disorders remains unclear, as the majority of placebo-controlled randomized controlled trials (RCTs) of antidepressant medication do not show a significant benefit of medication over placebo.4 The high frequency of these so-called “failed trials” in children and adolescents appears to be a consequence of elevated response to placebo rather than a reduced medication response.5 A recent meta-analysis found that response to placebo (with mean rate of 48%) explains most of the variability between positive and negative trials, as opposed to the response to active medication (mean rate of 59%).6 From a research perspective, identifying mechanisms of placebo response in children and adolescents might produce methodological changes in RCTs to minimize placebo response and facilitate signal detection for active treatments. Conversely, from a clinical perspective, maximizing placebo response may safely and effectively enhance the treatment of depression in children and adolescents.
In adult populations, evidence suggests that patient expectancy, which refers to patients' beliefs about how treatment will affect them, is the major determinant of placebo effects in antidepressant RCTs.7 A series of meta-analyses have evaluated antidepressant response in comparator (i.e., one medication vs. a different medication) as opposed to placebo-controlled antidepressant RCTs.8–9 In 48 placebo-controlled and 42 comparator trials of antidepressants for MDD in adults aged 18–65, the odds of responding to medication in comparator trials were 1.8 times the odds in placebo-controlled trials (95% CI = 1.45 – 2.17, p < 0.001).8 Similarly, in RCTs for patients with late life depression, the odds of medication response in comparator trials were nearly two times the odds in placebo-controlled trials.9 The elevated medication response rates in comparator trials may be explained by increased expectancy among participants in these studies,10 since greater expectancy has been linked to the improvement of depressive symptoms in clinical trials.11–12 Patients in comparator trials know they have a 100% chance of receiving an active medication despite not knowing the exact agent, whereas patients in placebo-controlled trials do not know whether they are receiving an active medication.
Whether patient expectancy of improvement influences the outcome of antidepressant treatment in children and adolescents is unknown. Given that expectancy powerfully affects antidepressant response in both younger and older adults with MDD, it is reasonable to hypothesize that it also influences medication response in children and adolescents with depression. However, treatment expectancies are cognitions that may be less developed in younger people. In addition, adults entering an antidepressant RCT receive information about the study design, the history and past effectiveness of the drugs used in the study, and the investigator's opinions of the treatment options. In RCTs for pediatric depression, the parent or legal guardian provides informed consent, while the patient assents to research participation.
Another source of the large observed placebo response in younger patients may be the therapeutic effects of meeting with health professionals and research staff. Patients in research studies are provided with diagnoses that conceptualize and explain their symptoms, psycho-education about the causes and course of depression, medical work up and monitoring, and encouragement to continue treatment.13 These actions foster patients' confidence in the ability of study clinicians and medication to help them, which may influence their reported symptoms. Recent studies of antidepressants for pediatric MDD have attempted to account for these effects by utilizing extended screening phases to identify participants whose symptoms resolve with the initial therapeutic contacts, but the utility of these approaches for diminishing placebo response is unclear.
In this study we examined the influence of study design and contact with research staff on response to antidepressant medication and placebo in RCTs for pediatric MDD. Consistent with the results of our prior meta-analyses in adults, we expected antidepressant response would be significantly higher in comparator vs. placebo-controlled RCTs and that significantly higher rates of response to antidepressant medication would be observed in open studies than in either placebo-controlled or comparator trials. We further hypothesized that there would be positive main effects of patient age and contact with research staff on response rates to medication and placebo. Lastly, we anticipated finding a significant age × study type interaction, such that greater effects of expectancy on medication response would be found with increasing patient age.
METHOD
Search strategy and selection criteria
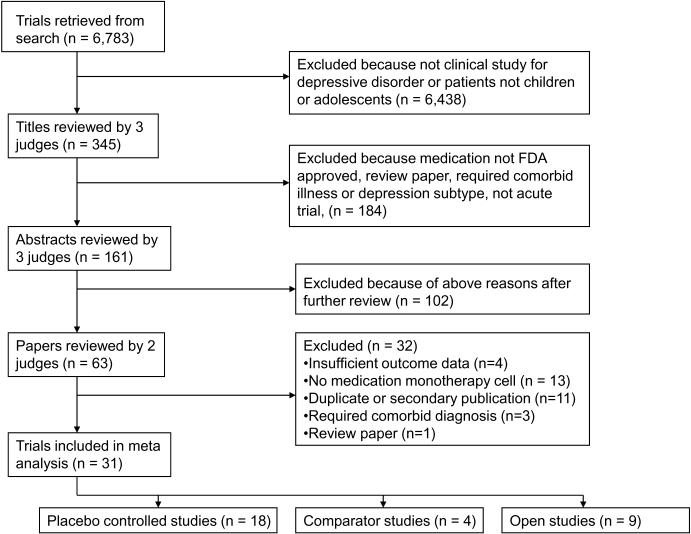
A Medline search was conducted to identify clinical studies of antidepressants in children and adolescents with depressive disorders. The search strategy comprising (“depression” OR “depressive disorder” OR “major depressive disorder” OR “dysthymia”) AND (“antidepressant” OR the class and individual generic name of all Food and Drug Administration (FDA) approved antidepressants) returned 55,353 results (see Figure 1). These results were limited to 1) English language articles, 2) age group 18 years or younger, and 3) human studies, yielding 6,783 journal articles. Two authors (BRR and JT) conducted a review of these titles to exclude those that were not clinical studies of antidepressants for depressive disorders, yielding 345 titles.
Figure 1.
Literature review and selection of studies.
The remaining journal articles were then sequentially reviewed, proceeding from article title, to abstract, and finally full paper text, to determine whether they met inclusion and exclusion criteria. These evaluations were pooled, and any differences between judges were resolved by discussion. To further ensure all relevant papers were reviewed, the references of all meta-analyses and review articles published since the year 2000 among the 6,783 journal articles were searched for pertinent references. In addition, the Cochrane Database of Systematic Reviews was electronically searched using the topics depression AND (child OR adolescent). This yielded 221 protocols and completed reviews, among which the relevant references were reviewed to ensure they were captured by our search.
Included studies were required to have at least one treatment arm being monotherapy with an FDA approved antidepressant medication. Further criteria required trials to enroll patients with MDD, Dysthymia, or Depressive Disorder Not Otherwise Specified (NOS), last between 5 and 12 weeks (inclusive), be written in English, and have response rates specified using a standardized outcome measurement. Studies were excluded for enrolling patients with psychosis, bipolar disorder, or those defined to have treatment resistant depression. Also excluded were antidepressant augmentation studies and trials requiring as inclusion criteria a specific medical illness or an Axis I disorder other than depression.
Data extraction
For each included study, demographic characteristics of the participants, details of the treatment condition, duration of active treatment in each study, and response rates to medication and placebo were entered into a database. We used the total number of study visits made by participating patients (i.e., visits during the evaluation, placebo lead-in, and active treatment phases combined) as the best available measure of the amount of therapeutic contact occurring in each study, We considered other possible metrics for contact (e.g., duration of active medication treatment) but we judged these to be less reliable in light of the amount of methodological detail provided in published articles and the potential for confounding with active medication treatment.
Since there was variability in the criteria different studies used to judge depression response, we standardized the response rate data to the extent that was possible. If studies reported multiple response rates based upon different outcome measures, we selected one response rate for extraction according to the following priority list: Children's Depression Rating Scale—Revised (CDRS-R) ≥ 40% decrease from baseline,14–15 Hamilton Rating Scale for Depression (HRSD) ≥ 50% decrease from baseline,16 Montgomery-Asberg Depression Rating Scale (MADRS) ≥ 50% decrease from baseline,17 Clinical Global Impression (CGI) Improvement score of 1 or 2,18 and no longer meeting diagnostic criteria on the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS).19 Two judges (BRR and JT) extracted the data, and any differences were resolved by consensus.
Data analyses
Data analyses followed those in two prior manuscripts, where the procedures are described in greater detail.8–9 Mixed effects logistic regression models were used, similar to the approach taken by Bryk and Raudenbush,20 Hox,21 and Haddock, Rindskopf, and Shadish.22 The multilevel logistic regression model is described by two equations: a within-studies equation and a between-studies equation, which accommodates the hierarchical structure of patients nested within medication conditions nested within studies. The first set of models described below was restricted to treatment cells involving medication monotherapy. `Medication response' in the following paragraphs refers to the reported response rate for the medication treatment cells in the studies comprising the sample.
The initial step was to determine whether there is significant variability in medication response rates across studies. To do this, we ignored the nesting within study (medication group) and fit an unconditional model (Model 1). The within-studies equation for Model 1 is
where ln (p/[1-p]) is the log odds of medication response and B0 is a constant that is assumed initially to be the same for all groups within a study. At the between-studies level, the equation is
which describes the true medication response rates as varying around a grand mean (G00) with error (U0). To determine whether there were genuine differences between the studies (heterogeneity) or whether the variation in findings was compatible with chance alone (homogeneity), we examined the Birge ratio, which is calculated by dividing a chi-square by its degrees of freedom.23–24 The value of the Birge ratio is near 1 when there is only random variation between studies, and as the value exceeds 1, the results of a set of studies lack homogeneity (i.e., they are more varied than expected based on sampling error alone).
If there is significant variability in medication response rates across studies, it is possible to test the hypothesis that medication response rates vary depending on study type (Model 2). To do this, we included study type as a fixed effect in the between-studies equation:
`Comparator' is a dummy variable coded one for comparator trials and zero otherwise, and `open' is a dummy variable coded one for open studies and zero otherwise. The dummy variables can be recoded to make comparator studies the reference group and test for differences in medication response between open and comparator studies. Using this method, odds ratios and estimated probabilities of response to antidepressant medication in the different study types were computed.
The analysis proceeded to test whether mean patient age and the number of study visits in which patients met with research staff (i.e., contact amount) influence antidepressant response (Model 3). We added these two variables to the between-studies equation, centering each mean on the overall grand mean for age and number of study visits in our sample:
If studies comprised more than one active treatment condition, the mean ages of patients in each cell were combined to calculate a single mean age for each study. We were interested in the effect of patient age because individuals gain increasing cognitive capacities with increasing age. Phase of cognitive development may influence how patients generate expectancies based on study design and how they experience contact with research staff.
Finally, a full model was constructed by adding two-way interactions between study type, patient age, and contact with research staff:
Two-way interaction terms were generated by multiplying the centered mean ages, number of study visits, and the dummy variables encoding study type. We considered testing the three-way interaction terms but decided against this due to concerns about multicollinearity between the predictors and the absence of a compelling theoretical justification for these interactions.
Because active medication was administered in all of the treatment cells analyzed in the above models, true medication effects may confound estimates of the influence of contact amount on antidepressant response rates. Individual studies with greater numbers of study visits are likely to be those with longer durations of active treatment and therefore more time for effective medication to work. In order to determine the effect of contact with research staff in the absence of true medication effects, we analyzed response rates from treatment cells within placebo-controlled studies in which placebo was administered. By definition, these treatment cells came from placebo-controlled studies, so it was not possible to examine the effect of study type on response to placebo. Model 1 tested whether there was significant variability in response to placebo across studies, Model 2 tested for main effects of patient age and contact with research staff, and Model 3 tested for a two-way interaction between patient age and the amount of contact. `Placebo response' in this second set of models refers to the reported response rate for the placebo cells in the placebo-controlled studies.
All of the regression models were estimated using HLM 6. Differences in study characteristics, patient demographics, and clinical features across the different study types were investigated using two-tailed independent samples t-tests for continuous variables and chi-square (X2) tests for categorical variables (SPSS version 18).
RESULTS
Characteristics of included studies and participants
Nine open studies, 18 placebo-controlled trials, and 4 comparator trials met the study's inclusion and exclusion criteria (Table 1). As shown in Table 2, these included 9 medication monotherapy conditions enrolling 228 participants in the open studies, 5 medication monotherapy conditions enrolling 325 participants in the comparator studies, and 19 medication monotherapy conditions enrolling 1709 participants in the placebo-controlled RCTs. Among the comparator trials, 1 out 4 studies (25%) demonstrated significant differences in depression response rates between active treatment groups. Among the placebo controlled trials, 7 out of 18 studies (38.9%) demonstrated significant differences in depression response rates between medication and placebo. There were no significant differences between the three study types in the criteria used to judge depression response (Pearson X2 = 13.266, df 8, p = 0.103).
Table 1.
Summary of included studies and participants.
| Open Studies | ||||||
|---|---|---|---|---|---|---|
| Author | Treatment | Weeks | Diagnosis | N | Mean age | Response |
| Emslie 200725 | Venlafaxine | 6 | MDD | 85 | 10.1 | 45.0 |
|
| ||||||
| Findling 200026 | Nefazodone | 8 | MDD | 26 | 12.1 | 76.9 |
|
| ||||||
| Geller 198627 | Nortriptyline | 8 | MDD | 23 | 9.2 | 60.9 |
|
| ||||||
| Glod 200328 | Bupropion | 8 | MDD | 11 | 15.5 | 63.6 |
|
| ||||||
| Goodnick 200029 | Nefazodone | 8 | MDD | 10 | 15.0 | 70.0 |
|
| ||||||
| Nixon 200130 | Sertraline | 12 | MDD or DD | 21 | 15.0 | 76.2 |
|
| ||||||
| Nobile 200031 | Paroxetine | 12 | DD | 7 | 14.4 | 71.0 |
|
| ||||||
| Shirazi 200532 | Citalopram | 6 | MDD | 30 | 13.6 | 73.3 |
|
| ||||||
| Waslick 199933 | Fluoxetine | 8 | DD or MDD/DD | 15 | 15.9 | 73.0 |
|
| ||||||
| Comparator Studies | ||||||
|
| ||||||
| Braconnier 200334 | Paroxetine | 8 | MDD | 63 | 15.9 | 65.1 |
| Clomipramine | 58 | 16.2 | 48.3 | |||
|
| ||||||
| Clarke 200535 | SSRI | 12 | MDD | 75 | 15.3 | 72.1 |
| SSRI + CBT | 77 | 15.3 | 77.0 | |||
|
| ||||||
| Goodyer 200736 | Fluoxetine | 12 | MDD | 103 | 14.0 | 42.7 |
| Fluoxetine + CBT | 105 | 14.0 | 40.0 | |||
|
| ||||||
| Melvin 200637 | Sertraline | 12 | MDD,DD,D | 26 | 15.5 | 46.0 |
| CBT | NOS | 22 | 15.0 | 86.0 | ||
| Sertraline + CBT | 25 | 15.3 | 45.0 | |||
|
| ||||||
| Placebo-Controlled Studies | ||||||
|
| ||||||
| Berard 200638 | Paroxetine | 12 | MDD | 182 | 15.5 | 58.8 |
| Placebo | 93 | 15.8 | 57.3 | |||
|
| ||||||
| Emslie 199739 | Fluoxetine | 8 | MDD | 48 | 12.2 | 56.0 |
| Placebo | 48 | 12.5 | 33.0 | |||
|
| ||||||
| Emslie 200240 | Fluoxetine | 8 | MDD | 109 | 12.7 | 65.1 |
| Placebo | 110 | 12.7 | 36.8 | |||
|
| ||||||
| Emslie 200641 | Paroxetine | 8 | MDD | 101 | 11.9 | 48.5 |
| Placebo | 102 | 12.1 | 46.0 | |||
|
| ||||||
| Emslie 200742 | Venlafaxine ER | 8 | MDD | 169 | 12.2 | 50.3 |
| Placebo | 165 | 12.3 | 55.0 | |||
|
| ||||||
| Emslie 200943 | Escitalopram | 8 | MDD | 154 | 14.7 | 59.1 |
| Placebo | 157 | 14.5 | 52.9 | |||
|
| ||||||
| Geller 199244 | Nortriptyline | 8 | MDD | 26 | 9.7 | 30.8 |
| Placebo | 24 | 9.7 | 16.7 | |||
|
| ||||||
| Geller 199045 | Nortriptyline | 8 | MDD | 12 | 14.0 | 8.3 |
| Placebo | 19 | 14.4 | 21.1 | |||
|
| ||||||
| Keller 200146 | Paroxetine | 8 | MDD | 90 | 14.8 | 66.7 |
| Imipramine | 94 | 14.9 | 58.5 | |||
| Placebo | 87 | 15.1 | 46.0 | |||
|
| ||||||
| Klein 199847 | Desipramine | 6 | MDD | 23 | 15.7 | 52.2 |
| Placebo | 22 | 15.7 | 50.0 | |||
|
| ||||||
| Kutcher 199448 | Desipramine | 6 | MDD | 30 | 17.8 | 26.7 |
| Placebo | 30 | 17.8 | 35.0 | |||
|
| ||||||
| Kye 199649 | Amitriptyline | 8 | MDD | 18 | 14.6 | 72.2 |
| Placebo | 10 | 15.1 | 90.0 | |||
|
| ||||||
| Puig-Antich 198750 | Imipramine | 5 | MDD | 20 | 9.0 | 45.0 |
| Placebo | 22 | 9.2 | 68.0 | |||
|
| ||||||
| TADS Team 200451 | Fluoxetine | 12 | MDD | 109 | 14.6 | 60.6 |
| CBT | 111 | 14.6 | 43.2 | |||
| Fluoxetine + CBT | 107 | 14.6 | 71.0 | |||
| Placebo | 112 | 14.6 | 34.8 | |||
|
| ||||||
| Von Knorring 200652 | Citalopram | 12 | MDD | 121 | 16.0 | 61.0 |
| Placebo | 112 | 16.0 | 49.0 | |||
|
| ||||||
| Wagner 200353 | Sertraline | 8 | MDD | 185 | a | 69.0 |
| Placebo | 179 | a | 59.0 | |||
|
| ||||||
| Wagner 200454 | Citalopram | 8 | MDD | 89 | 12.1 | 36.0 |
| Placebo | 85 | 12.1 | 24.0 | |||
|
| ||||||
| Wagner 200655 | Escitalopram | 8 | MDD | 129 | 12.2 | 45.7 |
| Placebo | 132 | 12.4 | 37.9 | |||
Note: CBT = Cognitive Behavior Therapy; DD = Dysthymic Disorder; D NOS = Depressive Disorder Not Otherwise Specified; ER = Extended Release; MDD = Major Depressive Disorder; SSRI = Selective Serotonin Reuptake Inhibitor; TADS = Treatment for Adolescents with Depression.
Study did not report mean age of participants.
Table 2.
Clinical characteristics of included patients and methodological features of studies included in the multilevel meta-analysis.
| Characteristic | Open Studies | Comparator Studies | Placebo-Controlled Studies | |||
|---|---|---|---|---|---|---|
| N studies | 9 | 4 | 18 | |||
| N medication treatment groups | 9 | 5 | 19 | |||
| N patients in medication treatment groups | 228 | 325 | 1709 | |||
| Mean age | 13.5 ± 2.4 | 15.4 ± 0.8 | 13.6 ± 2.2 | |||
| Mean study visits | 10.8 ± 2.6 | 13.3 ± 3.1 | 10.0 ± 1.9 | |||
| Mean drop out rate | 19.0 ± 10.2 | 23.0 ± 14.3 | 27.7 ± 8.0 | |||
| Mean N ITT | 19 ± 12.7 | 65 ± 27.9 | 89.4 ± 59.0 | |||
| Mean pre-treatment HRSD | 21.4 ± 4.7 | 22.9 ± 1.2 | 18.6 ± 3.7 | |||
| N active treatment conditions | N patients | N active treatment conditions | N patients | N active treatment conditions | N patients | |
|---|---|---|---|---|---|---|
| Study duration | ||||||
| 5–7 weeks | 2 | 115 | 0 | 0 | 3 | 69 |
| 8–9 weeks | 2 | 98 | 2 | 121 | 12 | 1033 |
| 10–12 weeks | 5 | 15 | 2 | 129 | 4 | 597 |
|
| ||||||
| Medications used | ||||||
| SSRI | 2 | 85 | 0 | 0 | 1 | 169 |
| SNRI | 2 | 85 | 0 | 0 | 1 | 169 |
| TCA | 1 | 23 | 1 | 58 | 7 | 213 |
| Atypical AD | 4 | 47 | 0 | 0 | 0 | 0 |
| MAOI | 0 | 0 | 0 | 0 | 0 | 0 |
Note: Atypical AD = Atypical antidepressant (e.g., bupropion, nefazodone, mirtazipine, trazodone); HRSD = Hamilton Rating Scale for Depression; ITT = Intent to treat; MAOI = Monoamine Oxidase Inhibitor; SNRI = Serotonin Norepinephrine Reuptake Inhibitor; SSRI = Selective Serotonin Reuptake Inhibitor; TCA = Tricyclic antidepressant.
Mean response rates to medication ranged from 45–77% in the open studies to 40–86% in the comparator and 8–72% in the placebo-controlled trials. For the purpose of comparison, mean response rates to placebo in the placebo-controlled trials ranged from 17–90%. Although we originally intended to analyze remission rates in addition to response rates, there was not sufficient information provided in the publications examined to permit this analysis. Only 2/9 open studies, 1/4 comparator studies, and 5/18 placebo-controlled studies provided information about antidepressant remission rates.
Open, comparator, and placebo-controlled studies differed in the frequencies of medication classes used (Pearson X2 = 10.447, df 4, p = 0.034), with open studies more often investigating combined serotonin norepinephrine reuptake inhibitors (SNRIs) and atypical antidepressants (e.g., bupropion, nefazodone) compared to placebo-controlled studies (Pearson X2 = 7.841, df 2, p = 0.020). The medication class under investigation did not significantly differ between open and comparator studies (Pearson X2 = 3.329, df 2, p = 0.189) or between placebo-controlled and comparator trials (Pearson X2 = 0.909, df 2, p = 0.635). Furthermore, the three study types did not differ significantly in the distribution of funding sources (Pearson X2 = 9.423, df 8, p = 0.308) or years of publication (Pearson X2 = 34.037, df 32, p = 0.370). Enrolled patients did not differ in mean age (F(2,29) = 1.515, p = 0.235), type of depressive disorder (Pearson X2 = 8.088, df 4, p = 0.088), mean drop out rate (F(2,29) = 2.927, p = 0.68), or baseline depression severity (F(2,14) = 1.526, p = 0.251).
Information about the amount of contact with research staff in the three study types is also shown in Table 2. The number of study visits ranged from 8–14 in the open studies, 10–16 in the comparator, and 7–13 in the placebo-controlled trials, and study visits did not differ significantly across study designs (Pearson X2 = 25.625, df 14, p = 0.060). In like fashion, the duration of active medication treatment did not differ across study types (Pearson X2 = 7.282, df 8, p = 0.507).
Analysis of response rates in medication treatment cells
Coefficients and odds ratios for the predictor variables in the models describing response to medication are tabulated in Table 3. In Model 1, the unconditional model of antidepressant response rates, variability between studies was over 3 times that expected by chance alone (Birge ratio: X2 / df = 90.3 / 28 = 3.23). Therefore, the null hypothesis that antidepressant response rates are homogeneous across studies was rejected, and the analysis proceeded with the conditional models.
Table 3.
Coefficients and odds ratios for predictor variables at each step of the multilevel meta-analysis of response rates in medication treatment cells.
| Medication Response Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | |
| Intercept | 0.15 (0.10) | 1.17 (0.96–1.42) | 0.031 (0.11) | 1.03 (0.82–1.30) | 0.03 (0.11) | 1.03 (0.81–1.30) | 0.01 (0.10) | 1.01 (0.81–1.25) |
| Comparator | -- | -- | −0.067 (0.28) | 0.94 (0.53–1.66) | −0.099 (0.29) | 0.91 (0.49–1.67) | −0.16 (0.65) | 0.84 (0.22–3.30)) |
| Open | -- | -- | 0.62 (0.23)* | 1.87 (1.17–2.99)* | 0.64 (0.23)* | 1.90 (1.19–3.03)* | 0.74 (0.22)* | 2.09 (1.31–3.32)* |
| Age | -- | -- | -- | -- | 0.048 (0.047) | 1.05 (0.95–1.16) | 0.035 (0.052) | 1.04 (0.93–1.15) |
| Contacts | -- | -- | -- | -- | −0.02 (0.048 | 0.98 (0.89–1.08) | −0.078 (0.054) | 0.93 (0.83–1.36) |
| Age x Contacts | -- | -- | -- | -- | -- | -- | −0.0082 (0.023) | 0.99 (0.95–1.04) |
| Age x Comparator | -- | -- | -- | -- | -- | -- | 0.13 (0.32) | 1.14 (0.59, 2.21) |
| Age x Open | -- | -- | -- | -- | -- | -- | 0.041 (0.11) | 1.04 (0.83–1.32) |
| Contacts x Comparator | -- | -- | -- | -- | -- | -- | 0.0064 (0.19) | 1.01 (0.68–1.49) |
| Contacts x Open | -- | -- | -- | -- | -- | -- | 0.19 (0.097) | 1.22 (0.99–1.49) |
| Variance component | 0.165 | 0.136 | 0.124 | 0.072 | ||||
| −2LL | 131.2 | 124.0 | 123.0 | 118.0 | ||||
| df | 28 | 26 | 24 | 19 | ||||
Note: CI = confidence interval; LL = log likelihood; SE = standard error.
p < 0.05
Including study type in Model 2 accounted for 17.6% of the variability observed in antidepressant response rates and significantly improved model fit (Likelihood ratio test ~X2(2) = 7.20, p < 0.05). The odds of responding to medication in open studies were 1.87 times the odds of responding in placebo-controlled trials (95% CI 1.17 – 2.99, p = 0.012) and 2.01 times the odds in comparator trials (95% CI 1.16 – 3.48, p = 0.015). However, the odds of responding to medication in comparator trials were not significantly different from the odds of responding in placebo-controlled trials (OR 0.93, 95% CI = 0.53 – 1.67, p = 0.814). Estimates of average medication response rates derived from Model 2 were 64.7% for open studies, 51.2% for comparator trials, and 52.0% for placebo-controlled trials. For the purposes of comparison, the mean response rate to placebo in the placebo-controlled trials was 46.8%.
In Model 3, including patient age and the amount of contact reduced the variability in antidepressant response rates by 8.8% but did not significantly improve model fit (Likelihood ratio test ~X2(2) = 1.0, p = 0.61). Across the different study types and contact amounts, the odds of responding to medication did not significantly change with increasing mean patient age (OR 1.05, 95% CI = 0.95 – 1.16, p = 0.318). Medication response rates also did not change significantly with increasing number of study visits (OR 0.98, 95% CI = 0.89– 1.08, p = 0.676), controlling for study type and age. Finally, including two-way interaction terms in Model 4 reduced the variance component by 41.9% but did not significantly improve model fit (Likelihood ratio test ~X2(5) = 5.0, p = 0.42). No significant interactions between study type, age, and contact amount were found for the data on medication response rates. However, there was a trend toward a significant contact × open interaction, such that the difference in medication response between open and placebo-controlled trials was greater in studies having more study visits (OR 1.22, 95% CI = 0.99 – 1.49, p = 0.059).
To supplement our investigation of the effects of therapeutic contact, we examined studies that reported providing supportive psychotherapy as part of the psychopharmacologic management protocol. Three out of 9 open, 1/4 comparator, and 3/18 placebo-controlled studies provided some type of supportive psychotherapy, which did not differ significantly across study types (Pearson X2 = 0.616, df 2, p = 0.735). Details of the therapy provided were often lacking in publications describing the studies, so it was unclear when therapy was provided during a screening phase vs. the active treatment period, whether all patients received the supportive therapy, and the quantity of therapy provided. Consequently, we were not able to determine whether supportive treatment influenced response rates to placebo and medication.
Finally, we conducted a number of subgroup analyses in order to assess the robustness of the results obtained. Limiting the analyses to Selective Serotonin Reuptake Inhibitors (SSRIs) resulted in a similar overall pattern of medication response to that found in the larger sample, with the exception that the difference between open and placebo-controlled studies became non-significant given the smaller number of studies (OR 1.53, 95% CI = 0.89 – 2.73, p = 0.140). Excluding participants with non-MDD diagnoses did not change the pattern of results. Finally, excluding studies lasting less than 7 weeks resulted in similar differences between open vs. comparator and placebo-controlled studies, but we also found a significant effect for mean patient age (OR 1.09, 95% CI = 1.01 – 1.19, p = 0.039) across study types and contact amounts.
Analysis of response rates in placebo cells within placebo-controlled studies
Coefficients and odds ratios for the predictor variables in the models describing response to placebo are summarized in Table 4. In Model 1, the unconditional model, variability between studies was over 4 times that expected by chance alone (Birge ratio: X2 / df = 71.4 / 16 = 4.46). Therefore, the null hypothesis that response to placebo is homogeneous across studies was rejected, and the analysis proceeded with the conditional models.
Table 4.
Coefficients and odds ratios for predictor variables at each step of the multilevel meta-analysis of response rates in placebo treatment cells.
| Placebo Response Analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 3 | |||
| Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | Coefficient (SE) | Odds Ratio (CI) | |
| Intercept | −0.23 (0.13) | 0.79 (0.60–1.05) | −0.30 (0.14) | 0.75 (0.55–1.01) | −0.35 (0.13) | 0.71 (0.53–0.94) |
| Age | -- | -- | 0.027 (0.064) | 1.03 (0.90–1.18) | 0.10 (0.068) | 1.11 (0.96–1.28) |
| Contacts | -- | -- | −0.075 (0.076) | 0.93 (0.79–1.09) | −0.044 (0.070) | 0.96 (0.82–1.11) |
| Age * Contacts | -- | -- | -- | -- | 0.072 (0.031)* | 1.07 (1.01–1.15)* |
| Variance component | 0.212 | 0.192 | 0.145 | |||
| −2LL | 74.64 | 73.99 | 69.10 | |||
| df | 16 | 14 | 13 | |||
Note: CI = confident interval; LL = log likelihood; SE = standard error.
p < 0.05
The main effect model (Model 2) including patient age and the amount of contact reduced the observed variability in response rates to placebo by 9.4% but did not significantly improve model fit (Likelihood ratio test ~X2(2) = 0.65, p = 0.72). The full model (Model 3) including the interaction between patient age and amount of contact with research staff reduced the observed variability in response rates by 31.6% over the unconditional model but did not significantly improve model fit (Likelihood ratio test ~X2(3) = 5.54, p = 0.14). In terms of the main effects, the odds of responding to placebo did not change significantly for each unit change in age (OR 1.03, 95% CI = 0.90 – 1.18, p = 0.686) or number of study visits (OR 0.93, 95% CI = 0.79 – 1.209, p = 0.342). However, there was a significant age × contact interaction (OR 1.07, 95% CI = 1.01 – 1.15, p = 0.038), indicating that effect of increasing numbers of study visits on response to placebo was greater for older as compared to younger patients.
In a post hoc analysis, we also examined the influence of placebo lead-in periods, which are designed to diminish the response to placebo observed in a trial by identifying likely placebo responders and removing them from the efficacy analyses. In our sample 11/18 placebo-controlled studies utilized a placebo lead-in period. Response rates to placebo were not significantly different in studies utilizing a placebo lead-in period compared to those that did not (OR 0.93, 95% CI = 0.55 – 1.60, p = 0.800). No significant interactions were found between the presence of placebo lead-in periods and mean patient age (OR 1.16, 95% CI = 0.92 – 1.47, p = 0.194) or the amount of contact with research staff (OR 1.12, 95% CI = 0.79 – 1.59, p = 0.503).
DISCUSSION
This meta-analysis examined 31 studies of antidepressant medication for depressive disorders in children and adolescents across open, placebo-controlled, and active comparator study designs. Consistent with our hypotheses, open studies of antidepressant medications were associated with a significantly higher medication response rate compared to placebo-controlled and comparator RCTs. Contrary to our hypotheses, response rates to antidepressant medication did not differ significantly between placebo-controlled and active comparator study designs. Medication response rates also did not depend upon mean patient age or the amount of contact with research staff.
Mechanisms for change in clinical trials can be conceptualized as comprising medication effects, placebo effects, and non-specific study effects. Medication effects are the specific physiological effects of the medication being studied on the target disorder (e.g., the effect of serotonin reuptake inhibition on MDD). Placebo effects are the psychological and physiological effects on a patient of receiving a treatment that is believed by the patient to be effective for the target disorder. In clinical trials, patient expectancy of therapeutic improvement is believed to be the primary mechanism of placebo effects. Non-specific study effects occur by virtue of studying a patient over time in a research study, and they include the natural history of the patient's condition, therapeutic aspects of the health care context, and the expectations of clinicians and raters in research studies.
Since `effect' denotes a conceptual cause of patient change and the term `response' denotes what is actually observed in a research study, it follows that antidepressant response results from a combination of medication, placebo, and non-specific study effects. Differences in placebo and non-specific study effects across studies may explain differences in observed clinical responses to the same medications. For example, adult participants in comparator trials have enhanced expectancy of therapeutic benefit and consequently higher antidepressant response rates based on their certainty of receiving active medication (as opposed to participants in placebo-controlled trials being aware they may receive placebo).8,10,56 This appears not to be the case for children and adolescents with depression, since in this analysis of antidepressant studies for pediatric MDD, medication response in placebo-controlled and comparator trials did not differ significantly.
One interpretation of these findings is that children and adolescents do not generate the same treatment expectancies as do adult RCT participants, and consequently differences in patient expectancy do not cause differential medication response rates across study designs. Younger patients entering clinical studies may not receive the same information disclosure as adults and may not be as cognitively capable of understanding what information they do receive. We attempted to evaluate this interpretation by testing whether increasing patient age was associated with greater differences in medication response between placebo-controlled and comparator trials, but we found no evidence for a patient age × study type interaction. Even greater levels of cognitive sophistication than that possessed by adolescents may be required to generate expectancies about therapeutic improvement based on study design.
Rather than expectancy-based placebo effects, high placebo response rates in pediatric MDD trials may be driven by other factors, such as the therapeutic aspects of the trial protocol. The possibility has been discussed in the literature that children and adolescents with depression respond well to frequent and regular meetings with clinicians who foster the therapeutic alliance and bolster confidence and self-esteem.57 Data from this meta-analysis are partially consistent with this hypothesis, since there was an increasingly positive effect of contact with research staff on response to placebo with increasing patient age. Older youths may benefit more from contact with research staff due to their enhanced capacities for self-evaluation, which may improve the therapeutic alliance by facilitating therapist and patient agreement on treatment goals and the tasks of therapy.58–59 However, the effects of therapeutic contact on antidepressant and placebo response appeared to be limited in our analyses, since we did not observe main effects of contact with research staff on antidepressant and placebo response rates.
Some of the studies in our sample included as part of their design extended screening phases and placebo lead-in periods to identify participants whose symptoms responded quickly to contact with health professionals or placebo. While we did not find sufficient information in the published articles to evaluate the effect of extended screening phases and supportive therapy, we did not determine placebo response to be significantly different between studies having a placebo lead-in period and those that did not. This is consistent with findings in studies of adult patients with MDD, where single-blind placebo lead-in periods have not been found to reduce placebo response or influence the detection of drug-placebo differences.60
Another notable finding of this meta-analysis was that medication response rates were higher in open vs. placebo-controlled and comparator studies. One possible explanation for these differences is that open treatment induces more optimistic treatment expectancies than even comparator studies, though patients in comparator studies are also assured of receiving an active treatment for their condition. Since parents may contribute to the outcome ratings on some measures (e.g., CDRS and KSADS), their expectations about treatment outcome also may influence medication response rates in open studies. Furthermore, it is possible that patients entering combined studies of medication and psychotherapy (3/4 comparator studies in this sample had a psychotherapy arm) preferred randomization to psychotherapy, and they were disappointed (and experienced a corresponding “nocebo” effect) when they were randomized to medication. However, the most cogent explanation of this overestimation of treatment effects in open studies may be increased rater bias.61 Given that open studies are conducted without blinding by investigators who are attempting to find a significant effect of medication in order to support further investigation, rater bias may play a significant role.
Finally, a number of limitations should be considered when interpreting the findings of this study. The use of trial-level summary data limited the data available for analysis in this study, as not all authors reported complete information about patient and trial characteristics in their published article. Four studies were excluded because of inadequate reporting of outcome data, and we were not able to analyze antidepressant remission rates (only 8/31 studies provided remission rate data). Additionally, publication bias may have affected which studies were included in these analyses, since some open label studies as well as RCTs failing to demonstrate significant differences between medication and placebo may not have been published. However it is not the efficacy of medication compared to placebo that was investigated in this analysis, so publication bias seems unlikely to have affected the overall patterns of response observed across trials.
A second limitation of this study concerns the analyses of patient age, which were based on the mean ages of participants provided in each study, centered around the grand mean for age across studies. There may be important differences between the responses of children (aged 7–12 years) and adolescents (aged 13–18 years) to antidepressant medications and placebo that would be useful to investigate with a dichotomous variable for patient age (i.e., child vs. adolescent).62 Unfortunately, it was not possible to conduct the analyses in this fashion due to the characteristics of the study sample. Age-grouped data for both children and adolescents were available for some (but not all) of the placebo-controlled trials in our sample, but the comparator trials only enrolled adolescents. Thus, a dichotomous variable made it impossible to assess the primary question of interest regarding patient age, which was whether there was a patient age × study type interaction indicating increased expectancy effects as cognitive development proceeds.
Thirdly, patient and study characteristics other than expectancy, patient age, and contact with research staff may influence response to antidepressant medication and placebo. Family history of affective disorder, severity of depressive symptoms, greater number of depressive episodes, age, sex, ethnicity, number of concurrent psychiatric comorbidities, length of illness, length of current episode, global functioning of the child and family, and suicidal behaviors have all been suggested to influence medication response in children and adolescents.63–65 The number of treatment sites and the duration of the depressive episode at baseline have been found to be associated with increased response to placebo.6 We chose not to include these demographic, clinical, and methodological characteristics in our modeling of antidepressant and placebo response rates. Rather, our primary concern was to determine whether these predictors of medication and placebo response significantly differed between the study types we analyzed, which could confound our primary comparisons. However, we found that only the distribution of medication classes used between studies differed between study types, with open studies more frequently using SNRIs and atypical antidepressants than placebo-controlled (but not comparator) trials. When we repeated the analyses excluding SNRI and atypical antidepressant treatment cells, the overall pattern of results did not change.
In summary, there are many sources of patient change apart from antidepressant medication in clinical trials for depression. In adult depressed patients, expectancy of improvement is a major determinant of responses rates to antidepressants and placebo. In contrast, findings from this multilevel meta-analysis of antidepressant and placebo response rates in pediatric MDD trials suggest that patient expectancy plays a minor role in children and adolescents. Other non-specific study effects, such as the therapeutic effects of contact with research staff, may explain more of the variability in response rates. However, it is impossible for any retrospective analysis to fully differentiate the contributions of medication, expectancy-based placebo effects, and non-specific study effects to clinical outcome. The execution of prospective studies will be critical to answering these questions, and a major significance of this meta-analysis is to suggest novel clinical trial designs with this capability.
Acknowledgments
This work was supported by a National Institute of Mental Health grants K23 MH085236 (BRR), K23 MH075006 (JRS), R21 MH087774 (JRS), MH36197 (BSP), K02-74677 (BSP), a Hope for Depression Research Foundation grant (BRR), and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (BRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Roose has served on a Data and Safety Monitoring Board for Medtronics, Inc.
Drs. Rutherford, Sneed, Rindskopf, and Peterson, and Ms. Tandler report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 2.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 3.Birmaher B, Ryan ND, Williamson DE, et al. Childhood and adolescent depression: a review of the past ten years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Emslie GJ. Understanding Placebo Response in Pediatric Depression Trials. Am J Psychiatry. 2009;166:1–3. doi: 10.1176/appi.ajp.2008.08101541. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Deniau E, Maturana A, et al. Are Child and Adolescent Responses to Placebo Higher in Major Depression than in Anxiety Disorders? A Systematic Review of Placebo-Controlled Trials. PLOS One. 2008;7:1–8. doi: 10.1371/journal.pone.0002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo Response in Randomized Controlled Trials of Antidepressants for Pediatric Major Depressive Disorder. Am J Psychiatry. 2009;166:42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford BR, Wager TD, Roose SP. Expectancy Effects in the Treatment of Depression: A Review of Experimental Methodology, Effects on Patient Outcome, and Neural Mechanisms. Current Reviews in Psychiatry. 2010;6:1–10. doi: 10.2174/157340010790596571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford BR, Sneed JR, Roose SP. Does Study Design Affect Outcome? The Effects of Placebo Control and Treatment Duration in Antidepressant Trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design Makes a Difference: Antidepressant Response Rates in Placebo-controlled versus Comparator Trials in Late Life Depression. Am J Geri Psychiatry. 2008;16:65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford BR, Sneed JR, Eisenstadt R, Roose SP. Antidepressant Study Design Affects Patient Expectancy: A Pilot Study. Psychol Med. 2010;40:781–788. doi: 10.1017/S0033291709991085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer B, Pilkonis PA, Krupnick JL, Egan MK, Simmens SJ, Sotsky SM. Treatment Expectancies, Patient Alliance, and Outcome: Further Analyses from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 2002;70:1051–1055. [PubMed] [Google Scholar]
- 12.Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject Expectations of Treatment Effectiveness and Outcome of Treatment with an Experimental Antidepressant. J Clin Psychiatry. 2004;65:1174–1179. doi: 10.4088/jcp.v65n0904. [DOI] [PubMed] [Google Scholar]
- 13.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The Problem of the Placebo Response in Clinical Trials for Psychiatric Disorders: Culprits, Possible Remedies, and a Novel Study Design Approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- 14.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 15.Poznanski EO, Mokros HB. Manual: Children's Depression Rating Scale—Revised. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Guy W. New Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. 1976. National Institute of Mental Health; Rockville, MD: 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- 19.Puig-Antich J, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS) New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- 20.Bryk AS, Raudenbush SW. Hierarchical linear models. Sage Publications; Newbury Park, CA: 1992. [Google Scholar]
- 21.Hox J. Multilevel Analysis: Techniques and applications. Lawrence Erlbaum Publishers; Mahwah, NJ: 2002. [Google Scholar]
- 22.Haddock CK, Rindskopf D, Shadish WR. Using odds ratios as effect sizes for meta-analysis of dichotomous data: A primer on methods and issues. Psychological Methods. 1998;3:339–353. [Google Scholar]
- 23.Birge RT. The calculation of errors by the method of least squares. Rev Mod Physics. 1932;40:207–227. [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Emslie GJ, Findling RL, Yeung PP, Kunz NR, Li Y. Venlafaxine ER for the Treatment of Pediatric Subjects with Depression: Results of Two Placebo-Controlled Trials. J Am Acad Child Adolesc Psychiatry. 2007;46:479–488. doi: 10.1097/chi.0b013e31802f5f03. [DOI] [PubMed] [Google Scholar]
- 26.Findling RL, Preskorn SH, Marcus RN, et al. Nefazodone Pharmacokinetics in Depressed Children and Adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39:1008–1016. doi: 10.1097/00004583-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Geller B, Cooper TB, Chestnut EC, Anker JA, Schluchter MD. Preliminary Data on the Relationship Between Nortriptyline Plasma Level and Response in Depressed Children. Am J Psychiatry. 1986;143:1283–1286. doi: 10.1176/ajp.143.10.1283. [DOI] [PubMed] [Google Scholar]
- 28.Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ. Open Trial of Bupropion SR in Adolescent Major Depression. J Child Adolesc Psych Nurs. 2003;16:123–130. doi: 10.1111/j.1744-6171.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 29.Goodnick PJ, Jorge CA, Hunter T, Kumar AM. Nefazodone Treatment of Adolescent Depression: An Open-Label Study of Response and Biochemistry. Ann Clin Psychiatry. 2000;12:97–100. doi: 10.1023/a:1009024214337. [DOI] [PubMed] [Google Scholar]
- 30.Nixon MK, Milin R, Simeon JG, Cloutier P, Spenst W. Sertraline Effects in Adolescent Major Depression and Dysthymia: A Six-Month Open Trial. J Child Adolesc Psychopharm. 2001;11:131–142. doi: 10.1089/104454601750284036. [DOI] [PubMed] [Google Scholar]
- 31.Nobile M, Bellotti B, Marino C, Molteni M, Battaglia M. An Open Trial of Paroxetine in the Treatment of Children and Adolescents Diagnosed with Dysthymia. J Child Adolesc Psychopharm. 2000;10:103–109. doi: 10.1089/cap.2000.10.103. [DOI] [PubMed] [Google Scholar]
- 32.Shirazi E, Alaghband-Rad J. An Open Trial of Citalopram in Children and Adolescents with Depression. J Child Adolesc Psychopharm. 2005;15:233–239. doi: 10.1089/cap.2005.15.233. [DOI] [PubMed] [Google Scholar]
- 33.Waslick BD, Walsh BT, Greenhill LL, Eilenberg M, Capasso L, Lieber D. Open trial of fluoxetine in children and adolescents with dysthymic disorder or double depression. J Affect Disord. 1999;56:227–236. doi: 10.1016/s0165-0327(99)00032-4. [DOI] [PubMed] [Google Scholar]
- 34.Braconnier A, Coent R, Cohen D. Paroxetine Versus Clomipramine in Adolescents with Severe Major Depression: A Double-Blind, Randomized, Multicenter Trial. J Am Acad Child Adolesc Psychiatry. 2003;42:22–29. doi: 10.1097/00004583-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Clarke G, Debar L, Lynch F, et al. A Randomized Effectiveness Trial of Brief Cognitive-Behavioral Therapy for Depressed Adolescents Receiving Antidepressant Medication. J Am Acad Child Adolesc Psychiatry. 2005;44:888–898. [PubMed] [Google Scholar]
- 36.Goodyer I, Dubicka B, Wilinson P, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomized controlled trial. BMJ. 2007;332:1–8. doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melvin GA, Tonge BJ, King NJ, Heyne D, Gordon MS, Klimheit E. A Comparison of Cognitive-Behavioral Therapy, Sertaline, and Their Combination for Adolescent Depression. J Am Acad Child Adolesc Psychiatry. 2006;45:1151–1161. doi: 10.1097/01.chi.0000233157.21925.71. [DOI] [PubMed] [Google Scholar]
- 38.Berard R, Fong R, Carpenter DJ, Thomason C, Wilkinson C. An International, Multicenter, Placebo-Controlled Trial of Paroxetine in Adolescents with Major Depressive Disorder. J Child Adolesc Psychopharm. 2006;16:59–75. doi: 10.1089/cap.2006.16.59. [DOI] [PubMed] [Google Scholar]
- 39.Emslie GJ, Rush AJ, Weinberg WA, et al. A Double-blind, Randomized, Placebo-Controlled Trial of Fluoxetine in Children and Adolescents with Depression. Arch Gen Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 40.Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Emslie GJ, Wagner KD, Kutcher S, et al. Paroxetine Treatment in Children and Adolescents with Major Depressive Disorder: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2006;45:709–719. doi: 10.1097/01.chi.0000214189.73240.63. [DOI] [PubMed] [Google Scholar]
- 42.Emslie GJ, Yeung PP, Kunz NR. Long-Term, Open-Label Venlafaxine Extended-Release Treatment in Children and Adolescents with Major Depressive Disorder. CNS Spectr. 2007;12:223–233. doi: 10.1017/s1092852900020940. [DOI] [PubMed] [Google Scholar]
- 43.Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the Treatment of Adolescent Depression: A Randomized Placebo-Controlled Multisite Trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- 44.Geller B, Cooper TB, Graham DL, Fetner HH, Marsteller FA, Wells JM. Pharmacokinetically Designed Double-blind Placebo-controlled Study of nortriptyline in 6- to 12-Year-Oids with Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:34–44. doi: 10.1097/00004583-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Geller B, Cooper TB, Graham DL, Marsteller FA, Bryant DM. Double-Blind Placebo-Controlled Study of Nortriptyline in Depressed Adolescents Using a “Fixed Plasma Level” Design. Psychopharm Bull. 1990;26:85–90. [PubMed] [Google Scholar]
- 46.Keller MB, Ryan ND, Strober M, et al. Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial. J Am Acad Child Psychiatry. 2001;40:762–772. doi: 10.1097/00004583-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Klein RG, Mannuzza S, Koplewicz HS, et al. Adolescent Depression: Controlled Desipramine Treatment and Atypical Features. Depress Anx. 1998;7:15–31. doi: 10.1002/(sici)1520-6394(1998)7:1<15::aid-da3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 48.Kutcher S, Boulos C, Ward B, et al. Response to Desipramine Treatment in Adolescent Depression: A Fixed-Dose, Placebo-Controlled Trial. J Am Acad Child Adolesc Psychiatry. 1994;33:686–694. doi: 10.1097/00004583-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Kye CH, Waterman GS, Ryan ND, et al. A Randomized, Controlled Trial of Amitriptyline in the Acute Treatment of Adolescent Major Depression. J Am Acad Child Adolesc Psychiatry. 1996;35:1139–1144. doi: 10.1097/00004583-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Puig-Antich J, Perel JM, Lupatkin W, et al. Imipramine in Prepubertal Major Depressive Disorders. Arch Gen Psychiatry. 1987;44:81–89. doi: 10.1001/archpsyc.1987.01800130093012. [DOI] [PubMed] [Google Scholar]
- 51.Treatment for Adolescents with Depression Study Team Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents with Depression: Treatment for Adolescents with Depression Study (TADS) Randomized Controlled Trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 52.Von Knorring AL, Olsson GI, Thomsen PH, Lemming OM, Hulten A. A Randomized, Double-blind, Placebo-controlled Study of Citalopram in Adolescents with Major Depressive Disorder. J Clin Psychopharm. 2006;26:311–315. doi: 10.1097/01.jcp.0000219051.40632.d5. [DOI] [PubMed] [Google Scholar]
- 53.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of Sertraline in the Treatment of Children and Adolescents with Major Depressive Disorder: Two Randomized Controlled Trials. JAMA. 2003;290:1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 54.Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE. A Randomized, Placebo-Controlled Trial of Citalopram for the Treatment of Major Depression in Children and Adolescents. 2004;161:1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- 55.Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K. A Double-Blind, Randomized, Placebo-Controlled Trial of Escitalopram in the Treatment of Pediatric Depression. J Am Acad Child Adolesc Psychiatry. 2006;45:280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- 56.Rutherford BR, Rose S, Sneed JR, Roose SP. Study Design Affects Participant Expectations: A Survey. J Clin Psychopharm. 2009;29:179–181. doi: 10.1097/JCP.0b013e31819a9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen D, Deniau E, Maturana A, et al. Are Child and Adolescent Responses to Placebo Higher in Major Depression than in Anxiety Disorders? A Systematic Review of Placebo-Controlled Trials. PLOS One. 2008;7:1–8. doi: 10.1371/journal.pone.0002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirk S, Gudmundsen G, Kaplinski H, et al. Alliance and Outcome in Cognitive-Behavioral Therapy for Adoloscent Depression. J Clin Child Adolesc Psychol. 2008;37:631–639. doi: 10.1080/15374410802148061. [DOI] [PubMed] [Google Scholar]
- 59.DiGiuseppe R, Linscott J, Jilton R. Developing the therapeutic alliance in child-adolescent psychotherapy. Appl Prev Psychol. 1996;5:85–100. [Google Scholar]
- 60.Trivedi M, Rush J. Does a Placebo Run-In or a Placebo Treatment Cell Affect the Efficacy of Antidepressant Medications? Neuropsychopharmacology. 1995;11:33–43. doi: 10.1038/npp.1994.63. [DOI] [PubMed] [Google Scholar]
- 61.Marcus SM, Gorman JM, Tu X, et al. Rater bias in a blinded randomized placebo-controlled psychiatry trial. Stat Med. 2006;25:2762–2770. doi: 10.1002/sim.2405. [DOI] [PubMed] [Google Scholar]
- 62.Bridge JA, Iyengar A, Salary CB, et al. Clinical Response and Risk for Reported Suicidal Ideation and Suicide Attempts in Pediatric Antidepressant Treatment: A Meta-Analysis of Randomized Controlled Trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 63.Tao R, Emslie G, Mayes T, Nakonezny P, Kennard B, Hughes C. Early Prediction of Acute Antidepressant Treatment Response and Remission in Pediatric Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:71–78. doi: 10.1097/CHI.0b013e318190043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowatch RA, Carmody TJ, Emslie GJ, Rintelmann JW, Hughes CW, Rush AJ. Prediction of response to fluoxetine and placebo in children and adolescents with major depression: a hypothesis generating study. J Affect Disord. 1999;54:269–276. doi: 10.1016/s0165-0327(98)00205-5. [DOI] [PubMed] [Google Scholar]
- 65.Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]