Abstract
Objective
Apolipoprotein E (APOE) genotypes are associated with variable risk of developing late onset Alzheimer’s disease (LOAD), with APOE ε4 having higher risk. A variable poly-T length polymorphism at rs10524523, within intron 6 of the TOMM40 gene has been shown to influence age of onset in LOAD, with very long poly-T length associated with earlier disease onset, and short poly-T length associated with later onset. In this study, we tested the hypothesis that brain and cognitive changes suggestive of presymptomatic LOAD may be associated with this TOMM40 polymorphism.
Methods
Among N=117 healthy APOE ε3 homozygous adults (mean age 55), we compared those homozygous for very long (VL/VL; n=35) TOMM40 poly-T lengths (who are presumably at higher risk) to those homozygous for short (S/S; n=38) poly-T lengths, as well as those with heterozygous (S/VL; n=44) poly-T length polymorphisms, on measures of learning and memory and on structural brain imaging.
Results
The VL/VL group exhibited lower performance than the S/S TOMM40 group on primacy retrieval from a verbal list learning task, a finding which is also seen in early AD. A dose-dependent increase in the VL TOMM40 polymorphism (from no VL alleles, to S/VL heterozygous, to VL/VL homozygous) was associated with decreasing gray matter volume in the ventral posterior cingulate and medial ventral precuneus, a region of the brain affected early in LOAD.
Conclusions
These findings among APOE ε3/ε3 late middle-aged adults suggest that a subgroup with very long TOMM40 poly-T lengths may be experiencing incipient LOAD-related cognitive and brain changes.
Apolipoprotein E (APOE) epsilon 4 (ε4) is considered the primary genetic risk factor (1–3) for late-onset Alzheimer’s Disease (LOAD; Alzheimer’s Disease, AD). The other two APOE variants, APOE ε2 and ε3, are considered low and neutral risk respectively. However, the influence of ε4 only accounts for a portion of the heritability. In population studies of frequency of APOE variants among AD cases, the proportion without an ε4 allele is 36% to 50% (4–6); thus approximately 40% to 50% of AD cases require an explanation beyond APOE ε4 (7).
Another gene, TOMM40 (translocase of the outer mitochondrial membrane), has been proposed as a genetic risk factor in LOAD (8, 9). The TOMM40 gene is adjacent to APOE, and in a genome-wide association study (GWAS) of 381 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a TOMM40 risk allele (rs2075650, distinct from the poly-T polymorphism being reported in this paper) was twice as frequent in persons with AD compared with normal controls (10), and was associated with hippocampal atrophy (though not at a genome-wide level of signficance, and not significantly different from APOE). In a larger ADNI sample of normal controls, persons with mild cognitive impairment (MCI) and AD, a quantitative trait analysis demonstrated a significant association between this same TOMM40 allele and bilateral hippocampal and left amygdalar gray matter densities and volumes (11). In these studies, both APOE and TOMM40 genes were associated with gray matter loss in regions known to be vulnerable to AD pathology.
More recently, a variable length poly-T sequence (rs10524523) within intron 6 of the TOMM40 gene has been shown to predict age of onset of LOAD (8). In that study, the length distributions of the poly-T alleles were found to have a distinct relationship to APOE alleles (ε3 or ε4). Based on the number of the T residues, TOMM40 alleles were categorized as short (<21), long (21–29) or very long (≥30). For APOE ε3/e3 genotypes, TOMM40 appeared to be bimodally distributed into either short or very long alleles. Among APOE ε3/ε4 persons, the linkage of a short or very long poly-T allele to the APOE ε3 allele was associated with a later or earlier age of onset of AD, respectively.
The purpose of this study was to determine the potential contribution of poly-T length at TOMM40 rs10524523 to cognition and brain atrophy among the most common APOE genotype in a late middle-aged cohort by comparing verbal memory measures and MRI gray matter volumes in areas of the brain known to be affected in early AD. We therefore only studied people with an APOE ε3/3 genotype (who ostensibly have neutral risk for AD under the prevailing view). Because poly-T length has been associated with age of onset of LOAD, we hypothesized that AD-related differences might be detectable between S/S, S/VL, and VL/VL TOMM40 poly-T groups. Specifically, we hypothesized that the VL/VL group would most resemble AD on quantitative measures of verbal memory and brain volume in the posterior cingulate, a region that is involved in recognition memory and metacognition (12, 13), is known to have early AD pathology (14), and has been previously associated with risk for AD (15, 16).
METHODS
Participants
This study was conducted with the approval of the University of Wisconsin Institutional Review Board and all subjects provided signed informed consent prior to participation. The individuals in this study were participants in the Wisconsin Registry for Alzheimer’s Prevention (WRAP), a longitudinal cohort study of late middle-aged persons with or without a parental family history (FH) of AD (17, 18). Recruiting and assessment procedures for WRAP have been previously described (17). Briefly, enrollment in WRAP is limited to English speaking persons between the ages of 40 and 65 years and who have or had a parent with autopsy-confirmed or probable AD as defined by National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) research criteria (19). Persons without a FH are eligible for WRAP as controls if their mothers survived to at least age 75 and fathers to at least age 70 without AD, other dementia or significant memory deficits.
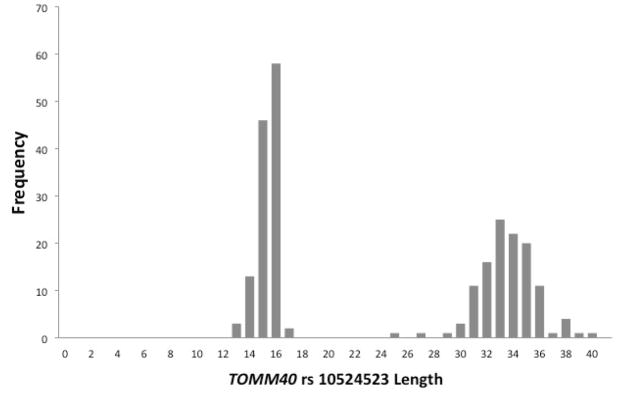
The present analyses were conducted on a subset of these participants who had successfully completed cognitive and imaging studies, had been genotyped for both APOE and TOMM40 poly-T, and had been found to have an APOE genotype of ε3/ε3 and a TOMM40 poly-T genotype of either homozygous Short (S/S), homozygous Very Long (VL/VL), or heterozygous (S/VL) TOMM40 genotype. This design was chosen based on the bimodal distribution of TOMM40 alleles among APOE ε3/ε3 subjects reported in (8) which was also evident in the present sample. The histogram in Figure 1 depicts the bimodal distribution of poly-T length among all available APOE ε3/ε3 subjects who met the criteria described above (n=120). Almost all poly-T lengths in this cohort (98%) were Short, S, (<21) or Very Long, VL, (≥30). Of those in Figure 1, three subjects with the L allele (poly-T lengths from 21 to 29) were excluded prior to the analysis due to their low frequency among APOE ε3/ε3 individuals (two with S/L and one with L/VL genotypes were excluded), leaving n=117 who fell into three separate TOMM40 rs10524523 genotypes: S/S n=38; S/VL n=44; VL/VL n=35. Demographic features are shown in Table 1.
Figure 1.
TOMM40 allele Frequency Distributions. Frequency distributions of the TOMM40 poly-T repeat length for 120 APOE3/3 subjects. The histogram clearly shows a bimodal distribution with most (98%) occurring at <= 20 repeats (short), or >= 30 repeats (very long). Each individual provides two poly-T lengths, representing each chromosome. Three individuals with poly-T repeat lengths >20 and <30 were classified by definition as an L allele, and excluded from further analysis.
Table 1.
Primers for PCR Amplification
| SNP | Primer Sequence |
|---|---|
| rs429358 | Outer: 5′-ACTGTGCGACACCCT-3′ and 5′-CTGAGGCCGCGCT-3′ Inner: 5′-GCGCTGATGGACG-3′ and 5′-CCTGGTACACTGCCA-3′ |
| rs7412 | Outer: 5′-CTGGAGGAACAACTGAC-3′ and 5′-CCCATCTCCTCCATC-3′ Inner: 5′-GACATGGAGGACGTG-3′ and 5′-GCTCCTGTAGCGG-3′ |
| rs8106922 | Outer: 5′-AGGCCCCTATTCCTG-3′ and 5-GTGCAAGACTTCATCTCAAA-3′ Inner: 5′-CATGCTAGCAGGCCA-3′ and 5′-ATCTGCAGAGACAGAAATG-3′ |
| rs10524523 | Outer: 5′-CTGGGCTCAAATGAACC-3′ and 5′-CAAATGTGATTTTATAGGGCCA-3′ Inner: 5′-GAGATGGGGTCTCACT-3′ and 5′-AGAGAGGGAGGGACA-3′ |
PCR, polymerase chain reaction; SNP, Single nucleotide polymorphism
Procedures
DNA samples and genotyping
DNA was extracted from blood. Samples were aliquoted on 96 well plates for determination of APOE and TOMM40 genotypes at Polymorphic DNA Technologies (polymorphicdna.com, Alameda, CA). Four different DNA polymorphisms were determined for each subject. Three of these polymorphisms, rs429358, rs7412, and rs8106922 are single nucleotide polymorphisms (SNPs), and the fourth, rs10524523, is a poly-T length polymorphism located in an intronic region of TOMM40. SNPs rs429358 and rs7412 define the APOE genotype, and rs8106922, within TOMM40, has been associated with risk of AD. For all four polymorphisms, PCR amplification of the region surrounding each variant site was employed to create short DNA amplicons that were subsequently used as templates for Sanger sequencing. The APOE genotype and TOMM40 rs10524523 poly-T length for each DNA strand were reported.
Nested PCR
For each target polymorphism, human genomic DNA samples were first PCR-amplified with a pair of “outside” oligonucleotide primers, and those amplification products were then re-amplified with a second pair of “inside” primers. The primers used for these amplifications are shown in Table 1.
The first “outer” PCR reaction was performed in a 384-well plate by combining in each well approximately 10 ng of genomic DNA with 1 unit of KlenTaq1™ (Ab Peptides, Inc., St Louis, MO), 0.5 μL of 10 X PCR Buffer, 1.0 μL of 5.0 M betaine, 0.175 μL DMSO, 0.8 μL of a mixture of dNTPs (2.5 mM each), 0.5 μL of a mixture of the two outer oligonucleotide primers (2 μM each), plus water to bring the entire reaction volume per well to a total of 5.0 μL. Plates were placed in a thermocycler and subjected to 40 cycles of the following conditions: 94° C for 20 s., 55° C for 25 s., and 72° C for 60 s.
The second “inner” PCR reaction was performed in a different 384-well plate by combining in each well 0.8 μL of the product from the first PCR reaction with 0.25 units of Klen Taq, 0.5 μL of 10 X PCR Buffer, 1.0 μL of 5.0 M betaine, 0.175 μL DMSO, 0.2 μL of a mixture of dNTPs (2.5 mM each), 1.0 μL of a mixture of the two inner oligonucleotide primers (2 μM each), plus water to bring the entire reaction volume per well to a total of 5.0 μL. Plates were placed in a thermocycler and subjected to 40 cycles of the following conditions: 94° C for 20 s, 55° C for 25 s, and 72° C for 60 s. These reaction products were then purified using PCR Cleanup Plates (MultiScreen384 PCR, Millipore Corp., Billerica, MA), with a final elution of each sample with 16 μL of water.
Sanger Sequencing Reactions
For each inner PCR product template, two separate Sanger sequencing reactions were performed using either the forward or reverse “inner” PCR primer as a sequencing primer. Reactions were carried out in a new 384-well plate by combining in each well 0.8 μL of the eluent from the PCR cleanup plates with 0.125 μL Big Dye Mix (Applied BioSystems, Inc., Foster City, CA), 0.215 μL 5X Dilution Buffer (Applied BioSystems, Inc.), 0.25 μL of a 1 μM solution of one of the “inner” primers, 0.105 μL DMSO, 0.6 μL of 5 M betaine, plus water to bring the entire volume to 3.0 μL. Plates were then placed in a thermocycler and subjected to 30 cycles of the following conditions: 96° C for 10 s., 50° C for 15 s., and 60° C for 120 s. These reaction products were then purified using Sequencing Reaction Cleanup Plates (MultiScreen384 SEQ, Millipore Corp.), with a final elution of each sample with 20 μL of 0.3 mM EDTA solution. From each well, this eluent was transferred to a fresh 384-well plate and loaded onto a 3730XL DNA Analyzer (Applied BioSystems, Inc.) running with a 36 cm capillary array using the “Rapid” run module. This instrument created a forward and reverse electropherogram data file for the region of each target variant for each genomic sample.
Data Analysis for the Three SNP Targets
For each SNP target and genomic sample, the forward and reverse electropherograms were processed by a proprietary sequence analysis system called Agent (Paracel, Celera Genomics, Alameda, CA). This system performs a bi-directional alignment of the sequencing data to the known reference sequence of each target region and reports the actual nucleotide calls found at each sequence position, along with “quality values” (QVs) indicating the relative confidence in each call. This system is tuned to recognize heterozygous nucleotide mixtures and it provides QVs for mixed-base calls as well as single-base calls. For this particular set of targets, nucleotide calls were made for the entire 101-bp region surrounding each target SNP (50 bp flanking each side of each SNP), but only the genotype calls at the actual SNPs of interest were reported. APOE genotype was determined from the rs429358, rs7412 SNPs according to the following allele mapping: APOE e2 if rs429358=T, rs7412=T, APOE e3 if rs429358=T, rs7412=C, APOE e4 if rs429358=C, rs7412=C.
Data Analysis for the Poly-T Length Polymorphism Target
Simple polymeric repeats such as poly-T tracts are difficult to analyze with methods that employ PCR due to slippage that may occur during each PCR cycle causing the newly polymerized strand to have either one fewer or one more residue than the template strand. After numerous cycles of PCR, the amplification product contains a complex mixture of products. The direct sequencing of such mixed templates is therefore very problematic because the sequencing electropherograms for such regions will be the superposition of the electropherograms of the numerous components of that mixture. For poly-T regions of length greater than 10 T residues, the degree of signal degradation becomes significant, and for N=15 and greater, an accurate interpretation of the number of T’s is difficult if not impossible. Since the rs10524523 poly-T target has known alleles longer than N=40, a conventional interpretation of the electropherograms was not a practical way to determine these genotypes. An analysis strategy was developed based on known properties of the sequencing reactions. The DNA polymerase adds an extra dye-labeled deoxyadenosine residue right after the end of a PCR template as part of the dye-terminator sequencing reaction. In a sequencing read of a PCR template without a homopolymer present, this produces one very large “A-peak” at the end of the electropherogram. In the case of a sequencing read that contains a homopolymer, multiple A-peaks are observed, with each such peak corresponding to the end of the various component templates present. This variation in template length is caused by the variation in the value of N for each component. The position of the A-peaks correspond to the N values (poly-T length) of each component. The reverse PCR primer used to produce the template was chosen in a region that was completely devoid of A-residues so that any A-peaks observed in that region would represent fully-extended products and not internal A-peaks. The following amplification product was produced:
GGGGTCTCACTATGTCACCTAGGCTTGTCTCCAACTGCTGACCTCAAGCTGTCCTCTTGCCCCAGCCCTCCAAAGCATTGGGATTACTGGCATGAGCCATTGCATCTGGCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGAGATGGGGTCTCACCATGTTGCCCGGGCTGGCTTCGAATGCCTAGGCTCAAGCAATCCTCCCTTCTCAGCCTCCCAAATAGCTGGGATTACAGGCACGTGCCACCACGCCAGGCTTGTTTTCTTTCCCTGTCCCTCCCTCTCT_addedA
When the forward sequencing reaction was carried out, the extra A-peaks were added at the position shown above as “addedA”. In the forward read, unambiguous sequence data was obtained up to the poly-T region, however, a complex mixture of peaks was observed following the poly-T stretch. The absolute length of the poly-T region was obtained by using the “C-peak” (just upstream from the poly-T) as a reference base relative to the region of “added A”. The number of T residues (N) represented by a given A-peak, was calculated by taking the peak number in the electropherogram for the A-peak and subtracting the peak position of the C-peak and lastly, subtracting 145 (the length of the sequence from the end of the poly-T to the end of the reference sequence.) The value of N for each component is therefore: N = A – C – 145, where A is the peak number for a given A-peak and C is the peak number for the reference C-peak. The approach was validated by cloning a variety of templates and sequencing the clones in order to determine the actual N-value in each clone. The poly-T regions in cloned templates do not suffer from PCR slippage and so the poly-T length can be unambiguously determined. These clones were used as calibration standards. Without any adjustments to the calculations, the Standard Error in this method was approximately 1 T-residue. The main source of error was that the nucleotide position numbering determined by the base-calling software is sometimes erroneous. These position-numbering errors were spotted by manual review of each electropherogram, and when this error is adjusted, the SE in the method was less than 0.5 T-residue. If the genomic sample contains two rs10524523 alleles with similar N-values, for example N=33 and N=35, the two sets of A-peaks may overlap to such a degree that it is difficult to ascertain specific peaks by visual inspection (e.g. differentiating 33 and 35, 32 and 36 or possibly homozygous 34. This problem was addressed by using several known authentic standard mixtures with corresponding rules for visually determining the most likely composition of each possible overlapping peak pattern. The estimated error in this assessment may be as much as 1 additional T residue, there the genotype calls made on samples with two similar rs10524523 alleles are somewhat less accurate.
Neuropsychological and other Laboratory Assessment
WRAP participants complete a health history form and a battery of laboratory and neuropsychological tests which are described in detail elsewhere (17). Because impairment in secondary memory is often the earliest cognitive indicator of AD pathology (20), performance on the Rey Auditory Verbal Learning Test (AVLT) was examined. The AVLT entails learning and recall of a list of 15 unrelated nouns. We have previously reported serial position effects on the AVLT, where WRAP participants with parental FH of AD exhibit a reduced primacy effect (e.g. fewer recalled words from the beginning of the list) compared to persons without a FH. For analyses in the present report, the primacy region was defined as serial position items number 1 through 4, the middle region as items 5 through 11, and recency region as items 12 through 15 (18).
Imaging
MRI (General Electric 3.0 Tesla, Waukesha, WI) was performed as part of related research on three scanners (90 on scanner 1; 19 on scanner 2; 8 on scanner 3) with comparable parameters. TOMM40 genotype distributions did not differ between scanners. Imaging included a volumetric T1-weighted inversion recovery prepared fast SPGR sequence. T2 weighted imaging was also done and visually inspected by a neuroradiologist to rule out overt pathology that would exclude subjects from further analysis. For scanner 1, the 3D T1-weighted volume was acquired in the axial plane with a 3D fast spoiled gradient echo (3D EFGRE) sequence using the following parameters: TI = 600 ms; TR = 8.4 ms; TE = 1.7 ms; flip angle = 10°; acquisition matrix = 256 × 192× 124, interpolated to 256 × 256 × 124; FOV = 240 mm; slice thickness = 1.2 mm (124 slices); acquisition time ~7.5 min. For scanner 2, the whole brain was imaged in the sagittal plane with the 3D EFGRE sequence using the following parameters: TI = 900 ms; TR = 6.6 ms; TE = 2.8 ms; flip angle = 8°; acquisition matrix = 256 × 256 × 166, FOV = 260 mm; slice thickness = 1.2 mm (166 slices). For scanner 3, the images were acquired in axial plane with the 3DEFGRE sequence using the following parameters: TI = 450 ms; TR = 8.1 ms; TE = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 × 156, FOV = 260 mm; slice thickness = 1.0 mm (156 slices).
Voxel-based morphometry (VBM)
Processing of the T1-weighted images for the cross-sectional analysis was performed using a six class segmentation tool in Statistical Parametric Mapping software http://www.fil.ion.ucl.ac.uk/spm (SPM8). The procedure involved segmentation of the original anatomical images into classes including gray matter (GM), white matter, cerebrospinal fluid, skull, fat tissue and image background using spatial priors; normalization (12-parameter affine transformation and nonlinear deformation with a warp frequency cutoff of 25) of the segmented maps to the Montreal Neurological Institute template (MNI); bias correction; and modulation which scaled the final GM maps by the amount of contraction or expansion required to warp the images to the template. The final result was a GM volume map for each participant, where the total amount of GM remained the same as in the original images. The spatially normalized GM maps were smoothed using an 8 mm isotropic Gaussian kernel to optimize signal to noise and facilitate comparison across participants. Analysis of GM employed an absolute threshold masking of 0.1.
Statistics
Sample characteristics were compared across TOMM40 groups using one-way analysis of variance and chi-square tests. Cognitive outcomes were compared across TOMM40 groups via analysis of covariance using age, sex, and education level as covariates, as these are known to influence verbal memory performance. Given the a priori expectation that the S/S and VL/VL groups represented the lowest and highest risk of earlier age of onset of AD (8, 9), separate ANCOVAs were also performed and effect sizes (21) were calculated for these two groups. Variables were transformed as needed to normalize distributions; alpha was .05 for all tests described here.
Gray matter maps were also compared between the three genetically stratified groups. For voxel-wise univariate statistics, we conducted an analysis of covariance. Age was treated as a covariate of interest and was allowed to interact with brain volume. Total intracranial volume was included as a covariate of no interest. The voxel level threshold was p < 0.005 with a minimum cluster size of 200 contiguous voxels. Because of the specific a priori hypothesis regarding the posterior cingulate, we used a predefined search region limited to the posterior cingulate and precuneus and used the FDR corrected cluster statistic for group inference. Plots of significant group differences and group by age effects were made by extracting the first principal component of the whole cluster using the Volume of Interest tool in SPM8.
RESULTS
Sample characteristics are shown in Table 2 and other health characteristics are shown in Table 3. There were no significant differences between the two TOMM40 Poly-T groups on gender, education, or Verbal or Performance (VIQ, PIQ). The VL/VL group was significantly older, and the S/VL group had a higher percentage of participants with a parental family history of AD than the other two groups. The groups did not differ on cholesterol level, systolic or diastolic blood pressure (BP), body mass index (BMI), or self-reported diagnosis of heart disease, hypertension, hypercholesterolemia, or diabetes (see Table 3). Homocysteine levels were normal across all groups, but significantly higher (p < .05) in the VL/VL group compared to the S/S group, and there was a trend (p = .052) for more S/VL participants to report a history of hypertension compared to the other groups.
Table 2.
Characteristics of APOE ε3/ε3 Study Population by TOMM40 group
| Group characteristic | TOMM40 S/S (n = 38) | TOMM40 S/VL (n = 44) | TOMM40 VL/VL (n = 35) |
|---|---|---|---|
| Age, mean (SD)1 | 54.39 (6.25) | 53.43 (6.08) | 58.69 (5.67) |
| Education, % with ≥ BA | 73.7 | 68.2 | 77.1 |
| Gender, % female | 71.1 | 56.8 | 60.0 |
| Family history, % positive2 | 50.0 | 77.3 | 54.3 |
| Verbal IQ | 114.87 (12.0) | 113.41 (10.0) | 116.71 (6.4) |
| Performance IQ | 114.61 (10.5) | 114.55 (10.6) | 113.20 (9.4) |
Bolded values represent significant group differences, p < .05.
VL/VL > (S/S, S/VL);
S/VL > (S/S, VL/VL).
APOE, Apolipoprotein E; TOMM40, translocase of the outer mitochondrial membrane 40; S, short; VL, very long.
Table 3.
Health Attributes of APOE ε3/ε3 Study Population by TOMM40 group
| Group characteristic | TOMM40 S/S (n = 38) | TOMM40 S/VL (n = 44) | TOMM40 VL/VL (n = 35) |
|---|---|---|---|
| Heart disease (%) | 7.9 | 15.9 | 11.4 |
| Hypertension (%) | 5.3 | 22.7 | 8.6 |
| Hypercholesterolemia (%) | 31.6 | 36.4 | 31.4 |
| Diabetes (%) | 0 | 0 | 5.7 |
| Cholesterol | 214.21 (36.04) | 202.77 (32.93) | 196.12 (32.55)† |
| Homocysteine1 | 7.71 (2.14) | 8.28 (1.77) | 8.89 (2.54)† |
| Systolic BP | 128.47 (20.23) | 128.75 (17.11) | 126.60 (16.29)† |
| Diastolic BP | 75.05 (11.84) | 75.43 (10.67) | 73.06 (10.83) |
| BMI | 26.87 (3.87) | 27.48 (4.73) | 26.53 (4.26)† |
Bolded values represent significant group differences, p < .05; for hypertension, p = .052.
Raw data were log-transformed to reduce skewness for the purpose of mean comparisons.
VL/VL > S/S.
APOE, Apolipoprotein E; TOMM40, translocase of the outer mitochondrial membrane 40; S, short; VL, very long.
Serial position data for the AVLT, summarized in Table 4, were available for 112 participants (95.7%). After adjusting for age, gender and education, the main effect of TOMM40 poly-T length in the three group model was marginally significant for two memory measures: retrieval from the primacy region (p = .075) and total recall (p = .055), and in each case, the order of performance was S/S > S/VL > VL/VL. In the exploratory ANCOVA comparing S/S and VL/VL genotypes, TOMM40 poly-T length effects were significant for both primacy [F(1,65) = 4.48, p = .038] and total recall [F(1,65) = 5.77, p = .019]. The effect sizes were medium to large for these comparisons and small for the nonsignificant comparisons. No between group differences in midposition and recency data were observed for either the three or two level TOMM40 analyses.
Table 4.
Performance of Low (S/S), Intermediate (S/VL), and High (VL/VL) Risk Groups on the Rey Auditory Verbal Test
| TOMM40 S/S (n = 38) | TOMM40 S/VL (n = 42) | TOMM40 VL/VL (n = 32) | Cohen’s D for S/S vs VL/VL | |
|---|---|---|---|---|
| Primacy (max = 20) | 15.84 (2.82) | 14.88 (2.28) | 14.22 (2.56) | 0.599 |
| Midposition (max = 35) | 23.11 (5.30) | 20.16 (5.09) | 21.76 (5.37) | 0.253 |
| Recency (max = 20)† | 16.18 (2.74) | 15.25 (2.40) | 15.76 (2.61) | 0.157 |
| Total correct (5 trials) (max = 75) | 55.13 (7.73) | 52.02 (7.52) | 49.63 (7.57) | 0.718 |
Bold values denote statistically significant group differences (p < .05).
Distribution transformed for statistical comparison.
TOMM40 Poly-T VL Dose Effect on Brain Volume
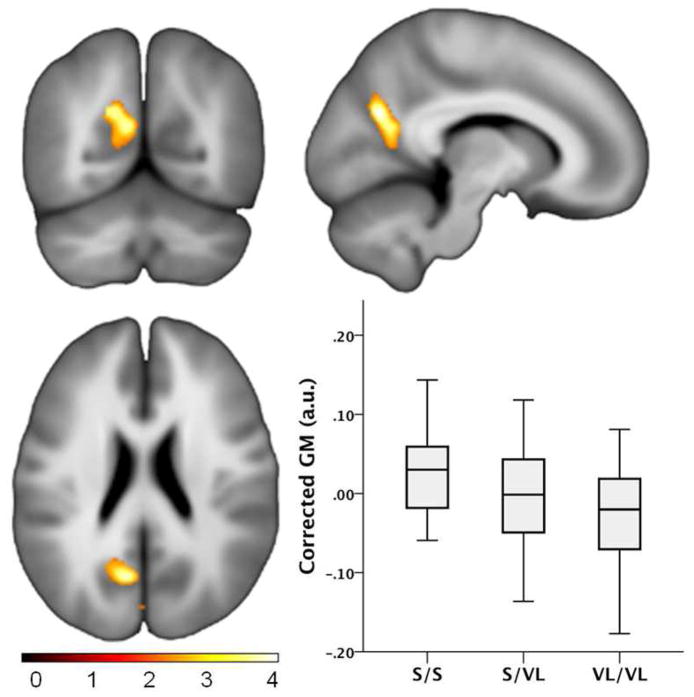
A voxel-wise analysis for group differences was done to identify brain regions where there was a dose effect of VL, that is, decreasing volume with more VL TOMM40 alleles after accounting for age and TICV. Regions where significant group effects were observed are shown in Figure 2 and included the left ventral posterior cingulate (voxel coordinate x,y,z: −9, −66, 25; t=4.10, p<.0001; cluster size 796 voxels, p=.005) and the cuneus (x,y,z: −2, −85, 13; t=3.43, p<.0001; cluster size 376 voxels, p=.042).
Figure 2.
Gray Matter Group Differences. Voxel-wise gray matter volume comparison between the S/S, S/VL and VL/VL TOMM40 groups depicting a “dose effect”. The boxplots depict the mean effect graphically, showing the first principle component scaled in arbitrary units extracted from the cluster in the posterior cingulate. The S/S group had the greatest volume, followed by the S/VL group, and the VL/VL group had the lowest volume.
VBM Age by Group Interaction
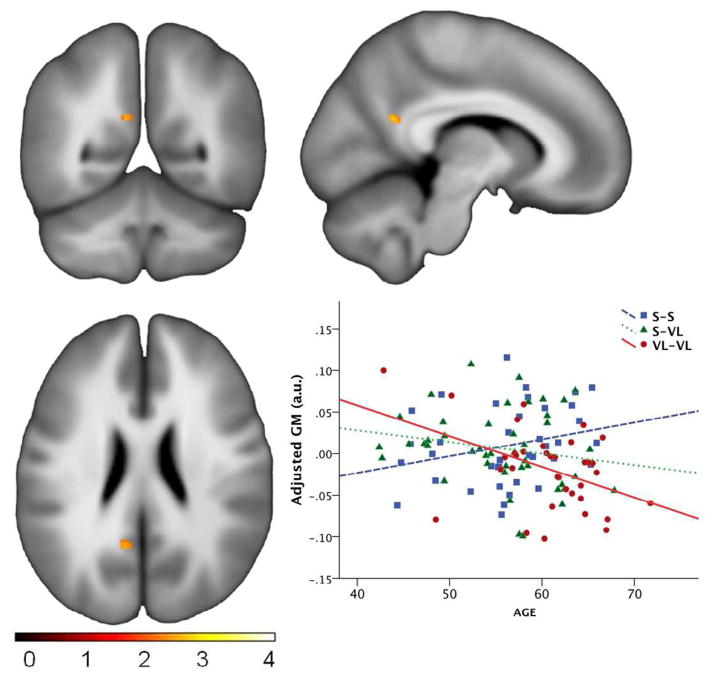
Next we conducted a whole-brain analysis to determine whether age-related atrophy differed by TOMM40 poly-T group. Rather than interrogating the entire brain, this comparison was restricted to regions previously shown to be vulnerable to AD including the amygdala, hippocampus, parahippocampal gyrus, posterior cingulate and precuneus. No effects were found at the pre-specified spatial threshold of 200 voxels. However, two smaller clusters were noted in the retrosplenial area (−9, −54, 22; t=3.21; p=.001; cluster size 35 voxels; see Figure 3), and the right anterior mesial temporal lobe (36, −1, −23; t=2.83; p=.003; cluster size 23 voxels) indicating a relatively stronger VL associated negative slopes with age.
Figure 3.
Gray Matter by Age Slope Differences. (A) voxel-wise group by age interaction was found in a small cluster in the retrosplenial aspect of the posterior cingulate, indicating that the effect of age on gray matter atrophy differs as a function of group. (B) Plot of the effect indicating a greater negative slope with age in the VL/VL group. Note the y-axis is statistically adjusted GM, and thus should not be interpreted strictly as absolute gray matter.
DISCUSSION
Although presence of one or more APOE ε4 alleles has been the most robust genetic risk factor for LOAD, this study found that in asymptomatic late middle-aged ε3/ε3 individuals, another genetic polymorphism in TOMM40, poly-T length at rs10524523, is associated with cognitive and gray matter volume differences that are suggestive of incipient AD. This same TOMM40 poly-T polymorphism has previously been shown to be associated with age of onset in LOAD (8), and the present study suggests that AD-related brain and cognitive changes may be occurring years before the onset of clinical symptoms in APOE ε3 homozygotes.
The TOMM40 homozygous Short and homozygous Very Long groups had different serial position curves on a list learning task. Deficits in learning and memory are distinguishing features of mild and very mild LOAD and amnestic MCI. Further, mild learning and memory dysfunction have been shown to presage development of clinical AD in community and population cohorts (20, 22, 23). Normal aging does not affect the basic shape of the serial position curve, even though the total amount recalled is typically lower for older persons (24). By contrast, patients with AD, even in very mild stages, show an increase in recency and decrease in primacy (25–27), likely reflecting AD-associated changes in episodic memory systems for encoding and retrieval that are seen in the hippocampus (28, 29), entorhinal cortex (28, 30), and ventral posterior aspects of the cingulate including the isthmus and retrosplenial area (13–15, 31). Although there was a medium effect size in the primacy effect, no group was abnormally low, which is to be expected in an asymptomatic population. Replication of these preliminary cognitive trends and MRI findings with independent samples is needed, as is longitudinal study to determine whether variation in intronic poly-T length at TOMM40 rs10524523 is associated with progressive changes in cognition and regional brain volume in this cohort.
The difference in ventral posterior cingulate volume in this study is particularly intriguing as this region is not only implicated in memory retrieval, it is also atrophic and hypometabolic in mild AD (14) and MCI (32). Dose-dependent hypometabolism in asymptomatic APOE ε4 carriers (15, 33) has been found in this region. It is also one of the earliest regions to show increased amyloid burden measured using Positron Emission Tomography with Pittsburgh Compound B (34–36). The gray matter finding in this study is complementary to GWAS studies using the chronologically older ADNI cohort in which a separate TOMM40 polymorphism was associated with gray matter volume differences in normal persons and those with a diagnosis of mild cognitive impairment or AD (10, 11).
The VL/VL group was 4 years older than the S/S group and thus age was included as a covariate. To further alleviate the potential concern that age is unduly influencing the result, we conducted a secondary analysis in which we trimmed the tails of the age distribution to include only those subjects between ages 56 and 66. Age was no longer significantly different, while the group effect in the posterior cingulate remained significant at an uncorrected voxel level alpha of .005 and 200 contiguous voxels.
In prior studies, we and others have found laboratory, cognitive and neuroimaging evidence for presumptive AD in asymptomatic persons with a first degree family history of AD (13, 16, 18, 35, 37–39). Some studies report interactions with APOE genotype, others report independent family history effects, suggesting the presence of an unknown gene(s) that may be responsible for the family history effect. In this study the S/S and VL/VL groups did not differ on the proportion of subjects with parental FH. However the S/VL group had a greater proportion with FH. Persons with a FH have a high prevalence of APOE ε4 alleles (17, 40), which may interact in some way with TOMM40 to explain FH genetic risk. The interplay of FH, TOMM40 and APOE genotype is the topic of ongoing studies.
In conclusion, this study suggests that a subgroup of late middle-aged APOE ε3/3 homozygotes who carry the TOMM40 VL/VL genotype may be at higher risk for AD than current paradigms for AD risk would predict. These asymptomatic subjects performed worse on a measure of episodic learning and also exhibited smaller volume in the posterior cingulate, a region well-known to be affected early in the course of AD. The study is limited by the relatively small number of subjects who were APOE ε3/ε3 and had both TOMM40 genotyping and structural imaging. The VL/VL group was also older than the other groups, though the analyses were statistically adjusted for age. If confirmed in independent studies, these findings suggest that TOMM40 poly-T length may help to explain residual AD risk among the most common APOE genotype in the population. The clinical significance of TOMM40 variants in this population will be elucidated with further longitudinal study to clinical endpoints of MCI and AD.
Acknowledgments
We gratefully acknowledge the assistance of Shawn Bolin, Maggie Kengott, Gail Lange, Kimberly Mueller, Christine Pire-Knoche, Janet Rowley, and Susan Schroeder for WRAP data collection. We especially thank the WRAP participants.
Study Sponsorship: This study was supported by grants for the NIH: R01 AG027161; R01 AG021155; P50 AG033514 Alzheimer’s Disease Research Center; and UL1RR025011 Clinical and Translational Science Award. Dr. Roses and Dr. Lutz are supported in part by RC1AG035635-01. Portions of this research were supported by the Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation and from the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI.
Footnotes
Financial Disclosures:
Sterling Johnson, PhD: No disclosures
Asenath La Rue: No disclosures
Bruce Hermann: No disclosures
Guofan Xu: No disclosures
Rebecca Koscik: No disclosures
Erin Jonaitis: No disclosures
Barbara Bendlin: No disclosures
Kirk Hogan: No disclosures
Allen Roses: Dr. Roses is the President of three companies filed as S-Corporations in the state of North
Carolina: Cabernet Pharmaceuticals, Inc. is a pipeline pharmacogenetic consultation and project management company that has other pharmaceutical companies as clients; Shiraz Pharmaceuticals, Inc., is focused on the commercialization of diagnostics, including companion diagnostics, for universities, pharmaceutical companies, and biotechnology companies; Zinfandel Pharmaceuticals is the sponsor of OPAL (Opportunity to Prevent Alzheimer’s Disease) which is a combined clinical validation of a diagnostic and a pharmacogenetic-assisted delay of onset clinical trial.
Ann Saunders: No disclosures
Michael Lutz: No disclosures
Sanjay Asthana: No disclosures
Robert Green: No disclosures
Mark Sager: No disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sterling C Johnson, Email: scjohnson@wisc.edu.
Asenath La Rue, Email: larue@wisc.edu.
Bruce P Hermann, Email: Hermann@neurology.wisc.edu.
Guofan Xu, Email: gxu@medicine.wisc.edu.
Rebecca L Koscik, Email: rekoscik@wisc.edu.
Erin M Jonaitis, Email: jonaitis@wisc.edu.
Barbara B Bendlin, Email: bbb@medicine.wisc.edu.
Kirk J. Hogan, Email: khogan@wisc.edu.
Allen D Roses, Email: allen.roses@duke.edu.
Ann M Saunders, Email: ann.saunders@duke.edu.
Michael W Lutz, Email: michael.lutz@duke.edu.
Sanjay Asthana, Email: Sa@medicine.wisc.edu.
Robert C Green, Email: rcgreen@bu.edu.
Mark A Sager, Email: masager@wisc.edu.
References
- 1.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 2.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 3.Sleegers K, Lambert JC, Bertram L, Cruts M, Amouyel P, Van Broeckhoven C. The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet. 2010;26:84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 6.van Duijn CM, de Knijff P, Cruts M, et al. Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer’s disease. Nat Genet. 1994;7:74–78. doi: 10.1038/ng0594-74. [DOI] [PubMed] [Google Scholar]
- 7.Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55:964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 8.Roses AD, Lutz MW, Amrine-Madsen H, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2009 doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Dement. 2010;6:125–131. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potkin SG, Guffanti G, Lakatos A, et al. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, Kim S, Risacher SL, et al. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson SC, Ries ML, Hess TM, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Arch Gen Psychiatry. 2007;64:1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, McLaren DG, Ries ML, et al. The influence of parental history of Alzheimer’s disease and apolipoprotein E epsilon4 on the BOLD signal during recognition memory. Brain. 2009;132:383–391. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 16.Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sager MA, Hermann B, La Rue A. Middle-Aged Children of Persons With Alzheimer’s Disease: APOE Genotypes and Cognitive Function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 18.La Rue A, Hermann B, Jones JE, Johnson S, Asthana S, Sager MA. Effect of parental family history of Alzheimer’s disease on serial position profiles. Alzheimers Dement. 2008;4:285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA workgroup under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JD. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 22.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 23.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith G, Kokmen E, Ivnik RJ, Tangalos EG. Memory function in normal aging. Neurology. 1992;42:396–401. doi: 10.1212/wnl.42.2.396. [DOI] [PubMed] [Google Scholar]
- 25.Bayley PJ, Salmon DP, Bondi MW, et al. Comparison of the serial position effect in very mild Alzheimer’s disease, mild Alzheimer’s disease, and amnesia associated with electroconvulsive therapy. J Int Neuropsychol Soc. 2000;6:290–298. doi: 10.1017/s1355617700633040. [DOI] [PubMed] [Google Scholar]
- 26.Bigler ED, Rosa L, Schultz F, Hall S, Harris J. Rey-Auditory verbal learning and the Rey-Osterrieth complex figure design performance in Alzheimer’s disease and closed head injury. Journal of Clinical Psychology. 1989;45:277–280. doi: 10.1002/1097-4679(198903)45:2<277::aid-jclp2270450216>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Gainotti G, Marra C. Some aspects of memory disorders clearly distinguish dementia of the Alzheimer’s type from depressive pseudo-dementia. J Clin Exp Neuropsychol. 1994;16:65–78. doi: 10.1080/01688639408402617. [DOI] [PubMed] [Google Scholar]
- 28.Dickerson BC, Feczko E, Augustinack JC, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chetelat G, Desgranges B, de la Sayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 33.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 35.Mosconi L, Rinne JO, Tsui WH, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SC, Schmitz TW, Trivedi MA, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Exel E, Eikelenboom P, Comijs H, et al. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- 39.van Vliet P, Westendorp RG, Eikelenboom P, et al. Parental history of Alzheimer disease associated with lower plasma apolipoprotein E levels. Neurology. 2009;73:681–687. doi: 10.1212/WNL.0b013e3181b59c2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]