Abstract
Mantle cell lymphoma (MCL) is a malignancy of mature B cells characterized by the translocation t(11;14) that leads to aberrant expression of cyclin D1. Response to first-line chemotherapy is good but most patients relapse resulting in a median survival of 5-7 years. The important PI3K/AKT/mTOR pathway can be targeted with small molecules. mTOR inhibitors have clinical activity and temsirolimus has been approved in Europe. Second generation mTOR inhibitors and the PI3K inhibitor CAL-101 offer additional means to target the pathway. Promising results with the BTK inhibitor PCI-32765 suggest that B-cell receptor signaling could play a role. For unknown reasons, MCL appears to be particularly sensitive to disruption of protein homeostasis. The proteasome inhibitor bortezomib achieves responses in up to 50% of relapsed patients. Much work has been done in elucidating the mechanism of its cytotoxicity, its incorporation into combination therapies, and the development of second generation proteasome inhibitors. Deacetylase and HSP90 inhibitors are also promising classes of drugs that can synergize with proteasome inhibitors. Finally, BH3 mimetics are emerging as tools to sensitize tumor cells to chemotherapy. Participation in clinical trials offers patients an immediate chance to benefit from these advances and is essential to maintain the momentum of progress.
Introduction
It was in the late 1800s that the German physician Paul Ehrlich developed the idea of a “magic bullet”, referring to a substance that directly aims at a pathogenic microorganism. It has been argued that the “magic bullet concept” of specifically targeting tumor cells while sparing normal tissues has been proven in cancer treatments.1 While the concept may have been proven in select circumstances, most agents we discuss here as targeted approaches to MCL are not magic bullets. Imatinib (Gleevec/Glivec, Novartis), which targets the BCR-ABL kinase in chronic myeloid leukemia, often serves as a proof for the existence of these famed magic bullets. The proteasome inhibitor bortezomib, approved for relapsed MCL, may serve as a counterpoint. It universally inhibits proteasome activity in both normal and malignant cells and a consistent explanation why certain tumor cells are hypersensitive to proteasome inhibition remains elusive. Thus, the development of targeted agents, which we here use synonymously with “non-traditional-chemotherapy” based agents, is as much driven by insights into tumor biology generated with the tools of modern biology and genetics as it is helped by serendipity and empiric testing of agents across multiple tumor types.
MCL is a disease in urgent need of novel agents. There is no consensus on a standard of care.2 The disease is heterogeneous with some patients suffering from an aggressive cancer, while others may go for years without requiring treatment. Initial response to many first-line treatments is typically good but relapse is virtually certain.3 Furthermore, median age at diagnosis is around 65, making intensive treatment approaches, especially stem cell transplantation, difficult for a significant proportion of patients. The convergence of all these factors may be the reason that MCL is one of the most difficult to treat B-cell lymphomas with a median survival of 5-7 years. However, one may be hopeful for the future looking at the impressive breadth of agents with novel mechanisms of action that currently are in clinical development. Here we will focus entirely on small molecule drugs, their mechanism of action, and the stage of their clinical development but not touch on monoclonal antibody therapies.
Targeting the cell cycle
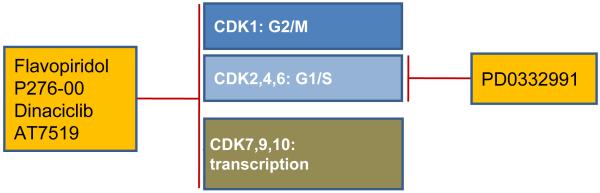
Dysregulated proliferation is one of the hallmarks of cancer and hence pharmacological intervention of cell cycle regulatory molecules appears as an attractive therapeutic strategy. In addition, high tumor proliferation is the best predictor of poor survival in MCL.4 The genetic hallmark of MCL is the translocation t(11;14)(q13;q32) that leads to constitutively high expression of cyclin D1 (reviewed in 5,6). Additional mutations in the cyclin D1 transcript delete regulatory elements that shorten mRNA half-life and thereby further increase cyclin D1 expression.7 Targeting cyclin D1 using shRNA-mediated knockdown has only minimal effects on the viability of MCL cells in vitro. This has been explained by the compensatory induction of cyclin D2.8 Cyclins are regulatory elements of holoenzymes formed with cyclin-dependent kinases (CDKs). Some CDKs, such as the interphase CDKs CDK2, CDK4 and CDK6 promote cell cycle progression, while others such as CDK7 and CDK9 primarily function to regulate transcription (Figure 1).9
Figure 1. Targeting cyclin-dependent kinases.
The cyclin-dependent kinases (CDKs) 1, 2, 4 and 6 are directly involved in the progression through G1/S and G2/M phases of the cell cycle. CDKs 7, 9 and 10 regulate transcription. Pan-CDK inhibitors exhibit activity against both groups, whereas PD0332991 selectively targets interphase CDKs.
One of the first CDK inhibitors to be tested in the clinic is flavopiridol (alvocidib, HMR-1275), a flavonoid that has broad activity against most CDKs. Consequently, flavopiridol not only inhibits cell cycle progression but in addition inhibits RNA synthesis by interfering with RNA polymerase II.9 However, early clinical trials in untreated or relapsed MCL have shown unsatisfactory results with minimal antitumor activity.10 More recently, flavopiridol achieved a response rate of 45% in a phase I trial in chronic lymphocytic leukemia (CLL).11 Key to this improved efficacy has been the recognition that flavopiridol is highly protein bound in human plasma but not by serum used in preclinical model systems. The Ohio state group implemented a modified dosing scheme, consisting of a 30-minute bolus dose followed by a 4-hour infusion that led to sustained concentrations of free drug and marked clinical efficacy.11 The group then went on to combine flavopiridol with fludarabine and rituximab for the treatment of relapsed B-cell non Hodgkin’s lymphomas (NHL) including MCL. In this study an overall response rate (ORR) of 82% (CR, 50%; CRu, 5%; PR, 26%) has been achieved. The ORR for patients with MCL was 80% (7 CR, 1 PR).12 Several studies using flavopiridol according to the modified dosing regimen are ongoing (Table 1). Other broad CDK inhibitors include P276-00, a second generation synthetic flavone, SCH 727965 (dinaciclib), and AT7519. All these compounds inhibit CDK9 and appear to primarily exert their antitumor effects through inhibition of transcription.13-15 With demonstrated activity in preclinical models they have entered clinical testing (Table 1).
Table 1. Targeted approaches in mantle cell lymphoma.
| Class | Drug | Studies |
|---|---|---|
| CDK inhibitors | ||
| Targeting CDKs induces cell cycle arrest (e.g., CDK4/6) and/or inhibition of translation (e.g., CDK9) |
Flavopiridol (alvocidib) | Ongoing phase II studies using pharmacologically based schedules for relapsed MCL: NCT00445341, NCT00112723 |
| Dinaciclib (SCH 727965) | Phase II in MCL/CLL (Study P04715AM2) (NCT00871546) | |
| P276-00 | Phase II in MCL (NCT00843050) | |
| AT7519 | Phase I/II in combination with bortezomib in MM (NCT01183949); Phase I in solid tumors/NHLs (NCT00390117) |
|
| PD0332991 | Phase I in MCL (NCT00420056); plus bortezomib in MCL (NCT01111188) |
|
| BCR and PI3K/AKT/mTOR inhibitors | ||
| BCR signal transduction inhibitors | ||
| Inhibit signal transduction components downstream of BCR activation |
Fostamatinib (inhibits SYK) |
Phase I/II in B-cell lymphomas including MCL (NCT00446095) |
| Enzastaurin (inhibits PKCβ) |
Phase II in relapsed MCL | |
| PCI-32765 (inhibits BTK) | Phase II (PCYC-1104-CA) in relapsed/refractory MCL (NCT01236391) |
|
| mTOR inhibitors | ||
| Targeting mTOR/TORC1 complex inhibits translation/ribosome biogenesis |
RAD001 (everolimus) | PILLAR-1 and SAKK 36/06 as single-agent in MCL |
| CCI-779 (temsirolimus) | Phase III/IV in relapsed/refractory MCL (NCT00117598/NCT01180049) |
|
| AKT inhibitors | ||
| Targeting Akt kinase inhibits signal transduction |
Perifosine | Completed Phase I in refractory neoplasms testing different loading schedules (NCT00019656) |
| MK2206 | Phase II in relapsed lymphoma (NCT01258998) | |
| Triciribine Phosphate Monohydrate (TCN-PM, VD-0002) |
Phase I in adults with advanced hematologic malignancies (NCT00642031) |
|
| GSK2141795 | Phase I ‘First-Time-In-Human Study’ oral GSK2141795 (NCT00920257) |
|
| PI3K p110 inhibitors | ||
| Targeting p110/PI3K kinase inhibits signal transduction pathway |
CAL-101 | Phase I in select relapsed/refractory hematologic malignancies (NCT00710528) |
| SF1126/LY294002 | Phase I study in B-cell malignancies | |
| Targeting regulators of protein homeostasis | ||
| HSP90 inhibitors | ||
| Targeting HSP90 chaperone impairs stability of various oncoproteins |
17-AAG (tanespimycin) | Completed Phase II in lymphomas/MCL (NCT00117988) |
| IPI-504 and IPI-493 | Phase I in hematologic malignancies (NCT01193491); completed Phase I in relapsed/refractory MM (NCT00113204) |
|
| STA-9090 (ganetespib) | Phase I studies in hematologic malignancies | |
| SNX-5422 and SNX-2112 | 2 Phase I in hematological malignancies (NCT00595686) and lymphomas completed (NCT00647764); Phase I in solid tumor/lymphoma that has not responded to treatment (NCT00644072) |
|
| Inhibitors of small modifier enzymes | ||
| Targeting small modifier enzymes inhibits degradation of select proteins |
MLN4924 | Phase I in adults with lymphoma/MM (NCT00722488) |
| RG7112 | Phase I in relapsed/refractory leukemia (NCT00623870) | |
| Proteasome inhibitors | ||
| Targeting proteasome subunit/s inhibits global protein degradation |
MLN9708/MLN2238 | Phase I in adults with lymphoma (NCT00893464) |
| Carfilzomib (PR171) | Phase I in hematological malignancies (NCT00150462); Phase I in CLL/SLL/PLL (NCT01212380) |
|
| ONX0912 (PR047) | Phase I Study in advanced refractory/recurrent solid tumors (NCT01129349) |
|
| NPI-0052 | Phase I in relapsed/refractory MM (NCT00461045); Phase I in advanced solid tumor malignancies/refractory lymphoma (NCT00396864) |
|
| Bcl-2 family inhibitors | ||
| Targeting antiapoptotic Bcl-2 family members inhibits cell survival |
GX15-070MS (obatoclax mesylate) |
Completed Phase I/II study of GX15-070MS (obatoclax mesylate) plus bortezomib in refractory/relapsed MCL (NCT00407303) |
| AT-101 | Completed Phase II in relapsed/refractory B-cell malignancies/MCL (NCT00275431) |
|
| ABT-737 and ABT-263 | Phase I/II relapsed/refractory lymphoid malignancies (NCT00406809) |
|
| PARP inhibitors | ||
| Targeting PARP inhibits DNA damage repair |
ABT-888 (Veliparib) | Phase I in patients with refractory solid tumors/hematological cancers completed (NCT00387608) |
| MK-4827 | Phase II in relapsed MCL/MCL with inactive ATM (NCT01244009) |
|
| Inhibitors of lysine deacetylases | ||
| Targeting lysine deacetylases impairs gene expression and protein function |
Vorinostat (SAHA, Zolinza) |
Phase I/II studies including MCL in combination regimens |
| Romidepsin (FR901228) | Completed Phase II in relapsed/refractory NHL (NCT00077194) | |
| Belinostat (PXD101) | Completed Phase I plus 17-N-Allylamino-17- Demethoxygeldanamycin in solid tumors/lymphoma (NCT00354185); Phase I plus bortezomib in advanced solid tumors/lymphomas (NCT00348985) |
|
| Panobinostat (LBH589) | Phase II in relapsed/refractory NHL (NCT01261247); Phase I/II plus everolimus in MM/NHL/HL (NCT00918333); Phase II in relapsed/refractory CLL/MCL (NCT01090973) |
|
| Entinostat (MS-275) | Completed Phase I in solid tumors/lymphomas (NCT00020579); plus isotretinoin (NCT00098891) |
|
| JAK/STAT inhibitors | ||
| Targeting JAK kinase inhibits signal transduction |
SB1518 | Phase II in advanced lymphoid malignancies/MCL (NCT01263899) |
In contrast to the pan-CDK inhibitors, the pyridopyrimidine PD0332991 is a highly specific and potent inhibitor of CDK4 and CDK6 and thus indeed targets cell cycle progression. While potently inhibiting proliferation of MCL cells in vitro it had no effect on cell viability.16 PD0332991 has entered early clinical studies in MCL and solid tumors. Intriguingly, PD0332991-induced G1 arrest sensitized multiple myeloma (MM) cells to killing by bortezomib.17 This combination is now being tested in clinical studies in patients with relapsed MCL.
Targeting the PI3K/AKT/mTOR and B-cell receptor signaling pathway
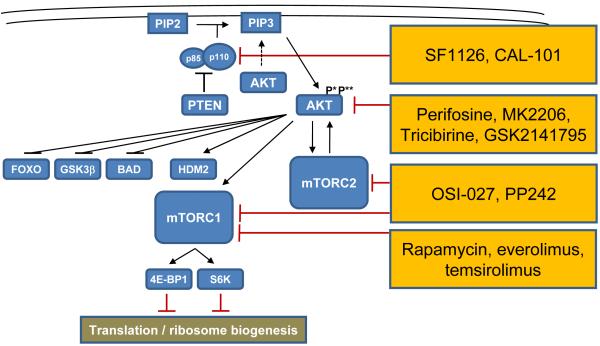
The PI3K/AKT/mTOR signaling pathway controls a variety of cellular processes including proliferation, metabolism, differentiation, apoptosis, and survival. The key signal transducers in this pathway, the lipid kinase PI3K and the serine/threonine kinases AKT and mTOR (Figure 2), have emerged as promising therapeutic targets as their constitutive activation is a key survival pathway in many cancers, including MCL (reviewed in 5,6). The mTOR kinase is the catalytic subunit of two distinct protein complexes with separate cellular functions, called mTORC1 and mTORC2. A biologic and pharmacologic important difference lies in the composition of the regulatory proteins within each complex. The regulatory subunit is called raptor in mTORC1 and rictor in mTORC2. mTORC1 regulates protein synthesis by phosphorylating proteins of the translation machinery such as 4E binding protein and S6 kinase. The main substrates of mTORC2 are AKT and related kinases.18
Figure 2. Targeting the PI3K/AKT/mTOR pathway.
PI3 kinases, heterodimers composed of p85 regulatory and p110 catalytic subunits, phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which leads to recruitment and activation of AKT. AKT activation requires both phosphorylation at threonine 308(P*) and at serine 473(P**) in order to activate mTOR-containing complexes mTORC1 and mTORC2 and HDM2 or inhibit FOXO, GSK3β, and BAD. Several small molecules targeting the PI3K/AKT/mTOR pathway at different levels are currently in clinical testing.
Rapamycin (sirolimus) and rapamycin analogs such as CCI-779 (temsirolimus) and RAD001 (everolimus), also referred to as rapalogs, are allosteric mTOR inhibitors that primarily inhibit mTORC1 (Figure 2). Single-agent temsirolimus achieved responses in up to 40% of patients with relapsed or refractory MCL, with CRs in less than 5% of patients.19,20 A phase III study of single-agent temsirolimus compared with physician’s-choice monotherapies found a superior ORR of 22% in the group with the highest temsirolimus dose as compared to 2% for alternative agents.21 Consequently, temsirolimus has been approved by the European Medicines Agency for the treatment of relapsed and refractory MCL. Everolimus has also been tested as single agent in relapsed and refractory MCL. The PILLAR-1 study reported PRs in 12% of patients refractory or intolerant to bortezomib (n=26),22 and the SAKK 36/06 reported two CRs (6%) and five PRs (14%; n=35).23 These studies also reported a relatively short median progression free survival (PFS) of 4-7 months and an equally short duration of response (DOR).19-21
The limited clinical activity of rapalogs may be explained by selective inhibition of mTORC1 and a negative feedback loop from mTORC2 to AKT that may antagonize some of the antitumor effects. Thus combined inhibition of both mTORC1 and mTORC2 is thought to increase efficacy.24 One such dual mTOR inhibitor is OSI-027. In vitro OSI-027 induced apoptosis in MCL cell lines and MCL patient cells ex vivo, against which rapamycin was ineffective. In the same study, OSI-027 has been shown to inhibit phosphorylation of AKT targets such as FOXO3A and BAD.25 PP242, a reversible competitive inhibitor of ATP binding to mTORC1 and mTORC2 has preclinical activity in acute leukemia.26,27
AKT is phosphorylated by PDK1 at threonine 308. However, full activation requires also phosphorylation at serine 473 by mTORC2. The clinically most advanced direct inhibitor of AKT is the oral alkylphospholipid perifosine, which targets the pleckstrin homology domain, thereby preventing its translocation to the plasma membrane.28 The results of clinical studies with single-agent perifosine in the treatment of solid tumors and hematological malignancies are modest, with few objective responses.29,30 MK2206, an allosteric inhibitor of AKT kinase has been shown to synergize with rapamycin against diffuse large B-cell lymphoma cell lines in vitro,31 and currently undergoes phase II testing as single agent in patients with relapsed and refractory lymphoma. Interestingly, nelfinavir, a protease inhibitor used in HIV therapy, also prominently inhibits AKT signaling, and was similarly able to synergize with rapamycin.31 Other AKT inhibitors in clinical development include tricibirine and GSK2141795 (Table 1).32
The PI3K kinase is a heterodimer composed of a p85 regulatory and a p110 catalytic subunit.28 There are many different PI3K isoforms subdivided in different classes. Among class IA, three isoforms are distinguished based on the catalytic subunit, p110α (PIK3CA), p110β (PIK3CB) and p110δ (PIK3CD). PI3K isoforms have both unique and redundant functions in several signal transduction pathways. While many MCL tumors show constitutive activation of the PI3K/AKT pathway, the relative importance of PI3K isoforms in MCL pathogenesis is ill-defined. Gains/amplifications of PI3KCA have been found in the majority of MCL cases analyzed.33 However, the PI3Kδ selective inhibitor CAL-101 has been shown to inhibit constitutive activation of the pathway and exert potent antitumor effects across a range of B-cell malignancies.34 In a phase I study of single-agent CAL-101 the ORR was 62% (10/16) in relapsed or refractory MCL.35 The pan-PI3K inhibitor SF1126 is a peptidic prodrug of LY294002 with improved pharmacokinetic properties. LY294002 is a first-generation PI3K inhibitor that was not suitable for in vivo applications but has been used extensively in vitro. SF1126 has been shown to inhibit serine 473 phosphorylation of AKT in CLL cells from patients undergoing treatment, and is currently in early development for CLL and NHL.36
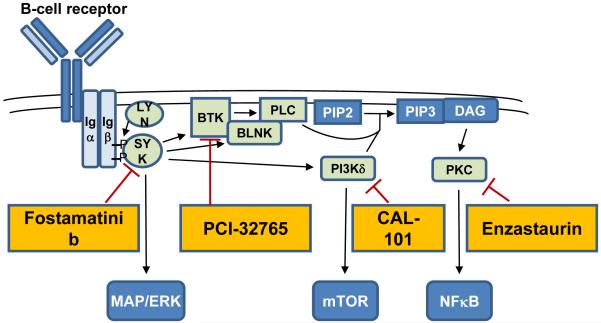
A key pathway activating PI3K/AKT is the B-cell receptor (BCR) signaling pathway (Figure 3). Recent studies reported constitutive activation of the BCR signal transduction components SYK and PKCβ II in MCL. However, inhibition of SYK with fostamatinib and PKCβ with enzastaurin induced rare or no objective responses in MCL, respectively. In contrast, a phase I study of the BTK inhibitor PCI-32765 reported an ORR of 43% across lymphoma subtypes with PRs in 3 of 4 MCL patients.37
Figure 3. Targeting the B-cell receptor signaling pathway.
The B-cell receptor signaling pathway is initiated through phosphorylation of co-receptors Igα (CD79a) and Igβ (CD79b), recruiting the tyrosine kinase SYK. In turn, SYK phosphorylates several downstream kinases including BTK and PI3Kδ. Activation of PI3Kδ and phospholipase C (PLC) generates PIP3 and diacylglycerol (DAG), respectively. DAG is necessary for activation of protein kinase C (PKC). Inhibitors targeting the signaling cascade downstream of the BCR in clinical development are indicated.
Targeting regulators of protein homeostasis
Many oncoproteins increase cell proliferation and metabolic activity causing increased cellular stress in transformed cells. While tumor cells adapt to these conditions in order to ensure cell survival, many homeostatic pathways are utilized to capacity. Thus, tumor cells are often more susceptible to pharmacologic disruption of homeostatic processes than normal cells. Protein homeostasis in particular has emerged as a key system that can be pharmacologically targeted at several points in the life cycle of a protein. Inhibitors of chaperones or enzymes responsible for posttranslational modifications and of the proteasome are in clinical use or undergoing clinical testing.
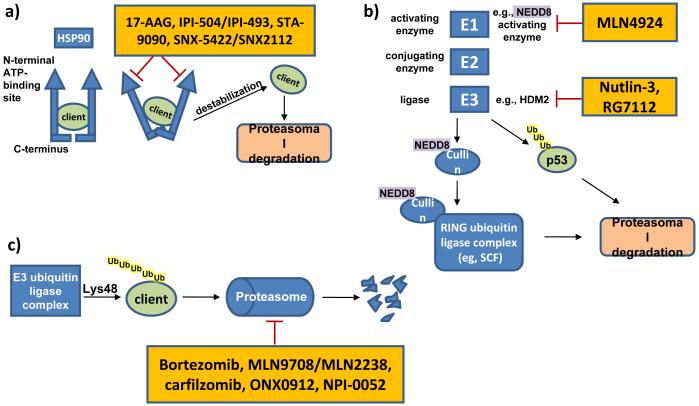
The heat shock protein 90kDa (HSP90) is a ubiquitously expressed chaperone that governs protein homeostasis of hundreds of substrate proteins, also referred to as clients.38 HSP90 forms complexes containing multiple proteins including the client protein and co-chaperones. Most pharmacological inhibitors target the N-terminal ATP-binding site of HSP90, preventing N-terminal dimerization, leading to release of clients that may then be degraded (Figure 4a). HSP90 is highly expressed in MCL and some of its client proteins are central to tumor biology, including cyclin D1, CDK4, AKT, and p53. The first HSP90 antagonist to enter the clinic more than 10 years ago is the ansamycin 17-allylamino-17-demethoxy-geldanamycin (17-AAG, tanespimycin). 17-AAG depletes cyclin D1, AKT, BID and caspase 9 and is cytotoxic against MCL cells in vitro.39 17-AAG and first-generation derivatives have generally shown moderate antitumor activity, which in part may be due to poor water solubility.40 IPI-504 (retaspimycin) a novel ansamycin, kills MCL cells in vitro and induced tumor regression in a human xenograft mouse model.41 A phase I study of IPI-504 in patients with relapsed or refractory MM has reported only disease stabilization in half of the patients.42 It has been noted that progress with this class of drugs may require further optimization of dosing schedules and formulation.38 In recent years, synthetic inhibitors of HSP90 with chemical structures distinct from ansamycins have been developed. Among these, STA-9090 (ganetespib) has entered clinical trials in hematologic malignancies, following promising preclinical results in myeloma and lymphoma xenograft models.43 In MM cells, SNX-5422, an orally bioavailable prodrug that is converted to SNX-2112 induced depletion of HSP90 clients more potently than 17-AAG.44 Clinical studies testing SNX-5422 in refractory hematologic malignancies are ongoing.
Figure 4. Targeting regulators of protein homeostasis.
A) HSP90 requires dimerization, mediated through the C-terminal domain, for full chaperone function. Blocking ATP-binding at the N-terminal domain, interferes with HSP90 dimerization and prevents chaperoning of client proteins resulting in their proteasomal degradation. B) Small ubiquitin-like proteins are sequentially conjugated to protein substrates and affect their localization, function and degradation. E1 enzymes such as the NEDD8 activating enzyme activate small NEDD8 proteins, which are then conjugated and ligated to acceptor proteins by E2 and E3 enzymes, respectively. NEDD8ylation of cullin proteins is essential for function of multi protein cullin-RING (E3 ubiquitin) ligase complexes. Inhibition of the E1 NEDD8 activating enzyme leads to inhibition of the SCF complex. Inhibition of the E3 ubiquitin ligase HDM2, leads to stabilization of select proteins, e.g., tumor suppressor protein p53. C) Proteins are marked with Lys48-linked ubiquitin chains for proteasomal degradation. Pharmacological inhibition of the enzymatic activity of catalytic proteasome subunits causes proteotoxic cellular stress and ultimately apoptosis.
The proteasome is a multiprotein complex with three proteolytic activities conferred by distinct subunits; peptidylglutamyl-like (β1-subunit), trypsin-like (β2-subunit), and chymotrypsin-like (β5-subunit). Polyubiquitination flags proteins for transport to and subsequent degradation through the proteasome, which is the major pathway for the non-lysosomal degradation of intracellular proteins (Figure 4c). The first in class proteasome inhibitor bortezomib (Velcade) is a peptide boronic acid that specifically and reversibly targets the chymotrypsin-like activity. Bortezomib has been approved by the FDA for the treatment of MCL and MM. An important mechanism of bortezomib-induced cell death is the induction of oxidative and endoplasmic reticulum (ER) stress that converge to upregulate the proapoptotic protein NOXA.45-47 The induction of NOXA is mediated by the concerted action of decreased ubiquitination of histone 2A and transcriptional activation by ATF3 and ATF4.48 Inhibition of the proteasome impacts many other pathways and this may be particularly important when considering combination therapies.49
Bortezomib monotherapy induces responses in up to half of patients with relapsed or refractory disease. Interestingly, patients who were responsive or refractory to their last treatment had equal response rates to bortezomib, indicating non-overlapping mechanisms of resistance. However, CR rates are low.50 Predictors of treatment response to bortezomib have started to emerge. We recently found that a partial plasmacytic differentiation characterized by upregulation of IRF4 and CD38 identifies a subset of MCL patients with inferior response to bortezomib.6
Several additional proteasome inhibitors are undergoing clinical testing. MLN9708, a second generation reversible boronic acid proteasome inhibitor, hydrolyzes to the pharmacologically active MLN2238. Compared with bortezomib it has demonstrated improved pharmacologic and antitumor activities in lymphoma xenograft models.51 Carfilzomib (PR171), an epoxyketone irreversible inhibitor of the chymotrypsin-like activity, appears to have fewer side effects than bortezomib, in particular less peripheral neuropathy.52,53 In a large phase II study in relapsed and refractory MM carfilzomib monotherapy achieved an overall response rate of 24% (1 CR, 12 VGPRs and 48 PRs; n=257).54 A phase I study in patients with hematologic malignancies reported a CRu in one MCL patient. ONX0912 (PR047) another epoxyketone, is an irreversible inhibitor of chymotrypsin-like activity that can be given orally.55 ONX0912 is in phase I studies recruiting patients with solid tumors. Another irreversible inhibitor of the proteasome is NPI-0052 (salinosporamide A, marizomib), a beta-lactone gamma-lactam that is structurally related to lactacystein and can be given orally.56,57 NPI-0052 inhibits all three catalytic subunits of the proteasome, possibly accounting for its higher potency and activity against bortezomib-resistant cells.58
In an attempt to maximize cytotoxicity, bortezomib is increasingly combined with standard chemotherapy regimens. As first line treatment, the combination of bortezomib with R-CHOP chemoimmunotherapy induced an ORR of 91% with 72% CR/CRu in MCL (n=32).59 HyperCVAD with bortezomib induced on ORR of 96% with 75% CRs in 76 previously untreated patients.60 In the absence of randomized studies and long term follow up it is currently not clear whether the addition of bortezomib improves outcome in MCL. However, an intriguing observation in diffuse large B-cell lymphoma has been that patients with the activated B-cell like subtype seem to benefit from the addition of bortezomib to standard chemotherapy.59,61
Many combinations of bortezomib with other targeted agents have been tested in preclinical models and increasingly make their way into the clinic.49 Bortezomib leads to an increase in MCL-1, an effect that can be antagonized by the BH3 mimetic obatoclax. This combination enhanced NOXA-mediated displacement of BAK and showed synergistic antitumor activity against MCL cells in vitro.62 A phase I/II study of obatoclax in combination with bortezomib for relapsed or refractory MCL has been completed but final results have not yet been released. At the end of the phase I portion a CR/CRu was recorded in 3 of 9 patients.63 Another class of drugs that appears promising in combinations with proteasome inhibitors are HSP90 inhibitors. The combination results in increased intracellular accumulation of ubiquitinated proteins and enhances ER stress.49 A direct mechanistic explanation for this effect has come from the observation that IPI-504 in combination with bortezomib could overcome resistance to proteasome inhibition in MCL by destabilizing the interaction of HSP90 with ER-resident BIP/GRP78.41 A phase II study of this combination in refractory MM has reported an ORR of 14% with 2 PRs but was closed prematurely.64
Many studies combining HDAC inhibitors and bortezomib have been done in MM. The combination does appear to have beneficial activity and several potential mechanisms that could result in synergistic antitumor activity are entertained, including enhanced ER-stress responses, disruption of aggresome formation, and inhibition of NFκB.49 This synergistic activity is not limited to bortezomib but appears to be shared with other proteasome inhibitors.65 Intriguingly, a recent study found that treatment of breast cancer cell lines with the HDAC inhibitor panobinostat increased acetylation of BIP/GRP78, causing it to dissociate from PERK.66 PERK then triggered the ER-stress response inducing cell death. Thus, inhibiting BIP function emerges as a common mechanism for how HSP90 and HDAC inhibitors can synergize with proteasome inhibitors.
In addition to its role in targeting proteins for degradation through the proteasome, ubiquitin also exerts regulatory functions.67 Other small ubiquitin-like molecules such as NEDD8 have been identified that play a role in regulation of transcription, replication, chromosome segregation, DNA repair and apoptosis. The modification of protein substrates with these small molecules involves three distinct sequential steps performed by enzymes that fall into three distinct classes: activation (E1-family of enzymes), conjugation (E2-family) and 3) ligation (E3-family). The specificity is imparted by the E3 ligase complex that directly binds the substrate. The E1 NEDD8-activating enzyme is the first enzyme in the neddylation cascade that modifies, for example, cullin proteins (Figure 4b). These proteins are themselves subunits of the E3 cullin-RING ubiquitin ligase (CRL) complex, and neddylation is required for activation of CRL activity.67 MLN4924 selectively inhibits the E1 NEDD8-activating enzyme and thereby prevents the ubiquitination and subsequent proteasomal degradation of CRL targets.68 CRL substrate proteins such as CDT1 and p27 accumulate in response to MLN4924 treatment. CDT1 initiates DNA replication and its prolonged expression induces DNA re-replication. The resulting DNA damage may ultimately lead to cell death.69 Neddylation also promotes the degradation of IκBα and thereby induces activation of the NFκB pathway. In human xenograft mouse models inhibition of E1 NEDD8-activating enzyme was able to inhibit NFκB signaling resulting in tumor regression.70 Clinical testing has only just begun but a PR in a patient with Hodgkin’s lymphoma has been reported.71
Another enzyme regulating the posttranslational modification of target proteins is HDM2, an E3 ubiquitin ligase that targets p53 for proteasomal degradation (Figure 4b). HDM2 protein is frequently overexpressed in MCL, and high expression correlates with inferior survival.6 The treatment of MCL cells with the HDM2 inhibitor nutlin-3 can restore p53 activity and induce cell cycle arrest and apoptosis in vitro.72,73 RG7112 is a clinical grade inhibitor of HDM2 that has improved potency and pharmacological properties compared to nutlin-3. RG7112 treatment stabilizes p53 protein and induces p53 target genes including p21, BAX, NOXA, PUMA and FAS. A phase I study of 47 patients with relapsed or refractory leukemia has reported one CR in a patient with acute leukemia and tumor regression in patients with CLL/SLL.74
Targeting regulatory proteins of cell death
Programmed cell death plays important roles in lymphocyte development and the regulation of immune responses. BCL-2 family molecules are key regulators of cell death and fall into two functional groups, inhibitors and promoters of apoptosis. The antiapoptotic proteins, including BCL-2, BCL-XL, BCL-W, A1 and MCL-1, inhibit activation of proapoptotic molecules BAX and BAK. Structurally, all antiapoptotic members of the BCL-2 family harbor two or more so-called BCL-2 homology (BH) domains. In contrast, proapoptotic molecules BIM, BID, PUMA, BAD, and NOXA only contain a BH3 domain. These BH3-only proteins bind to antiapoptotic proteins and cause the release of BAX and BAK that are then free to trigger apoptosis. While BIM, BID and PUMA inhibit all five antiapoptotic family members, BAD selectively inhibits BCL-2, BCL-XL, BCL-W, and NOXA selectively targets A1 and MCL-1 (Figure 5).75 Expression of several members of the BCL-2 family is altered in MCL. Increased expression of BCL-2, MCL-1, and BCL-XL and loss of BIM are common (reviewed in 5,6).
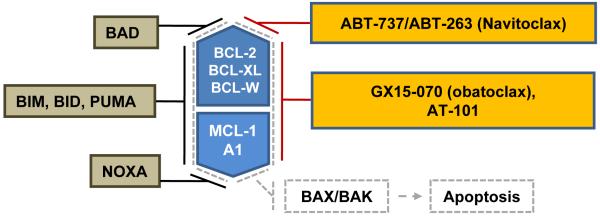
Figure 5. Targeting BCL-2 family proteins.
The BCL-2 proteins BCL-2, BCL-XL, BCL-W, MCL-1, and A1 are antiapoptotic proteins that sequester the apoptotic effectors BAX and BAK. Proapoptotic proteins BIM, BID, PUMA antagonize the function of all BCL-2 proteins, whereas BAD specifically antagonizes BCL-2, BCL-XL and BCL-W while NOXA antagonizes MCL-1 and A1. The small molecule BH-3 mimetic GX15-070 and AT-101 are pan-BCL-2 inhibitors, in contrast to ABT-737/ABT-263, which specifically inhibits BCL-2, BCL-XL and BCL-W but not MCL-1 and A1.
An important step in targeting antiapoptotic pathways for cancer therapy has been the synthesis of small molecules that antagonize BAX and BAK binding to BCL-2 family members.76 These molecules mimic the activity of BH3-only proteins and have been called “BH3 mimetics”. Similar to the BH3-only proteins, BH3 mimetics display variable degrees of specificity towards distinct BCL-2 family members (Figure 5). GX15-070 (obatoclax) is a pan-BCL-2 inhibitor that mimics both BAD- and NOXA-like activities. AT-101, a derivative of the natural product gossypol, is a pan-BCL-2 inhibitor that is available for oral administration. ABT-737 and its analog ABT-263 (navitoclax) that is used in clinical studies have BAD-like activity with high affinity for BCL-2 and BCL-XL but cannot target MCL-1 and A1. This relative selectivity may somewhat limit their antitumor activity. In vitro, MCL cell lines and primary tumor cells with high expression of BCL-2 relative to MCL-1 were sensitive to ABT-737, while cells with high MCL-1 expression were resistant.77 Consistently, upregulation of MCL-1 or A1 has been found to confer resistance to ABT-737 in lymphoma cell lines.78
Clinical studies with these BH3-mimetics are ongoing. In a phase I study of relapsed NHL and CLL, ABT-263 has been well tolerated and achieved an ORR of 22%.79 An objective reduction in lymphadenopathy has been observed in 46% of patients. Best responses have been seen in patients with CLL and SLL, while none of 4 MCL patients achieved an objective response. Single-agent activity of GX15-070 in patients with CLL has been modest.80 Results of a recently completed phase II study of AT-101 monotherapy in patients with relapsed or refractory B-cell malignancies including MCL have not yet been reported. Given their ability to sensitize cells to the effect of chemotherapy, radiation, and possibly immunotherapy, the best use of these agents may be in combination therapies and many such trials are ongoing. Moreover, BH3 mimetics that antagonize MCL-1 may be particularly useful to overcome chemoresistance mediated by tumor-microenvironment interactions.81,82
Other targeted approaches in MCL
Targeting poly(ADP-ribose) polymerase (PARP)
The family of ADP-ribosyl transferases (ARTs) consists of at least 17 enzymes including proteins with poly(ADP-ribose) polymerase (PARP) function such as PARP-1.83 PARP-1 is a highly conserved, abundant protein in the nucleus of cells and best known for its function in DNA damage response and repair, but it is also involved in the regulation of transcription. PARP-1 is required to maintain genomic integrity. Pharmacological inhibition of PARP activity has become an interesting therapeutic strategy as it can achieve “synthetic lethality” in tumors with dysfunctional DNA repair mechanisms.84 This is exemplified by the clinical activity of PARP inhibitors in breast and ovarian cancers with impaired DNA repair due to functional losses of BRCA1 and/or BRCA2. A characteristic of MCL is the high degree of genomic instability and the multiple aberrations in DNA damage response pathways,5,6 suggesting that MCL might be very responsive to PARP inhibitors. Indeed, the PARP inhibitor olaparib (AZD-2281/KU-0059436) has significant in vitro and in vivo activity against MCL cells with dysfunctional ATM.85 Several different PARP inhibitors are in advanced clinical development mostly for solid tumors but some studies include lymphoma as well.84
Targeting protein deacetylases
Acetylation of lysine residues on histones leads to decreased binding between DNA and histone and thereby facilitates transcription. Histone modifications are exerted by histone acetyltransferases and histone deacetylases (HDACs). HDACs fall into different classes: class I includes HDACs 1, 2, 3, and 8, class IIa includes HDACs 4, 5, 7 and 9, class IIb includes HDACs 6 and 10, class III includes non-classical sirtuins unrelated to HDACs, and class IV includes HDAC11. Acetylation regulates the function of important non-histone proteins including p53, HSP90, HIF-1α and c-MYC. Thus, the effects of HDAC inhibitors extend well beyond mere changes in gene expression.86 Their precise mechanisms of action on tumor cells are still under investigation, but include modulation of transcription, induction of oxidative stress, cell cycle arrest, and apoptosis.86 The class I and II HDAC inhibitors vorinostat (suberoylanilide hydroxamic acid, Zolinza) and romidepsin (FR901228) are approved for treatment of primary cutaneous T-cell lymphoma.87 Treatment of MCL cell lines with vorinostat inhibits translation of cyclin D1, possibly as a consequence of inhibition of the PI3K/AKT/mTOR pathway.88 In clinical studies of a combined 11 MCL patients vorinostat monotherapy achieved one CRu.89,90 There is good preclinical evidence for the combination of HDAC inhibitors with proteasome inhibitors91,92 and studies investigating this combination are ongoing in MCL and MM.
Targeting the JAK/STAT signaling pathway
The JAK/STAT signaling pathway regulates growth, proliferation, differentiation and survival in response to external stimuli especially cytokines.93 The JAK/STAT pathway is aberrantly activated in several B-cell lymphomas. In MCL, the active, phosphorylated form of STAT-3 was found in 47% of nodal cases94 and in 70% of leukemic cases.95 It has been hypothesized that STAT-3 activation may be through activation of the BCR and/or interleukins 6 and 10.95 Inhibition of JAK/STAT signaling with AG490 or degrasyn reduced phosphorylated STAT-3 levels and increased apoptosis in primary MCL cells in vitro.95 Degrasyn in combination with bortezomib had synergistic antitumor activity in an MCL mouse model.96 The oral JAK-2 inhibitor SB1518 is completing phase I testing. At the end of the dose escalation phase the ORR was 12% across multiple lymphoma subtypes but remarkably 2 of 3 MCL patients achieved PRs.97
Targeting the microenvironment
The immunomodulatory drugs thalidomide and lenalidomide are an integral part of MM regimens. While the precise mechanism of action is not understood, effects on the microenvironment either by disruption of tumor-stroma interactions or through activation of immune effector mechanisms are hypothesized to contribute.98 Phase II studies of single-agent lenalidomide in lymphoma have shown remarkable activity.99 In the NHL-002 study lenalidomide induced responses in 53% of 15 heavily pretreated patients. The PFS of 5.6 and the median DOR of 13.7 months compares favorably to other treatment options for these patients. A high ORR of 42% has been confirmed in the international NHL-003 study enrolling 57 MCL patients.100
Future directions
For most patients MCL remains an incurable disease. An impressive number of novel agents with widely different mechanisms of action have been developed. Increasingly, these drugs contribute to improved survival for patients with relapsed disease. A finding which is humbling enough since, for some of these “targeted agents” one would not have predicted efficacy in MCL, and for many we have not fully understood how they work or, for others, why they don’t work as well as one expected. However, the tools for further progress are in place. Drug development is rapidly progressing, an increased understanding of disease biology helps identify key pathways and dissect disease heterogeneity, and the application of molecular profiling techniques can reveal unexpected mechanisms of resistance. Undisputedly, many challenges remain. For one, considering the large number of different agents available one may wonder if there could be too much of a good thing as it seems daunting to efficiently sort out which agents will have the biggest clinical impact. In addition, it may be necessary to reconsider how one goes about developing combination therapies as there is potential for synergism as well as for antagonism of these agents and appropriate sequencing could play an important role. Finally, for some of these agents we may have to define realistic treatment goals and consider the heterogeneity of the disease. While adding to already intense treatment approaches might offer a chance for cure, long term control at low intensity may be a perfectly reasonable choice for many patients. Clinical trials obviously will be instrumental to answer these questions and improve treatment options. Referral of MCL patients to clinical trials is equally important as novel trial designs that optimize access of patients to the most effective agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR. Should there be a standard therapy for mantle cell lymphoma? Future Oncol. 2011;7:227–237. doi: 10.2217/fon.10.189. [DOI] [PubMed] [Google Scholar]
- 3.Dreyling M, Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009:542–551. doi: 10.1182/asheducation-2009.1.542. [DOI] [PubMed] [Google Scholar]
- 4.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 5.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood. 2007;109:4599–4606. doi: 10.1182/blood-2006-08-039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klier M, Anastasov N, Hermann A, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 9.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 10.Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–1745. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manohar SM, Rathos MJ, Sonawane V, Rao SV, Joshi KS. Cyclin-dependent kinase inhibitor, P276-00 induces apoptosis in multiple myeloma cells by inhibition of Cdk9-T1 and RNA polymerase II-dependent transcription. Leuk Res. 2011 doi: 10.1016/j.leukres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Santo L, Vallet S, Hideshima T, et al. AT7519, A novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3beta activation and RNA polymerase II inhibition. Oncogene. 2010;29:2325–2336. doi: 10.1038/onc.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squires MS, Cooke L, Lock V, et al. AT7519, a cyclin-dependent kinase inhibitor, exerts its effects by transcriptional inhibition in leukemia cell lines and patient samples. Mol Cancer Ther. 2010;9:920–928. doi: 10.1158/1535-7163.MCT-09-1071. [DOI] [PubMed] [Google Scholar]
- 16.Marzec M, Kasprzycka M, Lai R, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–1750. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menu E, Garcia J, Huang X, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 2008;68:5519–5523. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- 18.Janes MR, Limon JJ, So L, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 20.Ansell SM, Inwards DJ, Rowland KM, Jr., et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor OA, Popplewell L, Winter JN, et al. PILLAR-1: Preliminary Results of a Phase II Study of mTOR Inhibitor Everolimus In Patients with Mantle Cell Lymphoma (MCL) Who Are Refractory or Intolerant to Bortezomib. Blood. 2010;116:3963. ASH Annual Meeting Abstracts. [Google Scholar]
- 23.Renner C, Zinzani PL, Gressin R, et al. A Multi-Center Phase II Study (SAKK 36/06) of Single Agent Everolimus (RAD001) In Patients with Relapsed or Refractory Mantle Cell Lymphoma. Blood. 2010;116:2803. doi: 10.3324/haematol.2011.053173. ASH Annual Meeting Abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 25.Gupta M, Hendrickson AW, Han JJ, et al. Dual Inhibition of mTORC1/mTORC2 Induces Apoptosis of Mantle Cell Lymphoma by Preventing Rictor Mediated AKTS473 Phosphorylation by Potentiating AKT2-PHLPP1 Association. Blood. 2010;116:772. ASH Annual Meeting Abstracts. [Google Scholar]
- 26.Evangelisti C, Chiarini F, Ricci F, et al. Targeted Inhibition of mTORC1 and mTORC2 by the Active-Site mTOR Inhibitors, PP-242 and OSI-027, Has Cytotoxic Effects In T-Cell Acute Lymphoblastic Leukemia; ASH Annual Meeting Abstracts; 2010; p. 3242. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Z, Shi Y, Tsao T, et al. Targeting mTORC1/2 by a mTOR Kinase Inhibitor (PP242) induces Apoptosis In AML Cells Under Conditions Mimicking Bone Marrow Microenvironment; ASH Annual Meeting Abstracts; 2010; p. 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman DR, Davis PH, Lanasa MC, et al. Preclinical and Interim Results of a Phase II Trial of Perifosine In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL); ASH Annual Meeting Abstracts; 2010.p. 1842. [Google Scholar]
- 31.Petrich AM, Leshchenko VV, Kuo P-Y, Ye BH, Sparano JA, Parekh S. Genomic and Pathway Connectivity Analyses Identify Novel Strategies to Overcome mTOR Inhibitor Resistance In DLBCL; ASH Annual Meeting Abstracts; 2010.p. 436. [Google Scholar]
- 32.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3′-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15:5724–5732. doi: 10.1158/1078-0432.CCR-08-3215. [DOI] [PubMed] [Google Scholar]
- 34.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahl B, Byrd JC, Flinn IW, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma; ASH Annual Meeting Abstracts; 2010.p. 1777. [Google Scholar]
- 36.Garlich JR, Becker MD, Shelton CF, et al. Phase I Study of Novel Prodrug Dual PI3K/mTOR Inhibitor SF1126 In B-Cell Malignancies; ASH Annual Meeting Abstracts; 2010.p. 1783. [Google Scholar]
- 37.Fowler N, Sharman JP, Smith SM, et al. The Btk Inhibitor, PCI-32765, Induces Durable Responses with Minimal Toxicity In Patients with Relapsed/Refractory B-Cell Malignancies: Results From a Phase I Study. Blood. 2010;116:964. ASH Annual Meeting Abstracts. [Google Scholar]
- 38.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgakis GV, Li Y, Younes A. The heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9. Br J Haematol. 2006;135:68–71. doi: 10.1111/j.1365-2141.2006.06247.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim YS, Alarcon SV, Lee S, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roue G, Perez-Galan P, Mozos A, et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2010;117:1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- 42.Richardson PG, Chanan-Khan AA, Alsina M, et al. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. Br J Haematol. 2010;150:438–445. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs. 2010;11:1466–1476. [PubMed] [Google Scholar]
- 44.Okawa Y, Hideshima T, Steed P, et al. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113:846–855. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 47.Weniger MA. NRF2 at the Interface of Oxidative and Endoplasmic Reticulum Stress Pathways Is a Critical Integrator of Bortezomib Response in Mantle Cell Lymphoma in Vitro and In Vivo. Blood. 2009;114:727a. [Google Scholar]
- 48.Wang Q, Mora-Jensen H, Weniger MA, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JJ. Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy. Clin Cancer Res. 2010;16:4094–4104. doi: 10.1158/1078-0432.CCR-09-2882. [DOI] [PubMed] [Google Scholar]
- 50.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kupperman E, Lee EC, Cao Y, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 52.Parlati F, Lee SJ, Aujay M, et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 53.Arastu-Kapur S, Shenk K, Parlati F, Bennett MK. Non-Proteasomal Targets of Proteasome Inhibitors Bortezomib and Carfilzomib; ASH Annual Meeting Abstracts; 2008.p. 2657. [Google Scholar]
- 54.diCapua Siegel DS, Martin T, Wang M, et al. Results of PX-171-003-A1, An Open-Label, Single-Arm, Phase 2 (Ph 2) Study of Carfilzomib (CFZ) In Patients (pts) with Relapsed and Refractory Multiple Myeloma (MM); ASH Annual Meeting Abstracts; 2010.p. 985. [Google Scholar]
- 55.Zhou HJ, Aujay MA, Bennett MK, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J Med Chem. 2009;52:3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 56.Potts BC, Albitar MX, Anderson KC, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macherla VR, Mitchell SS, Manam RR, et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- 58.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Ruan J, Martin P, Furman RR, et al. Bortezomib Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 2010;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 60.Kahl BS, Li H, Smith MR, et al. The VcR-CVAD Regimen Produces a High Complete Response Rate in Untreated Mantle Cell Lymphoma (MCL): First Analysis of E1405 - A Phase II Study of VcR-CVAD with Maintenance Rituximab for MCL; ASH Annual Meeting Abstracts; 2009.p. 1661. [Google Scholar]
- 61.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 63.Goy A, Ford P, Feldman T, et al. A Phase 1 Trial of the Pan Bcl-2 Family Inhibitor Obatoclax Mesylate (GX15-070) in Combination with Bortezomib in Patients with Relapsed/Refractory Mantle Cell Lymphoma; ASH Annual Meeting Abstracts; 2007.p. 2569. [Google Scholar]
- 64.Richardson PG, Badros AZ, Jagannath S, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–437. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao R, Fiskus W, Balusu R, et al. Treatment with Histone Deacetylase 6-Specific Inhibitor WT-161 Disrupts hsp90 Function, Abrogates Aggresome Formation and Sensitizes Human Mantle Cell Lymphoma Cells to Lethal ER Stress Induced by Proteasome Inhibitor Carfilzomib. Blood. 2010;116:2856. ASH Annual Meeting Abstracts. [Google Scholar]
- 66.Rao R, Nalluri S, Kolhe R, et al. Treatment with panobinostat induces glucose-regulated protein 78 acetylation and endoplasmic reticulum stress in breast cancer cells. Mol Cancer Ther. 2010;9:942–952. doi: 10.1158/1535-7163.MCT-09-0988. [DOI] [PubMed] [Google Scholar]
- 67.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- 69.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–10320. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 71.Shah JJ, Harvey RD, O’Connor OA, et al. Phase 1 Dose-Escalation Study of Multiple Dosing Schedules of the Investigational Drug MLN4924, a Nedd8-Activating Enzyme Inhibitor, In Patients with Relapsed and/or Refractory Multiple Myeloma or Lymphoma; ASH Annual Meeting Abstracts; 2010.p. 2801. [Google Scholar]
- 72.Jin L, Tabe Y, Kojima K, et al. MDM2 antagonist Nutlin-3 enhances bortezomib-mediated mitochondrial apoptosis in TP53-mutated mantle cell lymphoma. Cancer Lett. 2010;299:161–170. doi: 10.1016/j.canlet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Tabe Y, Sebasigari D, Jin L, et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009;15:933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreeff M, Kojima K, Padmanabhan S, et al. A Multi-Center, Open-Label, Phase I Study of Single Agent RG7112, A First In Class p53-MDM2 Antagonist, In Patients with Relapsed/Refractory Acute Myeloid and Lymphoid Leukemias (AML/ALL) and Refractory Chronic Lymphocytic Leukemia/Small Cell Lymphocytic Lymphomas (CLL/SCLL); ASH Annual Meeting Abstracts; 2010.p. 657. [Google Scholar]
- 75.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 76.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 77.Touzeau C, Dousset C, Bodet L, et al. Rational for the Use of a Targeted-Therapy Using ABT-737 In Mantle-Cell Lymphoma. Blood. 2010;116:770. ASH Annual Meeting Abstracts. [Google Scholar]
- 78.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–153. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herishanu Y, Gibellini F, Njuguna N, et al. Activation of CD44, a receptor for extracellular matrix components, protects CLL cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.569962. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Annunziata CM, O’Shaughnessy J. Poly (ADP-ribose) polymerase as a novel therapeutic target in cancer. Clin Cancer Res. 2010;16:4517–4526. doi: 10.1158/1078-0432.CCR-10-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weston VJ, Oldreive CE, Skowronska A, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–4587. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 86.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 87.Copeland A, Buglio D, Younes A. Histone deacetylase inhibitors in lymphoma. Curr Opin Oncol. 2010;22:431–436. doi: 10.1097/CCO.0b013e32833d5954. [DOI] [PubMed] [Google Scholar]
- 88.Kawamata N, Chen J, Koeffler HP. Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood. 2007;110:2667–2673. doi: 10.1182/blood-2005-11-026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watanabe T, Kato H, Kobayashi Y, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II Study of Vorinostat for Treatment of Relapsed or Refractory Indolent Non-Hodgkin’s Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 92.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O’Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16:554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 93.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122–131. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Yared MA, Khoury JD, Medeiros LJ, Rassidakis GZ, Lai R. Activation status of the JAK/STAT3 pathway in mantle cell lymphoma. Arch Pathol Lab Med. 2005;129:990–996. doi: 10.5858/2005-129-990-ASOTSP. [DOI] [PubMed] [Google Scholar]
- 95.Baran-Marszak F, Boukhiar M, Harel S, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. 2010;95:1865–1872. doi: 10.3324/haematol.2009.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pham LV, Tamayo AT, Li C, Bornmann W, Priebe W, Ford RJ. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications. Mol Cancer Ther. 2010;9:2026–2036. doi: 10.1158/1535-7163.MCT-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Younes A, Fanale MA, McLaughlin P, Copeland A, Zhu J, de Castro Faria S. Phase I Study of a Novel Oral JAK-2 Inhibitor SB1518 In Patients with Relapsed Lymphoma: Evidence of Clinical and Biologic Activity In Multiple Lymphoma Subtypes. Blood. 2010;116:2830. doi: 10.1200/JCO.2012.42.5223. ASH Annual Meeting Abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 99.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 100.Witzig TE, Vose JM, Zinzani PL, Reeder A, Buckstein R. Durable Responses After Lenalidomide Oral Monotherapy in Patients with Relapsed or Refractory (R/R) Aggressive Non-Hodgkin’s Lymphoma (a-NHL): Results From An International Phase 2 Study (CC-5013-NHL-003) Blood. 2009;114:1676a. [Google Scholar]