Abstract
Gender-based differences in lipids have been noted in antiretroviral clinical trials. We present the metabolic and anthropometric data from the GRACE (Gender, Race And Clinical Experience) study by gender. Treatment-experienced adults received darunavir/ritonavir (DRV/r) 600/100 mg twice daily, plus a background regimen, over 48 weeks. Fasting blood samples were obtained for lipid, glucose, and insulin measurements at baseline and at weeks 24 and 48/early discontinuation. Anthropometric measurements were taken at baseline and at weeks 12, 24, and 48/discontinuation. The Assessment of Body Change and Distress questionnaire was administered at baseline and regular intervals. Descriptive statistics as well as comparisons using a Wilcoxon rank-sum test are reported. Four hundred twenty-nine patients (women, n=287; men, n=142) enrolled in GRACE; 94 women (32.8%) and 33 men (23.2%) discontinued the trial. Median changes in triglycerides from baseline to week 48 were higher in men (25 mg/dL versus 8 mg/dL; p=0.006); the mean change in triglycerides was higher in men than in women in all racial subgroups. Other lipid and glucose level changes were similar between genders. Anthropometric parameters increased for both genders, with larger increases in women. Patients' perceptions of body changes concurred with physical measurements. The proportion of women who were “satisfied” or “very satisfied” with their bodies increased from 45.2% to 57.8% from baseline to week 48 (p=0.005), while the proportion of men who were “satisfied” or “very satisfied” with their bodies increased from 56.3% to 61.5% from baseline to week 48 (p=0.317). DRV/r-based therapy was associated with small to moderate changes in metabolic parameters, and few between-gender differences were observed. Levels of self-reported, body-related distress improved for women and men over 48 weeks.
Introduction
Antiretroviral (ARV) agents have differing effects on metabolic and anthropometric parameters.1–3 Some protease inhibitors (PIs) have been independently associated with hyperglycemia, hypercholesterolemia, hypertriglyceridemia, and weight gain. Ritonavir, for example, has been more strongly associated with hypertriglyceridemia than have other PIs,2 and fosamprenavir/ritonavir and lopinavir/ritonavir have been associated with greater increases in total cholesterol and triglycerides than other boosted PIs.3
Gender-based differences in lipid parameters with ARV therapy have been noted in clinical trials in treatment-naïve and treatment-experienced HIV-infected patients.4–7 For instance, men receiving ARV therapy are more commonly reported to develop hypertriglyceridemia compared to women.5–7 Few of these trials, however, have enrolled a population of women large enough to draw definitive gender-based conclusions regarding metabolic effects of individual ARV agents.
Women, particularly women of color, have been historically underrepresented in ARV clinical trials.8,9 In order to allow for between-gender comparisons of efficacy and safety of darunavir/ritonavir (DRV/r)-based therapy in treatment-experienced patients, the GRACE (Gender, Race And Clinical Experience) study enrolled 67% (n/N=287/429) women.10 In GRACE, 50.9% of women and 58.5% of men in the intent-to-treat–time-to-loss of virologic response analysis achieved virologic response (HIV-1 RNA <50 copies per milliliter). In the time-to-loss of virologic response–nonvirologic failure censored analysis, which censored patients who discontinued for reasons other than virologic failure, 73% of women and 73.5% of men achieved a response. Few differences were seen between genders with regard to adverse events (AEs).10 A previous study has demonstrated that DRV has a favorable metabolic profile in treatment-naïve HIV-1–positive patients11; the results from this study, however, were not stratified by gender. Here we present the week 48 metabolic and anthropometric data from the GRACE study according to gender.
Methods
Study design and patients
GRACE was a multicenter, open-label, Phase IIIb study conducted over a 48-week period at 65 study sites primarily located within the United States, with a few sites in Canada and Puerto Rico. Treatment-experienced adults (HIV-1 RNA ≥1000 copies per milliliter at screening) received DRV/r 600/100 mg twice daily plus an optimized background regimen that was chosen based on treatment history and resistance testing (virco®TYPE HIV-1; Virco, Mechelen, Belgium). The use of antidiabetic agents and lipid-lowering agents other than lovastatin, simvastatin, and pravastatin was permitted during the study. Study visits and inclusion/exclusion criteria have been described.10
Study evaluations
Fasting blood samples were obtained for lipid, glucose, and insulin measurements at baseline, week 24, and week 48/early discontinuation (DC). Lipid assessments included total cholesterol, triglycerides, low-density lipoprotein (LDL; direct), and high-density lipoprotein (HDL). Lipid abnormalities were categorized as either above or below the United States National Cholesterol Education Program classifications for the borderline, or most conservative, cutoffs for each parameter.12
Anthropometric measurements were taken at baseline, week 12, week 24, and week 48/DC. Measurements included weight, body mass index (BMI), and waist and hip circumference. The Assessment of Body Change and Distress (ABCD) questionnaire was administered at baseline and at weeks 4, 12, 24, 36, and 48/DC. The questionnaire had a recall period of 4 weeks and comprised 27 self-reported questions, which measured patients' perceived body changes, physical and emotional distress, social concerns, and health behavioral changes.
Statistical methods
Descriptive statistics for lipid, glucose, and insulin analyses are reported for the intent-to-treat population. The change in lipid parameters was a predefined secondary end point and was additionally investigated using nonparametric methods with a Wilcoxon rank-sum test. All patients with fasting lipid values were included.
Results
Patient population and baseline characteristics
A total of 429 (women, n=287; men, n=142) patients were enrolled in GRACE and received at least one dose of study drug (Table 1). At baseline, women were on average younger and had a higher median BMI than men (Table 1). Women also had less advanced disease at baseline, demonstrated by higher median CD4+ counts and a lower proportion of US Centers for Disease Control and Prevention Class C disease, compared to men. Men had higher triglyceride levels than women at baseline (Table 2), and a higher proportion of men than women were on lipid-lowering therapy (Table 1). Additionally, a higher proportion of men were white, compared to women, while a higher proportion of women were black, compared to men. Overall, 94 women (32.8%) and 33 men (23.2%) were discontinued from the trial.10 No lipid-, glucose-, or anthropometric-associated AE led to study discontinuation, with the exception of weight gain in one woman.
Table 1.
Baseline Demographics and Disease Characteristics
| Women (n=287) | Men (n=142) | p Valuea | |
|---|---|---|---|
| Mean (SE) age, years | 41.7 (0.63) | 45.2 (0.75) | 0.0005b |
| Race, n (%) | 0.0110c | ||
| Black | 191 (66.6) | 73 (51.4) | |
| Hispanic | 60 (20.9) | 36 (25.4) | |
| White | 34 (11.8) | 31 (21.8) | |
| Other | 2 (0.7) | 2 (1.4) | |
| Disease and treatment characteristics | |||
| Mean (SE) duration of infection, years | 10.9 (0.32) | 12.2 (0.49) | 0.0279b |
| Mean (SE) viral load, log10 copies/mL | 4.65 (0.05) | 4.73 (0.07) | 0.8813b |
| Median (range) CD4+ count, cells/mm3 | 210 (1, 868) | 175 (2, 1125) | 0.0498d |
| CDC Class C, n (%) | 102 (35.5) | 67 (47.2) | 0.0202b |
| ≥2 prior PIs, n (%) | 168 (58.5) | 92 (64.8) | 0.2123b |
| Hepatitis B surface antigen (positive), n (%) | 12 (4.2) | 7 (4.9) | 0.7229b |
| Hepatitis C antibody (positive), n (%) | 39 (13.6) | 25 (17.6) | 0.2718b |
| Lipid-lowering therapy, n (%) | 18 (6.3) | 15 (10.6) | 0.1165b |
| Antidiabetic therapy, n (%) | 18 (6.3) | 7 (4.9) | 0.5765b |
Between-gender comparison.
t test (unequal variances).
χ2 test.
Kruskall-Wallis test.
SE, standard error; CDC, US Centers for Disease Control and Prevention; PI, protease inhibitor.
Table 2.
Changes in Fasting Lipid, Glucose, and Insulin Levels from Baseline Through Weeks 24 and 48
| |
Women (n=287) |
Men (n=142) |
|
||||
|---|---|---|---|---|---|---|---|
| Parameter,amedian (range) | BL | Change through week 24 | Change through week 48 | BL | Change through week 24 | Change through week 48 | p Value for the difference in change (women vs. men) through week 48 |
| Total cholesterol, mg/dL | 169 (79, 317) | 19 (−82, 211) | 21 (−101, 140) | 169 (80, 363) | 16 (−118, 204) | 20 (−103, 340) | 0.865 |
| Triglycerides, mg/dL | 120 (46, 411) | 17 (−181, 450) | 8 (−197, 267) | 139 (39, 768) | 30 (−404, 2562) | 25 (−438, 2200) | 0.006 |
| Low-density lipoprotein, mg/dL | 100 (22, 216) | 7 (−74, 126) | 11 (−86, 90) | 102 (25, 270) | 3 (−104, 109) | 1 (−135, 80) | 0.109 |
| High-density lipoprotein, mg/dL | 48 (10, 111) | 3 (−18, 60) | 3 (−39, 37) | 39 (17, 82) | 2 (−24, 34) | 0 (−19, 18) | 0.108 |
| Glucose, mg/dL | 86.4 (61.2, 351.0) | 1.8 (−187.2, 147.6) | 1.8 (−108.0, 117.0) | 88.2 (57.6, 419.4) | 0 (−72.0, 91.8) | 2.7 (−81.0, 115.2) | 0.206 |
| Apolipoprotein A1, mg/dL | 127 (71, 217) | 12 (−55, 133) | 9 (−77, 73) | 112 (64, 218) | −6 (−80, 80) | 11 (−44, 99) | 0.671 |
| Apolipoprotein B, mg/dL | 77 (31, 141) | 1 (−32, 110) | 4 (−46, 55) | 75 (23, 159) | 6 (−41, 70) | 3 (−60, 44) | 0.578 |
| Insulin, μIU/mL | 10.2 (1.9, 163.0) | 0.5 (−146.0, 118.8) | −0.2 (−145.0, 151.7) | 9.4 (1.9, 78.2) | 1.2 (−71.5, 160.4) | 0.7 (−65.0, 133.5) | 0.860 |
Sample sizes vary based on number of patients with recorded values at each time point.
BL, baseline.
Lipid- and glucose-associated laboratory abnormalities
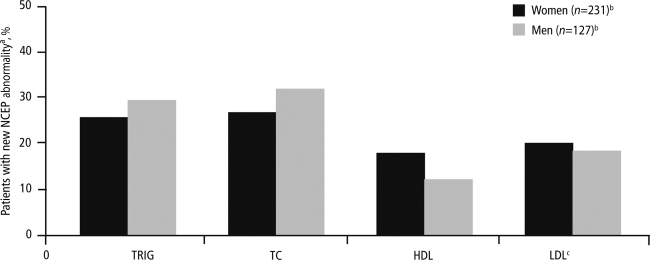
The median change in fasting lipid- and glucose-associated levels from baseline to week 48 was similar between women and men, with the exception of triglycerides, which were higher in men than in women (25 versus 8 mg/dL, p=0.006; Table 2); the mean change in triglycerides was higher in men than in women in all racial subgroups. The difference in changes from baseline to week 48 between genders was nonsignificant for other lipid- and glucose-associated levels. The majority of worst-grade lipid- and glucose-associated fasting laboratory abnormalities were grade 1–2 in women (168/185; 90.8%) and men (122/142; 85.9%). The incidence of grade 3 or 4 fasting triglyceride elevations was higher in men (n=11, 9.2%) versus women (n=1, 0.5%); the difference in incidence of grade 2–4 fasting triglyceride elevations between men and women was even more pronounced at 14.1% (n=20) and 1.4% (n=4), respectively. In total, 6 women (2.2%) and 4 men (2.9%) developed treatment-emergent grade 3 or 4 hyperglycemia, 10 women (4.6%) and 5 men (4.2%) developed grade 3 or 4 total cholesterol elevations, and 62 women (28.4%) and 26 men (21.7%) developed above-normal levels of LDL (directly measured). Rates of lipid levels above US National Cholesterol Education Program thresholds were similar between men and women (Fig. 1). Percentages of women and men starting lipid-lowering therapy while on study were 3.1% and 4.9%, respectively, and starting antidiabetic therapy were 1.0% and 2.1%, respectively.
FIG. 1.
Treatment-emergent, lipid-associated laboratory abnormalities graded according to the NCEP criteriaa. NCEP criteria12: triglycerides ≥150 mg/dL, total cholesterol ≥200 mg/dL, HDL ≤40 mg/dL for men and ≤50 mg/dL for women, and LDL ≥130 mg/dL. bNumber of subjects with indicated tests. cDirect. NCEP, National Cholesterol Education Program; TRIG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Anthropometric measurements and associated adverse events
Over 48 weeks, an increase in anthropometric parameters was seen in both genders, with larger increases observed in women compared to men (Table 3). Median (range) waist size increased by 2.5 (−50.8, 25.4) cm among women compared to 1.3 (−17.8, 24.1) cm for men. For women, changes in weight, BMI, and waist and hip circumference from baseline to week 48 were statistically significant (Table 3). There was no change in waist-to-hip ratio over 48 weeks in either gender. The most frequent anthropometric-associated AEs (observed in more than 5 patients) were increased weight in 11 women (3.8%) and 3 men (2.1%), anorexia in 3 women (1.0%) and 4 men (2.8%), and decreased weight in 2 women (0.7%) and 4 men (2.8%). The majority of these AEs were grade 1–2; 3 cases of grade 3–4 decreased weight (1 woman and 2 men) and 1 case of grade 3–4 increased weight (1 woman, 16.1 kg) were reported. In a post hoc analysis of covariance, both change in CD4+ cell count over 48 weeks and baseline BMI were independently associated with an increase in weight over 48 weeks. Interestingly, lower baseline BMIs were associated with larger increases in weight over the study period.
Table 3.
Changes in Anthropometric Measurements from Baseline Through Weeks 12, 24, and 48
| |
Women (n=287) |
Men (n=142) |
||||||
|---|---|---|---|---|---|---|---|---|
| Parameter,amedian (range) | BL | Change through week 12 | Change through week 24 | Change through week 48 | BL | Change through week 12 | Change through week 24 | Change through week 48 |
| Weight, kg | 72.6 (39.9, 198.7) | 1.4 (−9.1, 21.3) | 2.3 (−15.7, 29.2) | 3.2b (−18.1, 29.0) | 76.2 (48.8, 134.3) | 1.7 (−11.1, 15.0) | 2.3 (−9.1, 19.1) | 1.8 (−10.0, 17.7) |
| BMI, kg/m2 | 26.7 (15.5, 61.1) | 0.5 (−3.3, 8.3) | 0.9 (−5.7, 11.1) | 1.2b (−5.8, 10.1) | 24.7 (15.7, 45.1) | 0.5 (−4.0, 4.9) | 0.8 (−3.1, 6.2) | 0.5 (−3.4, 5.1) |
| Waist circumference, cm | 90.0 (63.5, 177.8) | 0.7 (−21.3, 22.3) | 2.5 (−15.2, 24.1) | 2.5b (−50.8, 25.4) | 89.0 (55.9, 129.5) | 0 (−47.6, 17.8) | 1.3 (−15.2, 25.0) | 1.3 (−17.8, 24.1) |
| Hip circumference, cm | 103.0 (67.3, 167.6) | 0.7 (−16.8, 22.9) | 2.5 (−17.8, 22.9) | 2.6b (−30.5, 24.1) | 95.1 (73.7, 129.5) | 0 (−46.7, 22.9) | 0.9 (−17.8, 22.9) | 0.7 (−14.0, 20.3) |
| Waist-to-hip ratio | 0.9 (0.7, 1.2) | 0 (−0.3, 0.4) | 0 (−0.4, 0.3) | 0 (−0.2, 0.3) | 0.9 (0.7, 1.1) | 0 (−0.1, 0.1) | 0 (−0.2, 0.2) | 0 (−0.2, 0.2) |
Sample sizes vary based on number of patients with recorded values at each time point.
Significant (p<0.05) change from baseline to week 48.
BL, baseline; BMI, body mass index.
Within the population enrolled in GRACE, some women and men experienced extreme changes in weight (range: −18.1, 29.0 kg) and anthropometrics (change in waist circumference, range: −50.8, 25.4 cm) from baseline to week 48. When the population of patients that gained greater than 7% of their baseline body weight (women, n=82 [28.6%]; men, n=34 [23.9%]) was compared to the overall population, few differences in baseline parameters were found. However, at baseline, patients with large weight gains had lower mean CD4+ counts (156 versus 219 cells/mm3), slightly higher mean log10 HIV RNA counts (4.8 versus 4.7 copies per milliliter), and lower mean weights (74.9 versus 76.2 kg) than the overall GRACE population.
Assessment of body change and Distress questionnaire
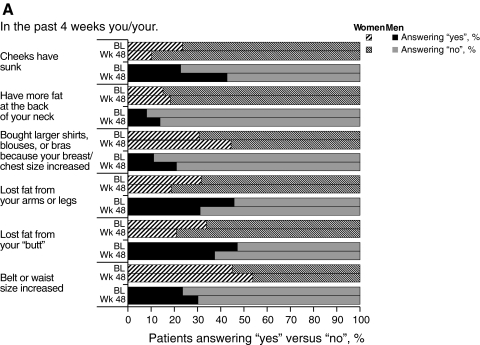
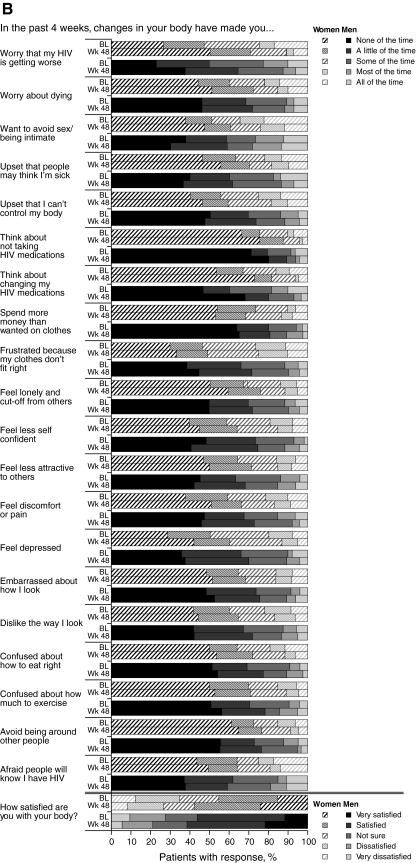
Patients' perceptions of body changes concurred with physical measurements, reflecting the weight gain reported during GRACE; at week 48, more women than men reported that their belt or waist size had increased (Fig. 2A). At week 48, a higher proportion of men and women reported that they had gained fat at the back of their necks, and that their chest or waist size increased, compared to the proportions reporting these effects at baseline. Additionally, the proportion of both genders reporting that their cheeks had sunken in or that they had lost fat from their arms/legs or “butt” was lower at week 48 compared to baseline. Overall, a higher proportion of both genders were “satisfied” or “very satisfied” with their bodies after 48 weeks compared to baseline (women, 57.8% versus 45.2% [p=0.005]; men, 61.5% versus 56.3% [p=0.317]; Fig. 2B). Self-reported, body-related distress generally decreased or remained constant during the study for women; for men, body-related distress remained relatively constant, although a greater proportion of men reported feeling less confident or afraid of disclosure of their HIV status at week 48 compared to baseline.
FIG. 2.
Results of the Assessment of Body Change and Distress questionnaire: A: Yes versus no. B: Categorical. BL, baseline; Wk, week.
Discussion
Overall, few differences were seen in GRACE between genders with respect to metabolic parameters or anthropometric measurements. The incidence of grade 3–4 fasting laboratory abnormalities or grade 3–4 anthropometric-associated AEs was low in both genders. Fasting triglyceride elevations were more common in men than women, as has been observed in other ARV trials that enrolled either treatment-naïve or treatment-experienced patients.4–7 The incidence of LDL elevations was numerically higher in women than men; this is supported by the cross-sectional FRAM (Fat Redistribution And Metabolic change) study, which recently demonstrated that HIV-infected women had higher LDL levels compared to uninfected women. In contrast, HIV-infected men had lower LDL levels than uninfected men,13,14 suggesting the LDL elevations may be more prevalent in HIV-infected women than men.
Women generally experienced larger increases in weight and other anthropometric measurements than men, in line with other ARV studies.15,16 Hormonal differences between genders may be a contributing factor to weight gain.17 A study that assessed the effect of PIs in mice with elevated cholesterol suggested that the observed gender-specific differences in weight changes were influenced by sex hormones; removal of the female sex hormones eliminated the discrepancies in weight change between genders.17 Regardless of the larger increases in some metabolic and anthropometric parameters in women, there was no change in waist-to-hip ratio for women or men over the 48-week study, suggesting that weight gain was proportional for both genders. The subset of GRACE patients who gained greater than 7% of their baseline body weight over 48 weeks were sicker at baseline than the overall population, as indicated by CD4+ counts, viral loads, and lower body weights. These findings are consistent with the post hoc analysis of covariance performed on all GRACE patients that showed that both change in CD4+ cell count over 48 weeks and lower baseline BMI were independently associated with an increase in weight over 48 weeks. Taken together, these data support that the large weight gain seen in this subpopulation may be an indication of a return to health for these patients.
Patients' perceptions of body change were consistent with changes in physical measurements; more women than men reported an increase in belt or waist size over the 48-week study. The management of weight gain and patients' perception of weight gain is important, particularly in women; an assessment of 1671 women from the Women's Interagency HIV Study has shown that women who perceive central fat gain, particularly in the abdomen, are at risk for decreased adherence to ARV therapy.18 Minimizing weight gain, therefore, may contribute to improving ARV adherence. It has been demonstrated that the reported prevalence of body shape changes and lipodystrophy varies greatly depending on the definition applied, as well as the individual or methods used to assess the changes.19,20 One study showed that although physician-based analyses of body changes in 159 HIV-infected men reported a lipodystrophy prevalence of 65%, only 19% and 21.3% of the patients themselves self-reported lipodystrophy and lipoatrophy, respectively.19 This suggests that it is important to not only monitor body changes in HIV-infected populations, but also to follow patients' perceptions of their body changes, as these may or may not align with clinical observations.
Despite increases in weight and other anthropometric measures, overall, self-reported, body-related distress, as reported by the ABCD questionnaire, remained the same or decreased over the course of DRV/r treatment for both women and men; more patients were satisfied or very satisfied with their bodies at week 48 compared to baseline. This may partially be explained by the decreased rate of reported arm/leg and butt fat loss at week 48 compared to baseline being viewed by some patients as a sign of improved overall health. Additionally, few patients considered discontinuing treatment or switching to an alternate ARV due to body-related distress. It is important to note, however, that close to 25% of the study patients were unsatisfied with their bodies at 48 weeks.
Although the assessment of gender-based differences in metabolic and anthropometric parameters and self-reported body distress were not the primary objectives of the GRACE study, the proportion of women enrolled, nonetheless, allows for a robust interpretation of gender-based outcomes. One limitation of this trial may be the relatively short duration of follow-up; it may be necessary to evaluate changes beyond 48 weeks in order to fully evaluate gender-based differences in the changes in anthropometric parameters. In addition, no adjustments were made for differences in baseline characteristics, which may have affected metabolic and anthropometric parameters. For example, the younger average age of women compared to men at baseline could be independently associated with lower triglyceride levels; likewise, the more advanced disease in men compared to women at baseline may suggest that their greater increase in triglyceride levels over 48 weeks is due to a return to health. Finally, this study did not investigate the impact of race/ethnicity, dietary intake, alcohol use, or other potential contributors to metabolic and anthropometric changes.
As weight gain and changes in anthropometric parameters have implications in tolerability and, possibly, adherence, future studies should be designed to detect gender-based differences and should further investigate the physiologic mechanisms leading to the larger increases in weight in women and triglycerides in men that were observed in GRACE and reported in other studies. A longer follow-up period for anthropometric parameters, as well as treatment adherence, would be beneficial.
In the GRACE study, DRV/r-based therapy was associated with small to moderate changes in metabolic parameters, and few between-gender differences were observed. The levels of self-reported, body-related distress improved for women and men over the course of the study, suggesting that DRV/r-based therapy is well tolerated by patients.
Acknowledgments
The authors would like to thank the patients and their families, the study sites, and the principal investigators for their participation in the trial. The authors would like to acknowledge Gilead for supplying emtricitabine, tenofovir, and emtricitabine/tenofovir. The authors would additionally like to acknowledge internal study support staff, as well as Cali Howitt, Ph.D., Medicus International New York, for her editorial assistance. Lori DeLaitsch of Tibotec Therapeutics Clinical Affairs reviewed and provided input on the manuscript.
Funding for the study and for editorial support was provided by Tibotec Therapeutics. J.S.C. is supported in part by 2K24AI056933. M.T.Y. is supported in part by K23 AI 059884.
Data from this manuscript were previously presented at the 11th International Workshop on Adverse Drug Reactions and Co-morbidities in HIV (ADRL), Philadelphia, PA, USA. October 26–28, 2009. Poster P31.
Author Disclosure Statement
J.S.C. has received research grants from Merck, Schering-Plough, Theratechnologies, and Tibotec; O.O. has received research grants from Merck, GlaxoSmithKline, Pfizer, and Tibotec; has served on the Speaker's Bureau and received honorarium from Gilead and Abbott; and has served as a consultant for Gilead. C.M. has served on a Speaker's Bureau and performed research for Tibotec Therapeutics, Bristol-Myers Squibb, ViiV Healthcare, and Gilead. M.T.Y. has received research grants from Gilead and Bristol-Myers Squibb. R.R., G.D.L.R., and J.M. are employees of Tibotec Therapeutics.
References
- 1.Walmsley S. Cheung AM. Fantus G, et al. A prospective study of body fat redistribution, lipid, and glucose parameters in HIV-infected patients initiating combination antiretroviral therapy. HIV Clin Trials. 2008;9:314–323. doi: 10.1310/hct0905-314. [DOI] [PubMed] [Google Scholar]
- 2.Tsiodras S. Mantzoros C. Hammer S. Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: A 5-year cohort study. Arch Intern Med. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 3.Hill A. Sawyer W. Gazzard B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials. 2009;10:1–12. doi: 10.1310/hct1001-001. [DOI] [PubMed] [Google Scholar]
- 4.Absalon J. Uy J. Rong Y. Mancini M. McGrath D. Gender-based differences in ARV-naive patients treated with boosted protease inhibitors (PIs): Results from the CASTLE study (AI424138) [Abstract TUPE0062]; Poster presented at the 17th International AIDS Conference; Mexico City, Mexico. Aug 3–8;2008 . [Google Scholar]
- 5.Hoffman RM. Umeh OC. Garris C. Givens N. Currier JS. Evaluation of sex differences of fosamprenavir (with and without ritonavir) in HIV-infected men and women. HIV Clin Trials. 2007;8:371–380. doi: 10.1310/hct0806-371. [DOI] [PubMed] [Google Scholar]
- 6.Kumar PN. Rodriguez-French A. Thompson MA, et al. A prospective, 96-week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: Effect of sex and ethnicity. HIV Med. 2006;7:85–98. doi: 10.1111/j.1468-1293.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 7.Thiebaut R. Dequae-Merchadou L. Ekouevi DK, et al. Incidence and risk factors of severe hypertriglyceridaemia in the era of highly active antiretroviral therapy: The Aquitaine Cohort, France, 1996–99. HIV Med. 2001;2:84–88. doi: 10.1046/j.1468-1293.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Evelyn B. Toigo T. Banks D, et al. Women's participation in clinical trials and gender-related labeling: A review of new molecular entities approved 1995–1999. USDA, June 2001. www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ParticipatinginClinicalTrials/ucm197788.htm. [Jan 25;2011 ]. www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ParticipatinginClinicalTrials/ucm197788.htm [PMC free article] [PubMed]
- 9.Zorrilla C. Agrait V. Why do we need the GRACE (Gender, Race, And Clinical Experience) study? Future HIV Ther. 2007;1:357–363. [Google Scholar]
- 10.Currier J. Averitt Bridge D. Hagins D, et al. Sex-based outcomes of darunavir-ritonavir therapy: A single-group trial. Ann Intern Med. 2010;153:349–357. doi: 10.1059/0003-4819-153-6-201009210-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills AM. Nelson M. Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 12.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. NIH Publication No. 02-5215. September 2002.
- 13.Currier J. Scherzer R. Bacchetti P, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48:35–43. doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohl D. Scherzer R. Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loutfy M. Andany N. Li M, et al. Gender and ethnicity differences in body change and distress of HIV-positive individuals taking antiretroviral therapy in Ontario [Poster P-08] Antivir Ther. 2009;14:A29. [Google Scholar]
- 16.Galli M. Veglia F. Angarano G, et al. Gender differences in antiretroviral drug-related adipose tissue alterations. Women are at higher risk than men and develop particular lipodystrophy patterns. J Acquir Immune Defic Syndr. 2003;34:58–61. doi: 10.1097/00126334-200309010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wilson ME. Allred KF. Kordik EM. Jasper DK. Rosewell AN. Bisotti AJ. Gender-specific effects of HIV protease inhibitors on body mass in mice. AIDS Res Ther. 2007;4:8. doi: 10.1186/1742-6405-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plankey M. Bacchetti P. Jin C, et al. Self-perception of body fat changes and HAART adherence in the Women's Interagency HIV Study. AIDS Behav. 2009;13:53–59. doi: 10.1007/s10461-008-9444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter VM. Hoy JF. Bailey M. Colman PG. Nyulasi I. Mijch AM. The prevalence of lipodystrophy in an ambulant HIV-infected population: It all depends on the definition. HIV Med. 2001;2:174–180. doi: 10.1046/j.1468-1293.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 20.Safrin S. Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]