Abstract
Rationale and objectives
The melanin-concentrating hormone 1 (MCH1) receptors play an important role in home-cage food consumption in rodents, but their role in operant high-fat food-reinforced responding or reinstatement of food seeking in animal models is unknown. Here, we used the MCH1 receptor antagonist SNAP 94847 to explore these questions.
Materials and methods
In experiment 1, we trained food-restricted rats (16 g/day of nutritionally balanced rodent diet) to lever press for high-fat (35%) pellets (3-h/day, every other day) for 14 sessions. We then tested the effect of SNAP 94847 (3–30 mg/kg, intraperitoneal (i.p.)) on food-reinforced operant responding. In experiments 2 and 3, we trained rats to lever press for the food pellets (9 to 14 3-h sessions) and subsequently extinguished the food-reinforced lever responding by removing the food (10 to 17 sessions). We then tested the effect of SNAP 94847 on reinstatement of food seeking induced by MCH (20μg, intracerebroventricular), noncontingent delivery of three pellets during the first minute of the test session (pellet-priming), contingent tone–light cues previously associated with pellet delivery (cue), or the pharmacological stressor yohimbine (2 mg/kg, i.p.).
Results
Systemic injections of SNAP 94847 decreased food-reinforced operant responding and MCH-induced reinstatement of food seeking. SNAP 94847 had no effect on pellet-priming-, cue-, or yohimbine-induced reinstatement.
Conclusions
Results indicate that MCH1 receptors are involved in food-reinforced operant responding but not in reinstatement induced by acute exposure to high-fat food, food cues, or the stress-like state induced by yohimbine. These results suggest that different mechanisms mediate food-reinforced operant responding and reinstatement of food seeking.
Keywords: Melanin-concentrating hormone, Extinction, Pellet priming, Cues, Reinstatement, Relapse, Self-administration, SNAP 94847, Stress, Yohimbine
Introduction
A major problem in the clinical treatment of obesity is the high rate of relapse to maladaptive eating habits (Peterson and Mitchell 1999). In humans, this relapse is often triggered by reexposure to palatable foods, food-associated cues, or stress (Drewnowski 1997; Freeman and Gil 2004; Grilo et al. 1989). Surprisingly, the mechanisms underlying relapse to palatable food have rarely been studied in animal models (Nair et al. 2009). Recently, we adapted a rat reinstatement model, commonly used to study relapse to abused drugs (Shaham et al. 2003) to investigate these mechanisms. In previous studies, we examined the role of peptides involved in stress responses and feeding—corticotropin-releasing factor (CRF), peptide YY3-36, and hypocretin (orexin)—in reinstatement of food seeking induced by acute noncontingent exposure to food pellets (pellet priming), exposure to cues previously associated with pellet delivery (cue), and the pharmacological stressor yohimbine. Yohimbine is an alpha-2 adrenoceptor antagonist that induces stress-like responses in humans and laboratory animals (Redmond and Huang 1979). We and others reported that yohimbine reliably reinstates food (Ghitza et al. 2006; Richards et al. 2008) and drug (Feltenstein and See 2006; Le et al. 2005; Lee et al. 2004; Shepard et al. 2004)seeking in laboratory animals.
We found that the CRF1 receptor antagonist antalarmin attenuates yohimbine-induced but not pellet-priming-induced reinstatement (Ghitza et al. 2006). We also found that peptide YY3-36 attenuates pellet-priming- and cue-induced but not yohimbine-induced reinstatement (Ghitza et al. 2007), while the hypocretin 1 receptor antagonist SB 334867 has no effect on either pellet-priming- or yohimbine-induced reinstatement (Nair et al. 2008). These results suggest that different mechanisms mediate pellet-priming- and cue-induced-reinstatement versus yohimbine-induced reinstatement of food seeking.
Here, we further explored the mechanisms underlying reinstatement of food seeking by studying the role of the melanin-concentrating hormone (MCH) system. MCH neurons are located in the lateral hypothalamus and project to many brain areas (Bittencourt et al. 1992; Zamir et al. 1986). MCH effects are mediated by MCH1 (the functional MCH receptor in the rodent brain) and MCH2 receptors (Chambers et al. 1999; Saito et al. 2001) that are expressed in hypothalamic and extrahypothalamic sites (Lembo et al. 1999; Saito et al. 1999). In rodents, ventricular MCH injections increase home-cage food intake and these effects are reversed by MCH1 receptor antagonists; MCH1 receptor antagonists also decrease home-cage food intake (Borowsky et al. 2002; Pissios et al. 2006; Qu et al. 1996).
Our study on the role of the MCH system in reinstatement of food seeking was inspired by the finding that nucleus accumbens injections of MCH and an MCH1 receptor antagonist reproduce the effects of ventricular and systemic injections of MCH and the MCH1 receptor antagonist, respectively, on home-cage food intake (Georgescu et al. 2005). The nucleus accumbens plays an important role in the appetitive motivational effects of food and drug rewards (DiLeone et al. 2003; Kelley and Berridge 2002; Wise 2006), cue-induced reinstatement of both drug and food seeking, and drug-priming-induced reinstatement (Bossert et al. 2005; Floresco et al. 2008; Schmidt et al. 2005; See 2005). Based on these previous findings, we assessed the effect of systemic injections of the MCH1 receptor antagonist SNAP 94847 on pellet-priming- and cue-induced reinstatement and also assessed the effect of SNAP 94847 on MCH-induced (ventricular injections) reinstatement. Because systemic injections of SNAP 94847 had minimal effects on pellet-priming- and cue-induced reinstatement, we did not assess the effect of accumbens SNAP 94847 injections.
We also examined the effect of SNAP 94847 on yohimbine-induced reinstatement because the MCH system plays a role in regulating stress responses (Hervieu 2003; Rokosz and Hobbs 2006). For example, systemic injections of SNAP 94847 or a related MCH1 receptor antagonist SNAP 7941 decrease behavioral stress responses in the rat social interaction test, guinea pig maternal-separation vocalization tests, chronic mild-stress-induced decreases in sucrose preference, stress-induced immobility in the rat (but not mice) forced-swim test, mouse light–dark test, and novelty suppressed feeding tests (Borowsky et al. 2002; David et al. 2007; Smith et al. 2009). Based on these results, we studied whether blockade of MCH1 receptors would decrease reinstatement of food seeking induced by the pharmacological stressor yohimbine. Finally, since prior studies only examined the effect of MCH1 receptor antagonists on home-cage food intake, we also studied the effect of SNAP 94847 on high-fat (35%) food-reinforced operant responding.
Materials and methods
Subjects and apparatus
Male Long–Evans rats (total n=76, Charles River, Raleigh, NC, USA; 300–385 g) were housed in self-administration chambers for the duration of the experiment under a reverse 12-h–12-h light–dark cycle (lights off at 9:30 a.m.). Five rats were excluded from the study due to poor health after guide cannulae surgery (n=2), failure to meet an extinction criterion (see below, n=1), loss of head cap (n=1), and lever responding that was greater than three standard deviations above the group mean (n=1). All rats were kept on a restricted diet of 16 g/day of Purina rat chow (about 60–65% of their daily food intake) during the training phase and 16–20 g/day during the extinction and reinstatement test phases to maintain their weights within a range of ±5 g of their body weight on the last training day. Body weights of all rats in the study were taken daily. All procedures followed the guidelines outlined in the “Principles of laboratory animal care” (NIH publication no. 85-23). The experiments were conducted in standard self-administration chambers (Med Associates, Georgia, VT, USA). Each chamber had two levers 9 cm above the floor, but only one lever (“active,” retractable lever) activated the pellet dispenser, which delivered 45-mg food pellets containing 35% fat and 45.2% carbohydrate (catalog # F05989, Bioserv, Frenchtown, NJ, USA).
Drugs
The MCH1 receptor antagonist SNAP 94847 (N-(3-{1-[4-(3,4-difluoro-phenoxy)-benzyl]-piperdin-4-yl}-4-methyl-phenyl)-isobutyramide; supplied by Lundbeck Research USA, Paramus, NJ, USA) and yohimbine hydrochloride (Sigma, St. Louis, MO, USA) were prepared daily prior to testing. SNAP 94847 (3, 10, 15, and 30 mg/kg, intraperitoneal (i.p.)) was dissolved in 20% 2-hydroxypropyl-β-cyclodextrin (encapsin) and yohimbine (2 mg/kg, i.p.) was dissolved in sterile water. The injection volumes were 1 ml/kg for SNAP 94847 and 0.5 ml/kg for yohimbine. MCH (2.5, 5, 10, and 20μg, intracerebroventricular (i.c.v.), injection volume 1 μL; Bachem, Torrance, CA) was dissolved in 0.9% saline on the first day of testing. The peptide solution was stored at 4°C for a maximum duration of 1 week. The doses for i.c.v. injections of MCH and for systemic injections of SNAP 94847 and yohimbine were based on previous studies (Duncan et al. 2005; Ghitza et al. 2007; Nair et al. 2008; Smith et al. 2009).
Intracranial surgery and intracranial injections
The rats were anesthetized with a mixture of sodium pentobarbital and choral hydrate (60 and 25 mg/kg, i.p.). They were then implanted with guide cannulae (23 gauge, Plastics One, Roanoke, VA) 2 mm above the ventricle (AP −0.9 mm, ML +1.4 mm, and DV −2.0 mm (Paxinos and Watson 2005)) using a stereotaxic instrument (Kopf, Tujunga, CA, USA). The analgesic buprenorphine (0.1 mg/kg, subcutaneous) was given after surgery and the rats were allowed to recover for 5–7 days. Injections (i.c.v.) of MCH were made with Harvard infusion pumps, using 10-μl Hamilton syringes that were connected to 30-gauge injectors (Plastics One, Roanoke, VA, USA) via polyethylene-50 tubing. Injections lasted 1 min and the injectors were left in place for an additional 1 min before being replaced with cannula blockers. As in our previous work (Erb et al. 1998; Shalev et al. 2001), at the end of the experiment, cannulae placements were verified by a positive dipsogenic response to angiotensin II (50 ng in 2μl, Sigma, St. Louis, MO, USA). Placements were considered accurate if a rat started drinking within 2 min of the injection and sustained drinking for 3–4 min (Johnson and Epstein 1975; Sakai et al. 1995).
Procedures
With the exception of our initial experiments on the effect of SNAP 94847 and MCH on ongoing food-reinforced responding, we used a reinstatement procedure that was based on our previous studies (Ghitza et al. 2006, 2007; Nair et al. 2008) and included three phases: training to lever press for food (9 to 14 sessions, until the rats demonstrated reliable and stable pellet intake for four to five daily sessions; see Fig. 1), extinction of the food-reinforced responding (10 to 17 sessions, until the active lever responding met the extinction criterion described below), and tests for reinstatement under extinction conditions (up to five sessions). During all phases, the sessions started 30 min after the beginning of the dark cycle (10 a.m.). Below, we first describe the training and extinction procedures for all experiments and then provide the specific details for the testing phase of each experiment. During testing, the experimental conditions were counterbalanced.
Fig. 1.
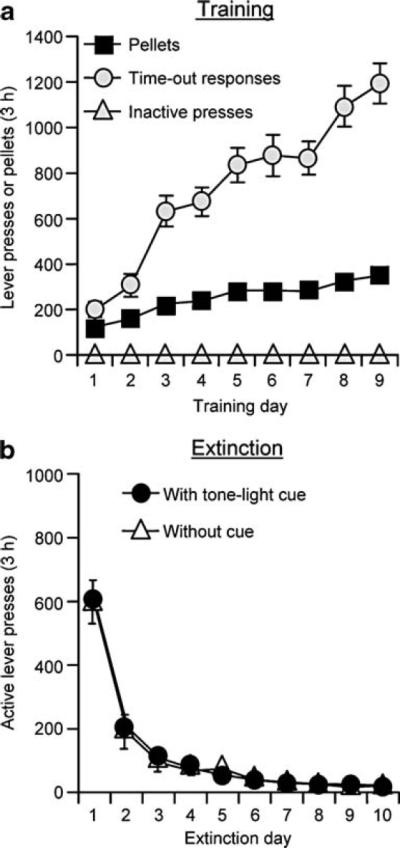
Training for food-reinforced responding and extinction of the food-reinforced lever responding. a Training: mean±SEM number of 35% fat pellets earned, timeout active lever presses, and inactive lever responses during the training sessions over nine alternating days (one 3-h session per day, every other day) for rats trained under a fixed-ratio 1 (FR-1) 20-s timeout reinforcement schedule (n=71). b Extinction: mean number of presses on the previously active lever in the presence or absence of adiscretetone-light cue during the extinction phase (n=53, extinction with cue; n=18, extinction without cue)
Training
All rats were given 3-h daily sessions of “autoshaping” for 2 to 3 days during which pellets were administered noncontingently every 5 min into a receptacle located near the active lever. Pellet delivery was accompanied by a compound 5-s tone (2,900 Hz, 20 dB above background)–light (a 7.5-W white light located above the active lever) cue. Subsequently, the rats were trained to lever press for the pellets on a fixed-ratio 1 20-s timeout reinforcement schedule. For experiment 1, the training sessions were conducted for 12–14 days every other day, 3 h/day (one 3-h session).
At the start of the session, the red house light was turned on and the active lever was extended. Following each pellet delivery, the compound tone–light cue (termed “cue” herein) was turned on for 5 s. At the end of the session, the house light was turned off and the active lever retracted. During the training days, regular food (16 g Purina rat chow) was given after the daily session (about 3.5 h into the dark cycle). During the off days, the 16 g regular food was given at the start of the dark cycle.
We chose to train the rats to lever press for the food pellets every other day under restricted feeding conditions because previous home-cage food consumption studies have shown that rats placed on a restricted diet and given intermittent access to palatable food develop binge-like eating behavior (Avena et al. 2008; Boggiano et al. 2007; Colantuoni et al. 2002; Corwinand Buda-Levin 2004; Figlewicz et al. 2007) and become hypersensitive to the effects of stress on palatable food intake (Hagan et al. 2003). In our previous studies, the duration of our training session was 6–9 h/day (Ghitza et al. 2006, 2007; Nair et al. 2006, 2008). However, in pilot studies, we found that the total pellet intake in the 3-h/day training sessions (~250–350 pellets per day or ~11.25 to 15.75 g/day) is similar to that observed in 6- or 9-h/day sessions. Thus, in the present study, rats were trained 3 h/day. We also chose our diet conditions based on two other considerations. First, there is evidence that humans are vulnerable to the effects of stress and food cues on relapse to maladaptive eating habits during dieting (Elfhag and Rossner 2005; Fedoroff et al. 2003; Herman and Polivy 1975). Second, as anticipated from these studies, the effects of our experimental manipulations (particularly pellet-priming-induced reinstatement) are less robust in rats trained, extinguished, and tested while given free access to regular food (unpublished observations).
Extinction of food-reinforced responding
For experiments 2 and 3, after training, the rats were given 10 to 17 daily extinction sessions until active lever responding was below a mean of 30 presses per 3 h for three consecutive sessions (the extinction criterion). For the rats that were subsequently tested for MCH-, yohimbine-, and pellet-priming-induced reinstatement, lever presses led to tone–light cue presentations but not pellet delivery. For the rats that were subsequently tested for cue-induced reinstatement, lever presses had no programmed consequences (i.e., neither the tone–light cue nor pellets were made available). During the extinction and reinstatement phases, regular food (16–20 g) was given approximately at the same time as during training (i.e., ~3.5 h after the onset of the dark cycle).
Experiment 1: Effect of SNAP 94847 on food-reinforced operant responding
We initially studied the effect of systemic injections of SNAP 94847 on ongoing food-reinforced responding. For 14 days, the rats (n=12) were given one 3-h training session as described above. We then assessed the effect of SNAP 94847 on lever presses for the pellets in four 3-h tests that were conducted every 48 h. We used a within-subjects experimental design with the factors of SNAP 94847 dose (vehicle, 3, 10, and 30 mg/kg) and session hour (hours 1, 2, and 3). Each rat was injected with vehicle or one of the doses of SNAP 94847, in a counterbalanced order. SNAP 94847 or its vehicle was injected 60 min prior to the test sessions because previous studies have demonstrated that SNAP 94847 doses of up to 30 mg/kg achieve significant brain penetration by 60 min (DGS unpublished data and Chen et al. 2007).
Experiment 2: Effect of SNAP 94847 on MCH-induced reinstatement of food seeking
To determine the effect of SNAP 94847 on MCH-induced reinstatement of food seeking, we initially assessed the effect of MCH on this reinstatement. Following our experiment on the effect of systemic injections of SNAP 94847 on food-reinforced responding, we implanted the rats with a guide cannula into the lateral ventricle. After a postoperative recovery period of 5 days, the rats were retrained to lever press for the high-fat food pellets for 3 days and the lever pressing response was extinguished in 13 daily extinction sessions. The rats (n=12) were injected with vehicle or MCH (2.5, 5, 10, and 20μg, i.c.v.) in five test sessions, every 48 h, in an ascending order of MCH dose, with extinction sessions on the intervening days. We used a within-subjects experimental design with the factors of MCH dose (vehicle, 2.5, 5, 10, and 20μg) and session hour.
In a different group of rats (n=10), we examined the effect of SNAP 94847 (30 mg/kg, i.p.) on MCH-induced (20μg) reinstatement. We used a within-subjects experimental design with the within-subjects factors of pretreatment condition (0 or 30 mg/kg SNAP 94847), MCH dose (0, 20μg), and session hour. On test days that were separated by 24–72 h, each rat was injected systemically with SNAP 94847 (30 mg/kg) or its vehicle 60 min before the test sessions and then injected with MCH or its vehicle 8–12 min before the sessions; the injections of MCH and its vehicle and SNAP 94847 and its vehicle were counterbalanced.
Experiment 3: Effect of SNAP 94847 on yohimbine-, pellet-priming-, and cue-induced reinstatement of food seeking
Pellet-priming-induced reinstatement
We tested the effect of SNAP 94847 on pellet-priming-induced reinstatement in four 3-h test sessions with two sessions run consecutively and one extinction day between the two sets of tests. During the test sessions, three food pellets were administered noncontingently within the first minute of the session (i.e., one pellet delivered every 20 s). We used a mixed experimental design that included the between-subject factor of SNAP dose (15 or 30 mg/kg, n=10 in each group) and the within-subjects factors of pretreatment condition (0 and SNAP 94847 [15 or 30 mg/kg]), priming condition (pellet, no pellet), and session hour. On test days that were separated by 24–72 h, each rat was injected systemically with the SNAP 94847 vehicle or one of the SNAP 94847 doses (15 or 30 mg/kg) 60 min before the test sessions and then exposed to the priming condition (three pellets or no pellets); the injections of SNAP 94847 and its vehicle and the priming conditions were counterbalanced.
Cue-induced reinstatement
As mentioned above, during the training phase, each pellet delivery was paired with a tone–light cue (cue); this cue was not presented during the extinction phase after lever pressing. During the tests for reinstatement, lever responding led to contingent presentations of the cue under the fixed-ratio 1 20-s timeout reinforcement schedule. We tested the effect of SNAP 94847 on cue-induced reinstatement in a total of four test sessions with two sessions run consecutively and five extinction days between test sets. We conducted five extinction days between sets of tests in accordance with previous experiments demonstrating that this procedure minimizes habituation to the presentation of conditioned cues (unpublished data and Bossert et al. 2006; Ghitza et al. 2007). We used a mixed design with between-subject factor of SNAP dose (15 or 30 mg/kg, n=8 in the 15 mg/kg group and n=10 in the 30 mg/kg group) and the within-subjects factors of pretreatment condition (0 and SNAP 94847 [15 or 30 mg/kg]), cue (cue, no cue), and session hour.
Yohimbine-induced reinstatement
We tested the effect of SNAP 94847 on yohimbine-induced reinstatement in four test sessions with two sessions run consecutively and one extinction day between sets of tests. We used a mixed experimental design that included the between-subject factor of SNAP dose (15 or 30 mg/kg, n=12 in the 15 mg/kg group and n=19 in the 30 mg/kg group) and the within-subjects factors of pretreatment condition (0 and SNAP 94847 [15 or 30 mg/kg]), yohimbine dose (0 or 2 mg/kg), and session hour. Ten rats each in the 15- and 30-mg/kg SNAP 94847 dose were rats previously tested for the effect of SNAP 94847 on pellet-priming-induced reinstatement. These rats were given 2 days of extinction prior to tests for yohimbine-induced reinstatement. On the test days that were separated by 24–72 h, each rat was injected systemically with the SNAP 94847 vehicle or one of the SNAP 94847 doses (15 or 30 mg/kg) 60 min before the test sessions and then injected with yohimbine 15 min later (i.e., 45 min prior to the test session); the injections of SNAP 94847 or its vehicle and yohimbine or its vehicle were counterbalanced.
Statistical analyses
Data were analyzed using SPSS version 15.0 statistical software. The data on the effect of SNAP 94847 on food-reinforced operant responding (experiment 1) were analyzed separately for the number of pellets earned and the number of timeout active lever presses divided by the number of pellets earned during the test sessions. Under our training conditions (Fig. 1), the number of non-reinforced lever presses during the 20-s timeout period after each pellet delivery is substantially higher than the number of reinforced lever presses. Thus, the ratio of timeout lever presses per pellet earned provides a measure of the effect of SNAP 94847 on timeout responding that is independent of the effect of the antagonist on pellet intake. The data from the reinstatement experiments (experiments 2 and 3) were analyzed for nonreinforced lever presses on the previously active lever. The factors used in the statistical analyses are described in the “Results” section. The experimental manipulations had minimal effects on inactive lever presses (a mean of less than four presses per 3 h), a potential measure of nondirected activity and/or response generalization (Shalev et al. 2002). Thus, the data for inactive lever presses are not reported in the “Results” section. Significant overall effects (p<0.05) in the different analyses of variance (ANOVAs) were followed by Fischer post hoc least significant difference tests.
Results
Experiment 1. Effect of SNAP 94847 injections on food-reinforced operant responding
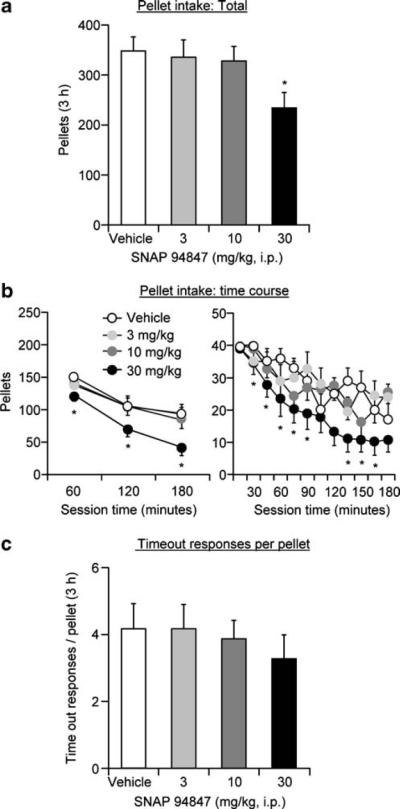
SNAP 94847 decreased the number of pellets earned at the highest dose (Fig. 2a, b). In contrast, SNAP 94847 had no effect on the ratio of timeout active lever presses per pellet delivery (Fig. 2c). The rats (n=12) were injected with vehicle or one dose of SNAP 94847 (3, 10, or 30 mg/kg) before four test sessions every 48 h, during which the rats lever-pressed for pellets. The statistical analyses for pellets earned included the within-subjects factors of SNAP 94847 dose (vehicle, 3, 10, and 30 mg/kg) and session hour (hours 1, 2, 3). This analysis revealed significant effects of SNAP 94847 dose (F3, 33=8.9, p<0.01) and session hour (F2, 22=39.1, p<0.01) but no interaction between the two factors. The statistical analysis for the ratio of timeout active lever presses per pellet delivery included the within-subjects factor of SNAP 94847 dose. This analysis revealed no significant effect of SNAP 94847 dose (p>0.3).
Fig. 2.
Systemic injections of SNAP 94847 decrease operant food-reinforced lever responding. a, b Mean±SEM number of 35% fat pellets earned after systemic injections of vehicle (20% encapsin) or SNAP 94847 (total per 3-, 1-h, and 15-min time course (n=12)). c Mean ratio of timeout lever presses per pellet delivery after vehicle or SNAP 94847 injections. Asterisk, different from vehicle condition, p<0.05
Experiments 2 and 3. Effect of SNAP 94847 injections on reinstatement
The rats in experiments 2 and 3 were trained for 9 to 14 sessions and demonstrated reliable food-reinforced responding and, as in our previous studies, a progressive escalation of timeout active lever presses across sessions (Ghitza et al. 2006, 2007; Nair et al. 2006, 2008; Fig. 1a). During training, the rats gained weight on the days when the pellets were available (mean±SEM 10.3±1.0 g/day) and lost weight on the days when pellets were not available (mean±SEM 7.3±0.4 g/day). After self-administration training, the rats were given 10 to 17 3-h extinction sessions, during which lever pressing decreased over time (Fig. 1b). During the training phase, the statistical analyses revealed significant increases over time for both pellets earned and timeout active lever presses (p values<0.01) but not for inactive lever presses. During the extinction phase, the analysis revealed a significant decrease in response rates for active lever presses over time (p values<0.01).
MCH-induced reinstatement (experiment 2)
Two groups of rats were tested. In the first group, we assessed the effect of ventricular injections of MCH (2.5, 5, 10, and 20μg) on reinstatement of food seeking (n=12). In the second group, we assessed the effect of SNAP 94847 (30 mg/kg, i.p.) on reinstatement induced by MCH (20μg; n=10). In the first group, ventricular injections of MCH significantly increased active lever responding; this effect was most pronounced in the first 60 min of the test session. The statistical analysis included the within-subjects factor of MCH dose (vehicle, 2.5, 5, 10, and 20μg) and session hour. This analysis revealed significant effects of MCH dose (F4, 44=7.6, p<0.01) and MCH dose×**session hour (F8, 88=7.0, p<0.01; Fig. 3a, b, left panel). In the second group, injections of SNAP 94847 60 min before MCH injections reversed MCH-induced reinstatement; injections of SNAP 94847 by itself had no effect on this reinstatement (n=10; Fig. 3a, b, right panel). The statistical analyses included the within-subjects factors of SNAP 94847 dose (vehicle, SNAP 94847), MCH dose (0, 20μg), and session hour. The ANOVA revealed significant effects of MCH dose (F1, 9=10.9, p<0.01), MCH dose×session hour (F2, 18=5.2, p<0.01), SNAP 94847 dose ×MCH dose (F1, 9=7.9, p<0.05), and SNAP 94847 dose×MCH dose×session hour (F2, 18=13.7, p<0.01).
Fig. 3.
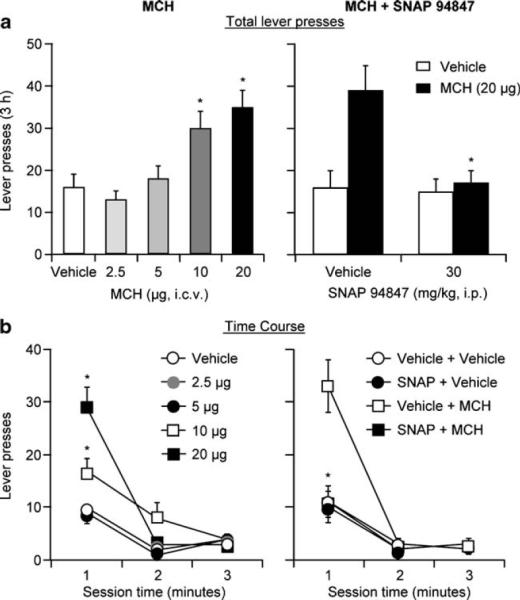
Ventricular injections of MCH reinstate food seeking and systemic injections of SNAP 94847 decrease MCH-induced reinstatement of food seeking. a Left panel: mean±SEM numberof active lever presses after ventricular injections of vehicle (saline) and MCH (2.5, 5, 10, and 20μg, n=12) during the reinstatement tests. Right panel: mean number of active lever presses in rats pretreated with SNAP 94847 or its vehicle (n=10) prior to ventricular injections of MCH or its vehicle. Asterisk, different from vehicle condition, p<0.05. b Corresponding 1-h session time course for the data depicted in a. Asterisk, different from vehicle-MCH condition, p<0.05
Pellet-priming-, cue-, and yohimbine-induced reinstatement (experiment 3)
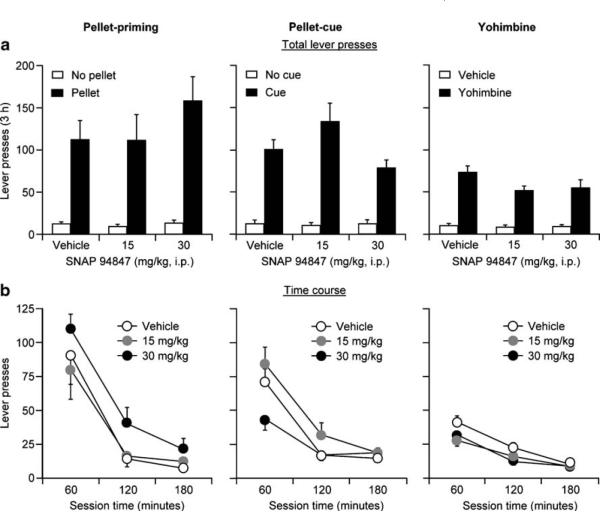
Systemic injections of SNAP 94847 (15 or 30 mg/kg) 60 min before exposure to the reinstating stimulus (pellet priming, cue, or yohimbine) had no effect on reinstatement of active lever responding (Fig. 4a, b). As previously described, to determine the effect of SNAP 94847 on pellet-priming-, cue-, or yohimbine-induced reinstatement, we pretreated groups of rats during four test sessions with the MCH1 receptor antagonist. These rats were injected with 15 or 30 mg/kg of SNAP 94847 or its vehicle and tested in extinction (no pellets, no cue, or yohimbine vehicle) and following a single exposure to food pellets, food-associated tone–light cues, or yohimbine. The statistical analyses included the between-subject factors of SNAP 94847 dose (15 or 30 mg/kg) and the within-subjects factors of session hour and the reinstating stimulus: pellet priming (pellet, no pellet; n=10 per dose), cue (cue, no cue; n=10 for 30 mg/kg; n=8 for 15 mg/kg), or yohimbine dose (0, 2 mg/kg; n=19 for 30 mg/kg; n=12 for 15 mg/kg). These analyses revealed significant effects of pellet priming (F1, 19=43.2, p<0.01), cue (F1, 17=117.0, p<0.001), and yohimbine dose (F1, 30=162.9, p<0.01) and session hour (p values<0.05 for each of the three reinstating stimuli). Neither the effect of SNAP 94847 dose nor the interaction between SNAP 94847 dose and pellet priming, cue, or yohimbine dose nor the triple interaction between SNAP 94847 dose, session hour, and the reinstating stimulus (pellet priming, cue, yohimbine) was significant (p values>0.1). For cue-induced reinstatement, while the rats that received 30 mg/kg SNAP 94847 decreased (p<0.05) lever pressing in the first hour of the test session compared with the rats that received 15 mg/kg (but not vehicle), the lack of both a dose–response relationship and statistically significant interactions (described above) led us to tentatively conclude that MCH1 receptor blockade with SNAP 94847 does not decrease cue-induced reinstatement.
Fig. 4.
Systemic injections of SNAP 94847 have minimal effect on pellet-priming-, cue-, or yohimbine-induced reinstatement. Mean± SEM number of active lever presses (total lever presses per 3-h (a) and 1-h session time course (b) after pretreatment of SNAP 94847 (15 or 30 mg/kg)) or its vehicle and subsequent exposure to pellet-priming (left panel, n=10 per dose) and cue (middle panel, n=8-10 per dose) and yohimbine (2 mg/kg, i.p., n=12-19 per dose)injections (right panel). During tests for pellet priming, the rats received three noncontingent pellets every 20 s during the first minute of the session. During tests for cue-induced reinstatement, lever presses led to contingent presentations of a tone-light cue that was paired with pellet delivery during training and was not presented during the extinction phase
Discussion
We found that systemic injections of the MCH1 receptor antagonist SNAP 94847 decreased MCH-induced reinstatement but overall had a minimal effect on pellet-priming-, cue-, or yohimbine-induced reinstatement of food seeking. These data suggest that while MCH1 receptors are critical for MCH-induced reinstatement, these receptors play a minimal role in reinstatement by the other reinstating stimuli. We also found that SNAP 94847 injections decrease food-reinforced operant responding, extending previous findings on the role of MCH1 receptors in food intake. Below, we discuss these findings.
Role of MCH 1 receptors in high-fat food-reinforced operant responding
Our finding that SNAP 94847 decreased operant high-fat food-reinforced responding is consistent with previous results on the effect of MCH1 receptor antagonists on home-cage food intake (Kowalski et al. 2004, 2006; Luthin 2007). We speculate that the effect of SNAP 94847 on food-reinforced operant responding is due to acceleration of satiety after initiation of food intake because the highest SNAP 94847 dose had no effect on pellet intake in the first 15 min of the test sessions; its effect on pellet intake only emerged at later time points (Fig. 2b right column). It is unlikely that the lack of effect of SNAP 94847 in the first 15 min was due to pharmacological issues related to drug bioavailability because SNAP 94847 achieves significant brain penetration within 60 min after injections (Daniel G. Smith unpublished and Chen et al. 2007). Additional potential evidence that MCH1 receptor antagonism accelerates food satiety is the finding of Kowalski et al. (2004) that the MCH1 antagonist T-226296 decreases home-cage meal size but not the number of meals.
Alternatively, the effect of SNAP 94847 on food-reinforced responding could be due to decreased motivation to seek food during dieting. This possibility is unlikely for two reasons. As mentioned above, SNAP 94897 had no effect on initiation of food-reinforced responding early in the test session, suggesting that the motivation to seek food during dieting remained intact after MCH1 receptor blockade. Additionally, SNAP 94847 had minimal effect on the motivation to seek food in the reinstatement tests when resumption of nonreinforced food seeking was induced by pellet priming, cue, or yohimbine.
Another possibility is the SNAP 94847-induced food aversion or motor deficits that account for its effect on food-reinforced responding. These two possibilities are unlikely. Regarding food aversion, Borowsky et al. (2002) reported that a related MCH1 receptor antagonist (SNAP 7941) has no effect on conditioned taste aversion. Regarding motor deficits, SNAP 94847 had minimal effect on initiation of food-reinforced responding (Fig. 2b right column), timeout lever presses, and pellet-priming-, cue-, or yohimbine-induced reinstatement.
While we did not examine the brain sites that mediate the effect of SNAP 94847 on food-reinforced responding, we speculate that a likely candidate is the nucleus accumbens. The nucleus accumbens receives dense innervations from lateral hypothalamic MCH neurons (Bittencourt et al. 1992; Zamir et al. 1986) and high levels of MCH1 receptors are expressed in this brain region (Lembo et al. 1999; Saito et al. 1999). Additionally, accumbens shell injections of MCH1 receptor antagonists decrease home-cage food consumption (Georgescu et al. 2005).
Role of MCH1 receptors in reinstatement of food seeking during dieting
Ventricular injections of MCH reinstated lever presses in our food-restricted rats. An issue to consider in the interpretation of these data is that food restriction is a condition known to elevate endogenous MCH levels (Qu et al. 1996). Thus, a potential mechanism for the effect of MCH on reinstatement is the exacerbation of hunger signals in the food-restricted rats, leading to hunger-induced increases in lever responding. In a meal pattern analysis study, Morens et al. (2005) reported that MCH increases the number of meals, meal duration, meal size, and the rate of ingestion, suggesting an increased hunger state. However, under our experimental conditions of dietary restriction during training, extinction, and testing, acute 24 h food deprivation has no effect on reinstatement of lever responding in the food-restricted rats (unpublished observation). This suggests that an increase in hunger following ventricular injections of MCH is unlikely to be the mechanism underlying MCH-induced reinstatement.
Our finding that MCH1 receptor antagonism blocks MCH-induced reinstatement raises the possibility that the MCH system and the MCH1 receptors are involved in reinstatement of food seeking. To test the generality of the effect of SNAP 94847 on reinstatement of food seeking, we examined its effect on reinstatement induced by pellet priming, pellet cues, and yohimbine. However, the findings that SNAP 94847 had minimal effects on pellet-priming, cue-, and yohimbine-induced reinstatement do not support this notion. Before ruling out the role of MCH1 receptors in reinstatement of food seeking, however, several issues should be considered.
First, we injected SNAP 94847 intraperitoneally in our experiments while in other studies using SNAP 94847 investigators have used the oral route of drug administration (David et al. 2007; Marsteller et al. 2009; Smith et al. 2009). Intraoral doses of 3, 10, and 30 mg/kg achieve significant brain penetration by 60 min, have a brain to plasma ratio of 2.3 at 4 h postdosing, and have an elimination half-life of 5.2 h (Daniel G. Smith unpublished and Chen et al. 2007). However, it is unlikely that the route of drug administration influenced our results for two main reasons. One reason is that a drug administered by a parenteral route typically achieves higher plasma levels compared to oral administration (Golan et al. 2008). The second reason is that under our experimental conditions, intraperitoneal injections of 30 mg/kg SNAP 94847 decreased pellet self-administration, blocked MCH-induced reinstatement, and blocked MCH-induced increase in water consumption (data not shown). The second issue to consider is that the doses of SNAP 94847 used in the present study result in 30–90% MCH1 receptor occupancy in the rat brain (David et al. 2007; Marsteller et al. 2009; Smith et al. 2009). While this MCH1 receptor occupancy appears to be sufficient for SNAP 94847 to decrease food self-administration and block MCH-induced reinstatement, it is possible that antagonists with higher MCH1 receptor occupancy are required to attenuate reinstatement of food seeking.
The third issue is that it is possible that the MCH system is involved in reinstatement of food seeking induced by stimuli other than the ones used here: pellet priming, discrete cues, and the pharmacological stressor yohimbine. For example, a question for future research is whether blockade of MCH1 receptors would attenuate reinstatement induced by discriminative (Ciccocioppo et al. 2001; Ettenberg 2009; Weiss et al. 2000) or contextual (Bossert et al. 2006; Crombag et al. 2008;Hamlin et al. 2006) food cues.
Concluding remarks
We found that the MCH1 receptor antagonist SNAP 94847 decreased high-fat food-reinforced responding and MCH-induced reinstatement but had no effect on pellet-priming-, cue-, or yohimbine-induced reinstatement of food seeking. This pattern of results is similar to what we recently observed with the hypocretin 1 receptor antagonist SB 334867 (Nair et al. 2008). This drug decreased food-reinforced responding but had no effect on pellet-priming- and yohimbine-induced reinstatement. These data suggest that distinct mechanisms mediate food-reinforced operant responding and reinstatement of food seeking. This conclusion is in agreement with findings from our studies on the effect of peptide YY3-36 and the CRF1 receptor antagonist antalarmin, which decreased yohimbine-induced reinstatement or pellet-priming- and cue-induced reinstatement, respectively, but had no effect on ongoing food-reinforced responding (unpublished data and Ghitza et al. 2006, 2007). Finally, to the degree that the reinstatement model simulates relapse-related processes in humans (Epstein et al. 2006;Nair et al. 2009), our data suggest that MCH1 receptor antagonists will be ineffective in preventing resumption of high-fat food seeking during dieting.
Acknowledgments
The work was supported by the Intramural Research Program of the National Institute on Drug Abuse. We thank Evan Goldart for technical support.
Footnotes
Conflict of interest The authors state no conflict of interest.
References
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Chen CA, Jiang Y, Lu K, Daniewska I, Mazza CG, Negron L, Forray C, Parola T, Li B, Hegde LG, Wolinsky TD, Craig DA, Kong R, Wetzel JM, Andersen K, Marzabadi MR. Synthesis and SAR investigations for novel melanin-concentrating hormone 1 receptor (MCH1) antagonists part 2: a hybrid strategy combining key fragments of HTS hits. J Med Chem. 2007;50:3883–3890. doi: 10.1021/jm060383x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3, 4-difluorophenoxy) phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Taste preferences and food intake. Annu Rev Nutr. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/ quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–7. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Gil KM. Daily stress, coping, and dietary restraint in binge eating. Int J Eat Disord. 2004;36:204–212. doi: 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci. 2007;27:11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan DE, Tashjian AH, Jr, Armstrong EJ, Armstrong AW. Principles of pharmacology: the pathophysiologic basis of drug therapy. 2nd edn. Lippincott Williams and Wilkins; Philadelphia: 2008. [Google Scholar]
- Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. J Consult Clin Psychol. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:66–72. [PubMed] [Google Scholar]
- Hervieu G. Melanin-concentrating hormone functions in the nervous system: food intake and stress. Expert Opin Ther Targets. 2003;7:495–511. doi: 10.1517/14728222.7.4.495. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res. 1975;86:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Spar BD, Weig B, Farley C, Cook J, Ghibaudi L, Fried S, O'Neill K, Del Vecchio RA, McBriar M, Guzik H, Clader J, Hawes BE, Hwa J. Effects of a selective melanin-concentrating hormone 1 receptor antagonist on food intake and energy homeostasis in diet-induced obese mice. Eur J Pharmacol. 2006;535:182–191. doi: 10.1016/j.ejphar.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, St-Onge S, Pou C, Labrecque J, Groblewski T, O'Donnell D, Payza K, Ahmad S, Walker P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- Luthin DR. Anti-obesity effects of small molecule melanin-concentrating hormone receptor 1 (MCHR1) antagonists. Life Sci. 2007;81:423–440. doi: 10.1016/j.lfs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Marsteller DA, Gerald CP, Kong R, Cajina M, Craig DA, Swanson CJ. The MCH(1) receptor antagonist SNAP 94847 induces sensitivity to dopamine D(2)/D(3) receptor agonists in rats and mice. Eur J Pharmacol. 2009;602:66–72. doi: 10.1016/j.ejphar.2008.10.051. [DOI] [PubMed] [Google Scholar]
- Morens C, Norregaard P, Receveur JM, van Dijk G, Scheurink AJ. Effects of MCH and a MCH1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062:32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE. Role of food type in yohimbineand pellet-priming-induced reinstatement of food seeking. Physiol Behav. 2006;88:559–566. doi: 10.1016/j.physbeh.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DE, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009 doi: 10.1016/j.pneurobio.2009.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edn. Elsevier Academic; Dordrecht: 2005. [Google Scholar]
- Peterson CB, Mitchell JE. Psychosocial and pharmacological treatment of eating disorders: a review of research findings. J Clin Psychol. 1999;55:685–697. doi: 10.1002/(sici)1097-4679(199906)55:6<685::aid-jclp3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Redmond DEJ, Huang YH. Locus coeruleus and anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokosz LL, Hobbs DW. Biological examination of melanin concentrating hormone receptor 1: multi-tasking from the hypothalamus. Drug News Perspect. 2006;19:273–286. doi: 10.1358/dnp.2006.19.5.985938. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Sakai RR, Ma LY, He PF, Fluharty SJ. Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept. 1995;59:183–192. doi: 10.1016/0167-0115(95)00111-n. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatr. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Smith DG, Hegde LG, Wolinsky TD, Miller S, Papp M, Ping X, Edwards T, Gerald CP, Craig DA. The effects of stressful stimuli and hypothalamic-pituitary-adrenal axis activation are reversed by the melanin-concentrating hormone 1 receptor antagonist SNAP 94847 in rodents. Behav Brain Res. 2009;197:284–291. doi: 10.1016/j.bbr.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1458. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Jacobowitz DM. Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res. 1986;373(1–2):240–245. doi: 10.1016/0006-8993(86)90337-9. [DOI] [PubMed] [Google Scholar]