Abstract
Gene therapies for retinal degeneration have relied on subretinal delivery of viral vectors carrying therapeutic DNA. The subretinal injection is clearly not ideal as it limits the viral transduction profile to a focal region at the injection site and negatively affects the neural retina by detaching it from the supportive retinal pigment epithelium (RPE). We assessed changes in adeno-associated virus (AAV) dispersion and transduction in the degenerating rat retina after intravitreal delivery. We observed a significant increase in AAV-mediated gene transfer in the diseased compared with normal retina, the extent of which depends on the AAV serotype injected. We also identified key structural changes that correspond to increased viral infectivity. Particle diffusion and transgene accumulation in normal and diseased retina were monitored via fluorescent labeling of viral capsids and quantitative PCR. Viral particles were observed to accumulate at the vitreoretinal junction in normal retina, whereas particles spread into the outer retina and RPE in degenerated tissue. Immunohistochemistry illustrates remarkable changes in the architecture of the inner limiting membrane, which are likely to underlie the increased viral transduction in diseased retina. These data highlight the importance of characterizing gene delivery vectors in diseased tissue as structural and biochemical changes can alter viral vector transduction patterns. Furthermore, these results indicate that gene delivery to the outer nuclear layer may be achieved by noninvasive intravitreal AAV administration in the diseased state.
Kolstad et al. evaluate the distribution of vector particles and transduction of AAV administered intravitreally in diseased versus healthy retinas. Whereas healthy retinas are not very receptive to vector penetration and transduction following intravitreal injection, in retinal degenerations the authors show improved and more extensive gene transfer.
Introduction
Success in the clinical application of adeno-associated virus (AAV)-mediated gene therapy for Leber's congenital amaurosis, an inherited blinding disease, has established proof-of-concept for this mode of treatment (Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008). All three clinical trials used similar methodology, a single subretinal injection of a therapeutic AAV2 vector. Although the remarkable clinical success in correcting the disease phenotype demonstrates that this method is effective, the methodology for these trials may be too invasive to be applied in advanced retinal disease states. In many cases, the risk of tearing the retina is too high to attempt a subretinal injection. Moreover, retinal detachment causes dramatic alterations in retinal cell morphology and survival (Lewis et al., 2002, 2003; Fisher and Lewis, 2003; Arroyo et al., 2005; Fisher et al., 2005; Wickham et al., 2006). Subretinal injection also restricts viral transduction to the region of detachment. Therefore, intravitreal delivery of AAV would provide a clinically safer and more efficacious approach to ocular gene therapy.
AAV is currently the most successful vector for gene therapy, with its various serotypes capable of stably transducing multiple retinal cell types with minimal immunogenicity (Auricchio, 2003; Surace and Auricchio, 2003; Buning et al., 2008; Buch et al., 2008). At present, eight AAV serotypes distinguished by the amino acid sequence of the viral capsid have been evaluated for ocular use. The capsid determines initial receptor attachment, cellular entry, and trafficking (Choi et al., 2005). Expression profile studies in the wild-type, adult rodent retina have shown that AAV serotypes 1, 2, 5, 8, and 9 are extremely efficient at transducing the retinal pigment epithelium (RPE) and photoreceptors after subretinal delivery. Unfortunately, delivery of most AAV serotypes into the vitreous results in poor retinal transduction, with the exception of AAV2, which efficiently transduces retinal ganglion cells (RGCs) (Harvey et al., 2002; Martin et al., 2002; Auricchio, 2003; Buch et al., 2008; Lebherz et al., 2008; Rolling, 2004). The lack of infection from the vitreous is likely due to barriers to diffusion into the retina. Previous work from our laboratory has shown that AAV injected into the vitreous either binds and accumulates at the inner limiting membrane (ILM) (AAV2, 5, and 9) or diffuses away from the retina if binding sites are unavailable (AAV1 and AAV5) (Dalkara et al., 2009). In addition, mild digestion of the ILM allows for enhanced AAV transduction of outer retinal cells from the vitreous (Dalkara et al., 2009).
During degeneration, the retina undergoes dramatic physical changes (Marc and Jones, 2003; Marc et al., 2003, 2007). Photoreceptor outer segments shorten and Müller cells become hypertrophic (Marc et al., 2003). Remodeling events are consistent among various animal models of photoreceptor degeneration (Jones and Marc, 2005). We have also observed disorganization of the ILM, where AAV particles accumulate in the healthy retina (our unpublished data; and Dalkara et al., 2009). We hypothesized that changes in the structural and biochemical makeup of the diseased retina, particularly at the ILM and extracellular matrix, would alter AAV transduction patterns and efficiency.
The purpose of this study was to explore changes in vector diffusion and infectivity in the diseased retina when virus is delivered from the vitreous and thereby to identify key structural changes that correlate with variations in viral transduction. We characterized the onset of expression and transduction profile of AAV1, 2, 5, 8, and 9 after intravitreal injection at various stages of degeneration in the TgS334ter-3 rat model of autosomal dominant retinitis pigmentosa (Steinberg et al., 1996). Our results clearly demonstrate that, unlike what is observed in the healthy retina, several AAV serotypes are capable of efficiently transducing Müller cells, photoreceptors, and RPE from the vitreous in diseased tissue. The changes in viral transduction correspond with structural changes at the inner limiting membrane that occur during degeneration. Taken together, these results point to the importance of considering structural and biochemical changes in diseased tissue when developing gene-targeting and vector-engineering strategies.
Materials and Methods
Generation of rAAV vectors
Adeno-associated virus was produced by triple plasmid transfection of 293T cells (Grieger et al., 2006). After ultracentrifugation, the interphase between the 54 and 40% iodixanol fraction, and the lower three-quarters of the 40% iodixanol fraction, were extracted and diluted with an equal volume of phosphate-buffered saline (PBS) plus 0.001% Tween 20. Amicon Ultra-15 centrifugal filter units were (Millipore, Bedford, MA) preincubated with 5% Tween in PBS and washed with PBS plus 0.001% Tween. The diluted iodixanol fractions were buffer-exchanged and concentrated to 250 μl in these filter units. Virus was washed three times with 15 ml of sterile PBS plus 0.001% Tween. Vector was then titered for DNase-resistant vector genomes by real-time PCR relative to a standard. Finally, the purity of the vector was validated by silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
Cyanine 3 labeling of rAAV vectors
Purified and concentrated rAAV was labeled as described previously (Bartlett et al., 2000). Amine-reactive cyanine 3 (Cy3) dye (GE Healthcare Life Sciences, Piscataway, NJ) was resuspended in 0.2 M NaCO3/NaHCO3 buffer at pH 9.3. Viral stock was diluted 1:2 with the dye suspension in a total volume of 400 μl. The reaction proceeded for 2 hr at room temperature and was quenched with 4 μl of 1 M Tris-HCl at pH 8.0. Buffer exchange and concentration were done with Amicon Ultra-5 centrifugal filter units (Millipore).
Intraocular administration
Adult wild-type Sprague-Dawley rats or TgS334ter-3 rats were used. All animal procedures were conducted according to the Association for Research in Vision and Ophthalmology (ARVO, Rockville, MD) Statement for the Use of Animals in Ophthalmic and Visual Research and the guidelines of the Office of Laboratory Animal Care at the University of California at Berkeley (Berkeley, CA). Rats were anesthetized with ketamine (72 mg/kg) and xylazine (64 mg/kg) by intraperitoneal injection before ocular injection. Pupils were dilated with tropicamide (1%). An ultrafine 30.5-gauge disposable needle was passed through the sclera into the vitreous cavity to create a hole. A 10-μl Hamilton syringe with a blunt-ended needle was passed through this opening to inject 5 μl, at 5 × 1012 vector genomes (VG)/ml, of Cy3-labeled AAV (n = 3 per serotype) or unlabeled AAV (n = 6 per serotype per time point). The AAV transgene encodes enhanced green fluorescent protein (eGFP) driven by the ubiquitous chicken β-actin (CBA) promoter.
Fundus imaging
In vivo retinal imaging of rats was performed 5 days to 4 weeks after injections with a fundus camera (Retcam II; Clarity Medical Systems, Pleasanton, CA) equipped with a wide-angle 130° retinopathy of prematurity (ROP) lens to monitor eGFP expression in live, anesthetized rats.
Cryosections
One day (for studies with labeled virus) to 4 weeks (for expression analysis) after vector injection, rats were humanely killed by CO2 overdose and cervical dislocation. Eyes were enucleated, a hole was made in the cornea, and eyes were fixed with 10% neutral buffered formalin overnight. The eyecups were washed in PBS and the cornea and lens were removed. The cups were then placed in 30% sucrose in PBS overnight. Eyes were then embedded in Tissue-Tek optimal cutting temperature (O.C.T.) embedding compound (Sakura Finetek USA, Torrance, CA) and oriented for 5- to 10-μm-thick transverse retinal sections.
Immunolabeling and histological analysis
Tissue sections were rehydrated in PBS for 5 min and then incubated in blocking solution (1% bovine serum albumin [BSA], 0.5% Triton X-100, and 2% normal donkey serum in PBS) for 2–3 hr. Slides were then incubated with commercial rabbit monoclonal antibody raised against intact capsids of AAV2 (ARP American Research Products, Belmont, MA), green fluorescent protein (Molecular Probes/Invitrogen, Carlsbad, CA), or laminin (L9393; Sigma-Aldrich, St. Louis, MO) diluted 1:100 in blocking solution. Mouse monoclonal antibody raised against vimentin (Dako, Cambridgeshire, UK) was used at a 1:1000 dilution in blocking buffer for 24 hr at 4°C. Tissue was then washed in two changes of PBS for 3 hr and secondary antibody (Alexa Fluor 488-conjugated anti-mouse diluted 1:100 in blocking buffer; Molecular Probes/Invitrogen) was applied overnight at 4°C. The tissue was washed with PBS before mounting in VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA) with 4′,6-diamidino-2-phenylindole (DAPI). The results were examined by fluorescence microscopy, using an Axiophot microscope (Carl Zeiss, Thornwood, NY) equipped with an X-cite PC200 light source (EXFO Life Sciences, Ontario, Canada) and QCapturePro camera (QImaging, Surrey, BC, Canada) or by confocal microscopy (LSM5; Carl Zeiss Microimaging, Jena, Germany). Images were prepared with Bitplane Imaris image-processing and manipulation software (Bitplane, St. Paul, MN).
Quantitative PCR
Five microliters of AAV5 (5 × 1012 VG/ml) was injected into the vitreous of anesthetized wild-type (n = 10) or TgS334ter-3 animals (n = 10). Five days postinjection, eyes were enucleated and fixed overnight. The cornea and lens were removed and the retina was dissected away from the RPE. The RPE was then scraped away from the choroid. Separate, autoclaved tools were used for each retina and RPE dissection to avoid contamination. Total DNA was extracted from tissue (DNeasy kit; Qiagen, Valencia, CA). A 5-μl sample of the extracted DNA was used for quantitative PCR (qPCR). Briefly, a master mix was prepared containing 1 × Platinum Taq buffer (Invitrogen, Carlsbad, CA), 5 mM MgCl2, 0.2 mM dNTPs, 0.32 mM forward hGFP primer (AAGATGACGGGAACTACAA), 0.32 mM reverse hGFP primer (TCCATCCTCCTTAAAGTCA), 0.32 mM qPCR hGFP probe (ACCCGCGCTGAAGTCAAGTTCG) (FAM-BHQ), and 1 × Platinum Taq (Invitrogen) and mixed at 20 μl for 5 μl of each DNA sample or 5 μl of qPCR standard (a dilution series from 1010 to 103 hGFP plasmid copies was prepared to be used as DNA standards). Reactions were set up on a real-time PCR system (Stratagene/Agilent Technologies, La Jolla, CA). The PCR cycles were set as follows: 10 min at 95°C, 30 sec at 95°C, followed by 2 min at 56°C for 40 cycles. The slope of the standard curve was −3.363 and the correlation coefficient R2 was 0.997. The standard curve was used to quantify total transgene number in the RPE and retina of wild-type and TgS334ter-3 animals compared with uninjected controls.
Results
Changes in viral transduction profile in the degenerating rodent retina
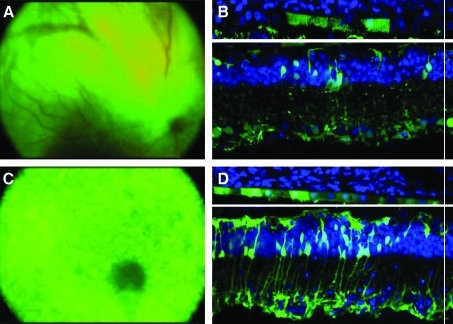
Wild-type Sprague-Dawley rats and TgS334ter-3 transgenic rats overexpressing a rhodopsin mutant causing autosomal dominant retinitis pigmentosa and subsequently photoreceptor degeneration (http://www.ucsfeye.net/mlavailRDratmodels.shtml) were injected via the vitreous with AAV1, 2, 5, 8, and 9 vectors carrying eGFP cDNA under the control of the ubiquitous CBA promoter. Intraocular injections were performed at three ages corresponding to three different stages of degeneration: P20 (early), P30 (intermediate), and P60 (advanced). As previously described, intravitreal injection of AAV1, 5, 8, and 9, at all ages tested, resulted in no measurable transgene expression in healthy rat retina, whereas AAV2 led to robust gene expression in the inner retina 3 weeks postinjection (Ali et al., 1998; Harvey et al., 2002; Auricchio, 2003; Buch et al., 2008; Lebherz et al., 2008). GFP expression begins rapidly in the TgS334ter-3 retina (5 days postinjection) for all serotypes except AAV2, which gave visible expression after 3 weeks. AAV2, 5, 8, and 9 led to GFP expression after injection at P20 and all later time points, whereas AAV1 expression was not seen until later stages of degeneration (P60). Strong transgene expression was evident in RPE, Müller glia, and remaining photoreceptors for all serotypes (Table 1). AAV5 and AAV1 exhibited the greatest level of expression (Fig. 1A–D), whereas AAV8- and AAV9-mediated transgene expression was limited to sporadic RPE, photoreceptors, and retinal Müller cells around the optic nerve head (data not shown). AAV2 expression was evident in inner retinal neurons, similar to the expression exhibited in the wild-type retina; however, Müller cell transduction was greatly increased in diseased tissue.
Table 1.
Transduction Patterns of Adeno-Associated Virus (Serotypes 1, 2, 5, 8, and 9) When Injected at Early, Intermediate, and Advanced Stages of Retinal Degenerationa
| AAV serotype | P20 | P30 | P60 |
|---|---|---|---|
| AAV1 | — | — | MC, INL, RPE |
| AAV2 | RGC, MC | RGC, MC, some INL, some RPE | RGC, MC, some INL, some RPE |
| AAV5 | MC, PR, RPE | MC, PR, RPE | MC, PR, RPE |
| AAV8 | MC, PR | MC, PR | MC, PR |
| AAV9 | MC, PR | MC, PR | MC, PR, some RPE |
Abbreviations: INL, inner nuclear layer; MC, Müller cells; P20, P30, and P60: early, intermediate, and advanced stages of retinal degeneration, respectively; PR, photoreceptors; RGC, retinal ganglion cells; RPE, retinal pigment epithelium.
Expression was seen primarily in Müller cells, photoreceptors, and retinal pigment epithelium, with some inner nuclear layer and retinal ganglion cell transduction.
FIG. 1.
(A–D) AAV-mediated GFP expression in the inner retina after intravitreal administration into TgS334ter-3 rat eyes. Fundus images and histological cross-sections (original magnification, ×45) of (A and B) AAV1 and (C and D) AAV5. AAV1 and AAV5 exhibited the most robust expression profile of all serotypes in the degenerated retina (n = 10 per serotype per time point). Both vectors transduced RPE, photoreceptors, and retinal Müller glia. In addition, GFP was seen in some retinal ganglion cells as well as microglial cells.
Viral particle migration through the retina is altered during degeneration
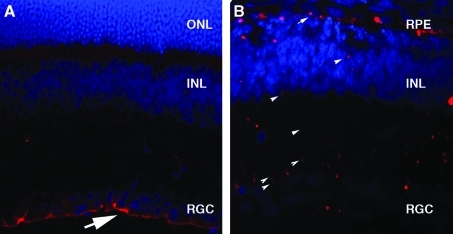
AAV viral capsids were labeled with Cy3 to allow visualization of their movement into and throughout the retina 24 hr after intravitreal injection. In the wild-type rat retina, AAV2, 8, and 9 accumulate at the vitreoretinal junction whereas labeled AAV1 and 5 particles are not visible, which is likely due to the absence of primary receptors at the vitreoretinal junction (Fig. 2A and B) (Dalkara et al., 2009). However, in TgS334ter-3 animals, viral particles from all serotypes tested (AAV1, 2, 5, 8, and 9) were visible throughout all layers of the retina, reaching the outer nuclear layer and RPE (Fig. 2B). The retina is a heterogeneous mixture of neurons, glia, and blood vessels arranged in a laminar structure. We observed the Cy3 AAV distribution pattern to be primarily radial, suggesting that the capsids traverse the retina along Müller cell processes.
FIG. 2.
Cy3-labeled virus dispersion in the P30 wild-type and degenerated retina. (A) AAV9–Cy3 accumulation at the vitreoretinal junction (arrow) in the wild-type retina after intravitreal injection. (B) AAV9–Cy3 dispersion (arrowheads) throughout the retina and into the inner retina and RPE when injected into the vitreous of P30 TgS334ter-3 rats. Eyes were taken 24 hr postinjection. INL, inner nuclear layer; ONL, outer nuclear layer; RGC, retinal ganglion cells; RPE, retinal pigment epithelium.
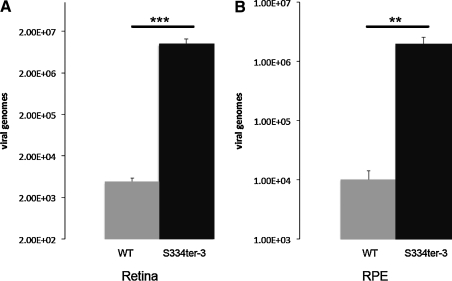
We employed qPCR to quantify viral movement and infectivity in the retina and RPE. We showed that vector genome levels in degenerated retinal tissue and the RPE is significantly enhanced compared with the wild-type retina (Fig. 3). In fact, transgene number is approximately 3 log units greater in the retina and 2.5 log units greater in the RPE.
FIG. 3.
Quantitative PCR demonstrates an increase in AAV transduction of the RPE and retina in degenerating tissue. AAV transgene copy number is significantly greater in (A) the retina and (B) RPE of TgS334ter-3 animals 5 days after intravitreal AAV5 injection (n = 10). Values represent average copy number normalized to uninjected control retina. WT, wild type.
Structural changes at the inner limiting membrane in transgenic rat retina
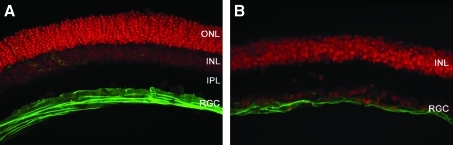
ILM disorganization during degeneration was visualized by immunostaining for laminin and vimentin in P30 TgS334ter-3 animals (Halfter et al., 2008). To account for changes in ILM structure within the retina, the regularity and thickness of the ILM matrix was examined by anti-laminin immunohistochemistry at comparable retinal eccentricities. The staining patterns observed were visibly different when contrasting healthy and degenerated retinas (Figs. 4 and 5), and such changes were similar at later stages of degeneration (P60) (data not shown). In addition to laminin, costaining for vimentin, a marker that exclusively labels intermediate filaments in Müller cells, showed substantial gliosis that results in the migration of Müller endfeet into and throughout the plane of the glycoconjugate layer of the ILM in TgS334ter-3 retinas (Fig. 5).
FIG. 4.
Agarose sections of (A) wild-type retina and (B) TgS334ter-3 retina stained for laminin (green) show disorganization of the inner limiting membrane in degenerated tissue. Animals were at degeneration stage P30 at the time of sacrifice, although disorganization was visible at all stages of degeneration (data not shown) (original magnification, × 25).
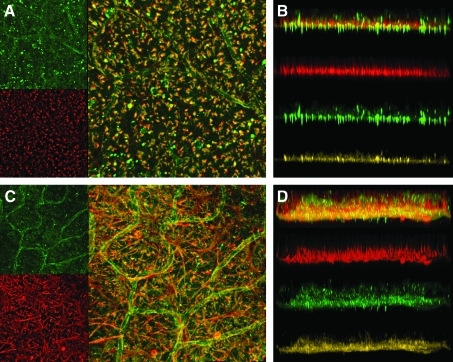
FIG. 5.
(A) Wild-type and (C) TgS334ter-3 flat-mount retinas stained for laminin (green) and vimentin (red). Z-stack projections of (B) wild-type retina and (D) TgS334ter-3 retina show the depth of Müller cell hypertrophy/ILM disorganization.
Discussion
Because subretinal injection poses a significant risk of retinal damage and limits the efficacy and extent of ocular gene therapy, we explored enhancement of AAV retinal transduction from the vitreous in rodents with disease-associated retinal degeneration. We hypothesized that changes in the structural and biochemical composition of the retina that occur during degeneration may allow for alterations in vector penetration and infection (Marc and Jones, 2003).
Previous work has shown that the enzymatic disruption of the interphotoreceptor matrix (Grüter et al., 2005) enhances viral infectivity. In another study, the viral transduction profile of AAV8 was shown to dramatically improve in a mouse model of retinoschisis (Park et al., 2009), a disease characterized by retinal tears arising because of the lack of schisin protein in the extracellular matrix. Similar changes take place during retinal degeneration. More specifically, extracellular matrix proteolysis occurs during neurite extension and Müller glial hypertrophy (Marc et al., 2003). As in the aforementioned studies, these changes in the ECM may contribute to the observed increase in AAV transduction in the degenerating retina.
We also reasoned that, like in the developing retina (Harvey et al., 2002), changes in the structural integrity and make-up of the inner limiting membrane could open access to previously masked AAV receptors. The ILM is a basement membrane that histologically defines the border between the retina and the vitreous humor and consists of 10 distinct extracellular matrix proteins including laminin, as well as Müller cell endfeet (Candiello et al., 2007). Data from our laboratory show that specific enzymatic digestion of the inner limiting membrane allows for AAV-mediated expression in the outer retina and RPE (Dalkara et al., 2009). Studies have shown that active matrix metalloproteinase-9 (MMP-9) levels are elevated in the retina during degeneration (Ahuja et al., 2006), causing laminin degradation at the ganglion cell layer/ILM (Zhang et al., 2004). These studies suggest that the ILM is a barrier to AAV infection and a region of the retina that could undergo great changes during retinal degeneration, perhaps opening access to deeper AAV penetration and transduction.
Laminin receptors found at the vitreoretinal junction, Müller cell endfeet, and RGCs are known to mediate viral transduction of AAV2, 8, and 9 (Akache et al., 2006). Also, AAV2 binds heparan sulfate proteoglycan as a primary receptor, which is found at the ILM (Chai and Morris, 1994, 1999; Summerford and Samulski, 1998), as well as fibroblast growth factor receptor-1 (FGFR1) as a secondary receptor (Qing et al., 1999). AAV1 and AAV5 bind sialic acid, which is not present at the wild-type ILM (Kaludov et al., 2001; Walters et al., 2001; Cho et al., 2002). In the degenerating retina, viral particles that would otherwise accumulate in receptor reservoirs at the ILM (AAV2, 8, and 9) or diffuse away into the vitreous when no receptors are present (AAV1 and AAV5) may now be recruited deeper into the retinal tissue by interacting with previously masked binding sites (Fig. 2) (Dalkara et al., 2009). For example, the increase in surface area and movement of Müller cell endfeet into and throughout the glycosaminoglycan layer of the ILM during gliosis is likely to expose receptors, such as the 32-kDa laminin receptor (a receptor for AAV2, 8, and 9), at the site of vector administration (Akache et al., 2006; Halfter et al., 2008) (Fig. 5).
Upregulation of AAV receptors throughout the retina could also assist in recruitment of viral particles. Fibroblast growth factor receptor-1 (FGFR1) has been shown to be upregulated during degeneration or after retinal stress due to injury or detachment, particularly in Müller glia (Wen et al., 1995; Guillonneau et al., 1998; Ozaki et al., 2000). Heparan sulfate proteoglycan expression also increases in the retina during degeneration (Landers et al., 1994). However, as the localization and identity of many receptors and coreceptors for specific AAV serotypes remain unknown, it is difficult to discern exactly how receptor recruitment of AAV changes in the degenerating retina and whether these changes are due to macroscopic structural changes and/or alterations in receptor expression patterns.
To apply our current data to improve ocular gene therapy in the clinic, it will be necessary to identify consistent patterns of vector transduction and associated retinal changes during disease across species and pathologies. It should be noted that the rate of retinal degeneration in the TgS334ter-3 rat is rapid, with rod degeneration beginning at postnatal day 8 and a single row of photoreceptor nuclei remaining at weaning age. Such rapid degeneration is likely to induce the described structural changes in the ILM and ECM at a faster pace with more pronounced effects than in many retinal diseases in patients, such as retinitis pigmentosa, which progress over decades. Furthermore, such rapid degeneration could amplify Müller cell reactivity or other retinal cell stress responses, enhancing the observed changes in infectivity and retinal structure. We may expect less dramatic structural changes and infectivity patterns at similar time points in other retinal disease models with slower photoreceptor degeneration rates. These will be assessed in future studies.
One common denominator among all retinal degenerations is glial reactivity. Research on glia throughout the central nervous system, including within the retina, has provided a more in-depth view of glial functions in health and disease. Glial cells have been recognized as being highly involved in disease states including neurodegenerative diseases. Müller cell endfeet are considered a part of the inner limiting membrane and therefore it is likely that some of the structural changes described here will apply across disease states and species. Furthermore, one natural function of Müller cells is to protect photoreceptors during degeneration or retinal stress by modulating neurotrophin expression (Harada et al., 2000; Zack, 2000). Therefore, targeting Müller glia from the vitreous with AAV carrying trophic factor cDNA is a promising method to improve ocular gene therapy for retinal diseases (Dorrell et al., 2009).
These data highlight the need to consider changes in the degenerating retina when developing therapeutic interventions for retinal diseases. This is particularly important, as patients who may benefit from gene therapy are likely to have undergone a degree of retinal degeneration before treatment is sought. Future experiments will be necessary to determine whether such dramatic changes in AAV transduction profiles are consistent across multiple retinal disease models. Identifying putative barriers to intravitreal introduction of AAV to the outer retina is the first step toward developing a safer and more efficacious approach to gene delivery.
Author Disclosure Statement
Kathleen D. Kolstad, none; Deniz Dalkara, none; Karen Guerin, none; Meike Visel, none; Natalie Hoffman, none; David V. Schaffer, none; John G. Flannery, none.
References
- Ahuja S. Ahuja P. Caffe A.R. Ekstrom P. Abrahamson M. Van Veen T. rd1 mouse retina shows imbalance in cellular distribution and levels of TIMP-1/MMP-9, TIMP-2/MMP-2 and sulfated glycosaminoglycans. Ophthalmic Res. 2006;38:125–136. doi: 10.1159/000090533. [DOI] [PubMed] [Google Scholar]
- Akache B. Grimm D. Pandey K. Yant S.R. Xu H. Kay M.A. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R.R. Reichel M.B. Byrnes A.P. Stephens C.J. Thrasher A.J. Baker D. Hunt D.M. Bhattacharya S.S. Co-injection of adenovirus expressing CTLA4-Ig prolongs adenovirally mediated lacZ reporter gene expression in the mouse retina. Gene Ther. 1998;5:1561–1565. doi: 10.1038/sj.gt.3300761. [DOI] [PubMed] [Google Scholar]
- Arroyo J.G. Yang L. Bula D. Chen D.F. Photoreceptor apoptosis in human retinal detachment. Am. J. Ophthalmol. 2005;139:605–610. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Auricchio A. Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res. 2003;43:913–918. doi: 10.1016/s0042-6989(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Bainbridge J.W. Smith A.J. Barker S.S. Robbie S. Henderson R. Balaggan K. Viswanathan A. Holder G.E. Stockman A. Tyler N. Petersen-Jones S. Bhattacharya S.S. Thrasher A.J. Fitzke F.W. Carter B.J. Rubin G.S. Moore A.T. Ali R.R. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bartlett J.S. Wilcher R. Samulski R.J. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch P.K. Bainbridge J.W. Ali R.R. AAV-mediated gene therapy for retinal disorders: From mouse to man. Gene Ther. 2008;15:849–857. doi: 10.1038/gt.2008.66. [DOI] [PubMed] [Google Scholar]
- Buning H. Perabo L. Coutelle O. Quadt-Humme S. Hallek M. Recent developments in adeno-associated virus vector technology. J. Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- Candiello J. Balasubramani M. Schreiber E.M. Cole G.J. Mayer U. Halfter W. Lin H. Biomechanical properties of native basement membranes. FEBS J. 2007;274:2897–2908. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- Chai L. Morris J.E. Distribution of heparan sulfate proteoglycans in embryonic chicken neural retina and isolated inner limiting membrane. Curr. Eye Res. 1994;13:669–677. doi: 10.3109/02713689408999903. [DOI] [PubMed] [Google Scholar]
- Chai L. Morris J.E. Heparan sulfate in the inner limiting membrane of embryonic chicken retina binds basic fibroblast growth factor to promote axonal outgrowth. Exp. Neurol. 1999;160:175–185. doi: 10.1006/exnr.1999.7195. [DOI] [PubMed] [Google Scholar]
- Cho E.Y. Choi H.L. Chan F.L. Expression pattern of glycoconjugates in rat retina as analysed by lectin histochemistry. Histochem. J. 2002;34:589–600. doi: 10.1023/a:1026032005521. [DOI] [PubMed] [Google Scholar]
- Choi V.W. McCarty D.M. Samulski R.J. AAV hybrid serotypes: Improved vectors for gene delivery. Curr. Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V. Aleman T.S. Boye S.L. Schwartz S.B. Kaushal S. Roman A.J. Pang J.J. Sumaroka A. Windsor E.A. Wilson J.M. Flotte T.R. Fishman G.A. Heon E. Stone E.M. Byrne B.J. Jacobson S.G. Hauswirth W.W. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D. Kolstad K.D. Caporale N. Visel M. Klimczak R.R. Schaffer D.V. Flannery J. Inner limiting membrane barriers to AAV mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell M.I. Aguilar E. Jacobson R. Yanes O. Gariano R. Heckenlively J. Banin E. Ramirez G.A. Gasmi M. Bird A. Siuzdak G. Friedlander M. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J. Clin. Invest. 2009. (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Fisher S.K. Lewis G.P. Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: A review and reconsideration of recent data. Vision Res. 2003;43:887–897. doi: 10.1016/s0042-6989(02)00680-6. [DOI] [PubMed] [Google Scholar]
- Fisher S.K. Lewis G.P. Linberg K.A. Verardo M.R. Cellular remodeling in mammalian retina: Results from studies of experimental retinal detachment. Prog. Retin. Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Grieger J.C. Choi V.W. Samulski R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Grüter O. Kostic C. Crippa S.V. Perez M.T. Zografos L. Schorderet D.F. Munier F.L. Arsenijevic Y. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- Guillonneau X. Regnier-Ricard F. Laplace O. Jonet L. Bryckaert M. Courtois Y. Mascarelli F. Fibroblast growth factor (FGF) soluble receptor 1 acts as a natural inhibitor of FGF2 neurotrophic activity during retinal degeneration. Mol. Biol. Cell. 1998;9:2785–2802. doi: 10.1091/mbc.9.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W. Dong S. Dong A. Eller A.W. Nischt R. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye. 2008;22:1207–1213. doi: 10.1038/eye.2008.19. [DOI] [PubMed] [Google Scholar]
- Harada T. Harada C. Nakayama N. Okuyama S. Yoshida K. Kohsaka S. Matsuda H. Wada K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Harvey A.R. Kamphuis W. Eggers R. Symons N.A. Blits B. Niclou S. Boer G.J. Verhaagen J. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: A comparison with lentiviral vectors. Mol. Cell. Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. Aleman T.S. Kaushal S. Cideciyan A.V. Schwartz S.B. Wang L. Conlon T. Boye S.L. Flotte T.R. Byrne B. Jacobson S.G. Phase I trial of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.W. Marc R.E. Retinal remodeling during retinal degeneration. Exp. Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kaludov N. Brown K.E. Walters R.W. Zabner J. Chiorini J.A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers R.A. Rayborn M.E. Myers K.M. Hollyfield J.G. Increased retinal synthesis of heparan sulfate proteoglycan and HNK-1 glycoproteins following photoreceptor degeneration. J. Neurochem. 1994;63:737–750. doi: 10.1046/j.1471-4159.1994.63020737.x. [DOI] [PubMed] [Google Scholar]
- Lebherz C. Maguire A. Tang W. Bennett J. Wilson J.M. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.P. Charteris D.G. Sethi C.S. Fisher S.K. Animal models of retinal detachment and reattachment: Identifying cellular events that may affect visual recovery. Eye. 2002;16:375–387. doi: 10.1038/sj.eye.6700202. [DOI] [PubMed] [Google Scholar]
- Lewis G.P. Sethi C.S. Linberg K.A. Charteris D.G. Fisher S.K. Experimental retinal reattachment: A new perspective. Mol. Neurobiol. 2003;28:159–175. doi: 10.1385/MN:28:2:159. [DOI] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A. Pugh E.N., Jr. Mingozzi F. Bennicelli J. Banfi S. Marshall K.A. Testa F. Surace E.M. Rossi S. Lyubarsky A. Arruda V.R. Konkle B. Stone E. Sun J. Jacobs J. Dell'Osso L. Hertle R. Ma J.X. Redmond T.M. Zhu X. Hauck B. Zelenaia O. Shindler K.S. Maguire M.G. Wright J.F. Volpe N.J. McDonnell J.W. Auricchio A. High K.A. Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R.E. Jones B.W. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol. 2003;28:139–147. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- Marc R.E. Jones B.W. Watt C.B. Strettoi E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Marc R.E. Jones B.W. Anderson J.R. Kinard K. Marshak D.W. Wilson J.H. Wensel T. Lucas R.J. Neural reprogramming in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.R. Klein R.L. Quigley H.A. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- Ozaki S. Radeke M.J. Anderson D.H. Rapid upregulation of fibroblast growth factor receptor 1 (flg) by rat photoreceptor cells after injury. Invest. Ophthalmol. Vis. Sci. 2000;41:568–579. [PubMed] [Google Scholar]
- Park T.K. Wu Z. Kjellstrom S. Zeng Y. Bush R.A. Sieving P.A. Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. Mah C. Hansen J. Zhou S. Dwarki V. Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Rolling F. Recombinant AAV-mediated gene transfer to the retina: Gene therapy perspectives. Gene Ther. 2004;11(Suppl. 1):S26–S32. doi: 10.1038/sj.gt.3302366. [DOI] [PubMed] [Google Scholar]
- Steinberg R.H. Flannery J.G. Naash M. Oh P. Matthes M.T. Yasumura D. Lau-Villacorta C. Chen J. LaVail M.M. Transgenic rat models of inherited retinal degeneration caused by mutant opsin genes. Invest. Ophthalmol. Vis. Sci. 1996;37:S698. [Google Scholar]
- Summerford C. Samulski R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace E.M. Auricchio A. Adeno-associated viral vectors for retinal gene transfer. Prog. Retin. Eye Res. 2003;22:705–719. doi: 10.1016/s1350-9462(03)00052-1. [DOI] [PubMed] [Google Scholar]
- Walters R.W. Yi S.M. Keshavjee S. Brown K.E. Welsh M.J. Chiorini J.A. Zabner J. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Wen R. Song Y. Cheng T. Matthes M.T. Yasumura D. LaVail M.M. Steinberg R.H. Injury-induced upregulation of bFGF and CNTF mRNAs in the rat retina. J. Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham L. Sethi C.S. Lewis G.P. Fisher S.K. McLeod D.C. Charteris D.G. Glial and neural response in short-term human retinal detachment. Arch. Ophthalmol. 2006;124:1779–1782. doi: 10.1001/archopht.124.12.1779. [DOI] [PubMed] [Google Scholar]
- Zack D.J. Neurotrophic rescue of photoreceptors: Are Muller cells the mediators of survival? Neuron. 2000;26:285–286. doi: 10.1016/s0896-6273(00)81160-5. [DOI] [PubMed] [Google Scholar]
- Zhang X. Cheng M. Chintala S.K. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2004;45:2374–2383. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]