Abstract
Community-acquired pneumonia (CAP) is a serious condition associated with significant morbidity and potential long-term mortality. Although the majority of patients with CAP are treated as outpatients, the greatest proportion of pneumonia-related mortality and healthcare expenditure occurs among the patients who are hospitalized. There has been considerable interest in determining risk factors and severity criteria assessments to assist with site-of-care decisions. For both inpatients and outpatients, the most common pathogens associated with CAP include Streptococcus pneumoniae, Haemophilus influenzae, group A streptococci and Moraxella catarrhalis. Atypical pathogens, Gram-negative bacilli, methicillin-resistant Staphylococcus aureus (MRSA) and viruses are also recognized aetiological agents of CAP. Despite the availability of antimicrobial therapies, the recent emergence of drug-resistant pneumococcal and staphylococcal isolates has limited the effectiveness of currently available agents. Because early and rapid initiation of empirical antimicrobial treatment is critical for achieving a favourable outcome in CAP, newer agents with activity against drug-resistant strains of S. pneumoniae and MRSA are needed for the management of patients with CAP.
Keywords: antimicrobial therapy, PSI/CURB-65 score, clinical outcomes
Epidemiology of community-acquired pneumonia (CAP)
Pneumonia and influenza together are the eighth leading cause of death and the leading causes of infectious disease mortality in the USA.1 Although the majority of patients with CAP are treated as outpatients, the greatest proportion of pneumonia-related mortality and healthcare expenditure occurs among persons who are hospitalized.2,3 Furthermore, the risk of death during a pneumonia-related hospitalization is significantly higher than for many other major causes of hospitalization; in 2005, pneumonia was associated with 4.6 deaths per 100 discharges versus heart disease (3.7) or all-cause mortality (4.2).4 In addition, pneumonia is the second leading cause of hospitalization (after childbirth) in the USA, with >1.2 million hospitalizations in 2006.5 In the past two decades, patients aged 65–84 years have experienced a 20% increase in pneumonia-related hospitalizations. For individuals >65 years of age, the rates of hospitalization and death caused by pneumonia continue to increase even though rates are decreasing for all other age groups.6,7 Although mortality due to CAP decreased significantly with the introduction of antibiotics in the 1950s, it has remained stable since that time, whereas hospitalizations are increasing.8 In recent years there has been considerable interest in individual risk factors and risk-scoring systems associated with mortality for patients with pneumonia. Pneumonia is associated with significant morbidity and mortality; at the same time the host and pathogens are changing, thus there is a need to review this condition.
Risk score and severity criteria assessment
Determination of the site of care [i.e. outpatient, hospitalization or admission to an intensive care unit (ICU)] is typically the initial management decision following diagnosis of CAP. The decision to hospitalize a patient is one of the most costly assessments in the management of CAP because the cost of inpatient care for pneumonia is up to 25 times greater than for outpatient care.2,9 Thus, the 600 000 to 1.1 million annual admissions consume the vast majority of the estimated $8.4–10 billion spent on the treatment of CAP.2,5 Use of risk scores and severity criteria may help guide clinicians in the site-of-care decision.
The number of patients hospitalized with CAP can clearly be decreased with use of objective admission criteria.10 Fine et al.11 developed a pneumonia-specific severity of illness (PSI) score as part of the Pneumonia Patient Outcome Research Team (PORT) study. The 20 items in the PSI included 3 demographic variables, 5 co-morbid conditions, 5 physical examination findings and 7 laboratory/imaging results. Points were assigned and tallied for each variable present, and the final score was then broken into five risk classes. Patients in risk classes I–III were considered low risk and manageable as outpatients, whereas classes IV and V might require hospitalization. Marrie et al.12 conducted a randomized evaluation of a critical pathway for management of pneumonia using PSI to assist with site-of-care decisions. The critical pathway group demonstrated reduced utilization of institutional resources (e.g. fewer bed-days per patient managed and decreased admission of low-risk patients) with no differences in rates of complications, readmissions or mortality.
The Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines on the management of CAP in adults proposed criteria to identify patients with severe CAP.3,13 These criteria have been validated by several investigators.13–16 The most recent modification of the British Thoracic Society criteria relies on five easily measurable factors.17 Multivariate analysis identified Confusion (based upon a specific mental test or disorientation in person, place or time), Urea >7 mmol/L (20 mg/dL), Respiratory rate >30 breaths/min, Blood pressure (systolic <90 mm Hg or diastolic <60 mm Hg) and age >65 years. This scoring system was named CURB-65 based on the measurable factors included for analysis. In the derivation and validation cohorts, the risk of 30 day mortality among patients with zero to two factors was 0.7% for no risk factors, 2.1% for one risk factor and 9.2% for two risk factors. Mortality was higher when three to five factors were present and was reported as 14.5%, 40% and 14%, respectively. The authors suggested that patients with a CURB-65 score of 0 or 1 could be treated as outpatients, whereas those with a score of 2 should probably be admitted and treated on the wards. Patients with a CURB-65 score of ≥3 often required ICU care.
Because there are no other randomized comparative trials that evaluate alternative admission criteria for CAP, it is currently unclear whether the PSI or CURB-65 score would be preferred in clinical settings. Although PSI classifies a slightly larger percentage of CAP patients in the low-risk categories while maintaining a lower mortality in low-risk patients when compared with the CURB or CURB-65 score in the same population,18 several factors should be considered in this comparison. Severity assessment criteria are important in helping physicians to identify patients who need hospitalization or ICU admission, but they are not meant to remove physicians’ clinical judgment in the decision-making process.
Aetiology of CAP
Bacteria are the most common aetiological pathogens of CAP and have traditionally been divided into two groups designated ‘typical’ and ‘atypical’.3,19 The most common typical pathogens include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, group A streptococci and Moraxella catarrhalis. Atypical pathogens include bacteria that cannot be seen with a Gram's stain and cannot be cultured in regular sputum or blood culture medium. The most common atypical pathogens include Legionella spp., Mycoplasma pneumoniae and Chlamydophila pneumoniae. Atypical pathogens are rarely identified in clinical practice because special laboratory tests are necessary for their identification. PCR-based assays for the pathogens associated with atypical pneumonia are now considered the best approach for rapid diagnosis,19 although they are not yet widely available. Community-associated methicillin-resistant S. aureus (CA-MRSA) is increasingly emerging as another bacterial pathogen associated with CAP. Characteristically, these organisms belong to the USA-300 pulse-field electrophoresis type, contain the Panton–Valentine leucocidin (PVL) gene and tend to produce severe, necrotizing CAP.20–22 Other pathogens identified in severely ill patients include Gram-negative bacilli, especially Klebsiella pneumoniae and Pseudomonas aeruginosa.
Viruses are another well-recognized aetiology of CAP,3 but because of the lack of rapid standardized tests for diagnosis, their real impact as aetiological agents of CAP has not been well defined. Most recently, the availability of multiplex-PCR assays that have been approved by the US FDA, such as the Luminex Respiratory Viral Panel,23 have advanced the ability to more rapidly detect up to 12 viral targets in a respiratory specimen in a single reaction well. The role of influenza in the aetiology of CAP may also be increasing as a result of the emergence of the 2009 H1N1 influenza A virus pandemic. Other recognized viral aetiologies of CAP include respiratory syncytial virus, parainfluenza virus, adenovirus and metapneumovirus. The aetiology of CAP may be the result of a combination of typical pathogens, atypical pathogens or viral pathogens. During the 2009 H1N1 influenza A virus pandemic, a combined viral and bacterial aetiology of CAP was detected in ∼30% of hospitalized patients with influenza CAP.24 A particularly fulminant course of CAP is produced by the combination of influenza and CA-MRSA.
Clinical outcomes in patients with CAP
Clinical outcomes days after hospitalization
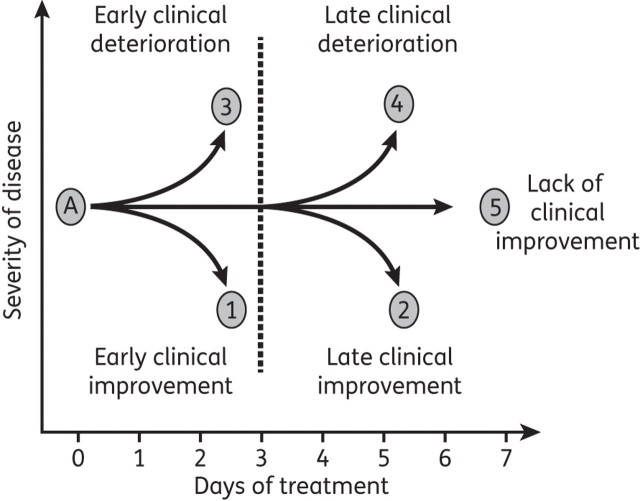
The clinical response of hospitalized patients with CAP during the first 7 days of therapy can be categorized into five possible outcomes.3 As shown in Figure 1, individuals with CAP may have early clinical improvement, defined as clinical improvement within the first 3 days of hospitalization (Figure 1, point 1), or late clinical improvement (Figure 1, point 2). Patients may develop early clinical deterioration, defined as clinical deterioration within the first 3 days of hospitalization (Figure 1, point 3), or late clinical deterioration (Figure 1, point 4). If after 7 days of therapy there is no evidence of clinical improvement or deterioration, the patient is categorized as having lack of clinical improvement (Figure 1, point 5). Several studies have evaluated clinical response in patients hospitalized with CAP to define time to clinical improvement and time to switch from intravenous to oral therapy.25–27 Patients were considered to have clinical improvement on the day they met certain criteria (Table 1). Patients who meet these criteria during the first 3 days of hospital treatment are considered to have early clinical improvement; those who meet the criteria during days 4–7 of hospital treatment are considered to have late clinical improvement. In one study,28 from a total population of 200 patients, 173 (86.5%) showed evidence of clinical improvement during the first 7 days of intravenous antibiotic therapy. Early clinical improvement (during the first 3 days of intravenous antibiotic therapy) was documented in 133 patients (66.5%). Late clinical improvement (from day 4 to day 7 of intravenous antibiotic therapy) was documented in 40 patients (20%).
Figure 1.
Clinical course of hospitalized patients with CAP.
Table 1.
| (1) Improvement in cough and shortness of breath |
| (2) Temperature of <37.8°C for at least 8 h |
| (3) Normalizing white blood cell count |
| (4) Adequate oral intake and gastrointestinal absorption |
Using a pathophysiological approach to characterize the aetiology of clinical failure, it was found that severe sepsis and cardiac deterioration are the primary aetiologies for clinical failure in hospitalized patients with CAP.29 The vast majority of clinical failures related to CAP occurred during the first 72 h of patient hospitalization. The association of clinical failure with cardiovascular events was reported in a recent article indicating that development of acute myocardial infarction is a common aetiology for clinical failure in hospitalized patients with CAP.30
Mortality rates in the elderly with CAP are higher than in younger populations, with estimates of up to 30%.31,32 A matched case–control database study of Medicare patients with CAP showed that elderly patients had an in-hospital mortality rate of 11% and a 1 year mortality rate of >40%.33 A recent study by Kothe et al.31 confirms these findings, showing that increased age was associated with increased mortality in CAP. It is unclear whether CAP is the reason why these patients die more frequently or if it is just a marker of illness in older populations. Several studies have attempted to identify risk factors for developing CAP in this age group. A population-based cohort study of 46 237 elderly patients was performed to determine risk factors associated with development of CAP in elderly patients.34 Jackson et al.34 found that chronic obstructive pulmonary disease (COPD), immunosuppression, smoking, congestive heart failure, diabetes, malignancy and previous hospitalizations for CAP were independent risk factors for developing the disease.
Clinical outcomes weeks after hospitalization
Patients with CAP typically have a follow-up clinic appointment within 30 days after hospital discharge. During the first month following the diagnosis of CAP, some patients may suffer clinical deterioration requiring re-hospitalization. The rate of re-hospitalization (for any reason) within 30 days after discharge for pneumonia was 20% in Medicare beneficiaries in the USA.35 These data indicate that re-hospitalization within 30 days is a common event in hospitalized patients with CAP. Early outpatient follow-up to evaluate resolution of pneumonia and optimal management of co-morbidities may reduce hospital readmission rates.
Clinical outcomes years after hospitalization
The final evaluation for a patient with CAP usually occurs during the 30 day follow-up visit, and patients who achieve clinical resolution of infection are considered to have survived the episode of CAP. This concept has started to change with recent studies indicating that CAP may impact survival long after the patient is considered clinically cured.36–38 In a recent study, a significant decrease in survival for hospitalized patients with CAP was observed over a 7.5 year average follow-up when compared with patients hospitalized for medical reasons other than CAP during the same period of time. Hospitalization for CAP was a significant predictor of decreased survival (hazard ratio 1.4; 95% confidence interval 1.2, 1.5; P < 0.0001), even after adjustment for age and co-morbidities, suggesting that having CAP is a marker for increased mortality risk over the long term.39
Empirical therapy and unmet needs
Empirical therapy for hospitalized patients with CAP
With the goal of improving clinical outcomes in hospitalized patients with bacterial CAP, the IDSA/ATS have developed guidelines for the treatment of hospitalized patients with CAP (Table 2).3 These guidelines recommend early initiation of empirical antibiotic therapy to cover the most likely typical and atypical bacteria associated with CAP. Currently recommended drugs for empirical therapy of hospitalized patients with CAP include a combination of a β-lactam antibiotic to cover typical pathogens and a macrolide antibiotic to cover atypical pathogens or monotherapy with a respiratory quinolone, because these agents have activity against both typical and atypical bacteria.3
Table 2.
Current guideline-recommendeda empirical antimicrobial regimens to treat severe CAP3
| Empirical treatment | Suspected pathogen |
|---|---|
| Intravenous β-lactam | Streptococcus pneumoniae, Haemophilus influenzae, enteric Gram-negative bacilli (Klebsiella spp.) |
| Third-generation cephalosporin (ceftriaxone or cefotaxime) | |
| or | |
| β-Lactam/β-lactamase inhibitor (ampicillin/clavulanate) | |
| plus | |
| Intravenous macrolide (erythromycin or azithromycin) | Legionella spp., Mycoplasma pneumoniae, Chlamydia pneumoniae and Chlamydophila psittaci |
| or | |
| Intravenous fluoroquinolone (as monotherapy)b (levofloxacin or moxifloxacin) | |
| if risk factors for Pseudomonas | |
| Intravenous, antipseudomonal β-lactam (cefepime, piperacillin/tazobactam, imipenem, meropenem) | Pseudomonas aeruginosa (and the other pathogens above) |
| plus either | |
| Intravenous aminoglycoside or intravenous ciprofloxacin or high-dose levofloxacin | |
| plus | |
| Intravenous macrolide (erythromycin or azithromycin) if aminoglycoside used, but not with the use of a fluoroquinolone | |
| if risk factors for CA-MRSA | |
| Linezolid or vancomycin | CA-MRSA |
aIDSA/ATS consensus guidelines.
bFluoroquinolones also cover S. pneumoniae (including DRSP), H. influenzae and enteric Gram-negative bacilli (Klebsiella spp.).
Unmet needs
The emergence of drug-resistant S. pneumoniae (DRSP) is well documented.40–42 However, the clinical relevance of DRSP for pneumonia is uncertain.9 Studies indicate that current levels of β-lactam resistance generally do not result in treatment failure for patients with CAP when appropriate agents (i.e. amoxicillin, ceftriaxone and cefotaxime) and doses are used, even in the presence of bacteraemia,3,43 although some data suggest that resistance to macrolides results in clinical failure.44 In an analysis of a large multi-hospital database, 41 699 patients hospitalized (but not admitted to the ICU) for CAP were identified from January 2000 to June 2009.45 Of these patients, 79% received 1 of the 40 mostly commonly used regimens for CAP. Fifteen percent of patients experienced ‘real-world’ failure of therapy, defined as receipt after 24 h of an antibiotic not used in the first 24 h. This study suggested that one in seven non-ICU CAP patients experience failure of initial antimicrobial therapy.
Risk factors for β-lactam-resistant S. pneumoniae include age <2 years or >65 years, β-lactam therapy within 3 months, alcoholism, medical co-morbidities, immunosuppressive illness or therapy, and exposure to a child in a daycare centre.46 Although the relative predictive value of these risk factors is unclear, recent antimicrobial treatment is probably most significant. Recent therapy or repeated courses with β-lactams, macrolides or quinolones are risk factors for pneumococcal resistance to the same class of antibiotic.47 One study demonstrated that treatment with either a β-lactam or macrolide within the previous 6 months predicted that, if pneumococcal bacteraemia is present, the organism would probably be penicillin resistant.47 Recommendations for use of highly active agents in patients at risk for penicillin-resistant S. pneumoniae (PRSP) are therefore not based solely on efficacy considerations, but also on the necessity to prevent additional resistance development by using the most active regimen. Increasing doses of certain agents, such as penicillins, cephalosporins or levofloxacin, may result in satisfactory outcomes; however, switching to more active agents may help stabilize or even decrease resistance rates.3
Recently, an increase in CA-MRSA pneumonia has been noted.48–52 CA-MRSA strains are epidemiologically, genotypically and phenotypically distinct from the original hospital-acquired strains. These new CA-MRSA are now incorporated into the hospital setting and are producing hospital-acquired infections. CA-MRSA tend to be resistant to antimicrobials, almost always contain a novel type IV SCCmec gene and include the gene for PVL,53–55 a toxin associated with necrotizing pneumonia, shock, respiratory failure and formation of abscesses and empyemas. A major concern with CA-MRSA is necrotizing pneumonia associated with PVL and other toxin production. Vancomycin clearly does not decrease toxin production, and the effect of trimethoprim/sulfamethoxazole and quinolones on toxin production is unclear. Addition of clindamycin or use of linezolid, both of which have been shown to impact toxin production, may warrant extra consideration for these necrotizing pneumonias.3 Unfortunately, emergence of clindamycin resistance has been reported (especially in erythromycin-resistant MRSA strains), and vancomycin would still be needed for adequate antibacterial effect.
Summary
CAP is a serious condition associated with significant morbidity and potential long-term mortality. Underlying co-morbid conditions, such as COPD, alcoholism, chronic heart disease and diabetes mellitus, are commonly noted in these patients. Severe CAP is defined by the presence of respiratory failure or symptoms of severe sepsis or septic shock. Approximately 10% of hospitalized patients present with severe CAP, which is associated with a significant mortality rate. The most common aetiological agents found in CAP include S. pneumoniae, H. influenzae, S. aureus, group A streptococci and M. catarrhalis; the most common atypical pathogens include Legionella spp., M. pneumoniae and C. pneumoniae. Other pathogens identified in severely ill patients include MRSA and Gram-negative bacilli. Early and rapid initiation of empirical antimicrobial treatment is critical for achieving a favourable outcome in CAP. Initial antimicrobial therapy should consist of an intravenous β-lactam antibiotic plus a macrolide or a respiratory fluoroquinolone. Modification of this regimen may be considered in the presence of co-morbid conditions and risk factors for specific pathogens. The emergence of resistant pathogens in CAP continually increases the challenge for appropriate management.
From a regulatory point of view, the US FDA classifies CAP caused by typical pathogens as community-acquired bacterial pneumonia (CABP), an updated description that aims to identify patients most likely to have CAP caused by a bacterial pathogen and for whom antimicrobial treatment would be appropriate.56 New antibiotics for therapy of CABP should demonstrate good activity against the most common typical pathogens. New agents with activity against drug-resistant pathogens are welcome additions for the empirical management of CAP. The articles in this Supplement provide information on ceftaroline (the active form of the prodrug ceftaroline fosamil; herein referred to as ceftaroline), a novel, broad-spectrum cephalosporin that has been approved for the treatment of CABP as well as acute bacterial skin and skin structure infections.57 In the next article, Joseph Laudano58 provides a concise review of the mechanism of action, spectrum of activity and basic pharmacokinetic and pharmacodynamic profile of ceftaroline. The results from the two Phase III clinical trials in CAP (FOCUS 1 and FOCUS 2; registration numbers: NCT00621504 and NCT00509106) are presented by Thomas File and Donald Low and colleagues.59,60 The FOCUS trials were designed as parallel, multicentre, randomized, double-blind comparative studies to evaluate the efficacy and safety of intravenous ceftaroline (600 mg every 12 h) compared with ceftriaxone (1 g every 24 h) for 5–7 days in adult patients with CAP. The FOCUS studies were identical in design except that patients received two 500 mg doses of oral clarithromycin every 12 h on day 1 of therapy in the FOCUS 1 study. Ian Critchley and colleagues61 examine the microbiological profile of ceftaroline against contemporary respiratory pathogens collected from the FOCUS trials; key CABP pathogens, such as S. pneumoniae, H. influenzae and S. aureus, were susceptible to ceftaroline with low MIC90s compared with ceftriaxone. In the article by Douglas Rank and colleagues,62 the integrated safety analysis from the two FOCUS trials is summarized and shows that ceftaroline provides the favourable safety profile expected of the cephalosporin class. George Drusano63 discusses the unique properties that make an antibiotic effective for treatment of CAP, including review of potency, binding affinity for penicillin-binding proteins and rapidity of penetration to the site of action. In the final article, Ronald Jones and colleagues64 present results from an international surveillance study that demonstrates the broad-spectrum coverage of ceftaroline for typical CAP pathogens, including DRSP and MRSA. The articles included in this Supplement provide a comprehensive overview of ceftaroline and suggest that this cephalosporin will provide a useful addition for the treatment of CABP.
Funding
Funding for editorial assistance was provided by Forest Laboratories, Inc.
Transparency declarations
This article was developed from a scientific panel of FOCUS investigators and experts in community-acquired bacterial pneumonia, held on 1–2 May 2010 in New York, NY, USA. This article is part of a Supplement sponsored by Forest Laboratories, Inc.
J. A. R. has participated as a speaker in scientific meetings or courses organized and financed by various pharmaceutical companies, including Astra-Zeneca, Bayer-Schering Pharma, Pfizer, GlaxoSmithKline and Cubist Pharmaceuticals. J. A. R. has been a consultant for Pfizer. J. A. R. has been the principal investigator for research grants sponsored by Bayer-Schering Pharma, Pfizer, Cubist Pharmaceuticals and Merck. A. R. A. has participated as a speaker in scientific meetings or courses organized and financed by various pharmaceutical companies, including Astra-Zeneca, Boehringer Ingelheim, Bayer-Schering Pharma, Pfizer, GlaxoSmithKline and Forest Laboratories, Inc. A. R. A. has been a consultant for Astra-Zeneca, Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Forest Laboratories, Inc. and Bayer-Schering Pharma. A. R. A. has been the principal investigator for research grants and the University of Texas Health Science Center at San Antonio was paid for participating in multicentre clinical trials sponsored by GlaxoSmithKline, Bayer-Schering Pharma, BARD, Lilly and NIH.
Grace Johnson, PharmD, of Scientific Therapeutics Information, Inc. (Springfield, NJ, USA) provided editorial assistance on this manuscript.
References
- 1.Heron M, Hoyert DL, Murphy SL, et al. National Vital Statistics Reports. Deaths: Final Data for 2006. April 17, 2009. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. (17 August 2010, date last accessed) [PubMed]
- 2.Niederman MS, McCombs JS, Unger AN, et al. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20:820–37. doi: 10.1016/s0149-2918(98)80144-6. doi:10.1016/S0149-2918(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. doi:10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Adv Data. 2007;((385)):1–19. [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality. Pneumonia is the Most Common Reason for Hospitalization. http://www.ahrq.gov/research/sep08/0908RA40.htm. (17 August 2010, date last accessed)
- 6.Curns AT, Holman RC, Sejvar JJ, et al. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165:2514–20. doi: 10.1001/archinte.165.21.2514. doi:10.1001/archinte.165.21.2514. [DOI] [PubMed] [Google Scholar]
- 7.Fry AM, Shay DK, Holman RC, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–9. doi: 10.1001/jama.294.21.2712. doi:10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert K, Fine MJ. Assessing prognosis and predicting patient outcomes in community-acquired pneumonia. Semin Respir Infect. 1994;9:140–52. [PubMed] [Google Scholar]
- 9.Metlay JP. Antibacterial drug resistance: implications for the treatment of patients with community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:777–90. doi: 10.1016/j.idc.2004.07.003. doi:10.1016/j.idc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.España PP, Capelastegui A, Quintana JM, et al. A prediction rule to identify allocation of inpatient care in community-acquired pneumonia. Eur Respir J. 2003;21:695–701. doi: 10.1183/09031936.03.00057302. doi:10.1183/09031936.03.00057302. [DOI] [PubMed] [Google Scholar]
- 11.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. doi:10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 12.Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA. 2000;283:749–55. doi: 10.1001/jama.283.6.749. doi:10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 13.Ewig S, Schäfer H, Torres A. Severity assessment in community-acquired pneumonia. Eur Respir J. 2000;16:1193–201. doi: 10.1034/j.1399-3003.2000.16f27.x. doi:10.1034/j.1399-3003.2000.16f27.x. [DOI] [PubMed] [Google Scholar]
- 14.Ewig S, Ruiz M, Mensa J, et al. Severe community-acquired pneumonia: assessment of severity criteria. Am J Respir Crit Care Med. 1998;158:1102–8. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 15.British Thoracic Society. Guidelines for the management of community-acquired pneumonia in adults admitted to hospital. Br J Hosp Med. 1993;49:346–50. [PubMed] [Google Scholar]
- 16.Cordero E, Pachón J, Rivero A, et al. Community-acquired bacterial pneumonia in human immunodeficiency virus-infected patients: validation of severity criteria. Am J Respir Crit Care Med. 2000;162:2063–8. doi: 10.1164/ajrccm.162.6.9910104. [DOI] [PubMed] [Google Scholar]
- 17.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82. doi: 10.1136/thorax.58.5.377. doi:10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrie TJ, Wu L. Factors influencing in-hospital mortality in community-acquired pneumonia: a prospective study of patients not initially admitted to the ICU. Chest. 2005;127:1260–70. doi: 10.1016/S0012-3692(15)34475-5. doi:10.1378/chest.127.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett JG. Is activity against ‘atypical' pathogens necessary in the treatment protocols for community-acquired pneumonia? Issues with combination therapy. Clin Infect Dis. 2008;47(Suppl 3):S232–6. doi: 10.1086/591409. doi:10.1086/591409. [DOI] [PubMed] [Google Scholar]
- 20.Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64:441–6. doi: 10.1093/jac/dkp241. doi:10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- 21.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–3. doi: 10.1126/science.1137165. doi:10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 22.Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005;128:2732–8. doi: 10.1378/chest.128.4.2732. doi:10.1378/chest.128.4.2732. [DOI] [PubMed] [Google Scholar]
- 23.xTAG RVP (Respiratory Viral Panel) Prescribing Information. Toronto, Ontario, Canada: Luminex Molecular Diagnostics, Inc.; 2008. [Google Scholar]
- 24.CDC. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4. [PubMed] [Google Scholar]
- 25.Ramirez JA, Srinath L, Ahkee S, et al. Early switch from intravenous to oral cephalosporins in the treatment of hospitalized patients with community-acquired pneumonia. Arch Intern Med. 1995;155:1273–6. doi:10.1001/archinte.155.12.1273. [PubMed] [Google Scholar]
- 26.Ramirez JA, Ahkee S. Early switch from IV antimicrobials to oral clarithromycin in patients with CAP. Infect Med. 1997;14:319–23. [Google Scholar]
- 27.Ramirez JA. Switch therapy in adult patients with pneumonia. Clin Pulm Med. 1995;2:327–33. doi:10.1097/00045413-199511000-00002. [Google Scholar]
- 28.Ramirez JA, Vargas S, Ritter GW, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med. 1999;159:2449–54. doi: 10.1001/archinte.159.20.2449. doi:10.1001/archinte.159.20.2449. [DOI] [PubMed] [Google Scholar]
- 29.Aliberti S, Amir A, Peyrani P, et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest. 2008;134:955–62. doi: 10.1378/chest.08-0334. doi:10.1378/chest.08-0334. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez J, Aliberti S, Mirsaeidi M, et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008;47:182–7. doi: 10.1086/589246. doi:10.1086/589246. [DOI] [PubMed] [Google Scholar]
- 31.Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32:139–46. doi: 10.1183/09031936.00092507. doi:10.1183/09031936.00092507. [DOI] [PubMed] [Google Scholar]
- 32.Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–24. doi: 10.1016/S1473-3099(04)00931-4. doi:10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man's friend? Arch Intern Med. 2003;163:317–23. doi: 10.1001/archinte.163.3.317. doi:10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 34.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–50. doi: 10.1086/425615. doi:10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. doi:10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 36.Mortensen EM, Kapoor WN, Chang CC, et al. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37:1617–24. doi: 10.1086/379712. doi:10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 37.Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:910–4. doi: 10.1164/rccm.200310-1448OC. doi:10.1164/rccm.200310-1448OC. [DOI] [PubMed] [Google Scholar]
- 38.Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore) 2008;87:329–34. doi: 10.1097/MD.0b013e318190f444. doi:10.1097/MD.0b013e318190f444. [DOI] [PubMed] [Google Scholar]
- 39.Bordon J, Wiemken T, Peyrani P, et al. Decrease in long-term survival for hospitalized patients with community-acquired pneumonia. Chest. 2010;138:279–83. doi: 10.1378/chest.09-2702. doi:10.1378/chest.09-2702. [DOI] [PubMed] [Google Scholar]
- 40.Gertz RE, Jr, Li Z, Pimenta FC, et al. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis. 2010;201:770–5. doi: 10.1086/650496. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs MR. Antimicrobial-resistant Streptococcus pneumoniae: trends and management. Expert Rev Anti Infect Ther. 2008;6:619–35. doi: 10.1586/14787210.6.5.619. doi:10.1586/14787210.6.5.619. [DOI] [PubMed] [Google Scholar]
- 42.Lynch JP, III, Zhanel GG. Streptococcus pneumoniae: does antimicrobial resistance matter? Semin Respir Crit Care Med. 2009;30:210–38. doi: 10.1055/s-0029-1202939. doi:10.1055/s-0029-1202939. [DOI] [PubMed] [Google Scholar]
- 43.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered and clinical outcome. Clin Infect Dis. 2003;37:230–7. doi: 10.1086/377534. doi:10.1086/377534. [DOI] [PubMed] [Google Scholar]
- 44.Lonks JR, Garau J, Gomez L, et al. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin Infect Dis. 2002;35:556–64. doi: 10.1086/341978. doi:10.1086/341978. [DOI] [PubMed] [Google Scholar]
- 45.Oster G, Berger A, Edelsberg J, et al. ‘Real-world' failure of initial antibiotic therapy in non-ICU community-acquired pneumonia (CAP) in US hospitals, 2000–2009. Abstracts of the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010; Washington, DC, USA: American Society for Microbiology; Poster #L1-1044. [Google Scholar]
- 46.Vanderkooi OG, Low DE, Green K, et al. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005;40:1288–97. doi: 10.1086/429242. doi:10.1086/429242. [DOI] [PubMed] [Google Scholar]
- 47.Ruhe JJ, Hasbun R. Streptococcus pneumoniae bacteremia: duration of previous antibiotic use and association with penicillin resistance. Clin Infect Dis. 2003;36:1132–8. doi: 10.1086/374556. doi:10.1086/374556. [DOI] [PubMed] [Google Scholar]
- 48.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–9. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hidron AI, Low CE, Honig EG, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis. 2009;9:384–92. doi: 10.1016/S1473-3099(09)70133-1. doi:10.1016/S1473-3099(09)70133-1. [DOI] [PubMed] [Google Scholar]
- 50.Lam AP, Wunderink RG. The role of MRSA in healthcare-associated pneumonia. Semin Respir Crit Care Med. 2009;30:52–60. doi: 10.1055/s-0028-1119809. doi:10.1055/s-0028-1119809. [DOI] [PubMed] [Google Scholar]
- 51.Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin- resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819–24. doi: 10.1086/342576. doi:10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 52.Mongkolrattanothai K, Boyle S, Kahana MD, et al. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis. 2003;37:1050–8. doi: 10.1086/378277. doi:10.1086/378277. [DOI] [PubMed] [Google Scholar]
- 53.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562–73. doi: 10.1086/427701. doi:10.1086/427701. [DOI] [PubMed] [Google Scholar]
- 55.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. doi:10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 56.FDA. Guidance for Industry. Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment. March 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm123686.pdf. (26 July 2010, date last accessed) [Google Scholar]
- 57.Teflaro™ [Ceftaroline Fosamil Prescribing Information (Draft)] New York, NY: Forest Laboratories, Inc.; 27 October 2010. [Google Scholar]
- 58.Laudano JB. Ceftaroline fosamil: a new broad-spectrum cephalosporin. J Antimicrob Chemother. 2011;66(Suppl 3):iii11–8. doi: 10.1093/jac/dkr095. [DOI] [PubMed] [Google Scholar]
- 59.File TM, Jr, Low DE, Eckburg PB, et al. on behalf of the FOCUS 1 investigators. FOCUS 1: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother. 2011;66(Suppl 3):iii19–32. doi: 10.1093/jac/dkr096. [DOI] [PubMed] [Google Scholar]
- 60.Low DE, File TM, Jr, Eckburg PB, et al. on behalf of the FOCUS 2 investigators. FOCUS 2: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother. 2011;66(Suppl 3):iii33–44. doi: 10.1093/jac/dkr097. [DOI] [PubMed] [Google Scholar]
- 61.Critchley IA, Eckburg PB, Jandourek A, et al. Review of ceftaroline fosamil microbiology: integrated FOCUS studies. J Antimicrob Chemother. 2011;66(Suppl 3):iii45–51. doi: 10.1093/jac/dkr098. [DOI] [PubMed] [Google Scholar]
- 62.Rank DR, Friedland HD, Laudano JB. Integrated safety summary of FOCUS 1 and FOCUS 2 trials: Phase III randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with community-acquired pneumonia. J Antimicrob Chemother. 2011;66(Suppl 3):iii53–9. doi: 10.1093/jac/dkr099. [DOI] [PubMed] [Google Scholar]
- 63.Drusano GL. What are the properties that make an antibiotic acceptable for therapy of community-acquired pneumonia? J Antimicrob Chemother. 2011;66(Suppl 3):iii61–7. doi: 10.1093/jac/dkr100. [DOI] [PubMed] [Google Scholar]
- 64.Jones RN, Farrell DJ, Mendes RE, et al. Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: results from an international surveillance study. J Antimicrob Chemother. 2011;66(Suppl 3):iii69–80. doi: 10.1093/jac/dkr101. [DOI] [PubMed] [Google Scholar]