Abstract
Background. PACTG (Pediatric AIDS Clinical Trials Group) 225, a multicenter, randomized, open-label trial in the United States evaluated reactogenicity and immunogenicity of 2 vaccination regimens: monovalent measles vaccine (Attenuvax) at 6 months of age and measles, mumps, and rubella, live attenuated (MMRII) vaccine at 12 months of age (2D), or only MMRII at 12 months of age (1D) in human immunodeficiency virus–infected (HIV-infected) (POS) and uninfected (NEG) children in the pre–highly active antiretroviral therapy (pre-HAART) period.
Methods. Plaque-reduction neutralization (PRN) of measles-neutralizing antibody titers were evaluated at study weeks 0, 6, 26, 32, 52, and 130 (∼3 years of age).
Results. The 110 subjects included: 65 2DNEG; 30 1DNEG; 7 2DPOS and 8 1DPOS. Vaccinations (n = 175) were associated with no adverse experiences >Grade 2 except for Grade 3 fever (n = 2, 1 1DPOS and 1 1DNEG). Six weeks after Attenuvax, all 2DPOS subjects (7/7) seroresponded (PRN titers ≥120 mIU/mL) with median titers significantly exceeding 2DNEG titers (2115 vs 628 mIU/mL, respectively; P = .023). At ∼3 years of age, 67% 1DPOS (4/6) and 83% 2DPOS (4/5) subjects maintained titers ≥120 mIU/mL. Prevaccination titers ≥25 mIU/mL among 2DNEG subjects correlated inversely with the likelihood of achieving titers ≥120 mIU/mL (56% vs 90%; P = .004).
Conclusions. Among HIV-infected children pre-HAART, Attenuvax at 6 months was well tolerated and immunogenic. These data support the current World Health Organization (WHO) recommendation to administer a first dose of measles vaccine at 6 months of age to HIV-infected children.
Measles infection may cause severe giant-cell pneumonia or disseminated disease in immune-compromised individuals [1]. Although live-attenuated virus vaccines are generally contraindicated in immune-compromised persons, measles vaccination of HIV-infected children has not been associated with serious life-threatening adverse events [2, 3]. A single report of fatal postvaccination measles pneumonia in an HIV-infected adult has resulted in a recommendation to restrict the use of live-attenuated measles vaccines to HIV-infected children with CD4+ T-lymphocyte counts >200 cells/mL [4, 5].
Despite the relative safety of measles vaccination in human immunodeficiency virus–infected (HIV-infected) children, seroconversion rates and antibody titers have been low [6]. During the pre–highly active antiretroviral therapy (pre-HAART) era, enzyme immunoassay (EIA) seroprevalence rates ranging between 12.5%–40% were observed in children after primary vaccination at approximately 15 months of age [6–10]. Postmeasles vaccination titers, measured at a mean age of 6.7 years, were significantly lower in HIV-infected children than healthy ones [7]. Additionally, HIV-infected infants (mean age 2.8 years) had measles antibody with a significantly lower avidity index than age-matched uninfected controls [8]. Low titers that declined rapidly and were not boosted by reimmunization suggested impaired primary vaccine responses or poor immunologic memory due to functional B-cell and/or memory T-cell defects, or possibly a lack of affinity maturation [7–8].
Issues in protecting HIV-infected infants against measles include: optimal immunization age, the safety profile of measles vaccine among infants below 12 months of age, and a vaccination schedule providing longest-term protection. Studies of HIV infected 6–12-month-old vaccinees suggest a trend toward higher seroresponse rates and higher postvaccination measles antibody seroprevalence compared to those receiving 1 primary dose >12 months [9–10]. This is consistent with results seen after diphtheria and tetanus toxoid vaccination. Since attrition in both lymphoproliferative and humoral responses may occur prior to the decline of helper T lymphocytes, early vaccination in HIV-infected children may be advantageous [11].
We hypothesized that HIV-infected children vaccinated at 6 months in the United States would develop a higher and more durable response to measles vaccine than HIV-infected children vaccinated after 12 months due to relative intactness of the immune system of the HIV-infected child in early life. We proposed to administer measles vaccine to HIV-infected infants at 6 months of age with revaccination at 12 months of age to protect those who did not respond to their first dose, per current recommendations [12–14]. We also proposed to compare the seroresponse rates after the 2-dose regimen with the seroresponse rates after 1 dose of measles, mumps, and rubella vaccine, live attenuated (MMRII, Merck & Co., Inc.) at 12 months. Responses in HIV-infected children and in HIV-exposed but uninfected children receiving the same regimen were also compared.
METHODS
Study Design
Pediatric AIDS Clinical Trials Group (PACTG) 225 was an open-label, randomized, pre-HAART era study. Children born to HIV-infected mothers and attending clinics based in the PACTG study centers throughout the United States were recruited. Eligible participants were randomized to 1 of 2 immunization schedules: monovalent measles vaccine (Attenuvax, Merck & Co., Inc.) at 6 months followed by MMRII, at 12 months of age, or MMRII at 12 months of age only. Subjects were stratified by HIV status and then randomized centrally using a computer-generated scheme into 1 of 4 treatment groups: 2DNEG (HIV-uninfected infants immunized at 6 months and 12 months of age), 2DPOS (HIV-infected infants immunized at 6 and 12 months of age), 1DNEG (HIV-uninfected infants immunized once at 12 months of age), and 1DPOS (HIV-infected infants immunized once at 12 months of age). All subjects were enrolled between 6–7 months of age. The 2DNEG and 2DPOS groups received 0.5 mL of Attenuvax at 6 months of age (study week 0), and all groups received 0.5 mL of MMRII at 12 months of age (study week 26). All subjects were followed for 24 months after their last vaccination for antibody persistence (study week 130). Local and systemic adverse reactions were evaluated in the clinic at 1 hour and at 7 days with phone follow-up on the 14th day after immunization. Laboratory parameters monitored before and 6 weeks after each immunization included: CD3+CD4+ lymphocyte counts and proportions, complete blood count (CBC) with differential white blood cell and platelet count, serum blood urea nitrogen (BUN), serum creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Adverse events were graded using the AIDS Clinical Trials Group (ACTG) toxicity scale for pediatric patients [15].
HIV-infected subjects were identified by the presence of 1 or more positive (noncord) blood test(s) for direct detection of HIV at any age (ie, HIV culture, HIV-DNA polymerase chain reaction [PCR], free P24 antigen performed in an ACTG-certified laboratory). HIV-uninfected subjects were defined by 1 or more negative (noncord) blood test(s) for direct detection of HIV at age 1 month or older. Serologic testing for HIV antibody (enzyme-linked immunosorbent assay [ELISA] and Western blot) at age 18 months confirmed the final assignment before analysis.
The study was reviewed and approved by the ethical and institutional review boards (IRB) at each participating site. Written informed consent was obtained from parents or legal guardians before any study-related procedures were performed. No measles cases were identified locally during the study period (1996–1998).
Inclusion and Exclusion Criteria
Children 6 months of age (but not over 7 months) born to HIV-infected mothers were eligible for enrollment if there was laboratory evidence of either maternal HIV-infection or a positive HIV antibody test in the infant. Children excluded were those with CD4+ T lymphocyte counts <750 cells/mm3 or <15% at 6 months of age; children with intercurrent illness, opportunistic infections, and/or unexplained fevers of >7 days' duration; and those with known exposure to measles within 14 days of randomization. Children receiving immunoglobulin products currently or in the previous 6 months, or steroid preparations (except dermatologic preparations) were also excluded, as were children with CD4+ T-cell lymphopenia and those with severe thrombocytopenia (platelet count <50,000) prior to any vaccine dose.
Objective
Two primary objectives of the study were to (1) compare measles seroresponse rates at 13 months of age (study week 32) in HIV-infected children immunized with the 2-dose regimen (2DPOS) with responses in children given a single dose of MMRII (1DPOS) and (2) to compare the measles seroresponse rates at 13 months of age among HIV-infected children to the response rates in HIV-uninfected children given the identical vaccine regimen.
The secondary objectives of the study were to assess the safety and immunogenicity of Attenuvax in 2DPOS subjects 7 months of age, to monitor adverse clinical effects and changes in CD4+ T lymphocyte proportions and counts following Attenuvax or MMRII vaccination, and to assess measles antibody decay.
Measles Serology
Measles seroresponses were evaluated using a plaque reduction neutralization (PRN) test as previously described [16]. The PRN titer was defined as the serum dilution that reduced the number of plaques by 50% calculated using the Spearman-Kärber method [16]. In this assay, a titer of 8 was equivalent to 8 mIU/mL of measles antibody. Serum samples without neutralizing antibody were assigned a titer of 2 to calculate mean titers. Seroresponse was defined as a PRN titer ≥120 mIU/mL [17].
CD3+CD4+ T-cell Counts and Laboratory Studies
Lymphopenia is known to occur after measles infection and sometimes transiently after measles immunization with measles vaccination [18, 19]. Therefore, CD3+CD4+ T-cell counts and percentages were evaluated pre- and postimmunization at study weeks 0, 6, 26, and 32, at either study sites or the ACTG central laboratory. Proportional change in CD4+ T lymphocyte counts or percentages were calculated by comparing pre- and postimmunization parameters (week 6 vs week 0, and week 32 vs week 26). The proportional change affecting CD4+ T lymphocytes was graded into 3 categories: grade 1 toxicity was defined as a CD4+% proportional change <10% and CD4+ count proportional change <20%; grade 2 was defined as CD4+% proportional change ≥10% but <40% and CD4+ count proportional change ≥20% but <50%; and grade 3 toxicity was defined as CD4+% proportional change ≥40% and CD4+ count proportional change ≥50%.
Changes in HIV-1 Viral Load After Measles Vaccination
Pre- and postvaccination HIV–type 1 (HIV-1) viral load data were collected post hoc. Plasma HIV-1 RNA measurements were performed by reverse transcriptase assay (Amplicor, Roche Diagnostics) at the ACTG certified laboratories as a part of disease monitoring and were not a part of study procedures. Data were available on 8 of the 15 HIV-infected subjects, including 5 2DPOS and 3 1DPOS subjects and were evaluated post hoc. Of the 5 2DPOS subjects, 2 had paired samples following the first and second vaccinations.
Maternal Measles Status and Baseline Measles Antibody Titers
Because low levels of passively acquired measles neutralizing antibody (≥1:25 titers or ≥25 mIU/mL) may interfere with vaccine take and inhibit seroconversion, the proportion of subjects with measles PRN antibody titers ≥25 mIU/mL at week 0 was examined [20–22].
Data Management and Statistical Methods
Data were collected on case report forms at each study site and collated into an SAS database by the ACTG Data Management Center, SAS Institute Inc. Logarithmic titers were not normally distributed, therefore a nonparametric test was used to compare pre- and postimmunization measles PRN titers. The Wilcoxon signed rank test was used to make pair-wise comparisons for continuous variables, and the Kruskall-Wallis test was used to make overall comparisons among all 4 groups for continuous variables, with Monte Carlo–estimated P values reported. The Fisher exact test was used to perform group comparisons for discrete variables. For comparisons across all 4 groups, post hoc pair-wise comparisons were performed only if the overall P value was significant among the 4 groups.
RESULTS
Subject Accrual, Demographics, and Clinical Characteristics at Baseline
The study opened to accrual on 11 September 1996. Since antiviral treatment during pregnancy significantly reduced transmission rates, fewer children were HIV-infected at birth, limiting the enrollment. In August 1997, the PACTG Research Priority subcommittee determined that it was not feasible to enroll 168 HIV-infected subjects; therefore, the study was closed in May 1998. A total of 110 subjects at 22 PACTG study sites, including 65 in 2DNEG, 7 in 2DPOS, 30 in 1DNEG, and 8 in the 1DPOS group were enrolled.
Selected baseline characteristics, including demographics, vital statistics measurements, and lymphocyte subsets are presented in Table 1. Median ages at enrollment for the 4 groups were similar, and there were no significant differences among the 4 groups with respect to weight (P = .34) or height (P = .58), gender (P = .42), or ethnicity (P = .42) (Table 1). At baseline, overall proportions of CD4+ T lymphocytes differed significantly among the 4 groups (P = .011), but this difference is explained by HIV status (Table 1). In contrast, baseline CD4+ counts were similar among the groups at baseline (P = .196).
Table 1.
Baseline Demographics
| Treatment group |
|||||
| Category | 2DNEG | 2DPOS | 1DNEG | 1DPOS | P value |
| Number | 65 | 7 | 30 | 8 | |
| Median age (weeks) | 28 | 26 | 28 | 28 | |
| (Min, Max) | (22, 32) | (26, 30) | (26, 30) | (26, 32) | NA |
| Sex | |||||
| Male | 35 | 3 | 13 | 2 | .4211 |
| Female | 30 | 4 | 17 | 6 | |
| Race/ethnicity | |||||
| Caucasian | 8 | 2 | 4 | 0 | .4213a |
| African American | 37 | 4 | 18 | 5 | |
| Hispanic | 19 | 1 | 8 | 3 | |
| Other | 1 | 0 | 0 | 0 | |
| Weight (kg) | |||||
| Number | 62 | 7 | 28 | 8 | .343 |
| Mean | 7.64 | 7.14 | 7.78 | 7.03 | |
| SD | 1.02 | 0.71 | 2.16 | 1.15 | |
| Height | |||||
| Number | 62 | 7 | 28 | 7 | .575 |
| Mean | 67.81 | 66.14 | 65.73 | 64.67 | |
| SD | 11.98 | 3.02 | 3.24 | 4.23 | |
| CD3+CD4+ (%) | |||||
| Mean percentage | 45 | 37 | 44 | 37 | .0105b |
| SD | 8 | 9 | 10 | 9 | |
| CD3+CD4+ count | |||||
| Mean count/mm3 | 3125 | 2074 | 3156 | 2826 | .1960 |
| SD | 1323 | 890 | 1251 | 808 | |
| Maternal historyc | |||||
| Measles infection | 6 | 1 | 2 | 1 | |
| Measles immunization | 27 | 1 | 6 | 3 | |
| History unknown | 32 | 4 | 23 | 5 | |
| Subjects with maternal antibody titer data (week 0) | n = 63 | n = 7 | n = 26 | n = 8 | |
| Titer ≥25 mIU/mL (%) | 18 (29) | 2 (29) | 7 (27) | 1(13) | .6595 |
| Number (%) completing study | 39 (56%) | 5 (71%) | 17 (65%) | 6 (75%) | |
NOTE. a The subject with “other race/ethnicity” was deleted when calculating the P value.
Breakdown for comparisons of CD3+CD4+ percentages were calculated by Wilcoxon signed rank test because the overall P value was <.05. They are: 2DNEG vs 1DNEG, P = .1477 2DPOS vs 1DPOS, P = .2809 2DPOS + 1DPOS vs 2DNEG + 1DNEG, P = .0008.
Numbers may not equal the total for each group because some reported histories of both infection and immunization while others did not report. Overall P value for categorical data used exact method. Overall P value for continuous data: Monte Carlo–estimated P values were calculated by Kruskall-Wallis test.
Adverse Reactions
During the study, no deaths were observed and there were no significant differences in premature discontinuation across the 4 study groups (Figure 1). No grade-4 adverse reactions were reported among 72 subjects who received Attenuvax at 6 months of age or 103 subjects receiving MMRII at 12 months of age. Grade-3 adverse reactions were limited to 2 reports of fever following MMRII at 12 months (Table 2). Transient changes in laboratory values, all ≤grade 2, were detected in a few children in each group (data not shown).
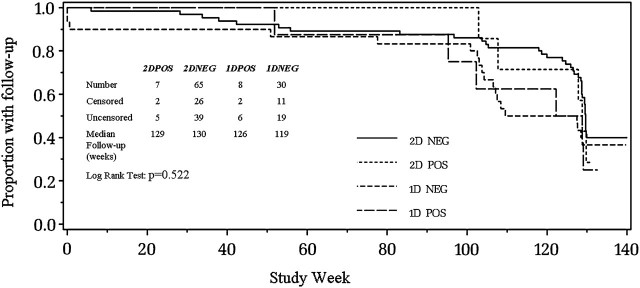
Figure 1.
HIV-infected (POS) and uninfected (NEG) children in PACTG Study 225 were randomized to 1 of 2 measles vaccine regimens: 2D children were given monovalent measles vaccine at 6 months of age (study week 0) followed by MMRII vaccine at 12 months of age; 1D children were only given MMRII at 12 months of age. Subjects were followed through 3 years of age (study week 130). The proportion with follow-up by study week for each group is shown: the inserted table shows the numbers of censored (follow-up <130 weeks) and uncensored (follow-up ≥week 130) subjects in each group and the median time for follow-up.
PACTG indicates Pediatric AIDS Clinical Trials Group; MMRII, measles, mumps, and rubella, live attenuated.
Table 2.
Adverse Event Reporting Following Immunization With Monovalent Measles Vaccine at 6 Months of Age and/or MMRII Vaccine at 12 Months of Age by Treatment Group
| Treatment group (number reporting AEa ≥ grade 3/number with any AE) |
||||
| Adverse event reporting following immunization with: | 2DNEG | 2DPOS | 1DNEG | 1DPOS |
| Monovalent measles vaccine at 6 months of age | ||||
| Number immunized | n = 65 | n = 7 | n = 0 | n = 0 |
| Subjects with follow-up | 65 | 7 | NAb | NA |
| Number of subjects reporting ≥1 adverse event | 19 | 4 | NA | NA |
| ≥Grade 3/any adverse event | ||||
| Local | 0/13 | 0/2 | NA | NA |
| Systemic | 0/4 | 0/1 | NA | NA |
| Feverc | 0/4 | 0/0 | NA | NA |
| Rash | 0/1 | 0/1 | NA | NA |
| Number of subjects with laboratory follow-up | n = 64 | n = 7 | NA | NA |
| Thrombocytopeniad | 0/0 | 0/0 | NA | NA |
| MMRII at 12 months of age | ||||
| Number immunized | n = 61 | n = 7 | n = 27 | n = 8 |
| Subjects with follow-up | 61 | 7 | 27 | 8 |
| Number of subjects reporting ≥1 adverse events | 17 | 2 | 9 | 4 |
| ≥Grade 3/any adverse event | ||||
| Local | 0/8 | 0/2 | 0/5 | 0/0 |
| Systemic | 0/5 | 0/0 | 0/5 | 0/4 |
| Fever | 0/7 | 0/0 | 1/5 | 1/2 |
| Rash | 0/1 | 0/0 | 0/0 | 0/0 |
| Number of subjects with laboratory follow-up | n = 60 | n = 7 | n = 25 | n = 8 |
| Thrombocytopenia | 0/0 | 0/0 | 0/0 | 0/0 |
NOTE. aAE indicates adverse event.
NA indicates not applicable.
Grade 3 fever = temperature of ≥104°F lasting between 2–5 days.
Thrombocytopenia = decline in platelet count to <150,000/mm3.
Change in Absolute CD4+ Counts or Percentages
After measles vaccination at 6 months of age, no significant differences in the proportional change in absolute CD4+ lymphocyte counts or percentages were observed at week 6 relative to the prevaccination counts (P = .97 and P = .078, respectively) (Table 3). Likewise, there were no significant differences in the proportional change at week 32 relative to week 26 CD4+ counts or percentages following MMRII vaccination at 12 months of age (P = .17 and P= .47, respectively) (Table 3). No toxicities ≥grade 3 in this category were noted after any vaccination.
Table 3.
Impact on CD4+ T-cell Percentages and/or CD4+ T-cell Counts Postimmunization With Monovalent Measles Vaccine or MMRII in HIV-Infected vs HIV-Uninfected Children
| Study week | Changes in CD4+ percentage and/or count | Total | Treatment group |
|||||||||
|
2DNEG |
2DPOS |
1DNEG |
1DPOS |
P valuea | ||||||||
|
n = 65 |
n = 7 |
n = 30 |
n = 8 |
|||||||||
| Number | % | Number | % | Number | % | Number | % | |||||
| Week 6 vs week 0 | Number vaccinated | n = 65 | n = 7 | n = 0 | n = 0 | |||||||
| Total number of subjects with data | n = 64 | n = 7 | n = 28 | n = 8 | ||||||||
| CD4+ toxicity category | ||||||||||||
| No toxicity | 19 | 29.7 | 2 | 28.6 | 11 | 39.3 | 1 | 12.5 | .3020 | |||
| Grade[1]b | 42 | 65.6 | 4 | 57.1 | 16 | 57.1 | 6 | 75.0 | ||||
| Grade[2]b | 3 | 4.7 | 1 | 14.3 | 0 | 0 | 1 | 12.5 | ||||
| Grade[3]b | 0 | 0 | 0 | 0 | 1 | 3.6 | 0 | 0 | ||||
| CD4+ count <750/mm3 Week 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Week 6 | 0 | 0 | 1 | 14.3 | 0 | 0 | 0 | 0 | ||||
| Mean proportional | ||||||||||||
| ▵CD4+ count (±SD)c | 0.01 (0.40) | 64 | 0.01 (.43) | 7 | 0.08 (0.39) | 28 | –0.01 (0.38) | 8 | 0.03 (0.16) | .9726 | ||
| ▵CD4+ % (±SD)c | 0.00 (0.14) | –0.03 (0.12) | 0.08 (0.14) | 0.05 (0.17) | –0.02 (0.16) | .0785 | ||||||
| Week 32 vs week 26 | Number vaccinated | n = 61 | n = 7 | n = 27 | n = 8 | |||||||
| Total number of subjects with data | n = 55 | n = 7 | n = 24 | n = 8 | ||||||||
| CD4+ toxicity category | ||||||||||||
| No Toxicity | 18 | 32.7 | 2 | 28.6 | 7 | 29.2 | 4 | 50 | .8574 | |||
| Grade[1]b | 36 | 65.5 | 5 | 71.4 | 17 | 70.8 | 4 | 50 | ||||
| Grade[2]b | 1 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Grade[3]b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| CD4+ count <750/mm3 | ||||||||||||
| Week 26 | 0 | 0 | 0 | 0 | ||||||||
| Week 32 | 0 | 0 | 0 | 0 | ||||||||
| Mean proportional | ||||||||||||
| ▵CD4+ count (±SD)c | –0.04 (0.29) | 55 | –0.02 (0.27) | 7 | –0.10 (0.22) | 24 | –0.13 (0.27) | 8 | 0.09 (0.47) | .1710 | ||
| ▵CD4+ % (±STD)c | 0.00 (0.18) | –0.02 (0.14) | –0.03 (0.13) | 0.03 (0.23) | 0.13 (0.27) | .4725 | ||||||
NOTE. aMonte Carlo–estimated P values were calculated using the Kruskall-Wallis test.
CD4+ toxicity category: grade[1] CD4+% proportional change <10% and CD4+ count proportional change <20%; grade[2] CD4+% proportional change ≥10% but <40% and CD4+ count proportional change ≥20% but <50%; grade[3] CD4+% proportional change ≥40% and CD4+ count proportional change ≥50%.
{[CD4+ post-CD4+pre]/[CD4+pre]} × 100 = proportional change, where “CD4+” refers to either the absolute count or the percentage of total lymphocytes.
P values for subgroups were calculated only if the overall P value was <.05 using the Wilcoxon signed rank test.
Changes in HIV-1 Viral Load After Measles Vaccination
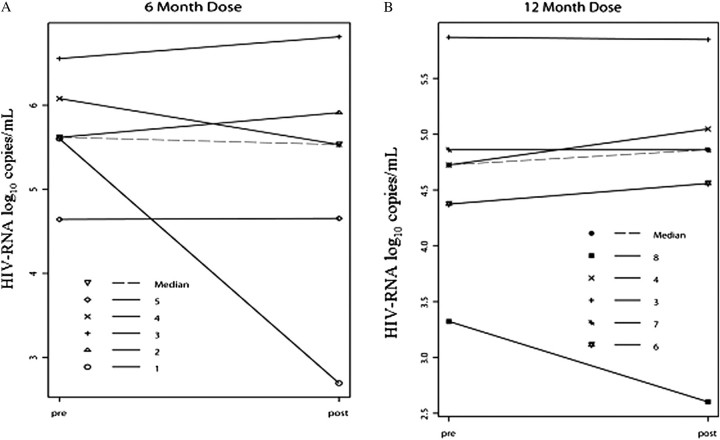
The mean interval between pre- and postvaccination HIV-1 viral load samples for 6-month vaccinees was 59 days, and for 12-month vaccinees was 112 days. No significant differences in postvaccination median HIV-1 RNA values were found following vaccination at 6 or 12 months of age (.0107 and –.0007, respectively; P = .458) (Figure 2).
Figure 2.
Plasma HIV RNA viral load measurements (HIV-RNA log10 copies/mL) pre- and postvaccination were available for 10 subjects enrolled in PACTG Study 225. Panel A shows the results for 5 2DPOS vaccinees given monovalent measles vaccine at 6 months of age (subjects 1, 2, 3, 4, and 5). Panel B shows the results following MMRII immunization at 12 months of age for 2 2DPOS vaccinees (subjects 3 and 4) and 3 1DPOS vaccinees (subjects 6, 7, and 8). Solid lines represent the pre- and postvaccination HIV RNA load for each subject. Dashed lines in each figure represent the median HIV RNA load for each group. The mean interval between pre- and postvaccination viral load measurements was 59 days after vaccination at 6 months of age, and 112 days after vaccination at 12 months of age. Plasma HIV RNA viral loads were measured using Amplicor assay (Roche Diagnostics) as previously described with a minimum detection level of 400 RNA copies per mL. The y-axis scale for Panel A differs from that of Panel B.
HIV indicates human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group.
Maternal Measles Status and Baseline Measles Antibody Titers
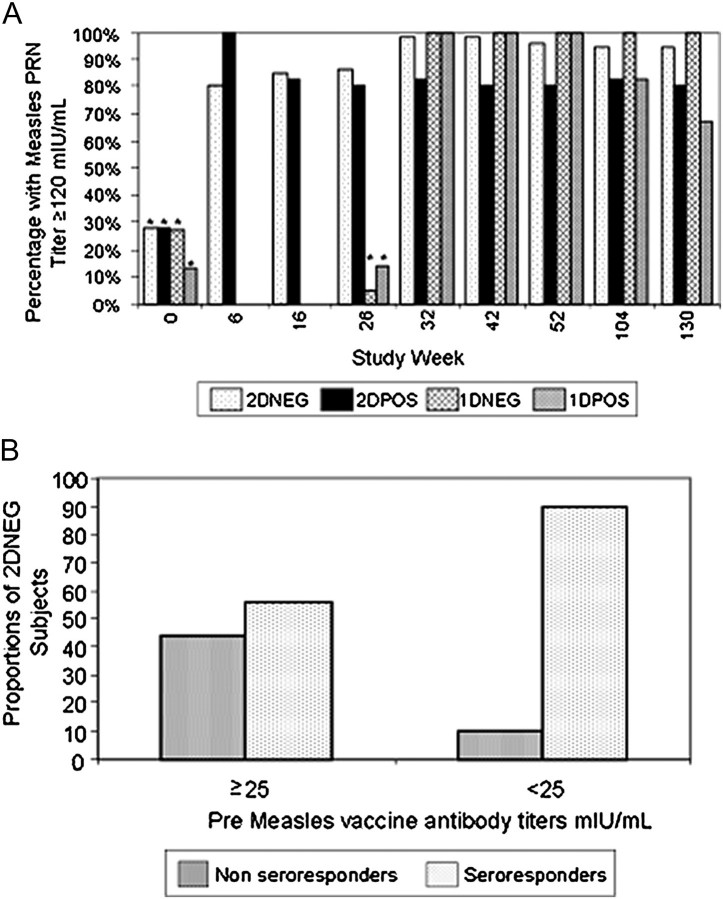
History of measles disease or vaccination were available for 47 of 110 (43%) of mothers. Ten mothers reported measles disease in childhood while 37 had documented measles vaccination (Table 1), and no differences in the proportion with ≥25 mIU/mL (P = .4) or prevaccine median measles antibody titers among the 4 groups at week 0 was found. For 2DNEG subjects, the presence of a measles PRN titer ≥25 mIU/mL at week 0 determined the probability of developing a PRN titer ≥120 mIU/mL at week 6. Only 10 of 18 children with week 0 baseline titers ≥25 mIU/mL seroresponded, while 38 of 42 with baseline titers <25 mIU/mL responded (56% vs 90%, P = .004) (Figure 3b). In contrast, all seven 2DPOS infants seroresponded after primary vaccination at 6 months of age and no differences in seroresponse based on baseline titers ≥25 mIU/mL were found in this group.
Figure 3.
HIV-infected (POS) and uninfected (NEG) children enrolled in PACTG 225 were randomized to 1 of 2 measles vaccine regimens: 2D children were given monovalent measles vaccine at 6 months of age (study week 0) followed by MMRII vaccine at 12 months of age (study week 26); 1D children were only given MMRII at 12 months of age only. Subjects were followed until ∼3 years of age (study week 130). Measles PRN titers were measured in parallel with the Measles International Reference Serum (WHO 66/202) and are expressed in mIU/mL with a titer of 1:8 equivalent to 8 mIU/mL in this assay. Panel A shows pre- and postvaccination measles PRN titers. Bars marked with an asterisk (*) designate the proportion of study subjects with measles PRN titers ≥25 mIU/mL. Bars not marked with an asterisk represent PRN titers ≥120 mIU/mL at study weeks 6, 16, 26, 32, 42, 52, 104, and 130 by treatment group. Panel B is a further analysis of the study week 6 postdose 1 data for 2DNEG subjects only and shows the percent of seroresponders (PRN titer ≥120 mIU/mL) stratified by prevaccination titer ≥25 mIU/mL or <25 mIU/mL.
PACTG indicates Pediatric AIDS Clinical Trials Group; MMRII, measles, mumps, and rubella, live attenuated; WHO, World Health Organization; PRN, plaque reduction neutralization.
Seroresponse Rates Following 1-Dose vs 2-Dose Regimens
Overall, 98% of subjects vaccinated developed measles PRN titers ≥120 mIU/mL by study week 32 (89/91). All seven (100%) 1DPOS subjects given MMRII vaccine at 12 months of age and 5 of 6 2DPOS (83%) subjects immunized at 6 and 12 months of age had measles PRN titers ≥120 mIU/mL when tested at study week 32 (Figure 3) albeit the median antibody titers among 2DPOS (2830 mIU/mL) and 1DPOS (2702 mIU/mL) groups were similar (Table 4). Interestingly, 7 of 7 (100%) 2DPOS subjects responded to primary measles immunization at 6 months of age, all with PRN titers ≥120 mIU/mL (median titer of 2115 mIU/mL) by study week 6. However, 1 2DPOS child failed to maintain the measles antibody levels and did not boost when revaccinated at 12 months of age despite a titer <8 mIU/mL before revaccination.
Table 4.
Median Measles PRNa Titers Pre- and Postimmunization With 1 or 2 Doses of Measles-Containing Vaccine in HIV-Infected and Uninfected Children Born to HIV-Infected Mothers
| Treatment group |
||||||||
|
2DNEG |
2DPOS |
1DNEG |
1DPOS |
|||||
| Study week | Number | Median PRN titer mIU/mL (Q1, Q3) | Number | Median PRN titer mIU/mL (Q1, Q3) | Number | Median PRN titer mIU/mL (Q1, Q3) | Number | Median PRN titer mIU/mL (Q1, Q3) |
| Week 0b | 63 | 8 (8, 39) | 7 | 8 (8, 43) | 26 | 8 (8, 32) | 8 | 8(8,12) |
| Week 6 | 61 | 628 (192, 1448) | 7 | 2115 (695, 2722) | NA | NA | NA | NA |
| Week 16 | 61 | 910 (311, 1907) | 6 | 966 (282, 2435) | NA | NA | NA | NA |
| Week 26c | 62 | 1090 (472, 2212) | 5 | 1569 (306, 2490) | 22 | 2 (2, 2) | 7 | 2 (2,2) |
| Week 32 | 56 | 3445 (1972, 5027) | 6 | 2830 (529, 4956) | 22 | 3240 (1525, 4371) | 7 | 2702 (1351, 5839) |
| Week 42 | 49 | 2353 (1297, 3566) | 5 | 321 (301, 3369) | 21 | 3273 (1360, 4467) | 7 | 2149 (969, 4562) |
| Week 52 | 54 | 1468 (779, 2947) | 5 | 353 (229, 2478) | 23 | 3125 (1693, 4565) | 6 | 2428 (237, 4290) |
| Week 104 | 42 | 1049 (715, 2353) | 6 | 1612 (293, 2628) | 16 | 5602 (1806, 8200) | 6 | 696 (171, 1687) |
| Week 130 | 41 | 9671 (628, 2364) | 5 | 867 (358, 1893) | 17 | 3465d (2395, 8200) | 6 | 573 (111, 2048) |
NOTE. aPRN indicates plaque reduction neutralization.
Time point at which 2DNEG and 2DPOS children were immunized with measles vaccine at 6 months of age (study week 0).
Time point at which all children (2DPOS, 2DNEG, 1DPOS and 1DNEG) were given MMRII at 12 months of age.
Median measles PRN titer in the 1DNEG group was significantly higher than the median PRN titer in the 2DNEG group with Monte Carlo–estimated P value <.001 calculated using the Wilcoxon signed rank test.
When first comparing the seroresponses of HIV-infected subjects with HIV-uninfected subjects in those given a single dose MMRII at 12 months of age, the seroresponse rates were identical in 1DPOS and 1DNEG subjects, as all achieved measles PRN titers ≥120 mIU/mL irrespective of HIV status (Figure 3). Median antibody titers at week 32 were also similar in 1DPOS and 1DNEG subjects (2702 vs 3240 mIU/mL, respectively, P = .4144; Table 4). Only 49 of 61 (80%) of 2DNEG subjects responded to Attenuvax as compared with 100% (7/7) of 2DPOS subjects 6 weeks after the first dose. Also, median titers after Attenuvax were significantly lower in 2DNEG subjects as compared to 2DPOS subjects (628 vs 2115 mIU/mL, respectively, P = .023). Following MMRII, 98% of 2DNEG subjects developed titers ≥120 mIU/mL as compared with 83% of 2DPOS subjects. Median titers were higher in 2DNEG subjects than 2DPOS subjects (3445 and 2830 mIU/mL, respectively) but not significantly different (P = .2).
Measles Antibody Titer Decline
By ∼3 years of age (study week 130), median PRN antibody titers among 2DPOS and 1DPOS subjects were low (867 vs 573 mIU/mL, respectively, P = .163; Table 4) and the proportion of subjects with antibody titers ≥120 mIU/mL had similarly declined with 4 of 5 (83%) 2DPOS subjects and 4 of 6 (67%) 1DPOS maintaining titers ≥120 mIU/mL.
In contrast, among HIV-uninfected subjects, the median titers in the 1DNEG subjects were significantly higher than those observed in 2DNEG subjects at ∼3 years of age (3465 vs 967 mIU/mL, respectively, P = .001) and 100% of 1DNEG and 95% of 2DNEG subjects maintained levels of measles PRN antibody ≥120 mIU/mL (Table 4). Due to the skewness of the data (P value for normality test <.01), median values for measles antibody titers are used for the above comparisons and presented in Table 4, while geometric mean titers are provided in a supplemental table.
DISCUSSION
This prospectively designed study assessed seroresponse rates following early versus conventional measles vaccination and monitored adverse clinical reactions and CD4+ subset changes in of HIV-infected infants not receiving antiviral therapy. Because HAART treatment during pregnancy inhibits HIV transmission to newborns, the trial did not enroll sufficient numbers of subjects to prove or disprove the primary study hypothesis regarding improved immunogenicity following early 2-dose measles vaccination in HIV-infected children in the United States. Nevertheless, this study yielded useful data with regard to the secondary objectives, and these findings are summarized herein. Measles vaccination at 6 months of age was well tolerated, as no clinical reactions >grade 3 were observed among HIV-infected groups and there were no significant adverse changes in CD4+ counts or percentages detected 6 weeks after early or conventional measles vaccination. Retrospective examination of viral load before and after measles vaccine in 8 HIV-infected subjects showed no apparent increase in HIV-RNA following immunization at 6 or 12 months of age. From these observations and within this limited cohort, it appears that early administered measles vaccine and the 2-dose vaccine regimen were well tolerated. A report from Malawi also suggests that Edmonston-Zagreb vaccine administered to 6-month-old HIV-infected infants is well tolerated, though immunosuppression after this vaccine strain has been reported elsewhere in HIV-uninfected infants [24, 25].
The study also showed that early vaccination was immunogenic in HIV-infected subjects with a 100% seroresponse rate and a high median measles PRN titer after a first dose at 6 months of age (2115 mIU/mL), confirming our previous observation that suggested a higher rate of seropositivity when subjects are vaccinated at <1 year of age [9–10]. Unfortunately, due to premature termination, PACTG 225 lacked statistical power to confirm the primary study hypothesis. Reports from sub–Saharan Africa indicate that immunity induced by vaccinating untreated HIV-infected children with Edmonston-Zagreb measles strain at 9 months or with 2 doses at 6 and 9 months achieved only 64% seropositivity rates [23, 24].
In this study, measles antibody persisted in the majority of HIV-infected, 2-dose recipients at or above titers associated with protection (median 867 mIU/mL) for at least 2 years after revaccination, suggesting a lower rate of secondary vaccine failure. Observational studies from United States, Thailand and sub–Saharan Africa document a median loss of detectable measles EIA antibody by ∼30 months after primary immunization [8, 14, 25–27]. The World Health Organization (WHO) therefore recommends a routine second dose of measles vaccine for all HIV-infected children [14].
Revaccinating HIV-infected children after provision of HAART (10.7–31.8 months) results in improved seroprotection rates (64%–90%) [28–31]. In one study, despite immune recovery and improved CD4+ counts, the seroprotection rate declined to 80% within 6 months of vaccination. The persistence of functional T lymphocyte and B lymphocyte defects despite HAART may be responsible for continued decline in antibody responses [32]. In our study, 2DPOS infants had high CD4+ T lymphocytes (2074 cells/mm3) at primary vaccination and 80% of vaccinees retained seroprotective measles titers for at least 2 years after their last vaccination, suggesting a better sustainability than previously reported. Additional benefits may be gained by early initiation of HAART in combination with early vaccination.
This study also compared 2 vaccine regimens in HIV-uninfected children. Similar to previous reports, median measles antibody titers remained significantly lower despite revaccination in 2DNEG subjects than in 1DNEG subjects receiving a single dose of MMRII at 12 months of age [33]. The significance of lower measles antibody titers in children immunized early in life, if any, is unknown [22].
Worldwide, measles case fatality rates are highest in children under 12 months of age [24]. Recent data from the developing world suggest that unlike infants born during the prevaccine era who derived protection from passively acquired measles antibody throughout the first year of life [34–35], the present cohort of infants are more likely to be born to measles-vaccinated mothers who have lower levels of measles antibody and thus are susceptible to measles infection earlier in life [36]. For this reason, in the developing world, consideration is now being given to vaccinating HIV-infected children against measles at 6 months of age to narrow the window of susceptibility [14, 37].
Sporadic importation of measles into the United States continues. Since measles was declared contained in 2000, 130 measles cases were reported in 2008 and 13% of these cases occurred in children under 12 months of age [38, 39]. In the present study, 73% of US-born infants had either lost or had low levels of passively acquired measles antibodies (<25 mIU/mL) by 6 months of age, making them vulnerable to severe measles infection but also likelier to respond to vaccine.
Although the number of HIV-infected children studied under PACTG 225 was small and the study lacks substantial power to distinguish significant differences between groups, these findings show that Attenuvax given to 6-month-old infants can be well tolerated, highly immunogenic, and can provide early protection to these children. These data provide support for the current WHO recommendation to vaccinate HIV-infected children as early as 6 months of age in measles-endemic regions. In the United States where vaccination coverage is currently high, herd immunity provides some protection for this vulnerable infant population. However, a decline in the vaccination coverage in the general population can result in increased mortality in this highly susceptible infant group [40]. In developing countries, a combination of early HAART to preserve immune system function and early vaccination with a 2-dose regimen may prove to be beneficial in providing sustained protection for these infants, but this requires further study. Similar trials of 1-dose and 2-dose regimens among HIV-infected infants receiving HAART are warranted in developing countries with high incidence of measles and HIV.
Supplementary Data
Supplementary data are available at (http://www.jid.oxfordjournals.org/) online.
Funding
This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the PACTG, and #1 U01 AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group, and was also supported in part by a grant from National Institutes of Health (NIH) and NICHD (K. K.). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support of the sites was provided by the NIAID and NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). Merck Vaccine Division supplied the Attenuvax and MMRII vaccines.
Supplementary Material
Acknowledgments
We thank Dr Illsoon Yang for the interim analyses. Drs Jinhai Wang and David Menschik for their critical review of the manuscript, and the principal investigators and participating families at the ACTG study sites.
PACTG 225 Site Acknowledgments
Site 5051-University of Florida Health Science Center; Site 60336-Baylor College of Medicine CTU; Site 3601-UCLA Med. CTR (Dept of Ped); Site-4401 NYU Med. Ctr., Dept. of Medicine (William Borkowsky, MD; Mary Minter, RN; Nagamah Deygoo; Aditya Kaul, MD); Site 5006-Harlem Hospital; Site 5038-Yale University School of Medicine; Site 5048-Los Angeles County Medical/University of Southern California; Site 5052-Univ. of Colorado Denver NICHD CRS (Myron J. Levin, MD; Elizabeth J. McFarland, MD; Carol Salbenblatt, RN, MSN; Jody Maes, MD; This work was supported in part by Grant Number UL1 RR025780 NIH/NCRR Colorado CTSI); Site 2901-Boston Children's Hospital; Site 5044-Howard University Hospital, Dept. of Pediatrics; Site 6801-UW School of Medicine-CHRMC (Lisa M. Frenkel MD; Ann J. Melvin MD, MPH; Kathleen Mohan, ARNP); Site 4501-UCSF Medical Center; Site 5003-Metropolital Hospital; Site 5057-University of Rochester-Pediatric Component; Site 7101-Children's Hospital, Washington, DC; Site 60446-Univ. Of Puerto Rico CTU (Irma L. Febo, MD; Licette Lugo MD; Ruth Santos, RN, MPH; Maritza Cruz); Site 2802-U. of Medicine & Dentistry of NJ, NJ Medical School; Site 5032-Robert Wood Johnson Univ. Hosp; Site 5040-Suny Health Science Center, Stony Brook, Ped. Inf.; Site 60466-UCLA-Los Angeles/Brazil AIDS Consortium (LABAC) CTU); Site 5028-Univ. of Illinois College of Medicine at Chicago, Dept. of Peds (Kenneth Rich, MD; Karen Hayani, MD; Julia Camacho, RN; Carolyn Everett, RN); Site 60318-UCSD IMPAACT CTU; Site 60341-Columbia Collaborative-HIV/AIDS CTU.
Members of the PACTG 225 Protocol Team include: Bonnie Zimmer, BS, Frontier Science & Technology Research Foundation, Amherst, NY; Elizabeth Hawkins, MA; Thomas Nevin, MA, MBA, IMPAACT Operations Center, Silver Spring, MD.
This study has been presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Diego, CA, 1998.
References
- 1.Enders JF, Carthy Mc, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in cases of giant-cell pneumonia without rash. N Engl J Med. 1959;261:875–81. doi: 10.1056/NEJM195910292611801. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Measles in HIV-1 infected children, United States. MMWR. 1988;37:183–6. [PubMed] [Google Scholar]
- 3.McLaughlin M, Thomas P, Onorato I, et al. Live virus vaccines in human immunodeficiency virus-infected children: a retrospective survey. Pediatrics. 1988;82:229–33. [PubMed] [Google Scholar]
- 4.Centers for Disease Control. General recommendation on immunization—Recommendation of the Advisory Committee on Immunization Practices (ACIP) MMWR. 1994;43:22–5. [Google Scholar]
- 5.Angel JB, Walpita P, Lerch RA, et al. Vaccine-associated measles pneumonitis in an adult with AIDS. Ann Intern Med. 1998;129:104–6. doi: 10.7326/0003-4819-129-2-199807150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Krasinski K, Borkowsky W. Measles and measles immunity in children with human immunodeficiency virus. JAMA. 1989;261:2512–6. [PubMed] [Google Scholar]
- 7.Palumbo P, Hoyt L, Demasio K, Oleske J, Connor E. Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr Inf Dis J. 1992;11:1008–14. doi: 10.1097/00006454-199211120-00004. [DOI] [PubMed] [Google Scholar]
- 8.Brunell PA, Vimal V, Sandhu M, Courville TM, Daar E, Israele V. Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J AIDS. 1995;10:540–8. [PubMed] [Google Scholar]
- 9.Arpadi SM, Markowitz LE, Baughman AL, et al. Measles antibody in vaccinated human immunodeficiency virus type 1-infected children. Pediatrics. 1996;97:653–7. [PubMed] [Google Scholar]
- 10.Rudy BJ, Rutstein RM, Pinto-Martin J. Responses to measles immunization in children infected with human immunodeficiency virus. J Pediatr. 1994;125:72–4. doi: 10.1016/s0022-3476(94)70125-3. [DOI] [PubMed] [Google Scholar]
- 11.Borkowsky W, Rigaud M, Krasinski K, Moore T, Lawrence R, Pollack H. Cell-mediated and humoral immune responses in children infected with human immunodeficiency virus during the first four years of life. J Pediatr. 1992;120:371–5. doi: 10.1016/s0022-3476(05)80899-6. [DOI] [PubMed] [Google Scholar]
- 12.Measles immunization in HIV-infected children. American Academy of Pediatrics. Committee on Infectious Diseases and Committee on Pediatric AIDS. Pediatrics. 1999;103:1057–60. [PubMed] [Google Scholar]
- 13.Murphy MD, Brunell PA, Lievens AW, Shehab ZM. Effect of early immunization on antibody response to reimmunization with measles vaccine as demonstrated by enzyme-linked immunosorbent assay (ELISA) Pediatrics. 1984;74:90–3. [PubMed] [Google Scholar]
- 14.Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84:349–60. [PubMed] [Google Scholar]
- 15.Appendix II, ACTG Division of AIDS toxicity table for grading severity of pediatric (3 months of age) adverse Experiences. 1994. [Google Scholar]
- 16.Albrecht P, Herman K, Burns GR. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–60. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch RL, Mokhtarian F, Griffin DE, Brooks BR, Hess J, Johnson RT. Measles virus vaccination of measles seropositive individuals suppresses lymphocyte proliferation and chemotactic factor production. Clin Immunol Immunopathol. 1981;21:341–50. doi: 10.1016/0090-1229(81)90223-3. [DOI] [PubMed] [Google Scholar]
- 19.Okada H, Sato TA, Katayama A, et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch Virol. 2001;146:859–74. doi: 10.1007/s007050170121. [DOI] [PubMed] [Google Scholar]
- 20.Sabin AB, Flores Arechiga A, Fernandez de Castro J, et al. Successful immunization of children with and without maternal antibody by aerosolized measles vaccine. I. Different results with undiluted human diploid cell and chick embryo fibroblast vaccines. JAMA. 1983;249:2651–62. [PubMed] [Google Scholar]
- 21.Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–8. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 22.Kumar ML, Johnson CE, Chui LW, Whitwell JK, Staehle B, Nalin D. Immune response to measles vaccine in 6-month-old infants of measles seronegative mothers. Vaccine. 1998;16:2047–51. doi: 10.1016/s0264-410x(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 23.Aaby P, Knudsen K, Whittle H, et al. Long-term survival after Edmonston-Zagreb measles vaccination in Guinea–Bissau: increased female mortality rate. J Pediatr. 1993;122:904–8. doi: 10.1016/s0022-3476(09)90015-4. [DOI] [PubMed] [Google Scholar]
- 24.Helfand RF, Witte D, Fowlkes A, et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. 2008;198:1457–65. doi: 10.1086/592756. [DOI] [PubMed] [Google Scholar]
- 25.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 26.al-Attar I, Reisman J, Muehlmann M, McIntosh K. Decline of measles antibody titers after immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:149–51. [PubMed] [Google Scholar]
- 27.Aurpibul L, Puthanakit T, Siriaksorn S, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against measles in HIV-infected children with immune recovery after highly active antiretroviral therapy. HIV Med. 2006;7:467–70. doi: 10.1111/j.1468-1293.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J. 2009;28:295–9. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children. Clin Infect Dis. 2001;32:1090–4. doi: 10.1086/319591. [DOI] [PubMed] [Google Scholar]
- 30.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Response to measles, mumps, and rubella revaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Clin Infect Dis. 2007;45:637–42. doi: 10.1086/520651. [DOI] [PubMed] [Google Scholar]
- 31.Sutcliff CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630–42. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Feyen O, Jebran AF, et al. Memory B-cell function in HIV-infected children—decreased memory B cells despite ART. Pediatr Res. 2009;66:185–90. doi: 10.1203/PDR.0b013e3181aa057d. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz LE, Albrecht P, Orenstein WA, Lett SM, Pugliese TJ, Farrell D. Persistence of measles antibody after revaccination. J Infect Dis. 1992;166:205–8. doi: 10.1093/infdis/166.1.205. [DOI] [PubMed] [Google Scholar]
- 34.Pabst HF, Spady DW, Marusyk RG, et al. Reduced measles immunity in infants in a well-vaccinated population. Pediatr Inf Dis J. 1992;11:525–9. doi: 10.1097/00006454-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado YA, Lawrence EC, DeHovitz R, Hatzell H, Albrecht P. Early loss of measles antibodies in infants of mothers with vaccine-induced immunity. Pediatrics. 1995;96:447–50. [PubMed] [Google Scholar]
- 36.Samb B, Aaby P, Whittle HC, et al. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr Infect Dis J. 1995;14:203–9. doi: 10.1097/00006454-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Markowitz LE, Albrecht P, Rhodes P, et al. Changing levels of measles antibody titers in women and children in the United States on response to vaccination. Kaiser Permanente Measles Vaccine Trial Team. Pediatrics. 1996;97:53–8. [PubMed] [Google Scholar]
- 38.Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–24. doi: 10.1086/522989. [DOI] [PubMed] [Google Scholar]
- 39.Update: Measles—United States. MMWR. 2008;57:893–6. [PubMed] [Google Scholar]
- 40.Immunizations. Studies affirm need for vaccinations in HIV-infected patients. IDS Policy Law. 2007;22:5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.