Abstract
The fate of antigen-specific T cells was characterized in myelin basic protein (MBP) T-cell receptor (TCR) transgenic (Tg) mice after oral administration of MBP. Peripheral Th cells are immediately activated in vivo, as indicated by upregulation of CD69 and increased cytokine responses (Th1 and Th2). Concurrently, surface TCR expression diminishes and internal TCR levels increase. When challenged for experimental autoimmune encephalomyelitis during TCR downmodulation, Tg mice are protected from disease. To characterize Th cells at later times after antigen feeding, it was necessary to prevent thymic release of naive Tg cells. Therefore, adult Tg mice were thymectomized before treatment. TCR expression returns in thymectomized Tg mice 3 days after MBP feeding and then ultimately declines in conjunction with MBP-specific proliferation and cytokine responses (Th1-type and Th2-type). The decline correlates with an increase in apoptosis. Collectively, these results demonstrate that a high dose of fed antigen induces early T-cell activation and TCR downmodulation, followed by an intermediate stage of anergy and subsequent deletion.
Introduction
Clinical, histopathological, and immunological similarities between experimental autoimmune encephalomyelitis (EAE) and the human central nervous system (CNS) autoimmune disorder multiple sclerosis (MS) allow EAE to be used to test immunotherapeutic strategies for MS. We and others have demonstrated amelioration of EAE via alteration in autoantigen-specific T-cell function after oral administration of a CNS protein (myelin basic protein, or MBP) (1–4). The mechanism by which the pathogenic T cells are rendered tolerant to self-antigen is determined by the dose of fed antigen. Repeated low doses (microgram to 1 mg range) are reported to induce TGF-β release from antigen-specific suppressor T cells (5, 6), or immune deviation from a “pro-inflammatory” Th1 to a “suppressive” Th2 cytokine profile (7). Alternatively, high doses of orally administered antigen (> 1 mg) may promote trafficking of antigen-specific T cells from the periphery to the gut (8), or anergy (9–11) and/or deletion of antigen-specific T cells (12, 13).
Deciphering the mechanisms of oral tolerance in conventional mice is hindered by the low frequency of antigen-specific cells. Therefore, we utilized MBP T-cell receptor (TCR) transgenic (Tg) mice, in which 95% of CD4+ T cells express a Vα4/Vβ8.2 TCR specific for the immunodominant NAc1-11 encephalitogenic epitope of MBP (14). Using these mice, we characterized MBP-specific Tg T cells over time after a high dose of orally administered MBP. The influence of new Tg thymic emigrants was eliminated by thymectomizing adult MBP TCR Tg mice before feeding. We found that a single high dose of MBP induces immediate TCR downmodulation and T-cell activation in vivo, which corresponds with protection from EAE. Subsequently, T cells enter an anergic state, followed by an eventual decline in T-cell number and function with evidence of apoptosis.
Methods
Animals.
B10.PL Vα4/Vβ8.2 MBP TCR Tg mice were obtained from C. Janeway (Yale University, New Haven, Connecticut, USA) and maintained in the Tg animal facility at The Ohio State University (OSU). Progeny were screened by flow cytometry for expression of the Vβ8.2 transgene on CD4+ blood lymphocytes. Tg+ mice were used in experiments at 6–8 weeks of age.
Neuroantigens.
MBP was extracted from guinea pig spinal cords (Harlan Sprague-Dawley, Indianapolis, Indiana, USA) using the method of Deibler et al. (15) or Swanborg et al. (16). NAc1-11 MBP peptide (ASQKRPSQRHG) was synthesized by the OSU peptide facility.
Induction of oral tolerance.
Mice were deprived of food, but not water, for 4–6 hours before oral antigen administration. MBP (in 0.5 ml PBS) was administered by gastric intubation to anesthetized mice. Control Tg mice were either untreated or were fed vehicle (PBS) or 100 mg ovalbumin (OVA). There were no observable differences between control groups.
Flow cytometry.
A total of 0.5 × 106 cells were stained with: FITC anti-Vβ 8.1/8.2; FITC anti–I-Ab; cychrome anti-CD4; FITC anti-CD62L; FITC anti-CD44; FITC anti-CD69; phycoerythrin (PE) anti-CD45RB; PE anti-FasL; and PE anti-Fas (PharMingen, San Diego, California, USA). Isotype controls: mouse IgG2a and IgG2b, rat IgG2a and IgG2b, and hamster IgG were run with each sample and matched for fluorochrome. After a 30-minute incubation, cells were washed and resuspended in 1% paraformaldehyde. Analysis was performed on an Epics XL flow cytometer (Beckman Coulter Inc., Miami, Florida, USA). Forward and right angle light scatter was used to gate the lymphocyte population and exclude monocytes/macrophages, granulocytes, and dead cells.
Analysis of intracellular TCR expression was performed using the Immunotech IntraPrep Permeabilization Reagent kit (Coulter Electronics Ltd.) with modifications. Briefly, 106 cells were incubated with FITC anti-Vβ 8.1/8.2 in 30% mouse serum (Sigma Chemical Co., St. Louis Missouri, USA). After washing, cells were incubated with a three- to fivefold excess of purified anti-Vβ 8.1/8.2 to saturate extracellular TCR sites. Cells were then washed, fixed, and permeabilized according to manufacturer’s instructions. Cells were incubated with PE anti-Vβ 8.1/8.2 for intracellular Vβ protein detection and then washed, resuspended in 0.5% paraformaldehyde, and analyzed (at least 10,000 events) by flow cytometry.
Induction of EAE.
Tg mice were injected subcutaneously over four sites on the back with 100 μl containing 200 μg MBP combined with CFA (containing 200 μg heat-killed Mycobacterium tuberculosis Jamaica strain). Mice also received 200 ng pertussis toxin (List Biological, Campbell, California, USA) intraperitoneally in 0.2 ml PBS, at the time of immunization and 48 hours later. For passive EAE, lymph node (LN) and spleen (SPL) cells were cultured in vitro with MBP and transferred intraperitoneally (10 × 106) to B10.PL recipients. Mice were also given pertussis toxin as already described here. All animals were observed daily for clinical signs and scored as follows: limp tail or waddling gait with tail tonicity, 1; waddling gait with limp tail (ataxia), 2; ataxia with partial limb paralysis, 2.5; full paralysis of one limb, 3; full paralysis of one limb with partial paralysis of second limb, 3.5; full paralysis of two limbs, 4; moribund, 4.5; and death, 5.
Adult thymectomy.
Thymectomy surgery was performed under a dissecting microscope as reported elsewhere (17). Adult Tg mice were given 20 μg gentocin intraperitoneally and were then anesthetized with ketamine (82–110 mg/kg) and xylazine (7.5 mg/kg) intraperitoneally. During surgery, mice were intubated and maintained on a rodent ventilator. An upper median sternotomy was performed, and both lobes of the thymus were dissected and removed. The thoracic cage was sutured closed and the skin was secured; mice were then extubated and provided with oxygen. Regular breathing resumed within 1 minute of extubation, and the entire procedure lasted approximately 10–15 minutes per animal. Sham surgery control mice were anesthetized and intubated; the thoracic cage was then opened and sutured closed. Mice were used in experiments 7–10 days after surgery.
Cytokine ELISPOT.
ELISPOT analysis was performed as described elsewhere (18). Plates (Whatman Polyfiltronics, Rockland, Massachusetts, USA) were coated overnight with: 2 μg/ml anti–IL-2, 4 μg/ml anti–IL-4, 5 μg/ml anti–IL-5, and 4 μg/ml anti-IFN-γ (PharMingen). Plates were blocked for 1 hour, and then LN cells (5 × 105 per 0.1 ml) were resuspended in HL-1 medium (BioWhittaker Inc., Walkersville, Maryland, USA) with 1% L-glutamine and 1:1,000 gentamicin and added to replicate wells with 40 μg/ml MBP, 40 μg/ml OVA (Sigma Chemical Co.), 1–5 μg/ml anti-CD3ε (PharMingen), or medium alone. Cultures were incubated for 24 (IL-2, IFN-γ) or 48 (IL-4, IL-5) hours and were then washed and secondary antibodies added: 2 μg/ml biotinylated anti–IL-2, anti–IL-4, and anti–IFN-γ, and 4 μg/ml biotinylated anti–IL-5 (PharMingen). After overnight incubation, plates were washed and incubated with alkaline phosphatase conjugated goat anti-biotin IgG (Vector Laboratories, Burlingame, California, USA) for 2 hours. Plates were washed, developed with BCIP/NBT phosphatase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA), and dried and analyzed by computer-assisted image analysis using a Series I Immunospot Image Analyzer (Cellular Technology, Cleveland, Ohio, USA).
TGF-β ELISA.
TGF-β analysis was performed as described elsewhere (19). Supernatants were harvested from SPL cells (4 × 106/ml) cultured in X-Vivo serum-free medium (BioWhittaker) for 72 hours ± MBP (40 μg/ml). Chicken anti–TGF-β (2.5 μg/ml) (R&D Systems Inc., Minneapolis, Minnesota, USA) was incubated in Immulon II plates (Dynatech Laboratories, Chantilly, Virginia, USA) at 4°C overnight. Plates were blocked with 0.25% gelatin (Bio-Rad Laboratories Inc., Hercules, California, USA) for 1 hour, and then sample (100 μl) or standard dilutions of hu rTGFβ (R&D Systems Inc.) were added for 2 hours. Mouse anti–TGF-β 1,2,3 (1 μg/ml) (Genzyme Pharmaceuticals, Cambridge, Massachusetts, USA) was added for 45 minutes, followed by 1 μg/ml of biotinylated horse anti-mouse IgG (Vector Laboratories). Avidin-peroxidase (Sigma Chemical Co.) was added and incubated, and then plates were developed with 2,2′azino-di-3ethyl-benzthiazoline sulfonate di-ammonium salt (ABTS) substrate (Boehringer Mannheim, Indianapolis, Indiana, USA). Samples were read at 405 nm on a Bio-Rad ELISA reader (Bio-Rad Laboratories Inc.).
Lymphocyte proliferation.
SPL, LN, or mesenteric lymph node (MLN) cells were cultured in RPMI 1640 containing 10% FBS, 25 mM HEPES, 2 mM L-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 5 × 10–5M 2-mercaptoethanol in round-bottom 96-well plates (5 × 105 cells per well). Cells were cultured with NAc1-11 (10 μg/ml) or medium alone in triplicate for 72 hours, including a final 18-hour pulse with [3H]thymidine. Cultures were harvested onto glass fiber filter mats using a Skatron harvester (Molecular Devices Corp., Sunnyvale, California, USA) and counted by liquid scintillation on a Wallac Betaplate (Wallac Inc., Gaithersburg, Maryland, USA).
Apoptosis analysis.
Analysis of DNA fragmentation was performed by TdT-mediated dUTP nick end labeling (TUNEL) (Boehringer Mannheim). Cells (106) were stained with PE–anti-Vβ8 and Cyc–anti-CD4, fixed with 4% paraformaldehyde, permeabilized with 0.1% TritonX-100/0.1% sodium citrate, and exposed to the TdT/biotin-dUTP mix for 1 hour at 37°C. FITC-streptavidin was added for 30 minutes, followed by washing. Positive control samples were treated with DNase (Genzyme Pharmaceuticals) for 10 minutes before TdT labeling, whereas negative control samples contained no TdT. Annexin V staining was performed according to the manufacturer’s instructions (Boehringer Mannheim). TUNEL and Annexin V stained samples (at least 10,000 events) were analyzed on an Epics XL flow cytometer.
Statistical analysis.
A nonparametric ANOVA with Kruskal-Wallis analysis was utilized to determine differences between groups. All values were considered significantly different at P ≤ 0.05.
Results
A single high dose of orally administered antigen induces immediate TCR downmodulation.
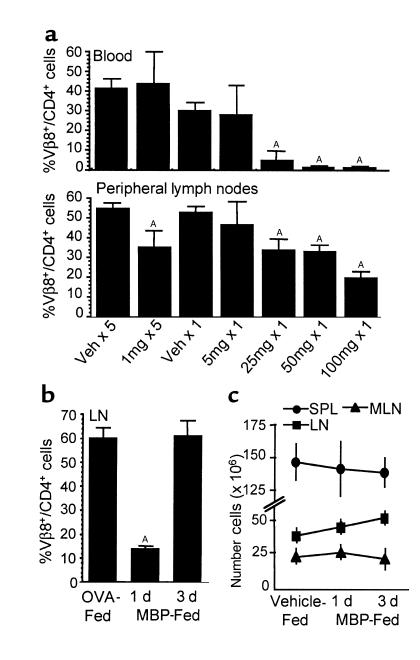
Considering the high frequency of MBP-specific T cells in MBP TCR Tg mice, it was first necessary to establish the appropriate dose for oral administration of MBP. Tg mice were fed either low doses (1 mg) of MBP every other day for 10 days (five feeds) or increasing single doses (5, 25, 50, or 100 mg) of MBP. One day after feeding, peripheral lymphocytes were analyzed by flow cytometry for Tg Vβ8 TCR expression on CD4+ cells. Repeated low oral doses of MBP decreased the percentage of Vβ8+/CD4+ cells in peripheral LNs (axillary, brachial, cervical, deep cervical, inguinal, mandibular, popliteal, periaortic) (Figure 1a). However, profound decreases were observed in the blood and LN after a single oral administration of 25, 50, or 100 mg of MBP. Similar reductions were observed in SPL and MLN cell preparations, but only following a single 100-mg dose of MBP (data not shown). These data suggest that a single high oral dose of MBP has the most significant effect on the MBP-specific population.
Figure 1.
A single high dose of orally administered antigen reduces TCR expression without affecting lymphoid organ cellularity. (a) MBP TCR Tg mice were fed vehicle or 1 mg MBP every other day for 10 days (five feeds), or vehicle or 5, 25, 50, or 100 mg MBP once. Lymphocytes were analyzed by flow cytometry 1 day after the last feeding for expression of the Tg Vβ8.2 TCR on CD4+ cells. Each bar is the mean percentage of Vβ8+/CD4+ cells ± SEM (n = 3). AValues are statistically different from the corresponding vehicle-fed controls at P ≤ 0.05. (b) Peripheral LN cells from MBP TCR Tg mice fed 100 mg MBP or 100 mg OVA were analyzed by flow cytometry 1 or 3 days after feeding. Each bar is the mean percentage of Vβ8+/CD4+ cells within the lymphocyte population ± SEM (n = 3). AValues are statistically different from OVA-fed control mice at P ≤ 0.05. (c) The total number of cells in single-cell suspensions from spleen (SPL), peripheral lymph nodes (LN), and mesenteric LN (MLN) of MBP- or vehicle-fed Tg mice was determined by trypan blue exclusion 1 or 3 days after feeding. Data are reported as the mean number of cells × 106 ± SEM (n = 3–11). Values were not statistically different from vehicle-fed control mice.
To determine if the early reduction of Vβ8+/CD4+ Tg cells was maintained, the MBP-specific T-cell population was assessed 1 or 3 days after MBP feeding (100 mg). Oral administration of an irrelevant antigen (OVA) had no effect on Vβ8+/CD4+ T-cell populations (Figure 1b). However, there was a significant reduction in Vβ8+/CD4+ T cells 1 day after MBP feeding, and there was a return of the Tg population by day 3. Similar observations were made in SPL and MLN (data not shown). It has been proposed that orally administered antigen can induce trafficking of antigen-specific T cells out of organized lymphoid compartments (8). However, despite a significant reduction in the percentage of Tg cells, the total cell numbers collected from SPL, LNs, and MLNs 1 and 3 days after MBP feeding were equivalent to vehicle-fed control mice (Figure 1c). Furthermore, immunohistochemical analysis did not demonstrate an increase of CD4+ Tg cells in the lamina propria, Peyer’s patches, or liver after MBP feeding (data not shown). These observations suggest that the immediate reduction of TCR+ Tg cells and their subsequent return is not due to T-cell trafficking.
It is possible that MBP-specific T cells undergo apoptosis within the first 24 hours of a high oral dose of MBP. However, TUNEL and Annexin V staining of lymphocyte populations isolated 1 day after MBP feeding did not demonstrate evidence for apoptosis of Vβ8+/CD4+ cells (data not shown). Furthermore, when the influence of newly derived Vβ8+/CD4+ cells was eliminated by thymectomizing adult MBP TCR Tg mice before feeding, Vβ8+/CD4+ expression was reduced by 78–94% in SPL, LNs, and MLNs 1 day after feeding, then completely restored by day 3. Given that the mice were thymectomized and unable to regenerate their T-cell repertoire, the Vβ8+/CD4+ cells present on day 3 must represent the original MBP-specific population. Collectively, these observations suggest that the reduction in Vβ8+/CD4+ cells observed 1 day after antigen feeding is not due to clonal deletion.
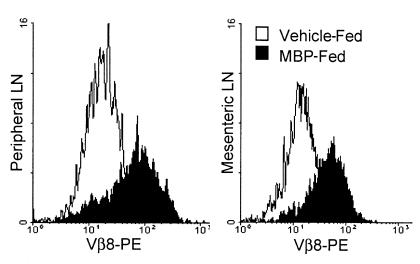
Another explanation for reduced Vβ8+/CD4+ expression is antigen-induced TCR downmodulation. To examine this possibility, surface versus internal TCR expression was analyzed. LN and MLN cells were surface-labeled with FITC anti-Vβ8.2 TCR antibody and then permeabilized, and internal TCR was labeled with PE anti-Vβ8.2. Surface FITC-Vβ8.2+ cells were analyzed for internal TCR expression, and Figure 2 demonstrates that MBP feeding increases levels of internal Tg TCR. The profound reduction in surface TCR+ cells 1 day after MBP feeding is also represented in Figure 2 by the reduced number of analyzed cells in the MBP-fed population. Although it cannot be determined whether the internal TCR is newly synthesized protein, these experiments suggest that TCR is internalized in vivo in response to oral administration of a high dose of MBP.
Figure 2.
Surface TCR downmodulation corresponds with TCR internalization. Peripheral and mesenteric LN cells from MBP or vehicle-fed Tg mice were analyzed by flow cytometry for surface (FITC) and internal (PE) Tg Vβ8.2 TCR expression 1 day after feeding. Surface TCR+ cells were gated and then analyzed for internal TCR staining. Histograms are representative of two separate experiments.
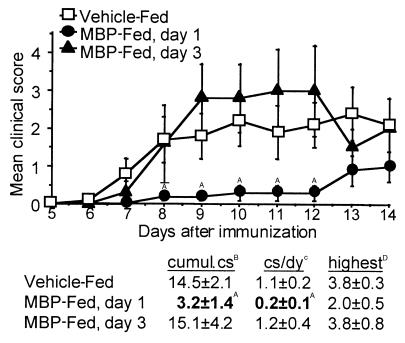
MBP TCR Tg mice are protected from EAE when challenged while TCR expression is reduced.
To assess the in vivo effects of oral antigen on Th cell function, Tg mice were challenged for EAE after MBP feeding. Figure 3 shows that Tg mice are protected from EAE when challenged during reduced TCR expression (day 1), as shown by a reduction in cumulative clinical score (cs), cs per day, and mean highest cs. However, MBP TCR Tg mice challenged when TCR expression is restored (day 3) are not protected from disease. When Tg cells are isolated 1 day after vehicle or MBP feeding and stimulated in vitro with MBP, only Tg cells from vehicle-fed mice passively transfer EAE to non-Tg recipients (EAE incidence: 2/4). EAE is not induced when cells from MBP-fed mice are transferred (EAE incidence: 0/7). Interestingly, adult Tg mice that were thymectomized, then challenged for EAE 1 day after MBP feeding, demonstrated a 14 day cumulative cs comparable to euthymic mice, but were not completely protected from disease (EAE mortality: 2/7) when compared with vehicle-fed thymectomized controls (EAE mortality: 3/4). Thus, onset of disease 13 days after immunization (Figure 3) most likely represents restored function of the Tg cells that would otherwise be rendered tolerant after high-dose MBP feeding.
Figure 3.
MBP TCR Tg mice are protected from EAE when challenged while TCR expression is reduced. MBP TCR Tg mice were fed vehicle or 100 mg MBP, and EAE was induced 1 or 3 days later by immunization with MBP/CFA/pertussis toxin. Mice were scored daily for disease, and the mean score of each group ± SEM is shown (n = 4–17). Clinical scores (cs) from vehicle-fed control mice were pooled regardless of the day of feeding. AValues are statistically different from vehicle-fed mice at P ≤ 0.05. BThe group mean of the sum of daily cs through day 14 ± SEM. CThe group mean of the cumulative cs divided by the number of days the animal was observed ± SEM. DThe group mean of the highest cs exhibited by mice with EAE ± SEM.
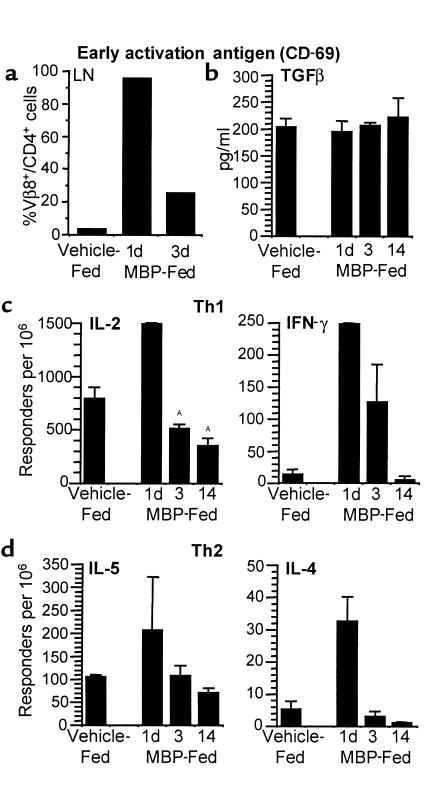
T-cell activation and cytokine responses are increased early, but later decline after MBP feeding.
Ligation of TCR by antigen in vitro can result in TCR downmodulation as the first step in activation (20). Therefore, the activation state of Tg Vβ8+/CD4+ T cells was examined by assessing the expression of early activation antigen (CD69), L-selectin (CD62L), CD45RB, and Pgp1 (CD44). Neither CD45RB nor CD44 expression was influenced by MBP feeding (data not shown), suggesting that Tg T cells were not triggered to differentiate from naive to memory cells (21). CD62L expression decreased on Tg cells from MLN 1 day after oral MBP and then returned to control levels by day 3 (data not shown). Simultaneously, CD69 expression was profoundly increased on Vβ8+/CD4+ cells 1 day after MBP feeding in peripheral LNs (Figure 4a), MLNs, SPL, and Peyer’s patches and could be detected within 6 hours of MBP feeding. An increase in CD69 and a decrease in CD62L are characteristic of T-cell activation (22). Therefore, concomitant with TCR downmodulation, MBP-specific T cells exhibit an activated phenotype.
Figure 4.
T-cell activation occurs early, and later it declines after MBP feeding. (a) MBP TCR Tg mice were fed vehicle or 100 mg MBP and sacrificed 1 or 3 days after feeding. Peripheral LNs were harvested from three animals per group, and pooled single-cell suspensions were analyzed by flow cytometry for early activation antigen (CD69) expression within Vβ8+/CD4+ Tg populations. (b–d) Adult MBP TCR Tg mice were thymectomized, then fed vehicle or 100 mg MBP, and sacrificed on days 1, 3, and 14 after feeding. TGF-β production was measured by ELISA from supernatants of splenocytes cultured with MBP. TGF-β concentration (pg/ml) was determined from a standard curve for duplicate cultures from individual animals, and the mean for each group ± SEM is shown (n = 2–4). Values were not statistically different from vehicle-fed control mice. IL-2, IFN-γ, IL-5, and IL-4 production was measured by ELISPOT from LN cells cultured with MBP. There was negligible production of any cytokine without in vitro restimulation with MBP. Responding cells per million were determined for replicate wells from individual animals, and the mean for each group ± SEM is shown (n = 2–6). AValues are statistically different from vehicle-fed mice at P ≤ 0.05.
To characterize further the functional capacity of the MBP-specific T-cell population, in vitro cytokine responses to MBP were assessed in lymphocytes from adult Tg mice that were thymectomized before MBP or vehicle feeding. Figure 4b shows that TGF-β levels are unchanged over time after MBP feeding. In contrast, there are profound increases in Th1 (IL-2, IFN-γ) and Th2 (IL-5, IL-4) MBP-specific cells 1 day after feeding (Figure 4, c and d). Interestingly, cytokine levels gradually declined to values lower than vehicle-fed control mice by 14 days after oral MBP.
Oral administration of MBP decreases Vβ8+/CD4+ cell numbers and function.
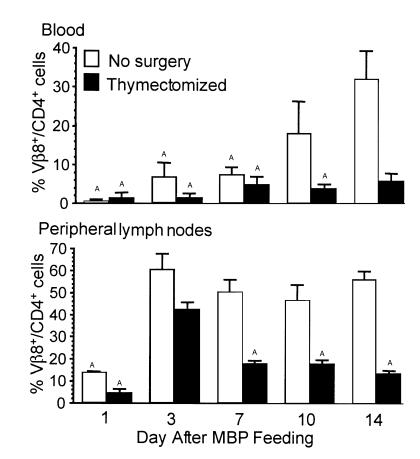
To monitor only those antigen-specific T cells exposed to a high dose of oral antigen, we compared Vβ8+/CD4+ cells from untreated and thymectomized Tg mice. Figure 5 shows that Vβ8+/CD4+ Tg cells in peripheral blood gradually returned in euthymic Tg mice, whereas Vβ8+/CD4+ cells did not return in thymectomized Tg mice fed MBP. In euthymic and thymectomized mice, Vβ8+/CD4+ Tg cells in peripheral LNs were profoundly reduced 1 day after MBP feeding and returned 2 days later (day 3). However, although Vβ8+/CD4+ Tg cells persist in euthymic Tg mice, thymectomized Tg mice fed MBP exhibit a gradual decline in Tg cells after day 3 (Figure 5b). Similar declines were observed in SPL and MLNs (data not shown). The absence of Vβ8+/CD4+ is a direct effect of oral administration of MBP because thymectomized mice fed vehicle maintained their Vβ8+/CD4+ population in all organs examined (data not shown). Furthermore, sham-surgery control mice fed MBP showed similar Vβ8+/CD4+ expression as euthymic MBP-fed mice (data not shown). These data demonstrate that in the absence of the release of naive Tg cells, the peripheral MBP-specific population diminishes 7–14 days after exposure to a high oral dose of MBP.
Figure 5.
The number of Vβ8+/CD4+ Tg T cells declines after MBP feeding. Euthymic and thymectomized MBP TCR Tg mice were fed 100 mg MBP and sacrificed 1, 3, 7, 10, and 14 days after feeding. Cells were analyzed by flow cytometry for the Tg Vβ8 TCR on CD4+ cells. Each bar is the mean percentage of Vβ8+/CD4+ cells ± SEM (n = 3–5). AValues are statistically different from corresponding control-fed mice at P ≤ 0.05.
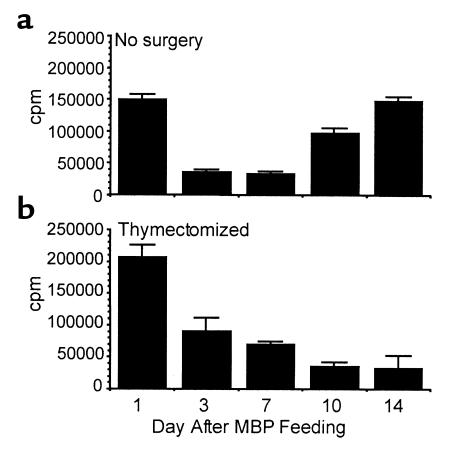
MBP-specific T-cell functional capacity was also assessed by measuring in vitro proliferation responses to MBP NAc1-11. The most robust proliferation occurred with cells isolated 1 day after MBP feeding. LN cells from euthymic Tg mice demonstrated decreased proliferation to NAc1-11 on days 3 and 7 after feeding (Figure 6a), despite the presence of control levels of Vβ8+/CD4+ Tg cells (Figure 5b). Together with the reduction in IL-2 production after feeding (Figure 4c), these observations are indicative of an anergic T-cell population (23). However, by days 10 and 14, proliferation returned to control levels. The recovery of the proliferation responses most likely represents new thymic emigrants, as proliferation responses were not restored in thymectomized Tg mice (Figure 6b). There was also evidence for anergy in thymectomized mice 3 days after feeding, when Vβ8+/CD4+ Tg cells are present (Figure 5b) but proliferation is reduced (Figure 6b). However, in contrast to early time points after MBP feeding (day 1), later analysis in MBP-fed thymectomized Tg mice shows that functional responses to MBP (cytokine production, proliferation) ultimately decline.
Figure 6.
Vβ8+/CD4+ Tg T-cell function declines after MBP feeding. Euthymic and thymectomized MBP TCR Tg mice were fed 100 mg MBP and sacrificed 1, 3, 7, 10, and 14 days after feeding. LN cell proliferative responses to NAc1-11 were measured by in vitro [3H]thymidine uptake. Data are representative of six separate experiments. (a) Each bar is the mean cpm of triplicate wells ± SEM. (b) Each bar is the mean cpm of cultures from two mice per group ± SEM.
Evidence for apoptosis of MBP-specific T cells after MBP feeding.
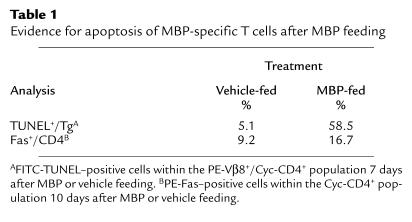
We assessed the possibility that MBP-specific T cells were undergoing apoptosis at later time points after exposure to a high oral dose of MBP. Adult MBP TCR Tg mice were thymectomized, fed vehicle or 100 mg MBP, and sacrificed 7 or 10 days later; MLNs were analyzed by flow cytometry for apoptosis. Thymectomized Tg mice fed MBP exhibited a dramatic increase in TUNEL+ Tg cells (Table 1). In addition, MBP feeding resulted in enhanced Fas expression on CD4+ cells (Table 1) and a fourfold increase in FasL expression on MHC class II–positive cells (data not shown). Therefore, these observations indicate that in addition to inducing anergy, high-dose oral antigen administration can result in apoptosis of antigen-specific T cells.
Table 1.
Evidence for apoptosis of MBP-specific T cells after MBP feeding
Discussion
Studies presented here explore the fate of MBP-specific T cells in vivo after high-dose MBP feeding by following the population over time in thymectomized Tg mice. To avoid the confounding influence of newly released T cells, we utilized thymectomized animals instead of Rag–/– mice due to the high level of spontaneous EAE that is observed when the Rag mutation is introduced onto the MBP TCR Tg background (14). A single high dose of MBP led to diminished function and deletion of MBP-specific Tg T cells. Preceding deletion, there is an early in vivo antigen-recognition event characterized by TCR downmodulation, CD69 upregulation, and heightened Th1 and Th2 cytokine responses that correlates with protection from EAE. Analysis of Th1-type (IL-2, IFN-γ), Th2-type (IL-4, IL-5), and TGF-β cytokines failed to demonstrate evidence for active suppression or immune deviation. Instead, we observed that the Vβ8+/CD4+ phenotype and functional responses ultimately declined within 14 days of feeding. TUNEL analysis then confirmed apoptosis of MBP-specific cells after a single high-dose MBP feeding. Interestingly, we also observed a brief period characteristic of anergy, when Vβ8+/CD4+ cells were demonstrable, but showed decreased IL-2 production and proliferation responses in vitro.
T-cell activation has also been shown to precede oral tolerance in nondisease model systems. Cytochrome (cyt) c TCR Tg mice fed repeated low doses of cyt c exhibited a reduction in Tg TCR+ cells and upregulation of CD69 in splenocyte populations (24). Similarly, when OVA-specific Tg TCR+ T cells were adoptively transferred to BALB/c recipients, a single oral dose of OVA resulted in activation and proliferation of OVA-specific T cells leading to T-cell anergy (25). In other studies, repeated oral administration of OVA resulted in reduced Tg TCR+ cells in SPL and LNs of BALB/c recipients concomitant with increased IFN-γ, IL-4, and TGF-β production upon in vitro re-stimulation with OVA (26). The level of TCR downmodulation in vivo has been shown to be directly related to the dose of tolerogen (27). However, to our knowledge, ours is the first demonstration of this in mucosal tolerance.
Two independent mechanisms of TCR downmodulation have been described previously (28). PMA-induced T-cell stimulation is believed to activate protein kinase C (PKC) and result in TCR internalization and then re-expression when the stimulus is removed (29). TCR downmodulation after specific ligand interaction is thought to be PKC independent and results in lysosomal degradation of the TCR (30). It is not known whether these pathways of TCR downmodulation are interrelated. In work presented here, MBP-fed animals with decreased surface TCR demonstrated increased intracellular expression of Vβ8 protein, resulting either from cytoplasmic localization of extracellular TCR or newly synthesized TCR. It is unlikely that the return of Vβ8+/CD4+ cells represents Tg cell progeny, as we did not observe evidence for in vivo proliferation of MBP-specific cells. Rather, the restoration of Vβ8+/CD4+ cells likely results from re-expression of the Tg TCR facilitated by factors provided in lymphoid tissue microenvironments.
Despite the profound reduction in TCR expression, a Vβ8+/CD4+ Tg population with an activated phenotype (CD69+) is present 1 day after antigen feeding in all lymphoid organs examined. This indicates that responses to high doses of fed antigen are not limited to the gut-associated lymphoid tissue. Indeed, in cyt c TCR Tg mice, splenocytes isolated 6 hours after cyt c feeding were able to stimulate naive Tg T cells when co-cultured in vitro (24). These authors concluded that orally administered cyt c was widely disseminated and was present in the SPL after feeding. Interestingly, a single low dose of cyt c (0.5 mg) induced increases in CD69 expression similar to results presented here. Thus, both high and low doses of orally administered antigen induce peripheral T-cell activation. Heightened in vitro cytokine responses to MBP most likely resulted from Vβ8+/CD4+/CD69hi/CD62Llo T cells, as TCR expression is required to ligate antigen. Given the high frequency of MBP-specific T cells in MBP TCR Tg mice, it is possible that this population did not encounter sufficient antigen in vivo to downmodulate TCR expression completely but did receive enough TCR ligation to signal changes in antigen responsiveness.
The ultimate fate of T cells, whether activated or hyporesponsive, is most likely determined by the antigen presenting cell (APC). Dendritic cells (DCs) as APCs have been shown to initiate active immunity and/or induce T-cell tolerance, depending on their maturation state. DC maturation can be triggered by CD40 ligand, inflammatory cytokines, the presence of necrotic cells, or viral and bacterial constituents (31, 32). Unlike immature DCs, mature DCs are potently immunogenic (31). We observed a short-lived period of protection after feeding MBP to Tg mice (Figure 3). Perhaps the bacterial products present during MBP/CFA immunization induce DC maturation and promote immunogenicity to MBP that overrides mucosally induced tolerance (Figure 2). The excessive number of MBP-specific T cells in MBP TCR Tg mice is noteworthy and can explain the fact that protection from EAE is less dramatic and more short-lived than observed in orally tolerized conventional rats and mice (1, 3). Because Liu et al. have recently demonstrated that DCs in the intestinal wall can acquire soluble protein antigen and migrate into peripheral lymph to prime antigen-specific T cells (33), we are currently investigating the role of DCs in the induction of oral tolerance.
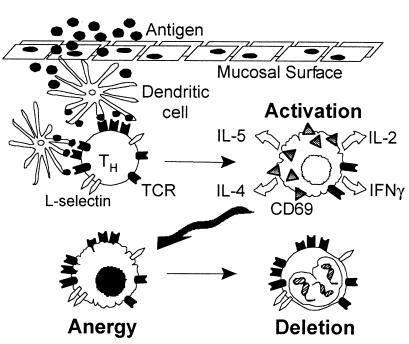
By integrating the data presented here with work by others, we propose a model for oral tolerance induction (Figure 7). Antigen traverses the gut epithelium and is presented by APCs, presumably DCs. Antigen-specific CD62L+/CD4+ T cells encounter a high dose of antigen presented on DCs, which triggers TCR downmodulation, CD62L downregulation, and CD69 upregulation within 24 hours. In vitro, these cells are hyper-responsive to antigen, as measured by IL-2, IFN-γ, IL-4, and IL-5 production. After 3 days, CD62L and TCR expression return, whereas CD69 expression and responses to antigen diminish, i.e., T cells are anergic. Finally (7–14 days after feeding), fewer antigen-specific T cells are detectable, in vitro responses decline, and apoptosis of Tg cells is seen. Collectively, these observations suggest that the mechanism of oral tolerance after high doses of antigen follows an in vivo continuum of activation, anergy, and then deletion.
Figure 7.
Proposed model for tolerance induction after a high dose of orally administered antigen.
Acknowledgments
The authors thank Phillip Popovich, Fei Song, Kennichi Dowdell, Karen Cox, Richard Wardrop, Carey Etling, and Dick Trezza for their assistance with these studies, and Charles Janeway for supplying the nucleus for our MBP TCR Tg breeding colony. This work was supported by NIH grant AI 35960, AI 43376, National Multiple Sclerosis Society grant RG 2302, and the Bremer Foundation.
Footnotes
Jacqueline M. Benson’s present address is: Stanford University School of Medicine, Stanford, California, USA.
References
- 1.Bitar DM, Whitacre CC. Suppression of EAE by the oral administration of MBP. Cell Immunol. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 2.Higgins PJ, Weiner HL. Suppression of EAE by oral administration of MBP and its fragments. J Immunol. 1988;140:440–445. [PubMed] [Google Scholar]
- 3.Meyer AL, Benson JM, Gienapp IE, Cox KL, Whitacre CC. Suppression of murine chronic REAE by the oral administration of MBP. J Immunol. 1996;157:4230–4238. [PubMed] [Google Scholar]
- 4.Benson JM, et al. Oral administration of MBP is superior to myelin in suppressing established REAE. J Immunol. 1999;162:6247–6254. [PubMed] [Google Scholar]
- 5.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to MBP suppress both in vitro and in vivo immune responses by the release of TGFβ after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of EAE. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 7.Fishman-Lobell J, Friedman A, Weiner HL. Different kinetic patterns of cytokine gene expression in vivo in orally tolerant mice. Eur J Immunol. 1994;24:2720–2724. doi: 10.1002/eji.1830241122. [DOI] [PubMed] [Google Scholar]
- 8.Hurst SD, Sitterding SM, Ji S, Barrett TA. Functional differentiation of T cells in the intestine of TCR transgenic mice. Proc Natl Acad Sci USA. 1997;94:3920–3925. doi: 10.1073/pnas.94.8.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in EAE. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 10.Van Houten N, Blake SF. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J Immunol. 1996;157:1337–1341. [PubMed] [Google Scholar]
- 11.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- 12.Chen Y, et al. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 13.Marth T, Strober W, Kelsall BL. High dose oral tolerance in OVA TCR-Tg mice: systemic neutralization of IL-12 augments TGF-β secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 14.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous EAE in immunodeficient anti-MBP TCR Tg mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 15.Deibler GE, Martenson RE, Kies MW. Large scale preparation of MBP from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 16.Swanborg RH, Swierkosz JE, Saieg RG. Studies on the species-variability of EAE in guinea pigs and rats. J Immunol. 1974;112:594–600. [PubMed] [Google Scholar]
- 17.DeMatteo RP, Markmann JF, Raper SE. An improved technique of thymectomy in the adult mouse. Transplantation. 1995;59:787–789. doi: 10.1097/00007890-199503150-00026. [DOI] [PubMed] [Google Scholar]
- 18.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 19.Chen YH, Weiner HL. Dose-dependent activation and deletion of antigen-specific T cells following oral tolerance. Ann NY Acad Sci. 1996;778:111–121. doi: 10.1111/j.1749-6632.1996.tb21120.x. [DOI] [PubMed] [Google Scholar]
- 20.Viola A, Lanzavecchia A. T cell activation determined by TCR number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 21.Carter LL, Swain SL. From naive to memory. Development and regulation of CD4+ T cell responses. Immunol Res. 1998;18:1–13. doi: 10.1007/BF02786509. [DOI] [PubMed] [Google Scholar]
- 22.Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins MK, Pardoll DM, Mizuguchi J, Chused TM, Schwartz RH. Molecular events in the induction of a nonresponsive state in IL-2-producing helper T-lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–673. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Dirden-Kramer B, Ito K, Ernst PB, Van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol. 1999;162:5868–5875. [PubMed] [Google Scholar]
- 26.Chen Y, Inobe J, Weiner HL. Inductive events in oral tolerance in the TCR Tg adoptive transfer model. Cell Immunol. 1997;178:62–68. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 27.Ferber I, et al. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674–676. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 28.Lauritsen JP, et al. Two distinct pathways exist for down-regulation of the TCR. J Immunol. 1998;161:260–267. [PubMed] [Google Scholar]
- 29.Salio M, Valitutti S, Lanzavecchia A. Agonist-induced TCR down-regulation: molecular requirements and dissociation from T cell activation. Eur J Immunol. 1997;27:1769–1773. doi: 10.1002/eji.1830270726. [DOI] [PubMed] [Google Scholar]
- 30.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of TCR-CD3-ζ complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by DCs that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory DCs. J Exp Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu LM, MacPherson GG. Antigen acquisition by DCs: intestinal DCs acquire antigen administered orally and can prime naïve T cells in vivo. J Exp Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]