Abstract
Background. Chronic hepatitis C virus (HCV)–induced liver fibrosis involves upregulation of transforming growth factor (TGF)–β and subsequent hepatic stellate cell (HSC) activation. MicroRNAs (miRNAs) regulate HCV infection and HSC activation.
Methods. TaqMan miRNA profiling identified 12 miRNA families differentially expressed between chronically HCV-infected human livers and uninfected controls. To identify pathways affected by miRNAs, we developed a new algorithm (pathway analysis of conserved targets), based on the probability of conserved targeting.
Results. This analysis suggested a role for miR-29 during HCV infection. Of interest, miR-29 was downregulated in most HCV-infected patients. miR-29 regulates expression of extracellular matrix proteins. In culture, HCV infection downregulated miR-29, and miR-29 overexpression reduced HCV RNA abundance. miR-29 also appears to play a role in HSCs. Hepatocytes and HSCs contribute similar amounts of miR-29 to whole liver. Both activation of primary HSCs and TGF-β treatment of immortalized HSCs downregulated miR-29. miR-29 overexpression in LX-2 cells decreased collagen expression and modestly decreased proliferation. miR-29 downregulation by HCV may derepress extracellular matrix synthesis during HSC activation.
Conclusions. HCV infection downregulates miR-29 in hepatocytes and may potentiate collagen synthesis by reducing miR-29 levels in activated HSCs. Treatment with miR-29 mimics in vivo might inhibit HCV while reducing fibrosis.
Hepatitis C virus (HCV) is an RNA virus infecting 170 million people worldwide. Persistent infection causes fibrosis, cirrhosis, end-stage liver disease, and ultimately, liver cancer in up to 30% of patients. Fibrotic progression is highly variable, and prognosis correlates with fibrotic stage. Fibrosis is morphologically characterized by increased deposition of extracellular matrix (ECM) proteins, including collagen types I/III, fibronectin and laminin. Transforming growth factor-β (TGF-β), released in response to HCV-mediated damage, activates hepatic stellate cells (HSCs), and strongly upregulates ECM protein production. Mechanisms regulating ECM gene expression in activated HSCs are of interest as potential therapeutic targets. Novel markers for fibrosis progression would also allow early treatment of rapid progressors.
MicroRNAs (miRNAs) are ∼22 nucleotide single-stranded non-coding RNAs (guide strands) that silence endogenous mRNA transcripts. Complementarity between miRNAs and the 3' untranslated regions (UTRs) of mRNAs directs gene silencing by stimulation of target mRNA degradation and/or translational repression. Individual mRNAs can be targeted by multiple miRNAs, and individual miRNAs often have hundreds of mRNA targets. The seed sequence (nucleotides 2–7) of the miRNA is especially important for target site recognition, and miRNAs that share the same seed sequence are grouped into families [1, 2]. Target mRNAs containing complementary seed matches as short as 6 nucleotides in their 3' UTRs can be targeted for repression [3, 4]. Algorithms, such as TargetScan (www.TargetScan.org), use seed matches in conjunction with additional sequence features and conservation to predict possible mRNA targets of miRNAs [1, 2].
HCV interacts with the miRNA machinery (reviewed in [5]). The most abundant liver miRNA, miR-122, binds the HCV 5' UTR, which surprisingly, leads to increased HCV RNA abundance [6]. Other miRNAs (miR-122, miR-199a*, miR-196, miR-296, miR-351, miR-431 and miR-448) can also regulate HCV genomic RNA abundance [7–10].
In this study, we used Multiplex RT and TaqMan miRNA Assays (Life Technologies) to profile 346 miRNAs by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) in control (uninfected) human liver and liver biopsy samples from patients with chronic HCV infection (C-HCV). Of interest, numerous miRNAs were dysregulated in C-HCV relative to uninfected controls. We used a novel strategy called pathway analysis of conserved targets (PACT) to conduct a pathway analysis of predicted targets with a 50% statistical chance of having a conserved miRNA/target interaction (see Materials and Methods). We identified 4 pathways associated with downregulated miRNA families. On the basis of the pathway analysis and miRNA profiling data, we selected the miR-29 family, which consists of miR-29a, miR-29b, and miR-29c, for further analysis. Hereafter, we will either indicate individual miR-29 members or refer to the miR-29 family as miR-29.
Our measurements of miR-29 in purified primary rat hepatocytes and HSCs indicated that both cell types contribute roughly equivalent levels of miR-29 to whole liver. We thus studied the role of miR-29 during HCV infection in hepatocytes and during HSC activation. This study expands the repertoire of miRNAs involved in the HCV lifecycle.
MATERIALS AND METHODS
Materials and methods contained in supplementary data available at http://jid.oxfordjournals.org.
RESULTS
C-HCV miRNA Expression Profiling
We analyzed the expression of 346 miRNAs in liver samples from 22 C-HCV–infected patients and 4 uninfected controls using multiplex stem-loop RT primers and miRNA qRT-PCR. Patient data and miRNA levels are listed in Supplementary Tables 1 and 2, respectively, available at http://genes.mit.edu/burgelab/Supplementary/Bandyopadhyay10/. All patients gave informed consent. After normalizing expression values, grouping miRNAs with shared seed regions and filtering for broadly conserved miRNA families [11], we selected miRNA families with ∼1.4-fold change between HCV-infected patients and the mean of the controls. We filtered for highly expressed miRNA families (top 50% of expression in controls) with consistent expression patterns (up or downregulated in >9 C-HCV–infected patients). These relatively strict criteria yielded 1 consistently upregulated miRNA family (miR-150) and 11 consistently downregulated families (Table 2).
Table 2.
Predicted miRNA Target Genes Identified by Pathway Analysis
| miRNA families | Predicted target genes |
| ECM-receptor interaction | |
| miR-101 | DAG1, ITGA3 |
| miR-148a; miR-152; miR-148b | TNXB, COL4A1, COL2A1, ITGB8, ITGA5, ITGA9, COL6A3, LAMA4 |
| miR-29a; miR-29b; miR-29c | COL5A3, COL4A4, COL1A1, COL3A1, COL5A2, COL4A1, COL4A2, COL2A1, HSPG2, ITGA6, COL1A2, ITGA11, LAMA2, LAMC1, COL6A3, COL4A6, COL11A1, COL5A1 |
| miR-133a; miR-133b | COL5A3, SV2A, CD47, COL6A3 |
| miR-140-5P | DAG1, TNN |
| miR-203 | ITGA2 |
| miR-210 | No predicted targets in pathway |
| miR-27a; miR-27b | SDC2, ITGA2, RELN, ITGA5, COL5A1 |
| miR-23a;miR-23b | No predicted targets in pathway |
| miR-204;miR-211 | No predicted targets in pathway |
| miR-19a; miR-19b | DAG1, SDC1, ITGB8, ITGA6, THBS1 |
| Focal adhesion | |
| miR-101 | CAV3, ITGA3, PIP5K1C, MAPK1, RAP1B |
| miR-148a; miR-152; miR-148b | PTEN, TNXB, COL4A1, PIK3R3, COL2A1, PPP1R12A, ITGB8, VAV2, MET, ITGA5, CAV2, ROCK1, ITGA9, IGF1, COL6A3, LAMA4, PPP1CB |
| miR-29a; miR-29b; miR-29c | COL5A3, COL4A4, VEGFA, COL1A1, COL3A1, PDGFC, PTEN, COL5A2, COL4A1, PIK3R3, COL4A2, COL2A1, AKT3, ITGA6, COL1A2, PIK3R1, ITGA11, PDGFB, LAMA2, CAV2, LAMC1, IGF1, COL6A3, COL4A6, COL11A1, COL5A1 |
| miR-133a; miR-133b | COL5A3, EGFR, IGF1R, CRK, COL6A3 |
| miR-140-5P | VEGFA, PPP1R12A, TNN, IGF1R, PPP1CC, PDGFRA, RAP1B |
| miR-203 | MAPK9, PPP1R12A, ITGA2 |
| miR-210 | No predicted targets in pathway |
| miR-27a; miR-27b | BIRC4, SHC4, VAV2, ITGA2, GRB2, RELN, MET, ITGA5, IGF1, PPP1CC, COL5A1, RAP1B |
| miR-23a; miR-23b | BIRC4, PIK3R3, PPP1R12A, PDPK1, PAK6, MET |
| miR-204; miR-211 | BCL2 |
| miR-19a; miR-19b | BIRC4, RAF1, ARHGAP5, RAP1A, PTEN, CCND1, PIK3R3, TLN2, PPP1R12A, ITGB8, VAV2, ITGA6, FLNC, MAPK1, CCND2, PAK6, THBS1, IGF1, RAP1B |
| Small cell lung cancer | |
| miR-101 | ITGA3, RXRB, PTGS2 |
| miR-148a; miR-152; miR-148b | MAX, PTEN, COL4A1, PIK3R3, CDKN1B, E2F3, LAMA4 |
| miR-29a; miR-29b; miR-29c | COL4A4, PTEN, COL4A1, PIK3R3, COL4A2, AKT3, ITGA6, PIK3R1, CDK6, LAMA2, LAMC1, TRAF4, COL4A6 |
| miR-133a; miR-133b | BCL2L1, RARB |
| miR-140-5P | BCL2L1 |
| miR-203 | ITGA2 |
| miR-210 | No predicted targets in pathway |
| miR-27a; miR-27b | RXRA, BIRC4, ITGA2, CDK6 |
| miR-23a; miR-23b | BIRC4, PIK3R3 |
| miR-204; miR-211 | BCL2 |
| miR-19a; miR-19b | BIRC4, PTEN, CCND1, PIK3R3, ITGA6 |
Functional Roles of Differentially Expressed miRNA Families
To identify potential roles for the downregulated miRNA families, we developed a general algorithm called PACT to determine functional categories enriched in targets of co-expressed miRNAs. Recently, we (RCF and CBB) refined the sensitivity and statistical power of TargetScan prediction sufficiently to rank individual targets using a metric called the probability of conserved targeting (PCT). The PCT controls for seed match type, dinucleotide content, and local background conservation. For the set of downregulated miRNA families, we pooled all predicted target mRNAs having PCT at least .5, corresponding to at least a 50% chance of the miRNA/mRNA target relationship being evolutionarily conserved. To correct for the biases caused by selecting predicted targets based on conservation, we generated 20 simulation cohorts of mock miRNAs and corresponding mock targets, which were used to estimate the number of predicted miRNA targets found in any particular pathway by chance (see Supplementary materials). We ranked pathways using the group enrichment P value, where enrichment is the tendency that the given set of miRNAs (eg, downregulated miRNAs) have more conserved targets in a pathway than expected by chance, controlling for relevant sources of bias in the PCT. Of interest, the predicted targets of downregulated miRNAs were highly enriched in ECM-receptor interaction (P = .0046) and focal adhesion pathways (P = .017) (Table 1, Table 2). We next tested whether these enrichments could be attributable to a general propensity for miRNAs to target these pathways. We calculated a random enrichment P value by repeating the analysis with randomly selected sets of actual miRNAs. We found that the enrichment for the ECM-receptor interaction and focal adhesion pathways was specific to the set of downregulated miRNA families in Table 2 (P = .0031 and P= .022, respectively).
Table 1.
Pathway Analysis Statistics
| Pathway | Group enrichment P valuea | # of predicted targets in pathway for mock miRNA controls |
# of predicted targets in pathway for miRNAs in Table 2b | |
| Mean controlb | Maximum controlb | |||
| ECM-receptor interaction | 0.0046 | 22 | 36 | 45 |
| Focal adhesion | 0.017 | 66 | 93 | 101 |
| Small cell lung cancer | 0.039 | 24 | 33 | 39 |
NOTE. aThe group enrichment P value represents the enrichment of predicted targets for a pathway relative to mock-predicted targets.
bTwenty control miRNA sets were generated that had the same characteristics as the test miRNA family. The mean and maximum number of miRNAs in simulations from these control sets that are predicted to target the pathway are indicated, which can be compared with the actual number of observed miRNAs predicted to target the pathway. See Supplementary Data for more details.
The focal adhesion category is composed mainly of integrins, laminins, platelet-derived growth factor (PDGF), and phosphatidylinositol 3 kinase family (PI3K) members. Laminins mediate ECM attachment, and integrins mediate signaling between the ECM and cell signaling pathways. PDGF is a powerful HSC mitogen in which signaling is mediated by PI3K. The ECM-receptor interaction category overwhelmingly consists of ECM components, integrins, and laminins. Thus, HCV infection is associated with an miRNA expression pattern in human liver that derepresses cellular adhesion and ECM genes, which could contribute to C-HCV–mediated fibrogenesis.
Downregulation of miR-29 in HCV-Infected Patients
Among downregulated miRNA families, miR-29 stood out in several respects: For each miRNA family and significant pathway, we calculated an individual enrichment P value representing the probability of a specific miRNA family having more conserved targets in the pathway than expected by chance. The two most significant pairs were miR-29 targeting ECM-receptor interactions and focal adhesion pathways (individual enrichment P=1 × 10−32 and P=2 × 10−7, respectively). miR-29 also had the largest number of predicted targets in the 3 significant pathways (Table 2), all of which are relevant to HCV pathogenesis. Lastly, all three miR-29 family members were downregulated in almost all C-HCV–infected patients (Figure 1A). Therefore, we further analyzed miR-29’s role in HCV pathogenesis.
Figure 1.
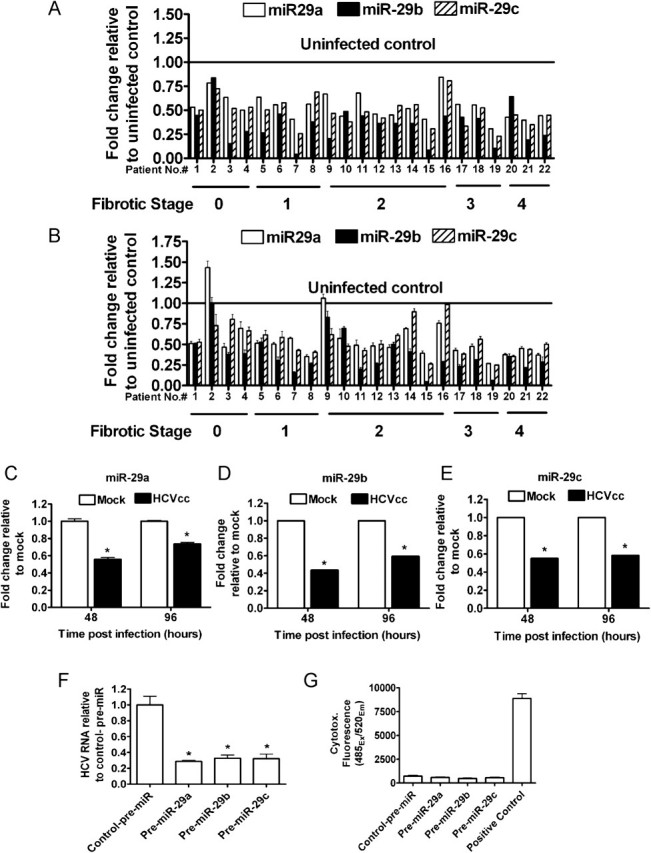
Downregulation of miR-29 in C-HCV. Fold change in mature miR-29 levels relative to control uninfected livers. A, miR-29 levels in C-HCV patients measured by multiplex qRT TaqMan miRNA profiling. B, Individual qRT-PCR validated miR-29 downregulation in HCV-infected needle biopsies (Pearson's correlation between A and B: miR-29a, r = .653 P = .0010; miR-29b, r = .623 P = .0019; miR-29c, r = .752 P < .0001). Mean ± standard error of the mean (s.e.m.); three independent measurements. (C-G) HCVcc infection reduces miR-29, and miR-29 inhibits HCV. Fold change of (C), miR-29a, (D) miR-29b and (E) miR-29c in HCVcc-infected cells relative to mock-infected cells. Average of two independent experiments in triplicate. Mean ± s.e.m. (F) Huh7.5 cells were treated with miR-29 mimic or negative control mimic (control-pre-miR) and infected with HCVcc. Fold change in HCV RNA relative to control-pre-miR. Representative experiment from three independent experiments in triplicate. Mean ± s.e.m. (G) Cytotox-Fluor cytotoxicity assay. Positive control, 30 μg/ml digitonin. Average of three independent experiments in triplicate. Mean ± s.e.m. * indicates a P value < .05 for all panels.
Individual TaqMan miRNA assays (Figure 1B) validated the multiplex data (Figure 1A) showing miR-29 downregulation of ∼2-fold in the majority of C-HCV samples irrespective of fibrotic stage. Previous results suggested that miRNA changes of this magnitude could be physiologically relevant; for example, deleting one of two copies of miR-1 in mice resulted in substantially increased fatality due to heart defects [12].
Contribution of Hepatocytes and HSCs to miR-29 Levels in Whole Liver
The liver is composed of many cell types that could serve as sources of miR-29 expression. Hepatocytes and HSCs are of special interest to HCV pathogenesis. Hepatocytes compose ∼70% of the cells in the liver and ∼78% of the liver mass. HSCs comprise 8%–14% of liver cells [13] but only ∼1.4% of liver mass because of their smaller size [14]. To determine the relative contribution of these 2 cell types to miR-29 levels in total liver, we measured miR-29 in purified primary rat hepatocytes and freshly isolated HSCs, compared with total liver (Table 3). HSCs expressed much higher levels of miR-29 than hepatocytes (6.8–53-fold higher). On the basis of cell number or cell mass, hepatocytes outnumber HSCs by ∼7-fold or ∼55-fold, respectively. Using these corrections for abundance and our measurements of miR-29 in isolated hepatocytes and HSCs, we calculated cell number and cell mass adjusted expression ratios for comparison (equal contribution) (Tables 3 and 1). We conclude that both of these cellular compartments likely contribute to miR-29 levels measured in the needle biopsy samples from patients. We therefore addressed the role of miR-29 during HCV infection of hepatocytes in culture and during stellate cell activation.
Table 3.
Expression of miR-29 in Primary Hepatocytes, HSCs, and Total Liver
| miR-29 Q-PCR quantification | |||
| miR-29a | miR-29b | miR-29c | |
| Total liver expression | 1.0 (± .17) | 1.0 (± .27) | 1.0 (± .27) |
| Primary hepatocytes expression relative to total liver | 1.9 (± .20) | 0.58 (± .15) | 2.6 (± .30) |
| Primary HSCs expression relative to total liver | 13 (± 7.4) | 31 (± 21) | 16 (± 10) |
| HSC/Hepatocyte miR-29 expression ratioa | |||
| miR-29 in HSC/miR-29 in hepatocytes | 6.8 | 53 | 6.2 |
| Cell number adjusted | 0.97 | 7.6 | 0.88 |
| Cell mass adjusted | 0.12 | 0.96 | 0.11 |
NOTE. a Expression ratio of 1 corresponds to an equal contribution of hepatocytes and HSCs to miR-29 abundance.
Downregulation of miR-29 After Acute HCV Infection In Vitro
First, we assessed the effects of HCV infection on miR-29 expression in hepatocytes with use of an HCV infectious cell culture model (HCVcc). HCVcc infects, replicates, and produces infectious virus in Huh7.5 hepatoma cells [15]. Huh7.5 cells were infected with HCVcc, and ∼40% and ∼100% of cells were infected at 48 and 96 h, respectively (data not shown). In agreement with recent reports [16–18], HCV infection of Huh7.5 cells down-regulated miR-29 by a factor similar to that observed in C-HCV–infected patients (Figure 1C–E). Thus, miR-29 downregulation in the livers of C-HCV–infected patients may be, at least in part, mediated by HCV infection of hepatocytes.
Modulation of HCV RNA Levels by miR-29
To assess whether miR-29 downregulation is advantageous to the virus, we determined the effects of miR-29 overexpression on levels of HCV genomic RNA in Huh7.5 cells after HCVcc infection. Of interest, miR-29 overexpression resulted in a ∼3-fold decrease in HCV RNA (Figure 1F), indicating an antiviral function. miR-29 overexpression did not cause significant cytotoxicity (Figure 1G). These data expand the small repertoire of miRNAs known to regulate HCV [8–10, 19] and raise the intriguing possibility that HCV may downregulate miR-29 to enhance its fitness.
miR-29 Downregulation During HSC Activation
As shown above, freshly isolated HSC express high levels of miR-29 family members, which target multiple transcripts relevant to HSC activation and fibrogenesis. We activated HSCs by plating them on plastic [13] and measured miR-29 expression. miR-29 was rapidly and dramatically downregulated after primary rat HSC activation (Figure 2A–C). Thus, miR-29 downregulation appears to be a consistent feature of early stellate cell activation. TGF-β, upregulated by HCV, is a primary inducer of HSC activation; therefore, we assessed whether TGF-β modulates miR-29 expression in HSCs. Indeed, after TGF-β treatment of LX-2 immortalized human HSCs, miR-29 was downregulated (Figure 3A). In contrast, TGF-β stimulation of hepatocytes (Huh7.5 cells) did not alter miR-29 levels (Figure 3B). These results suggest that TGF-β signaling may be a mechanism by which miR-29 is downregulated during C-HCV infection.
Figure 2.
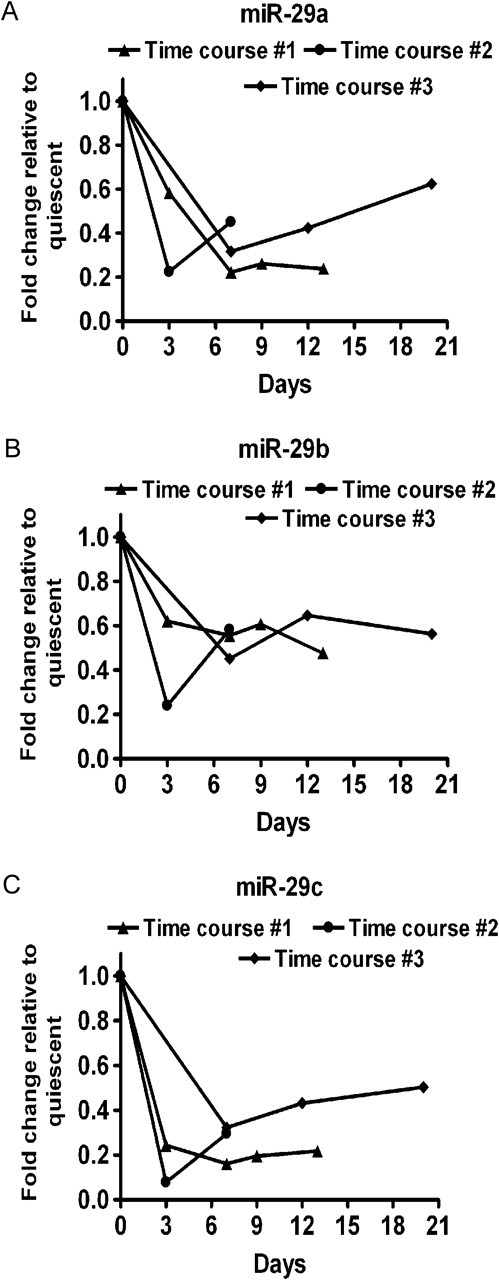
miR-29 downregulation in activated HSCs. Primary rat HSCs were isolated and activated in culture by plating on plastic. Data from three independent HSC isolations are shown (Time courses # 1, 2, and 3). Levels of (A) miR-29a, (B) miR-29b, and (C) miR-29c.
Figure 3.
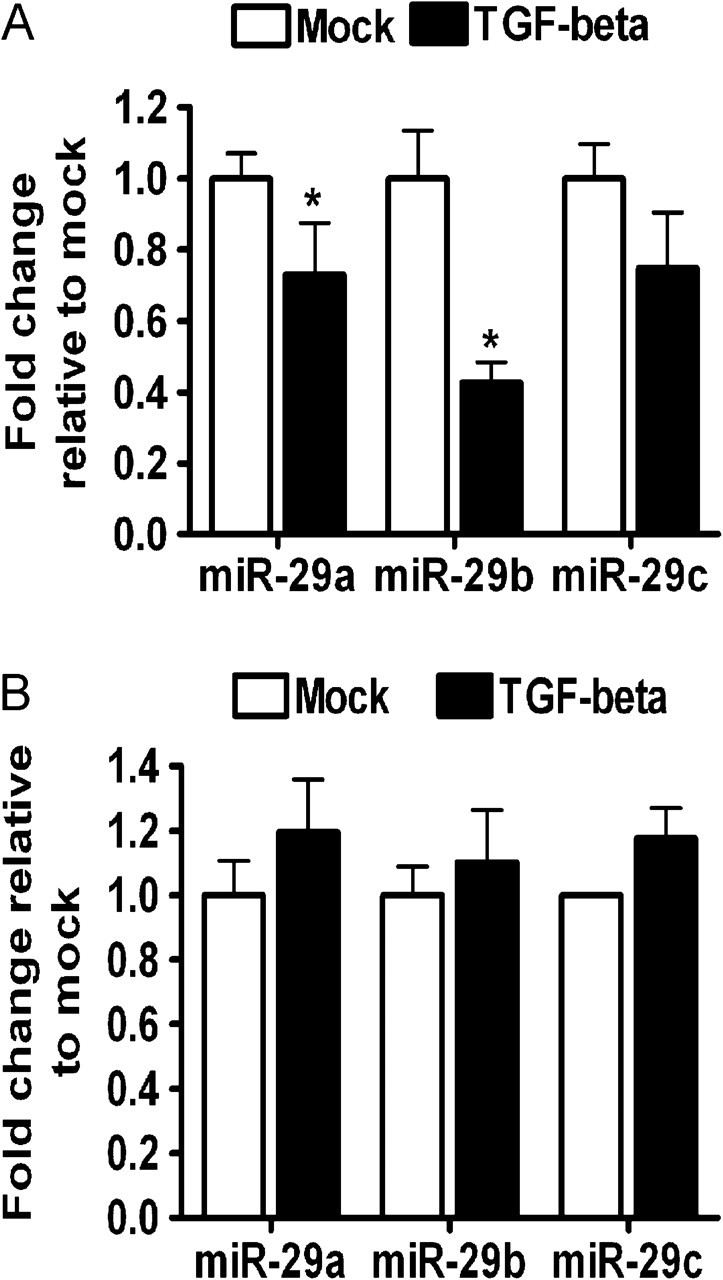
miR-29 downregulation in TGF-β-treated HSCs but not in hepatocytes. miR-29 levels in (A) LX-2 HSC cell line or (B) Huh7.5 cells treated with TGF-β (10 ng/ml) for 24 hours. Representative experiment from two independent experiments in quadruplicate. Mean ± s.e.m. * indicates a P value < .05 for all panels.
miR-29 Overexpression Alters HSC Proliferation and ECM Production
We next examined the effects of miR-29 restoration on features of the activated LX-2 phenotype by transfection of LX-2 cells with miR-29 mimics or negative control mimics. Transfection with Cy3-labeled siRNA indicated >90% transfection efficiency of LX-2 cells (data not shown). Restoration of miR-29 consistently resulted in a modest (∼20%) but statistically significant reduction in proliferation as determined by 2 separate assays (Figure 4A and B). Venugopal et al [28] observed similar decreases in HSC proliferation upon overexpression of miR-150 and miR-194 in LX-2 cells. miR-29 targets numerous collagen genes [19–21], including COL1A1 and COL3A1, but not the activation marker α-SMA. Consistent with these targeting relationships, miR-29 overexpressionresulted in profound suppression of COL1A1 and COL3A1 expression in LX-2 cells with no change in α-SMA expression as determined by qRT-PCR (Figure 4C). These results were not attributable to cell death, because neither cytotoxicity nor apoptosis were increased by miR-29 overexpression (Figure 4D and E). Thus, miR-29 over-expression alters two key features of HSC activation—proliferation and collagen expression—but not α-SMA expression.
Figure 4.
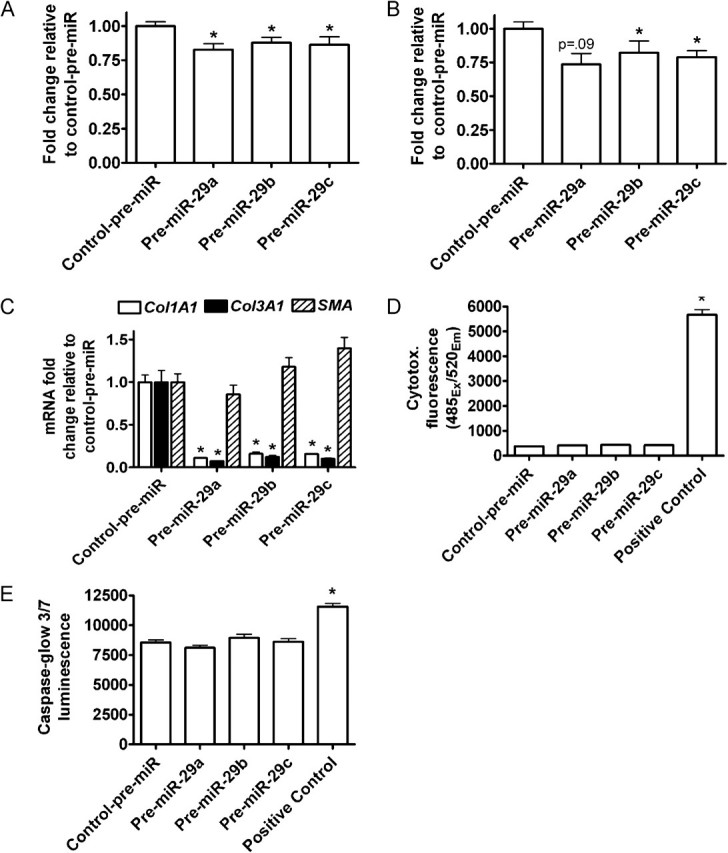
miR-29 overexpression in LX-2 cells inhibits proliferation and collagen expression. LX-2 cells were transfected with 25 nM miR-29 mimic or negative control mimic (control-pre-miR). A, WST-1 cell proliferation assay. Average of three independent experiments in quadruplicates. B, Ki67 mRNA levels. C, Col1A1, Col3A1, and α-SMA mRNA levels. Representative experiment from three independent experiments in triplicate. Mean ± s.e.m. D, Cytotox-Fluor cytotoxicity assay. Positive control, 30 μg/ml digitonin (E) Caspase-glow 3/7 apoptosis assay. Positive control, 2 μg/ml doxorubicin. Average of three independent experiments in quadruplicate. Mean ± s.e.m. * indicates a P-value < .05 for all panels.
DISCUSSION
miRNAs play important roles in HCV infection and associated pathological processes. Using quantitative miRNA profiling, we observed that miR-29 was the most consistently dysregulated miRNA in C-HCV–infected patient samples. Measurements of miR-29 in purified primary cell populations relevant to HCV pathogenesis indicated that both hepatocytes and HSCs contributed roughly equally to miR-29 expression in whole liver. Together, our results identify miR-29 as a potential new HSC activation marker and as a possible anti-HCV and antifibrotic therapeutic target.
Pathway analysis can identify biologically relevant signaling pathways coordinately regulated by miRNAs. Two groups previously used miRNA profiling in conjunction with pathway analysis to assess functional relationships in the context of HCV infection in humans. These studies relied on the fact that miRNA targeting reduces target mRNA stability. Ura et al and Peng et al measured miRNA and mRNA levels in HCV-infected and normal tissues and identified pathways associated with predicted target mRNAs [17, 22]. Ura et al [17] predicted cell adhesion, cell cycle, protein folding, and apoptosis pathways. Peng et al [22] used inverse expression between miRNAs and mRNAs to define targets and a graph theoretic approach to define miRNA/mRNA modules. They identified 38 HCV-associated miRNA/mRNA regulatory modules. Both approaches used inverse miRNA/mRNA relationships, which can be a powerful tool for uncovering biological relationships, although it does not necessarily identify direct targets. We chose a complementary pathway analysis approach that differed in several fundamental ways from these previous studies.
First, pathway analyses relying on miRNA/mRNA inverse relationships only interrogate interactions resulting in mRNA changes measurable by microarray. Because miRNAs typically have small effects on individual target mRNAs (<25% change and often much less) [23], many bona fide miRNA/mRNA interactions may not be observable by inverse expression (ie, small direct effects are easily masked by microarray noise or other biological effects). In addition, we (RCF and CBB) recently showed that 6-mer seed matches, which produce marginal mRNA abundance changes, were maintained in 3' UTRs in numbers similar to highly effective sites, such as 8-mer seed matches [11]. This observation suggests that many marginally effective sites are nevertheless important enough to be maintained by natural selection. Thus, we analyzed our miRNA expression data using an approach that was independent of inverse miRNA/mRNA relationships. Second, we predicted targets using the PCT conservation metric [11], which scores sites based on likelihood of preferential conservation over evolutionary time, specifically due to miRNA targeting. By using highly conserved seed matches to predict targets rather than inverse mRNA correlations, we select a relatively small number of targets that have been preserved through millions of years of evolution, providing a clearer opportunity for biological interpretability. Although species-specific targets cannot be identified using conservation, we can confidently detect targeting relationships of longer-term importance and, thus, identify pathways involved in miRNA function. Third, we proceed directly from predicted targets to pathway analysis without imposing a requirement on the network structure. Peng et al [22] explicitly selected bicliques (ie, completely connected sets of miRNAs and mRNAs); this could preclude detection of regulatory subnetworks in which, in aggregate (although perhaps not individually), a set of miRNAs targets many, but not all, members of a functional pathway. Finally, we rigorously controlled for biases in target prediction by explicitly controlling for UTR conservation, seed match number and type, seed match conservation, number of conserved targets specific to the particular miRNA family, and implicitly controlling for UTR length and nucleotide composition [11]. Of importance, target predictions that select for genes with seed matches and expression changes select for biased sets of genes with long UTRs (which have more miRNA seed matches by chance) and particular expression levels in the tissue of interest that are more readily detectable by array analysis and, therefore, render hypergeometric tests for function statistically invalid. Of importance, miRNA pathway analyses have not, to date, accounted for this critical bias. Taken together, our complementary approach was designed to find a small set of confident targets comprising conserved miRNA-pathway interactions in a rigorously controlled manner, leading to a clear biological interpretation and suggesting follow-up experiments.
Downregulated miRNA families were significantly associated with focal adhesion and ECM receptor interaction pathways (Tables 1 and 2); this is intriguing, because these pathways couple intracellular signaling pathways with ECM. Excess ECM deposition is central to HCV pathogenesis. The miR-29 family had the highest enrichment for these pathways with the most significant individual enrichment P value for any pathway of the up or downregulated miRNA families (P=1 × 10−32). Previous reports in other organs implicated miR-29 in numerous processes of potential relevance to C-HCV pathogenesis. miR-29 was previously shown to target ECM components [19–21]. Van Rooji et al [19] recently showed that miR-29 was downregulated during cardiac fibrosis. Two other reports showed that miR-29b regulates collagen production during osteoblast differentiation [21] and in immortalized LX-2 stellate cells [24]. Downregulated miRNAs were also associated with a cancer-related pathway (Tables 1 and 2). miR-29 is dysregulated in various cancers and targets important mediators of metastasis (reviewed in [25]). C-HCV can lead to fibrosis and hepatocellular carcinoma. Indeed, miR-29 is downregulated in hepatocellular carcinoma [18] and is negatively correlated with patient survival [26].
Our data support a role for HCV in downregulating miR-29 in hepatocytes. C-HCV and HCVcc infection in culture resulted in roughly similar miR-29 reductions. miR-29 was previously shown to have antiviral activity against human immunodeficiency virus [27, 28], raising the possibility that HCV might downregulate miR-29 to its advantage. Remarkably, miR-29 overexpression decreased HCVcc genomic RNA abundance. Thus, miR-29 can be deleterious to HCV, providing a possible motivation for the virus to downregulate miR-29. Several imperfect binding sites for miR-29 can be found in coding regions of the HCV genome. However, bulges in the central portion of putative miR-29/HCV duplexes make cleavage of the HCV genome by the RISC complex unlikely (data not shown).
In agreement with our observation, recent studies have established a role for miR-29 and other miRNAs in fibrogenesis of various organs, including heart [19, 29, 30], kidney [20, 31, 32], bone [21], and lung [33]; however, the role of miRNAs in liver fibrosis is only beginning to be explored (reviewed in [34]). HSC activation by enhanced TGF-β and subsequent fibrosis is a major consequence of C-HCV infection. Our pathway analysis pointed to a role for miR-29 in HSCs. A role for miRNAs during HSC activation is not without precedent. Several studies have implicated miRNAs in regulation of HSC activation, proliferation, and apoptosis [35–38]. We observe upregulation of miR-150 during C-HCV; this is interesting, because miR-150 was reported to negatively regulate HSC activation [39]. We also observed profound suppression of miR-29 upon HSC activation (Figure 2A–C).
Major aspects of HSC activation include cytoskeletal rearrangements (eg, α-SMA expression and stress fiber formation), increased proliferation, and increased ECM production (eg, COL1A1 and COL3A1). Venugopal et al [39] showed that miR-150 and miR-194 were reduced in bile duct-ligated rats, and overexpression of these miRNAs in LX-2 cells reduced proliferation (by ∼25%), increased apoptosis, and reduced α-SMA and collagen I levels. Another report showed miR-27 upregulated upon cell culture activation of rat HSCs [38]. miR-27 inhibition increased lipid droplet formation and decreased HSC proliferation. In contrast to the results of Venugopal et al [39], miR-27 inhibition did not alter type I collagen or α-SMA expression. These published results demonstrate that miRNAs can influence global stellate cell activation generally or affect only subsets of stellate cell activation markers.
Restoration of miR-29 expression in LX-2 HSCs reversed some but not all markers of HSC activation. miR-29 overexpression did not alter α-SMA levels. In contrast, although miR-29 overexpression had a very modest effect on proliferation (Figures 4A, 4B), miR-29 overexpression dramatically reduced COL1A1 and COL3A1 expression (Figure 4C). These data were not surprising, because COL1A1, COL3A1, and most other collagens expressed by HSCs are known miR-29 targets (references [19, 21] and Table 2). Recent studies [40, 41] also support independent expression of collagen and SMA in stellate cells.
TGF-β secreted by hepatocytes, Kupffer cells, and sinusoidal endothelial cells [42, 43] causes HSCs to activate, transdifferentiate, and secrete ECM [43]. TGF-β has been shown to downregulate miR-29 expression in cardiac fibroblasts [19]. Here, we show that TGF-β treatment of cultured HSCs resulted in miR-29 downregulation; this effect appears to be specific to HSCs, because TGF-β treatment of Huh-7.5 cells did not down-regulate miR-29. Recently, Roderburg et al [44] reported TGF-β–mediated downregulation of miR-29 in HSCs. Although it remains to be determined whether TGF-β is the sole factor leading to downregulation of miR-29 in HSCs in C-HCV–infected patients, these data raise the possibility that TGF-β–mediated suppression of miR-29 is a common feature of fibrotic liver injury. If so, miR-29 may be an important therapeutic target in chronic liver disease.
There is significant interest in pharmacological manipulation of miRNAs in humans. Van Rooji et al [19] showed that miR-29 inhibition in mice caused an upregulation of collagen expression in liver. Because fibrosis is a balance between ECM deposition and ECM degradation, miR-29–mediated suppression of ECM synthesis in HSCs could hopefully tip this balance toward reduced fibrosis in vivo. Our results clearly support further studies in animal models of HCV and fibrosis to determine the therapeutic usefulness of treatment with miR-29 mimics or small molecule drugs regulating miR-29 expression; this could have the dual benefit of inhibiting HCV while decreasing fibrosis. Of note, inhibition of miR-122 in primates has been shown to be safe [45], and clinical trials are currently underway to determine whether modulation of miR-122 levels in humans inhibits HCV.
Supplementary Data
Supplementary data are available at http://jid.oxfordjournals.org online.
Funding
This work was supported by the University of Iowa, College of Medicine Translational Pilot Grant (to R. T. M., W. N. S., and A. P. M.); University of Iowa, Levitt Center for Viral Pathogenesis Translational Pilot Grant (to R. T. M. and A. P. M.); University of Iowa Dean's Fellowship (to R. T. M.); National Institute of Allergy and Infectious Diseases Predoctoral Training Grant (T32AI007533 to R. T. M.); Veterans Administration Merit Review Grant (K. E. B. and W. N. S.); National Institutes of Health (to C. B. B. and R21 DK068453-01A1 to W. N. S.); and University of Iowa Carver Trust Foundation (to W. N. S. and M. S. I.); Department of Energy Computational Sciences Graduate Fellowship (to R. C. F.).
Supplementary Material
Acknowledgments
We thank Dr Steven Bloomer and Dr Anne Kwitek for rat primary hepatocytes and rat livers, respectively.
References
- 1.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 2.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen CB, Shomron N, Sandberg R, Hornstein E, Kitzman J, Burge CB. Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA. 2007;13:1894–910. doi: 10.1261/rna.768207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer S, Baumert TF. Unravelling the importance of microRNAs during hepatitis C virus infection in the human liver. J Hepatol. 2009;51:606–9. doi: 10.1016/j.jhep.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 7.Hou W, Tian Q, Zheng J, Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology. 2010;51:1494–504. doi: 10.1002/hep.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami Y, Aly HH, Tajima A, Inoue I, Shimotohno K. Regulation of the hepatitis C virus genome replication by miR-199a. J Hepatol. 2009;50:453–60. doi: 10.1016/j.jhep.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen IM, Cheng G, Wieland S, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–22. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166–74. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman RC, Farh KK, Burge CB, Bartel D. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 13.De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–55. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindenbach BD, Evans MJ, Syder AJ, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Wang T, Wakita T, Yang W. Systematic identification of microRNA and messenger RNA profiles in hepatitis C virus-infected human hepatoma cells. Virology. 398:57–67. doi: 10.1016/j.virol.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Ura S, Honda M, Yamashita T, et al. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098–112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- 18.Varnholt H, Drebber U, Schulze F, et al. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008;47:1223–32. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 19.van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Taylor NE, Lu L, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–82. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–84. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng X, Li Y, Walters KA, et al. Computational identification of hepatitis C virus associated microRNA-mRNA regulatory modules in human livers. BMC Genomics. 2009;10:373. doi: 10.1186/1471-2164-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun. 2010;391:316–21. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Li Y, Lai M. The microRNA network and tumor metastasis. Oncogene. 2010;29:937–48. doi: 10.1038/onc.2009.406. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Gusev Y, Aderca I, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–27. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahluwalia JK, Khan SZ, Soni K, et al. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology. 2008;5:117. doi: 10.1186/1742-4690-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 30.Duisters RF, Tijsen AJ, Schroen B, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8. doi: 10.1161/CIRCRESAHA.108.182535. 6p following 178. [DOI] [PubMed] [Google Scholar]
- 31.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:438–47. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato M, Putta S, Wang M, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pottier N, Maurin T, Chevalier B, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. pLoS One. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;15:5633–40. doi: 10.3748/wjg.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J Hepatol. 2009;50:766–78. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Guo CJ, Pan Q, Jiang B, Chen GY, Li DG. Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis. 2009;14:1331–40. doi: 10.1007/s10495-009-0401-3. [DOI] [PubMed] [Google Scholar]
- 37.Guo CJ, Pan Q, Cheng T, Jiang B, Chen GY, Li DG. Changes in microRNAs associated with hepatic stellate cell activation status identify signaling pathways. FEBS J. 2009;276:5163–76. doi: 10.1111/j.1742-4658.2009.07213.x. [DOI] [PubMed] [Google Scholar]
- 38.Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–66. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 39.Venugopal SK, Jiang J, Kim TH, et al. Liver fibrosis causes down-regulation of miRNA-150 and miRNA-194 in hepatic stellate cells and their over-expression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–9. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 41.de Meijer VE, Sverdlov DY, Popov Y, et al. Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. pLoS One. 2010;5:e11256. doi: 10.1371/journal.pone.0011256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 44.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2010;53(1):209–18. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 45.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


