Abstract
Haemophilus ducreyi 35000HP contains a homolog of the CpxRA 2-component signal transduction system, which controls the cell envelope stress response system in other gram-negative bacteria and regulates some important H. ducreyi virulence factors. A H. ducreyi cpxR mutant was compared with its parent for virulence in the human challenge model of experimental chancroid. The pustule formation rate in 5 volunteers was 33% (95% confidence interval [CI], 1.3%–65.3%) at 15 parent sites and 40% (95% CI, 18.1%–61.9%) at 15 mutant sites (P = .35). Thus, the cpxR mutant was not attenuated for virulence. Inactivation of the H. ducreyi cpxR gene did not reduce the ability of this mutant to express certain proven virulence factors, including the DsrA serum resistance protein and the LspA2 protein, which inhibits phagocytosis. These results expand our understanding of the involvement of the CpxRA system in regulating virulence expression in H. ducreyi.
The gram-negative bacillus Haemophilus ducreyi is the etiologic agent of chancroid, a sexually transmitted genital ulcer disease [1]. Despite being an obligate human pathogen, H. ducreyi has a broad transcriptional response to the human host [2] and regulates the expression of a variety of different virulence factors [3–5]. During the development of the chancroidal ulcer, H. ducreyi is likely to encounter a number of different conditions and host factors that could affect its gene expression. H. ducreyi expresses a functional homolog of the CpxRA 2-component signal transduction system (TCS), the only obvious intact TCS in its genome. In Escherichia coli, the CpxRA system deals with cell envelope stress and can be activated in response to aggregated and misfolded proteins in the periplasm [6], adhesion to surfaces [7], or changes in pH [8, 9] and osmolarity [10, 11]. In H. ducreyi, the CpxRA system controls expression of several important virulence factors, including DsrA, LspA2, and Flp [3–5]. The CpxRA TCS consists of the CpxA sensor (histidine) kinase, which functions as an autokinase, a kinase, and a phosphatase, and the cognate CpxR response regulator, which functions to regulate gene transcription [12]. When cpxA is deleted, CpxR probably accepts phosphoryl groups from other donors, such as acetyl phosphate, and cannot be dephosphorylated, resulting in constitutive activation of the system [3, 5, 13].
While the specific cues sensed by the CpxRA TCS in H. ducreyi have not yet been identified, transcription of cpxR and cpxA is downregulated in the presence of fetal calf serum (FCS) in vitro [4]. It is possible that a serum component(s) encountered by this bacterium during infection of the skin triggers the downregulation of the CpxRA system, which in turn could lead to the upregulation of proven virulence factors that would facilitate the infection process. The recent finding that a H. ducreyi cpxA mutant was highly attenuated in the human challenge model for experimental chancroid [3] raised the possibility that a functional CpxRA system is essential for virulence in this model. Alternatively, this attenuation could also be the result of loss of CpxA phosphatase activity and the consequent likely constitutive phosphorylation of CpxR, which in turn repressed the expression of several key H. ducreyi virulence factors [3, 5]. In this study, we evaluated a H. ducreyi cpxR deletion mutant for virulence in human volunteers and examined the effect of the deletion of cpxR on expression of virulence determinants that have roles in resistance to phagocytosis and serum killing.
METHODS
Bacterial and Mammalian Cell Growth Conditions
Bacterial strains used in this study are listed in Table 1. Two H. ducreyi cpxA mutants were used, one with an unmarked in-frame deletion in cpxA (35000HPΔcpxA) [3] and the other (35000HP cpxA::cm) containing a chloramphenicol resistance cartridge inserted in frame into a deletion within cpxA [4, 5]. H. ducreyi strains were routinely grown on chocolate agar plates and incubated at 33°C in a humidified atmosphere containing 95% air and 5% carbon dioxide (CO2). For in vitro experiments, H. ducreyi strains were grown in a Columbia broth-based medium (CB), as described elsewhere [14]. For phagocytosis assays, bacterial strains were grown overnight in 20 mL of CB, the cells were centrifuged at 3220 g for 10 min, and the pellet was resuspended in 2 mL of Dulbecco's modified Eagle medium (DMEM) containing 10% (vol/vol) FCS to an optical density at 600 nm (OD600) of .5. H. ducreyi cells used as inocula in the human challenge model were grown as described elsewhere [15]. The murine macrophage cell line J774A.1 (American Type Culture Collection TIB-67) was cultivated in high-glucose DMEM supplemented with GlutaMAX (Invitrogen) and 10% (vol/vol) heat-inactivated FCS and incubated at 37°C in a humidified atmosphere containing 90% air and 10% CO2.
Table 1.
Haemophilus ducreyi Strains Used in Studies
| Strain | Description | Reference |
| 35000HP | Human-passaged variant of 35000 | [15] |
| 35000HPΔcpxR | Isogenic 35000HP cpxR mutant with an internal deletion in cpxR containing a chloramphenicol resistance cartridge | [4] |
| 35000HPΔcpxA | Isogenic 35000HP mutant with an unmarked in-frame deletion in cpxA | [3] |
| 35000HPcpxA::cm | Isogenic 35000HP mutant with an internal deletion in cpxA containing a chloramphenicol resistance cartridge | [5] |
| 35000HPΩ12 | Isogenic 35000HP lspA1 lspA2 mutant | [34] |
| FX517 | Isogenic 35000HP dsrA mutant | [18] |
Phenotypic Comparisons
Outer membrane proteins (OMPs) and lipooligosaccharides expressed by selected H. ducreyi strains were analyzed as described elsewhere [16].
SDS-PAGE and Western Blot Analysis
Whole cell lysates of H. ducreyi were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 15% or 4%–20% (wt/vol) polyacrylamide gels and electroblotted onto nitrocellulose or polyvinylidene fluoride membranes, essentially as described elsewhere [4]. These membranes were subsequently blocked with either StartingBlock (phosphate-buffered saline [PBS]) Blocking Buffer (Thermo Scientific) containing 5% (vol/vol) normal goat serum or with 5% milk in 10 mmol/L Tris, 15 mmol/L sodium chloride, and 0.01% Tween and then probed with the primary antibodies. The LspA1-specific monoclonal antibody (mAb) 40A4 [14], the LspA2-specific mAb 1H9 [14], the LspB-reactive polyclonal mouse serum [17], the DsrA-reactive polyclonal mouse serum [18], the CpxR-reactive polyclonal rabbit serum [4], and the PAL-specific mAb 3B9 [19], have been described elsewhere. Western blots were developed using either the Western Lightning Chemiluminescence Reagent Plus (New England Nuclear) or the Enhanced Chemiluminescent Western Blotting Substrate (Thermo Scientific).
Phagocytosis Assay Using Opsonized Beads
To opsonize latex beads, a 40-μL portion of a 10% (vol/vol) suspension of latex beads (3.87-μm diameter; Bangs Laboratories) was added to 500 μL of PBS and centrifuged at 6000 g for 1 minute. The beads were washed twice with 500 μL of PBS and resuspended in 170 μL of PBS. A 30 μL portion of ChromPure human IgG (Jackson ImmunoResearch) was added, and the microcentrifuge tubes were rotated for 1 hour at room temperature. The beads were then rotated overnight at 4°C. The next day, the beads were washed 3 times with 500 μL of PBS and resuspended in 500 μL of PBS. A 5-μL portion of fluorescent donkey anti-human Cy3 antibody (Jackson ImmunoResearch) was added, and the microcentrifuge tube was rotated for 1 hour at room temperature. The beads were then washed 4 times with PBS and resuspended in 500 μL of DMEM containing 10% FCS. Particles were extruded through a 25-gauge needle to disrupt aggregates. For the phagocytosis assay, J774A.1 cells were plated 2 days before the experiment, at a density of 2.5 × 104 cells/well in 4-well chamber slides. The day before the experiment, the J774A.1 cells were washed with DMEM medium without serum, and 500 μL of DMEM medium without serum was added. On the day of the experiment, cells were washed, and 200 μL of bacterial suspension (OD600, .5) was added to each well. The slides were spun at 200 g for 5 min at room temperature and then placed in an incubator at 33°C with 95% air-5% CO2 for 1 hour. A 30 μL volume of the fluorescently-labeled opsonized beads was added and the slides were placed in a 37°C incubator with 90% air −10% CO2 for 15 min to allow for phagocytosis. Slides were then placed on ice and washed with ice-cold DMEM containing 10% FCS. A 5-μL portion of a 1.5 mg/mL stock of fluorescent donkey anti-human Cy2 antibody (Jackson ImmunoResearch) was added and the slides were incubated on ice for 10 min. Wells were washed 5 times with PBS and fixed with 4% paraformaldehyde (Electron Microscopy Services) in PBS for 20 min at room temperature. After 2 washes with PBS, 500 μL of 100 mmol/L glycine was added for 10 min. Cells were then stained with Hoechst 33342 in PBS for 10 min and washed with PBS. After aspirating, the plastic chamber was removed and the cells were dried. Vectashield (Vector Laboratories) and a coverslip were then added. J774A.1 cells were counted using Hoechst nuclear staining, total beads (external and ingested) were counted using cyanine (Cy) 3 staining (red), and external beads were counted using Cy2 staining (green). The phagocytosis index was determined as follows: (total beads−external beads)/total number of J774A.1 cells.
Bactericidal Assay
Survival of H. ducreyi strains in 50% (vol/vol) normal human serum (NHS) was determined using agar plate-grown bacteria, exactly as described elsewhere [3]. Data are reported as the percentage survival in NHS compared with that in heat-inactivated NHS ([geometric mean colony-forming units (CFUs) in active NHS/geometric mean CFUs in heat-inactivated NHS] × 100). Each experiment was repeated 5 times, and the mean and standard deviation (SD) of the survival percentage were calculated. The strains were compared performed using paired Student t tests. With the Bonferroni adjustment for multiple comparisons, differences for these assays were considered significant at P ≤ .012.
Human Inoculation Experiments
Healthy, H. ducreyi–naive volunteers >21 years of age gave informed consent for participation in the study and for human immunodeficiency virus serology, in accordance with the human experimentation guidelines of the US Department of Health and Human Services and the institutional review board of Indiana University–Purdue University of Indianapolis. Stocks of 35000HP and 35000HPΔcpxR used for the human inoculation experiments were prepared according to US Food and Drug Administration guidelines. The preparation and inoculation of the bacteria, calculation of the estimated delivered dose, experimental protocol, clinical observations, clinical end points, and treatment with antibiotics were described elsewhere [20, 21]. The trial design tested whether 35000HPΔcpxR was impaired in its ability to cause pustules relative to 35000HP in a multistage dose-ranging study with a minimum of 2 stages, as described elsewhere [20, 21]. Comparisons of papule and pustule formation rates for the 2 strains were performed using a logistic regression model with generalized estimating equations to account for the correlation among sites within the same individual, as described elsewhere [22]. The generalized estimating equation sandwich estimate for the standard errors was used to calculate 95% confidence intervals (CIs) for these rates. Individual colonies from the inocula, surface cultures, and biopsy specimens were scored for susceptibility to chloramphenicol on chloramphenicol-containing chocolate agar plates, as described elsewhere [23].
RESULTS
35000HPΔcpxR Is Virulent in Humans
The reduction in virulence of a 35000HPΔcpxA deletion mutant [3] suggested that an intact CpxRA system might be required for full virulence of H. ducreyi. Therefore, we evaluated a 35000HPΔcpxR mutant [4] for virulence in human volunteers.
Before performing the human inoculation experiments, we compared 35000HP with 35000HPΔcpxR and 35000HPΔcpxA. In both gonococcal broth and CB, the growth rates of the organisms and their lipooligosaccharide profiles were identical (data not shown). As reported previously, the OMP [3] and whole-cell protein profiles [5] of 35000HPΔcpxA showed up-regulation of OmpP2B and down-regulation of DsrA (data not shown). However, the OMP and whole cell protein profiles of 35000HP and 35000HPΔcpxR were identical (data not shown).
Seven healthy adults (6 male, 1 female; 6 black, 1 white; age range, 41–55 years; mean age ± SD, 45 ± 5 years) enrolled in the study. Two subjects withdrew on the day of inoculation. Two subjects were inoculated with an estimated delivered dose of 91 CFU of 35000HP at 3 sites and 25, 50, and 100 CFU of 35000HPΔcpxR at 3 sites (Table 2). Another group of 3 subjects was inoculated with 49 CFU of 35000HP at 3 sites and with 27, 54, and 109 CFU of 35000HPΔcpxR at 3 sites (Table 2). Overall, papules formed at 80% (95% CI, 65.7%–94.3%) of 15 parent sites and 73.3% of 15 mutant sites (95% CI, 39.3%–99.9%) (P = .38). After 24 h of infection, the mean ± SD of the area of papules was 15.4 ± 11.4 mm2 at parent-inoculated sites and 11.6 ± 10.2 mm2 at mutant-inoculated sites (P = .32). Pustules formed at 33% (95% CI, 1.3%–65.3%) of sites inoculated with the parent and 40% of sites inoculated with the mutant (95% CI, 18.1%–61.9%) (P = .35). Thus, an intact CpxRA system is not required for virulence in humans.
Table 2.
Response to Inoculation With Live Haemophilus ducreyi
| Volunteer (sex)a | Duration of observation, days | Strainb | Dose, CFU | Initial papules, no. | Final outcome, no. of sites |
|
| No disease | Pustule | |||||
| 370 (male) | 6 | Parent | 91 | 3 | 2 | 1 |
| Mutant | 25, 50, 100c | 0 | 3 | 0 | ||
| 371 (male) | 6 | Parent | 91 | 3 | 0 | 3 |
| Mutant | 25, 50, 100 | 3 | 1 | 2 | ||
| 369 (female) | 6 | Parent | 49 | 2 | 3 | 0 |
| Mutant | 27, 54, 109c | 3 | 1 | 2 | ||
| 373 (male) | 13 | Parent | 49 | 2 | 3 | 0 |
| Mutant | 27, 54, 109 | 2 | 2 | 1 | ||
| 375 (male) | 9 | Parent | 49 | 2 | 2 | 1 |
| Mutant | 27, 54, 109 | 3 | 2 | 1 | ||
NOTE. a Volunteers 370 and 371 were inoculated in the first group; volunteers 369, 373, and 375, in the second group.
The parent strain was 35000HP; the mutant strain, 35000HPΔcpxR.
One dose of each amount; CFU, colony-forming units.
All colonies tested from the broth cultures used to prepare the parent inocula (n = 71) and the mutant inocula (n = 71) had the expected antibiotic susceptibility (parent, chloramphenicol susceptible; mutant, chloramphenicol resistant). Surface cultures obtained after inoculation yielded H. ducreyi from 33% of the parent-inoculated and 27% of the mutant-inoculated sites. At the clinical end point, biopsy specimens were obtained from 1 parent-inoculated and 3 mutant-inoculated pustules and cultured; all biopsy specimens yielded H. ducreyi. All colonies recovered from surface cultures and biopsy specimens from parent sites (n = 161 and n = 35, respectively) and mutant sites (n = 126 and n = 74, respectively) had the expected antibiotic susceptibility. Thus, there was no evidence of cross-contamination between parent-inoculated and mutant-inoculated sites.
Two subjects (subjects 371 and 375) developed pustules at both mutant-inoculated and parent-inoculated sites. At the end point, subject 371 declined biopsy. Biopsy samples were obtained from 1 mutant and 1 parent-inoculated site from subject 375 and stained with hematoxylin-eosin and anti-CD3 antibodies, as described elsewhere. Both samples contained micropustules in the epidermis and a dermal infiltrate of perivascular CD3+ cells and were indistinguishable (data not shown).
35000HPΔcpxR Inhibits the Phagocytic Ability of Murine J774A.1 Macrophages In Vitro
CpxR was previously shown to negatively regulate expression of the lspB-lspA2 operon in H. ducreyi [4]. Lack of expression of the LspB secretion factor would in turn prevent secretion of both LspA1 and LspA2 [17], either of which can inhibit phagocytic activity of macrophagelike cell lines in vitro [24]. In the absence of CpxR, there is increased expression of LspB and LspA2 (Figure 1A, lane 3) relative to the wild-type parent strain [4] (Figure 1A, lane 1). Using J774.A1 murine macrophages, the ability of 35000HPΔcpxR to inhibit phagocytosis of opsonized fluorescent beads was compared with that of wild-type 35000HP, the lspA1 lspA2 mutant 35000HPΩ12, and 35000HP cpxA::cm. As would be predicted from the protein expression data in Figure 1A, the wild-type 35000HP strain (expressing LspA1, LspB, and a small amount of LspA2) inhibited phagocytosis effectively. In contrast, the 35000HPΩ12 mutant, which does not express either LspA1 or LspA2 (Figure 1A, lane 2), did not inhibit phagocytosis (Figure 1B). The 35000HPΔcpxR mutant (which expresses LspB, LspA1, and LspA2) inhibited phagocytosis (Figure 1B). Furthermore, the 35000HPcpxA::cm mutant did not express LspB or LspA2 (Figure 1A, lane 4) and did not inhibit phagocytosis (Figure 1B). This cpxA mutant does synthesize LspA1 (Figure 1A, lane 4), but the lack of detectable LspB protein precluded it from transporting any LspA1 to the bacterial cell surface [17].
Figure 1.
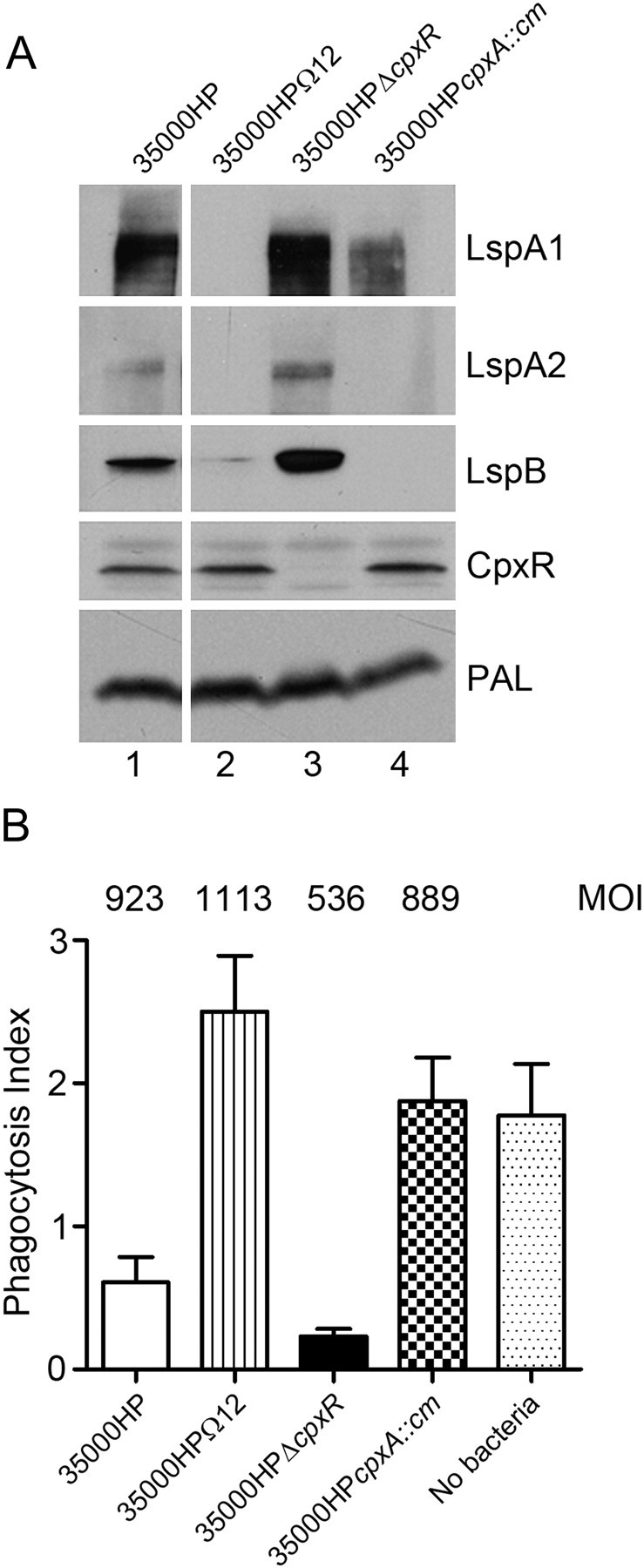
Protein expression and inhibition of phagocytosis by wild-type and mutant strains of Haemophilus ducreyi. A, Western blot analysis of protein expression by wild-type 35000HP (lane 1), the 35000HPΩ12 mutant (lane 2), the 35000HPΔcpxR mutant (lane 3), and the 35000HP cpxA::cm mutant (lane 4). The specific antigen reactive with each primary antibody is listed to the right of each panel. B, Ability of these 4 H. ducreyi strains to inhibit the phagocytic activity of murine J774A.1 macrophages, as measured by the uptake of immunoglobulin G–opsonized latex beads. A representative experiment is shown. The multiplicity of infection (MOI) used for each strain is listed at the top of each column.
35000HPΔcpxR Is Serum Resistant
The 35000HPΔcpxA mutant was previously shown to express low levels of DsrA and to be serum sensitive relative to 35000HP [3]. Here, we compared the level of expression of DsrA relative to PAL by Western blot analysis for 35000HP, 35000HPΔcpxR, and 35000HPΔcpxA (Figure 2A). 35000HPΔcpxA (Figure 2A, lane 2) expressed less DsrA than 35000HP (Figure 2A, lane 1), but 35000HPΔcpxR (Figure 2A, lane 3) expressed levels of DsrA that were similar to that of the parent strain. In bactericidal assays, 35000HPΔcpxA trended to be significantly more susceptible to serum killing than 35000HP (P = .014) (Figure 2B). 35000HPΔcpxA was as susceptible to the killing by NHS as the previously described dsrA mutant FX517 (P = .23) [18], whereas 35000HPΔcpxR was significantly more resistant to serum killing than both FX517 (P < .001) and 35000HPΔcpxA (P < .001) (Figure 2B). Thus, deletion of cpxR did not diminish or enhance expression of DsrA or resistance to serum killing (P = .093), relative to 35000HP.
Figure 2.
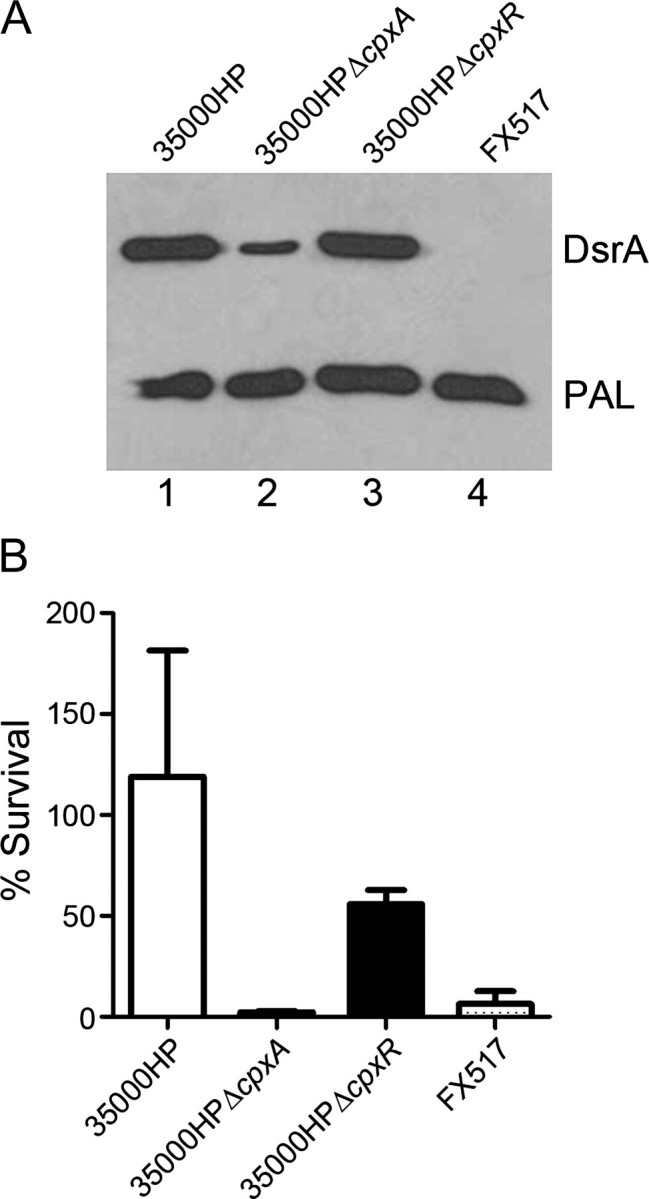
Expression of DsrA and serum resistance by wild-type and mutant strains of Haemophilus ducreyi. A, Representative Western blot analysis of whole cell lysates of 35000HP (lane 1), 35000HPΔcpxA (lane 2), 35000HPΔcpxR (lane 3), and the dsrA mutant FX517 (lane 4) probed with both polyclonal DsrA antiserum and the PAL-specific monoclonal antibody 3B9. The positions of the DsrA and PAL proteins are indicated. B, Serum bactericidal activity assays. The percentage survival of 35000HP, 35000HPΔcpxA, 35000HPΔcpxR, and FX517 in 50% normal human serum (NHS), calculated as (geometric mean colony-forming units [CFUs] in NHS/geometric mean CFUs in heat-inactivated NHS) × 100. Values represent means ± standard deviations of 5 independent experiments. After adjustment for multiple comparisons, differences in these assays were considered significant at P ≤ .012.
DISCUSSION
Although the importance of the CpxRA signal transduction system in dealing with envelope stress response in E. coli is well known, its involvement in controlling virulence expression is less well studied. Nonetheless, there is an emerging theme in microbial pathogenesis, in which this cell envelope stress response system controls expression of surface antigens and key virulence factors. For example, CpxR is required for the virulence of Xenorhabdus nematophila in its natural insect host [25]. Phosphorylated CpxR has been shown to activate virF transcription in vitro in Shigella sonnei [26], and the CpxRA system is involved in posttranscriptional processing of the InvE regulatory protein, which in turn affects the Shigella type III secretion system [27]. Studies have also shown that phosphorylated CpxR represses E. coli genes encoding surface appendages, including components of the curli fimbriae [28] and the pap pili [29]. In both enteropathogenic E. coli [30] and Yersinia enterocolitica [31], activation of the Cpx system by cpxA* mutations that result in constitutively active CpxA kinase activity caused decreased secretion of type III secretion system components. In their report of the most detailed study to date, which addressed temporal control of gene expression by CpxRA, Vogt and coworkers indicated that this TCS has a complex role in controlling expression of bundle-forming pili in enteropathogenic E. coli [32]. Taken together, these studies indicate that CpxR frequently functions as a repressor of virulence genes, and it has been proposed that repression of genes encoding certain envelope components might have a positive effect on the bacterial cell during times of stress [30].
A previous study [5] indicated that CpxR could bind in vitro to the putative promoter regions of a number of H. ducreyi genes, including lspB, dsrA, and the flp operon. These particular genes or other members of these operons encode surface proteins that are proven virulence factors of H. ducreyi [22, 33, 34]. The attenuation of the H. ducreyi cpxA deletion mutant in human volunteers [3], together with the lack of reduction in virulence of the H. ducreyi cpxR deletion mutant characterized in the present study, reinforce the findings of in vitro studies [3–5] which indicated that phosphorylated CpxR is responsible for repressing expression of several gene products essential for virulence of this pathogen in this model. The absence of the phosphatase activity of CpxA in the cpxA mutant would allow essentially constitutive phosphorylation of CpxR, presumably with small molecules like acetyl phosphate serving as phosphodonors [3, 5, 35]. This phosphorylated CpxR would effectively repress expression of the different virulence factors, resulting in the observed attenuation of this cpxA mutant. Conversely, in the cpxR deletion mutant, the complete absence of CpxR should allow expression of these virulence factors at levels at least equivalent to those found in the wild-type strain. In the present study, the ability of the cpxR mutant to inhibit phagocytosis in vitro (Figure 1) and resist killing by NHS (Figure 2) was found to be at least equivalent to that of the wild-type strain. By Western blot and densitometry analysis, the level of expression of CpxA in 35000HPΔcpxR was ∼30% that of the wild-type strain (data not shown). However, this lower level of CpxA expression did not affect the virulence of 35000HPΔcpxR, and the virulence phenotype of a H. ducreyi cpxR cpxA double mutant would be predicted to be the same as that of a cpxR null mutant.
The regulatory activities of the CpxRA TCS during the progression of natural bacterial diseases have not been defined, although recent studies in E. coli indicate that the function of this TCS can be complex [32]. The data presented here, as well as in previous reports, indicate that the H. ducreyi CpxRA system plays an important role in regulating the expression of multiple virulence factors needed for infection [3–5]. However, in the present study, inactivation of cpxR did not decrease the virulence of H. ducreyi. This result begs the question of the relevance of the CpxRA TCS to the infectious process. Although this human model clearly reproduces many aspects of naturally acquired infections, the introduction of H. ducreyi into the epidermis and dermis is necessarily artificial, both mechanistically and in its use of an in vitro–grown inoculum. Therefore, it is possible that the gene expression profile of the in vitro–grown bacterial inoculum is different from that of H. ducreyi during natural transmission (ie, from a lesion to uninfected epidermis). Consequently, important regulatory transition step(s) involving activation/deactivation of the CpxRA system may be precluded or circumvented. Another study has shown that, when the H. ducreyi CpxRA system is activated, there is an increase in the transcript levels of genes encoding putative fimbriae [5]. Expression of fimbrial genes has been shown to be important in the initial steps of colonization by other pathogens (for a review, see [36]), and it is possible that H. ducreyi fimbriae are also involved in the initial steps in natural infection. It should also be noted that the potential involvement of other regulatory molecules or systems cannot be overlooked. Consequently, studies are underway to clarify the role of the H. ducreyi CpxRA TCS in regulation of virulence determinant expression, the cues that modulate the activity of the only TCS in H. ducreyi, and whether additional signal transduction systems foster adaptation of this pathogen to the human host.
Funding
This work was supported by the National Institute of Allergy and Infectious Dieseases (grant AI32011 to E. J. H. and grants AI27863 and AI31494 to S. M. S.), and the Indiana Clinical and Translational Sciences Institute and Indiana Clinical Research Center (grant UL RR052761 to S. M. S.).
Acknowledgments
We thank Shelia Ellinger for preparing the regulatory documents for the human trials, and we thank the volunteers who participated in the trial.
References
- 1.Bong CT, Bauer ME, Spinola SM. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 2002;4:1141–8. doi: 10.1016/s1286-4579(02)01639-8. [DOI] [PubMed] [Google Scholar]
- 2.Bauer ME, Fortney KR, Harrison A, Janowicz DM, Munson RS, Jr., Spinola SM. Identification of Haemophilus ducreyi genes expressed during human infection. Microbiology. 2008;154:1152–60. doi: 10.1099/mic.0.2007/013953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinola SM, Fortney KR, Baker B, et al. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect Immun. 2010;78:3898–904. doi: 10.1128/IAI.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labandeira-Rey M, Mock JR, Hansen EJ. Regulation of expression of the Haemophilus ducreyi LspB and LspA2 proteins by CpxR. Infect Immun. 2009;77:3402–11. doi: 10.1128/IAI.00292-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labandeira-Rey M, Brautigam CA, Hansen EJ. Characterization of the CpxRA regulon in Haemophilus ducreyi. Infect Immun. 2010;78:4779–91. doi: 10.1128/IAI.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiGiuseppe PA, Silhavy TJ. Signal detection and target gene induction by the CpxRA two-component system. J Bacteriol. 2003;185:2432–40. doi: 10.1128/JB.185.8.2432-2440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto K, Silhavy TJ. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A. 2002;99:2287–92. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama S-I, Watanabe H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol. 1995;177:5062–9. doi: 10.1128/jb.177.17.5062-5069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama S, Kushiro A, Asahara T, et al. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology. 2003;149:2809–17. doi: 10.1099/mic.0.26229-0. [DOI] [PubMed] [Google Scholar]
- 10.Jubelin G, Vianney A, Beloin C, et al. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J Bacteriol. 2005;187:2038–49. doi: 10.1128/JB.187.6.2038-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclerc GJ, Tartera C, Metcalf ES. Environmental regulation of Salmonella typhi invasion-defective mutants. Infect Immun. 1998;66:682–91. doi: 10.1128/iai.66.2.682-691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raivio TL, Silhavy TJ. Sensing and responding to envelope stress. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, DC: ASM Press; 2000. pp. 19–32. [Google Scholar]
- 13.Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol. 2008;190:2314–22. doi: 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward CK, Lumbley SR, Latimer JL, Cope LD, Hansen EJ. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J Bacteriol. 1998;180:6013–22. doi: 10.1128/jb.180.22.6013-6022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Tawfiq JA, Thornton AC, Katz BP, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–7. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 16.Fortney KR, Young RS, Bauer ME, et al. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–8. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward CK, Mock JR, Hansen EJ. The LspB protein is involved in the secretion of the LspA1 and LspA2 proteins by Haemophilus ducreyi. Infect Immun. 2004;72:1874–84. doi: 10.1128/IAI.72.4.1874-1884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkins C, Morrow KJ, Jr., Olsen B. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–19. doi: 10.1128/iai.68.3.1608-1619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinola SM, Hiltke TJ, Fortney KR, Shanks KL. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect Immun. 1996;64:1950–5. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labandeira-Rey M, Janowicz DM, Blick RJ, et al. Inactivation of the Haemophilus ducreyi luxS gene affects the virulence of this pathogen in human subjects. J Infect Dis. 2009;200:409–16. doi: 10.1086/600142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janowicz DM, Ofner S, Katz BP, Spinola SM. Experimental infection of human volunteers with Haemophilus ducreyi: Fifteen years of clinical data and experience. J Infect Dis. 2009;199:1671–9. doi: 10.1086/598966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinola SM, Fortney KR, Katz BP, et al. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect Immun. 2003;71:7178–82. doi: 10.1128/IAI.71.12.7178-7182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks KE, Fortney KR, Baker B, et al. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J Infect Dis. 2008;197:1531–6. doi: 10.1086/588001. [DOI] [PubMed] [Google Scholar]
- 24.Vakevainen M, Greenberg S, Hansen EJ. Inhibition of phagocytosis by Haemophilus ducreyi requires expression of the LspA1 and LspA2 proteins. Infect Immun. 2003;71:5994–6003. doi: 10.1128/IAI.71.10.5994-6003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbert Tran EE, Goodrich-Blair H. CpxRA contributes to Xenorhabdus nematophila virulence through regulation of lrhA and modulation of insect immunity. Appl Environ Microbiol. 2009;75:3998–4006. doi: 10.1128/AEM.02657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama S, Watanabe H. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J Bacteriol. 1998;180:3522–8. doi: 10.1128/jb.180.14.3522-3528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitobe J, Arakawa E, Watanabe H. A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J Bacteriol. 2005;187:107–13. doi: 10.1128/JB.187.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorel C, Vidal O, Prigent-Combaret C, Vallet I, Lejeune P. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol Lett. 1999;178:169–75. doi: 10.1111/j.1574-6968.1999.tb13774.x. [DOI] [PubMed] [Google Scholar]
- 29.Hernday AD, Braaten BA, Broitman-Maduro G, Engelberts P, Low DA. Regulation of the pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol Cell. 2004;16:537–47. doi: 10.1016/j.molcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Macritchie DM, Ward JD, Nevesinjac AZ, Raivio TL. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect Immun. 2008;76:1465–75. doi: 10.1128/IAI.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlsson KE, Liu J, Edqvist PJ, Francis MS. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect Immun. 2007;75:3913–24. doi: 10.1128/IAI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt SL, Nevesinjac AZ, Humphries RM, Donnenberg MS, Armstrong GD, Raivio TL. The Cpx envelope stress response both facilitates and inhibits elaboration of the enteropathogenic Escherichia coli bundle-forming pilus. Mol Microbiol. 2010;76:1095–110. doi: 10.1111/j.1365-2958.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bong CT, Throm RE, Fortney KR, et al. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect Immun. 2001;69:1488–91. doi: 10.1128/IAI.69.3.1488-1491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janowicz DM, Fortney KR, Katz BP, et al. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect Immun. 2004;72:4528–33. doi: 10.1128/IAI.72.8.4528-4533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe AJ. Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr Opin Microbiol. 2010;13:204–9. doi: 10.1016/j.mib.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kline KA, Falker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–92. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]


