Abstract
The endogenous cannabinoids 2-arachidonoylglycerol (2-AG) and arachidonoyl ethanolamide (AEA or anandamide) play vital roles during nervous system development including regulating axonal guidance and synaptogenesis. The enzymatic degradation of 2-AG and AEA is highly susceptible to inhibition by organophosphate compounds in vitro. Furthermore, acute in vivo exposure of adult animals to the agricultural insecticide chlorpyrifos (CPS) caused moderate inhibition of both 2-AG and AEA hydrolysis. However, the effects of repeated exposure to lower levels of CPS, especially during development, on endocannabinoid metabolism in the brain is not known. To examine this, rat pups were orally exposed daily from postnatal days 10–16 to either 1.0, 2.5, or 5.0 mg/kg CPS. Body weight gain was reduced by 5.0 mg/kg on all days of treatment whereas 2.5 mg/kg reduced the weight gain only on the last two days of treatment. At 4-h postexposure on day 16, forebrain cholinesterase (ChE) activity and hydrolysis of 2-AG and AEA were inhibited in a dose-related manner, and the extent of inhibition from highest to lowest level was AEA hydrolysis > ChE activity > 2-AG hydrolysis. The extent of inhibition of AEA hydrolysis was approximately twice than that of ChE activity with AEA hydrolysis being virtually eliminated by 2.5 and 5.0 mg/kg and 1.0 mg/kg causing 40% inhibition. The sensitivity of AEA hydrolysis, compared with canonical targets such as ChE activity, suggests a potential alternative developmental target for CPS. Inhibition of AEA hydrolysis could result in accumulation of endocannabinoids, which could alter normal endocannabinoid transmission during brain maturation.
Keywords: chlorpyrifos, endocannabinoid, developmental neurotoxicology
The concern that exposure to organophosphate (OP) insecticides is exerting greater neurotoxic effects in children as compared with adults has led to the elimination of the household uses of several OP insecticides, such as chlorpyrifos (CPS) and diazinon. Even though this restriction proved to be beneficial in urban areas (Whyatt et al., 2004), the continued use of OP insecticides in agriculture still provides opportunities for childhood exposure. Indeed, children living in agricultural communities continue to be at risk of exposure to CPS as well as other OP insecticides (Koch et al., 2002). Childhood exposure to OP insecticides has been shown to have lasting negative impacts including decreased cognitive abilities and motor skills (Ruckart et al., 2004). Specifically, children exposed to CPS exhibit increased manifestation of attention disorder and attention-deficit/hyperactivity disorder problems (Rauh et al., 2006).
As for most compounds in the OP insecticide family, the neurotoxic action of CPS in pests is caused by the inhibition of acetylcholinesterase (ChE; acetylcholine hydrolase, EC 3.1.1.7), a serine esterase that degrades the widely distributed neurotransmitter acetylcholine (ACh). Significant inhibition of ChE activity leads to hyperactivity of the cholinergic system, which leads to disruption of normal physiological functioning. In juvenile animals and humans, the nervous system is in the process of maturing and establishing the correct pathways and synapses. Therefore, it is logical to assume that exposure to a neurotoxicant could impact the nervous system at a time when neurological pathways are being established, thus delaying mental development. However, neurochemical and behavioral aberrations have been observed at the levels of OP exposure that induce only minimal amounts of ChE inhibition and little hyperactivity in the cholinergic system (Levin et al., 2002; Slotkin et al., 2006, 2007; Timofeeva et al., 2008a,b). This has led to the hypothesis that the developmental toxicological effects of OP insecticides involve a currently unknown “noncholinergic mechanism of action.”
One potential target in the developing central nervous system (CNS) is the endocannabinoid system. The interaction of OP insecticides with components of the endocannabinoid system may cause developmental toxicity. The best characterized endogenous cannabinoid ligands are arachidonoyl ethanolamide (AEA or anandamide) and 2-arachidonoylglycerol (2-AG) (Devane et al., 1992; Di Marzo et al., 1994; Mechoulam et al., 1995; Stella et al., 1997; Sugiura et al., 1995). Both are lipid mediators that are synthesized on demand in an activity-dependent manner and are not stored in vesicles due to their lipophilic nature (Di Marzo et al., 1994). 2-AG is degraded in the CNS primarily by the action of monoacylglycerol lipase (MAGL), although other enzymes have been reported to contribute to its hydrolysis in the CNS (Blankman et al., 2007) and in the immune cells (Xie et al., 2010). AEA is degraded by the action of fatty acid amide hydrolase (FAAH), but FAAH has also been reported to degrade 2-AG in vitro. Previous studies have reported that acute OP treatment of adult mice results in greater levels of inhibition of endocannabinoid hydrolysis than of ChE activity (Quistad et al., 2001, 2002, 2006). However, the general conclusion of these studies was that inhibition of cannabinoid deactivation enzymes does not play a role in the toxicity of the OP unless it is administered at very high dose levels. Although this may be an appropriate conclusion for adults, it may not be so for juveniles that are exposed during time periods when significant developmental events are occurring, many of which are directly related to cognitive function (Casey et al., 2008; Liston et al., 2006; Nagy et al., 2004). It is known that the endocannabinoid system plays a pivotal role in normal brain development (for reviews see Anavi-Goffer and Mulder, 2009; Harkany et al., 2008), and inactivation of the enzymes responsible for endocannabinoid degradation could disrupt the appropriate signaling processes involved in brain development. In a recent report, Marco et al. (2009) reported that repeated exposure of adolescent rats to the specific FAAH inhibitor cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) resulted in altered levels of cannabinoid receptors in adults. These data suggest that OP-induced inhibition of the endocannabinoid hydrolytic enzymes during brain development has the potential to play a role in the developmental toxicity of these compounds. In addition, the greater in vivo sensitivity of the endocannabinoid metabolizing enzymes, as compared with ChE, to the inhibition by OPs in adults suggest that at low-level exposures to CPS, where inhibition of ChE activity is very low, there would still be moderate inhibition of endocannabinoid hydrolysis. Although this level of inhibition may not be important in adult animals, as was suggested by Quistad et al., it could be sufficient to produce deleterious effects on development. The goal of the current study was to initiate investigations into this area by determining the extent of inhibition of 2-AG and AEA hydrolysis in developing rats after being repeatedly exposed to different dosages of CPS.
The dosages used in this study were designed to induce inhibition of ChE activity in order to further understand the potential risk of exposure to OP insecticides in children. These dosages are above the oral repeated no observed effect level (NOEL) for inhibition of brain ChE activity (0.75 mg/kg) but below the oral repeated NOEL for signs of toxicity (4.5 mg/kg) for postnatal rats as reported by Zheng et al. (2000). However, it is unclear if these dosages recapitulate the exposure levels in children. Previous studies have used dosages as high as 5 mg/kg and claimed that this dosage falls within the range of estimated fetal and neonatal exposures from environmental exposure or home use of CPS (Aldridge et al., 2005c). Most likely, CPS exposure in children will occur at levels lower than those used in this study with the exception of situations of high levels of contamination.
MATERIALS AND METHODS
Chemicals.
CPS (> 99% purity) was a generous gift from DowElanco Chemical Company (Indianapolis, IN). All other chemicals were purchased from Cayman Chemicals (Ann Arbor, MI) or Sigma Chemical Co. (St Louis, MO).
Animal treatment.
Adult male and female Sprague Dawley rats were obtained from Harlan Laboratories (Prattville, AL) and used for breeding. All animals were housed in a temperature-controlled environment (22 ± 2°C) with a 12-h dark-light cycle with lights on between 0700 h and 1900 h in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility. LabDiet rodent chow and tap water were freely available during the experimentation. All procedures were approved by the Mississippi State University Institutional Animal Care and Use Committee. Following parturition, male and female rat pups within the same litter were assigned to different treatment groups. Only one pup representing each of the four treatments for a single sex was present in a single litter. Thus, there was always a representative control animal of the same sex present in each litter for the three CPS-treated animals. For this project, rats from six litters were used and each litter had an average of 6.33 ± 0.42 females and 7.00 ± 0.86 males. From two of these litters, both the males and females of the same litter were used. The remainder of the pups in the litter were used for other studies.
CPS was dissolved in corn oil and administered by oral gavage (per os) every day from postnatal day 10 (PND10) through PND16 (the day of birth was considered as PND0). The dosages selected were 1, 2.5, and 5 mg/kg. The latter dosage (5 mg/kg) was previously used in other developmental studies involving CPS (Aldridge et al., 2004, 2005b,c; Auman et al., 2000; Dam et al., 1999, 2000, 2003; Meyer et al., 2004a,b, 2005; Slotkin and Seidler, 2007). Oral gavage was performed using a 50-μl tuberculin syringe equipped with a 1-inch 24-gauge straight intubation needle (Popper and Sons, Inc., New Hyde Park, NY) to deliver the solution to the back of the throat.
Rat pups were sacrificed on PND16 at 4 h after the last exposure to CPS, a time utilized in our previous studies (Betancourt et al., 2007; Carr and Nail, 2011) and the one that exhibits peak blood CPS levels in PND12 and PND17 rats, as reported by Timchalk et al. (2006). Blood was collected by trunk bleeding to obtain serum. All brains were rapidly removed and dissected on ice to obtain the medulla-pons and the forebrain (remainder of the brain excluding the cerebellum), which were frozen on a stainless steel plate on top of dry ice and maintained at −80°C until assay.
Assays.
The forebrains were homogenized at 200 mg tissue weight/ml of cold 0.05 M Tris-HCl buffer containing 0.2mM EDTA (pH 7.4 at 37°C) in a glass mortar using a Wheaton motorized tissue grinder and a Teflon pestle. For determination of ChE activity, an aliquot was diluted in cold 0.05 M Tris-HCl buffer (pH 7.4 at 37°C) to 40 mg tissue/ml. The medulla-pons was homogenized at 40 mg tissue/ml in assay buffer. Forebrain and medulla-pons (1 mg tissue/ml final concentration) and serum (12.5 μl serum/ml final concentration) were diluted in assay buffer. The activity of ChE was measured spectrophotometrically using a modification (Chambers et al., 1988) of Ellman et al. (1961) with acetylthiocholine as the substrate (1mM final concentration) and 5,5′-dithiobis(nitrobenzoic acid) as the chromogen.
For the forebrain samples, an additional 1.0 ml aliquot of the 200 mg/ml homogenate was centrifuged at 1500 × g for 10 min to obtain a supernatant, which was diluted threefold for determination of AEA and 2-AG hydrolysis rates. AEA and 2-AG hydrolysis in the forebrain (2.667 mg tissue/ml final concentration) were determined by incubation with substrate (either 50μM AEA or 2-AG, respectively) for 10 min at 37°C. The reaction was terminated by the addition of four volumes of cold acetonitrile containing 2.5μM of internal standard (arachidonic acid-d8). Samples were placed in ice for 10 min and centrifuged at 21,000 × g for 10 min to obtain the supernatant. The amount of arachidonic acid formed was determined by liquid chromatography-mass spectrometry (Xie et al., 2010). Tissue blanks (no substrate) were included for each sample to correct for endogenous arachidonic acid amounts. For inhibition experiments involving the specific MAGL inhibitor JZL184, control forebrain homogenates were preincubated with increasing concentrations of JZL184 for 30 min at 37°C.
Protein concentrations of tissue extracts were quantified with the Folin phenol reagent using bovine serum albumin as a standard (Lowry et al., 1951). Specific activities were calculated as nmol (ChE) or pmol (AEA and 2-AG hydrolysis) product produced min−1 mg protein−1 (n = 8).
To verify inhibition of AEA hydrolysis, a serine hydrolase activity–based probe (Kidd et al., 2001), fluorophosphonate (FP)-biotin, was used to measure native serine hydrolase activity in male and female forebrains. Forebrains were homogenized in 0.1 M Tris-HCl (pH 7.5) containing 1mM EDTA. After a 1000 × g spin (15 min, 4°C), the supernatant was removed and a membrane fraction was produced by ultracentrifugation (100,000 × g; 60 min, 4°C). The membranes were resuspended in Tris-acetate (pH 7.4) buffer containing 20% (vol/vol) glycerol, and protein concentrations were determined using the bicinchoninic acid reagent (Pierce, Rockford, IL). Brain membranes were suspended in 0.05 M Tris-HCl (pH 8.0) buffer (1 mg protein/ml final concentration) and incubated with FP-biotin (4μM final concentration) for 60 min at room temperature. FP-biotin was added to reactions from a dimethyl sulfoxide (DMSO) stock (final DMSO amount in reactions, 2% vol/vol). Activity-based probe reactions were terminated by adding 10 μl of 6× SDS polyacrylamide gel electrophoresis (PAGE) loading buffer (reducing) and heated at 95°C (5 min). Samples were cooled and immediately loaded onto a 10% SDS-PAGE gel. Following electrophoresis, separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 3% (wt/v) nonfat milk, the membrane was probed with avidin-horseradish peroxidase (Sigma; 1:3000 vol/vol) for 60 min at room temperature. The membrane was then washed 4× (∼5–10 min each) with Tween buffer, and the enhanced chemiluminescent substrate (Pierce) was added for 5 min. The chemiluminescent signals on the membrane were captured on x-ray film. Negative control reactions consisted of proteomes that were heat denatured before treatment with FP-biotin; thus, inactivating the enzymes and preventing the reaction of serine hydrolases with the probe.
For Western blot analysis of MAGL, rat forebrain membrane proteins were separated by SDS-PAGE. Following electrophoretic transfer of proteins, PVDF membranes were probed with rabbit anti-MAGL (Cayman Chemicals, Ann Arbor, MI) for 1 h at room temperature. PVDF membranes were washed, followed by incubation with goat antirabbit secondary antibody conjugated to HRP (1:20,000 vol/vol). The chemiluminescent signal was recorded using X-OMAT photographic film (Eastman Kodak Co., Rochester, NY). PVDF were also probed with rabbit anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) to verify equal protein loading on gels.
Statistical Analysis.
The sphericity of the body weight data was initially tested by ANOVA using the general linear model with a repeated measures paradigm and was found to violate the assumption of sphericity. Therefore, subsequent analysis by ANOVA using the mixed model (Littell et al., 1996) was conducted with a repeat measures paradigm with a Huynh-Feldt covariance structure (Huynh and Feldt, 1970), followed by separation of means using least significant difference (LSD). The analysis identified differences in the main effects (sex and treatment) and all possible interactions. Enzyme-specific activities were analyzed by ANOVA using the Mixed procedure to determine significant sex, treatment, and sex × treatment interactions. All analyses included litter and sex × treatment × litter as random effects. There were no differences between sex and sex × treatment interactions with weight, weight gain, or enzyme activity data. Thus, males and females were pooled for subsequent analysis and mean separation was performed by LSD. The criterion for significance was set at p ≤ 0.05.
RESULTS
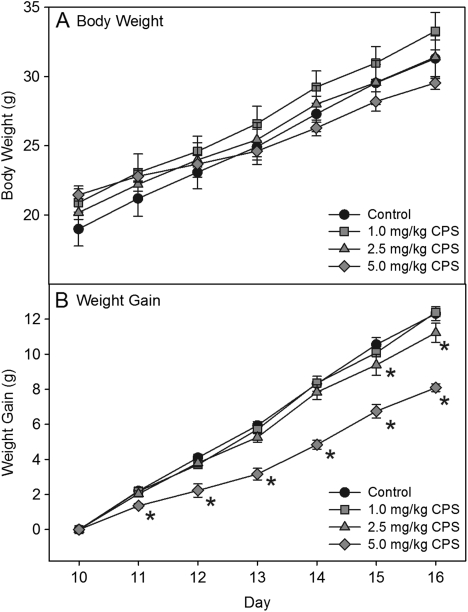
No signs of overt toxicity or cholinergic hyperstimulation, such as lacrimation, diarrhea, or tremors, were observed following CPS exposure. With respect to body weight, there were no significant differences between controls and any treatment group on any day (Fig. 1A). However, when weight gain was compared between groups, the high dosage resulted in significantly less weight gain at all time points, whereas the medium dosage resulted in significantly less weight gain on the last to days of treatment (Fig. 1B). The lowest dosage did not result in any effect on weight gain.
FIG. 1.
Plots of (A) body weight and (B) weight gain of rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control) or 1.0, 2.5, or 5.0 mg/kg CPS. Values are expressed as weight ± SE (n = 8). Values significantly different from their respective control (p ≤ 0.05) are indicated with an asterisk.
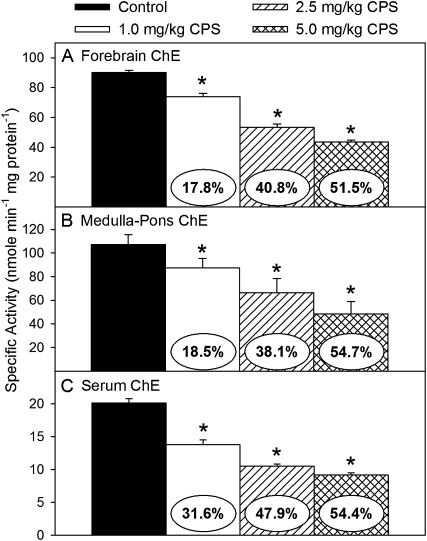
With respect to ChE activity levels, repeated exposure to CPS significantly decreased the specific activities of forebrain ChE (F(3,24) = 115.15, p < 0.0001), medulla-pons ChE (F(3,24) = 5.51, p < 0.0085), and serum ChE (F(3,24) = 72.15, p < 0.0001) in a dose-related manner (Figs. 2A–C, respectively). The level of inhibition of serum and forebrain ChE activity following exposure to the highest dosage of CPS was similar to that previously reported (Carr and Nail, 2011). The level of inhibition of ChE activity in serum was slightly greater than that in brain with 32, 48, and 54% at the low, medium, and high dosages, respectively. The level of inhibition of ChE activity was similar in the forebrain and medulla-pons with 18–19%, 38–41%, and 52–55% at the low, medium, and high dosages, respectively. Therefore, in future studies that may involve measuring other non–ChE-related parameters in the forebrain, the level of inhibition of ChE activity in the medulla, but not serum, can be used to estimate the level of inhibition of ChE activity in the forebrain.
FIG. 2.
Specific activity of ChE in the (A) forebrain, (B) medulla-pons, and (C) serum of rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control) or 1.0, 2.5, or 5.0 mg/kg CPS. Values are expressed as nmol product min−1 mg protein−1 ± SE (n = 8). Percent inhibition for each treatment group as compared with its respective control is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk are statistically significant (p ≤ 0.05) from control.
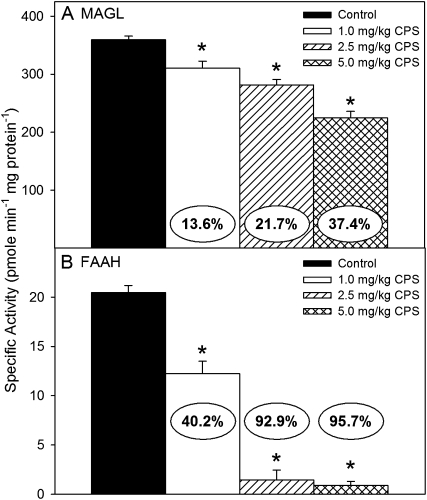
With respect to endocannabinoid hydrolysis, repeated exposure to CPS significantly decreased the amount of 2-AG hydrolysis (F(3,24) = 34.58, p < 0.0001) and AEA hydrolysis (F(3,24) = 100.51, p < 0.0001) in the forebrain of all treatment groups (Figs. 3A and B, respectively). Forebrain 2-AG hydrolysis was decreased in a dose-related manner, but the extent of inhibition observed at each dosage (14, 22, and 37%) was less than that of matching forebrain ChE. In contrast, the hydrolysis of AEA was almost completely eliminated by the medium and high dosages, with inhibition greater than 93%. The extent of inhibition of AEA hydrolysis induced by the lowest dosage (40%) was much greater than that observed for forebrain ChE.
FIG. 3.
Specific activity of (A) 2-AG hydrolysis and (B) AEA hydrolysis in the forebrain of rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control) or 1.0, 2.5, or 5.0 mg/kg CPS. Values are expressed as pmol product min−1 mg protein−1 ± SE (n = 8). Percent inhibition for each treatment group as compared with its respective control is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk are statistically significant (p ≤ 0.05) from control.
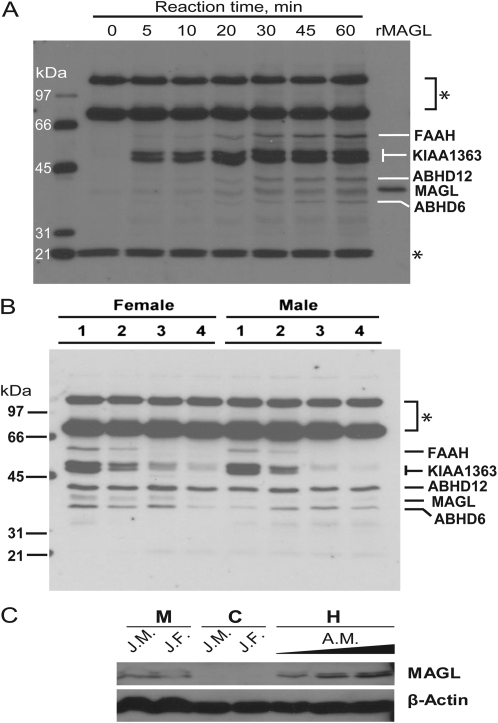
To further verify the inhibition of endocannabinoid hydrolysis induced by CPS exposure, serine hydrolase activity profiling using FP-biotin was performed on forebrain membrane fractions. A time course (0–60 min) verifying the incubation parameters for determination of FP-biotin activity using adult male rat brain is presented in Figure 4A. Five major serine hydrolases were detected including FAAH and MAGL. In the membranes of the CPS-treated male and female juvenile rats, the pattern of inhibition of FAAH corresponded to that observed in the AEA hydrolysis activity assays with total elimination of FAAH activity by the medium and high dosages of CPS and significant reduction of FAAH activity by the lowest dosage of CPS (Fig. 4B). In addition, CPS exposure reduced the activity of KIAA1363, a hydrolase known to be sensitive to inhibition following CPS (Nomura et al., 2005; Nomura and Casida, 2011), in a dose-related manner. Furthermore, ABHD12, which has been shown to hydrolyze 2-AG (Blankman et al., 2007), did not appear to be inhibited by CPS. However, the level of MAGL activity was barely detectable in the juvenile animals by serine hydrolase activity profiling. This raised the question regarding how much MAGL activity is present in juvenile brains and its contribution to 2-AG hydrolysis activity.
FIG. 4.
Serine hydrolase activity and Western blot profiling of rat brain. (A) Time course (0–60 min) of FP-biotin labeling of adult male rat brain membrane. Recombinant human MAGL was treated with FP-biotin for 60 min. (B) Representative gel using the FP-biotin activity probe to verify the inhibition of FAAH in the forebrain of male and female rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control; lane 1) or 1.0, 2.5, or 5.0 mg/kg (CPS; lanes 2–4). Numbers to the left of the gel indicate molecular weight markers. The asterisks indicate proteins that appear to be endogenously biotinylated; they also appear in samples not treated with FP-biotin (data not shown). (C) Western analysis of juvenile and adult brain extracts probed with either anti-MAGL or anti-β-actin. Juvenile brain subcellular fractions, 25 mg protein loaded per lane; adult brain homogenate, 8, 17, and 25 mg protein loaded per lane. AM, adult male; C, cytosol; H, whole-brain homogenate; JF, juvenile female; JM, juvenile male; M, membranes.
First, to verify the presence of MAGL protein in juvenile brains, the microsomal and cytosolic fractions of control juvenile brains were probed with either anti-MAGL or anti-β-actin antibodies by Western blot analysis and compared with adult animals. Although lower than adult levels, MAGL protein was detectable in the microsomal fraction of the juvenile brain with none detected in the cytosolic fraction (Fig. 4C), which is consistent with its subcellular location.
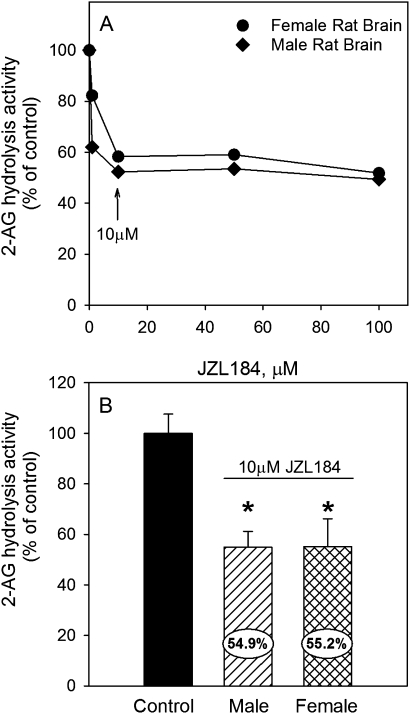
Second, to determine the quantitative contribution of MAGL to 2-AG hydrolysis, control juvenile rat brain (male and female) homogenates were titrated with increasing concentrations of the specific MAGL inhibitor JZL184 (Fig. 5A). Maximal inhibition of MAGL activity was observed at concentrations of ≥ 10μM JZL184. Using 10μM JZL184, it was determined that MAGL activity contributes about 45% of the observed 2-AG hydrolysis activity in control male and female juvenile rat brain (Fig. 5B). The remaining hydrolysis is performed by another enzyme, potentially ABHD6 and/or ABHD12 (Blankman et al., 2007).
FIG. 5.
Inhibition of 2-AG hydrolysis by the specific MAGL inhibitor JZL184 in control male and female juvenile rat brain homogenates. (A) Representative inhibition curve obtained by preincubating homogenates with increasing concentrations of JZL184. (B) Inhibition of 2-AG hydrolysis in homogenates preincubated with 10μM JZL184 (n = 4). Blank reactions (no 2-AG or inhibitor) or nonenzymatic reactions (2-AG in buffer only) were also used to assay the background levels of arachidonic acid in brain homogenate and nonenzymatic 2-AG hydrolysis rate.
DISCUSSION
With regard to the hypothesis that the adverse developmental effects of OP insecticides involves a currently unknown “noncholinergic mechanism of action,” we report here that repeated exposures of juvenile rats to low dosages of CPS caused marked dose-dependent inhibition of AEA hydrolysis. AEA hydrolysis appears to be a much more sensitive target for CPS-mediated inhibition than ChE activity. In contrast, the sensitivity of 2-AG hydrolysis appears to be equal to or slightly less than the sensitivity of ChE activity. The identification of a developmental toxicological target for CPS that is more sensitive than the traditional toxicological target (ChE) is significant. Contributing to this significance is the role of AEA hydrolysis in the appropriate functioning of the endocannabinoid system and the importance of that system in normal brain development (Anavi-Goffer and Mulder, 2009; Harkany et al., 2008).
With respect to the observed decrease in weight gain, increasing evidence has suggested that the endocannabinoid system regulates appetite, food intake, and body weight (Bermudez-Silva et al., 2010; Matias and Di Marzo, 2007). As first reported by Colombo et al. (1998) and subsequently by others, antagonism of the CB1 receptor results in weight loss. It has also been demonstrated that daily exposure of adult mice to the MAGL inhibitor JZL184 for 6 days results in the desensitization and functional antagonism of CB1 receptors (Schlosburg et al., 2010). Thus, it is possible that the inhibition of MAGL and/or FAAH induced by our 7-day exposure to the higher dosage of CPS could have been sufficient to result in a similar desensitization and functional antagonism of the CB1 receptors, thus leading to the observed decreased weight gain similar to what would be observed with CB1 antagonism.
The developmental neurotoxicity literature contains many studies demonstrating that OPs exert untoward effects during critical periods of brain maturation, including altered development of multiple neurotransmitter systems such as cholinergic, catecholaminergic, and seratonergic neurons (Aldridge et al., 2003, 2004, 2005c; Dam et al., 1999; Meyer et al., 2004a, 2005; Raines et al., 2001; Richardson and Chambers, 2005; Slotkin et al., 2001, 2002). Furthermore, a large number of studies have reported that developmental exposure to exogenous cannabinoids can also affect development of multiple neurotransmitter systems, including catecholaminergic, serotonergic, GABAergic, glutamatergic, and opioid systems (Fernandez-Ruiz et al., 2000, 2004; Garcia-Gil et al., 1997, 1999; Hernandez et al., 2000; Kumar et al., 1990; Molina-Holgado et al., 1996, 1997; Suarez et al., 2004; Vela et al., 1998; Wang et al., 2006). Although these collective studies suggested that developmental exposures to OP insecticides or exogenous cannabinoids can impact multiple neurotransmitter systems, there are important differences. Whereas exposure to exogenous cannabinoid receptor agonists would exert widespread effects in the brain, exposure to an OP insecticide, especially at low levels, would impact mainly AEA hydrolysis and exert actions only in specific brain regions, where AEA hydrolysis plays an important role in endocannabinoid metabolism (Bortolato et al., 2007; Fegley et al., 2005). With the exception of the work of Marco et al. (2009), we could find no literature reporting the effects of developmental inhibition of AEA hydrolysis on any neurotransmitter system.
Considering the large number of published reports, it is well established that developmental exposure to exogenous cannabinoids can have a negative impact on adult behavior. In the past, the major focus has been on prenatal exposure that mimics abuse of cannabis during pregnancy and how such an exposure scenario can lead to long-term behavioral alterations (Campolongo et al., forthcoming; Schneider, 2009). Although less well investigated, postnatal exposure to cannabinoids can also result in long-term behavioral alterations and that the timing of the exposure is important (Schneider et al., 2005). For example, it has been suggested that the earlier the exposure occurs in adolescence, the greater the potential for negative effects (Harte and Dow-Edwards, 2010; Moore et al., 2010). Although these studies demonstrated that developmental activation of brain endocannabinoid receptors by exogenous cannabinoid receptor agonists can cause long-term behavioral effects, it is unknown whether developmental exposure to a xenobiotic agent that alters the natural levels of the endocannabinoids can cause similar adverse effects. However, it is of interest that we, as well as others, have previously observed that developmental exposure to CPS results in anxiolytic behavior in adults (Aldridge et al., 2005a; Johnson et al., 2006). It is known that the cannabinoid system can modulate the anxiety response (Moreira and Lutz, 2008; Taber and Hurley, 2009). In addition, exposure of adult rodents to the FAAH inhibitor URB597 leads to anxiolytic-like effects, and FAAH knockout mice also exhibit anxiolytic behavior as adults (Haller et al., 2009; Kathuria et al., 2003; Moreira et al., 2008).
With respect to 2-AG hydrolysis activity, the reported contribution of MAGL to this activity in adult mice is ∼85% (Blankman et al., 2007). Here, we have demonstrated that the contribution of MAGL activity to 2-AG hydrolysis is only 45% in late preweanling rats. However, it is unclear what enzyme(s) contributes to the remaining 55% of 2-AG hydrolysis activity, although ABHD6 and ABHD12 are likely candidates.
In conclusion, several issues require clarification regarding the potential for inhibition of AEA metabolism to be a mechanistic target for developmental OP exposure. For instance, how fast is the recovery of FAAH activity following OP-mediated inhibition? Transient inhibition may be less likely to exert long-lasting effects than would persistent inhibition. Furthermore, does the inhibition of AEA hydrolysis result in accumulation of endocannabinoids in specific brain regions known to be associated with behaviors that are altered by developmental OP exposure? Previous studies have reported that the inhibition of FAAH and MAGL activity in adult mice exposed to a single high dosage of CPS results in increased levels of 2-AG and AEA in the brain (Nomura et al., 2008; Nomura and Casida, 2011). However, it is not clear if a similar situation exists in developing rats exposed to lower levels of CPS. If accumulation of the lipid mediators occurs, does this alter the signaling responses of the endocannabinoid system? Although we are proposing that the inhibition of FAAH activity may be a potential mechanism of toxic action following low-level OP exposure, future studies will address the issues listed above.
FUNDING
This study was funded in part by National Institute of Health (1P20-RR17661 and R01 ES 10386).
Acknowledgments
The authors wish to acknowledge the technical expertise of Antonio B. Ward and the statistical expertise of Dr Sumalee Chow. Research was also partially supported by the Mississippi Agricultural and Forestry Experiment Station (MAFES) under MAFES project MISV-384040 and the College of Veterinary Medicine, Mississippi State University. This paper is MAFES publication J-11999 and Center for Environmental Health Sciences publication 125.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol. Appl. Pharmacol. 2005c;203:132–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ. Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anavi-Goffer S, Mulder J. The polarised life of the endocannabinoid system in CNS development. Chembiochem. 2009;10:1591–1598. doi: 10.1002/cbic.200800827. [DOI] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Dev. Brain Res. 2000;121:19–27. doi: 10.1016/s0165-3806(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol. Biochem. Behav. 2010;95:375–382. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Filipov NM, Carr RL. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol. Sci. 2007;100:445–455. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V. Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology. doi: 10.1007/s00213-010-1892-x. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Nail CA. Effect of different administration paradigms on cholinesterase inhibition following repeated chlorpyrifos exposure in late preweanling rats. Toxicol. Sci. 2011;214:5–15. doi: 10.1093/toxsci/kfn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW. Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Commun. 1988;3:85–92. [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–L117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev. Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev. Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Transcriptional biomarkers distinguish between vulnerable periods for developmental neurotoxicity of chlorpyrifos: implications for toxicogenomics. Brain Res. Bull. 2003;59:261–265. doi: 10.1016/s0361-9230(02)00874-2. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox. Res. 2004;6:389–401. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil L, De Miguel R, Munoz RM, Cebeira M, Villanua MA, Ramos JA, Fernandez-Ruiz JJ. Perinatal Δ9-tetrahydrocannabinol exposure alters the responsiveness of hypothalamic dopaminergic neurons to dopamine-acting drugs in adult rats. Neurotoxicol. Teratol. 1997;19:477–487. doi: 10.1016/s0892-0362(97)00048-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil L, De Miguel R, Romero J, Perez A, Ramos JA, Fernandez-Ruiz JJ. Perinatal delta9-tetrahydrocannabinol exposure augmented the magnitude of motor inhibition caused by GABA(B), but not GABA(A), receptor agonists in adult rats. Neurotoxicol. Teratol. 1999;21:277–283. doi: 10.1016/s0892-0362(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol. Cell. Endocrinol. 2008;286:S84–S90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicol. Teratol. 2010;32:515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Berrendero F, Suarez I, Garcia-Gil L, Cebeira M, Mackie K, Ramos JA, Fernandez-Ruiz J. Cannabinoid CB(1) receptors colocalize with tyrosine hydroxylase in cultured fetal mesencephalic neurons and their activation increases the levels of this enzyme. Brain Res. 2000;857:56–65. doi: 10.1016/s0006-8993(99)02322-7. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurement designs have exact F-distributions. J. Am. Statist. Assoc. 1970;65:1582–1589. [Google Scholar]
- Johnson FO, Chambers JE, Carr RL. Effects of repeated postnatal exposure to chlorpyrifos or methyl parathion in the elevated plus-maze model of anxiety. Toxicologist. 2006;90:1445. [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kidd D, Liu Y, Cravatt BF. Profiling serine hydrolase activities in complex proteomes. Biochemistry. 2001;40:4005–4015. doi: 10.1021/bi002579j. [DOI] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ. Health Perspect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Haney M, Becker T, Thompson ML, Kream RM, Miczek K. Effect of early exposure to delta-9-tetrahydrocannabinol on the levels of opioid peptides, gonadotropin-releasing hormone and substance P in the adult male rat brain. Brain Res. 1990;525:78–83. doi: 10.1016/0006-8993(90)91322-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marco EM, Rubino T, Adriani W, Viveros MP, Parolaro D, Laviola G. Long-term consequences of URB597 administration during adolescence on cannabinoid CB1 receptor binding in brain areas. Brain Res. 2009;1257:25–31. doi: 10.1016/j.brainres.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol. Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ. Health Perspect. 2004a;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004b;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Alvarez FJ, Gonzalez I, Antonio MT, Leret ML. Maternal exposure to Δ9-tetrahydrocannabinol (Δ9-THC) alters indolamine levels and turnover in adult male and female rat brains regions. Brain Res. Bull. 1997;43:173–178. doi: 10.1016/s0361-9230(96)00434-0. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Amaro A, Gonzalez MI, Alvarez FJ, Leret ML. Effect of maternal Δ9-tetrahydrocannabinol on developing serotonergic neurons. Eur. J. Pharmacol. 1996;316:39–42. doi: 10.1016/s0014-2999(96)00753-4. [DOI] [PubMed] [Google Scholar]
- Moore NL, Greenleaf AL, Acheson SK, Wilson WA, Swartzwelder HS, Kuhn CM. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J. Pharmacol. Exp. Ther. 2010;335:294–301. doi: 10.1124/jpet.110.169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict. Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat. Chem. Biol. 2008;4:373–378. doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Casida JE. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J. Agric. Food Chem. 2011;59:2808–2815. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Leung D, Chiang KP, Quistad GB, Cravatt BF, Casida JE. A brain detoxifying enzyme for organophosphorus nerve poisons. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6195–6200. doi: 10.1073/pnas.0501915102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol. Appl. Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol. Appl. Pharmacol. 2001;173:48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol. Appl. Pharmacol. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Dev. Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol. Sci. 2005;84:352–359. doi: 10.1093/toxsci/kfi081. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environ. Health Perspect. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Cannabis use in pregnancy and early life and its consequences: animal models. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:383–393. doi: 10.1007/s00406-009-0026-0. [DOI] [PubMed] [Google Scholar]
- Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55, 212-2. Behav. Pharmacol. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ. Health Perspect. 2007;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev. Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res. Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Fernandez-Ruiz J, Ramos JA, Rubio M, Fernandez B. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and perinatal Δ9-tetrahydrocannabinol exposure. Cerebellum. 2004;3:66–74. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA. Endocannabinoids: stress, anxiety, and fear. J. Neuropsychiatry Clin. Neurosci. 2009;21:109–113. doi: 10.1176/jnp.2009.21.2.iv. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Poet TS, Kousba AA. Age-dependent pharmacokinetic and pharmacodynamic response in preweanling rats following oral exposure to the organophosphorus insecticide chlorpyrifos. Toxicology. 2006;220:13–25. doi: 10.1016/j.tox.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol. 2008a;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, et al. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008b;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Fernandez-Ruiz JJ, Garcia-Lecumberri C, Pelaprat D, Fuentes JA, Ramos JA, et al. Maternal exposure to Δ9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998;807:101–109. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 2006;6:255–264. doi: 10.1038/sj.tpj.6500375. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chem. Res. Toxicol. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Olivier K, Won YK, Pope CN. Comparative cholinergic neurotoxicity of oral chlorpyrifos exposures in preweanling and adult rats. Toxicol. Sci. 2000;55:124–132. doi: 10.1093/toxsci/55.1.124. [DOI] [PubMed] [Google Scholar]