Abstract
Exposure to genotoxic chemicals at a young age increases cancer incidence later in life. Aflatoxin B1 (AFB1) is a potent genotoxin that induces hepatocellular carcinoma (HCC) in many animal species and in humans. Whereas adult mice are insensitive to aflatoxin-induced carcinogenesis, mice treated with AFB1 shortly after birth develop a high incidence of HCC in adulthood. Furthermore, the incidence of HCC in adult male mice treated as infants is much greater than in females, reasons for which are unclear. In this study, treatment with AFB1 produced similar levels of DNA damage and mutations in the liver of newborn male and female gpt delta B6C3F1 mice. Twenty-four hours after dosing with AFB1 (6 mg/kg), the highly mutagenic AFB1-FAPY adduct was present at twice the level of AFB1-N7-guanine in liver DNA of males and females. A multiple dose regimen (3 × 2 mg/kg), while delivering the same total dose, resulted in lower AFB1 adduct levels. Mutation frequencies in the gpt transgene in liver were increased by 20- to 30-fold. The most prominent mutations in AFB1-treated mice were G:C to T:A transversions and G:C to A:T transitions. At this 21-day time point, no significant differences were found in mutation frequency or types of mutations between males and females. These results show that infant male and female B6C3F1 mice experience similar amounts of DNA damage and mutation from AFB1 that may initiate the neoplastic process. The gender difference in the subsequent development of HCC highlights the importance of elucidating additional factors that modulate HCC development.
Keywords: aflatoxin, neonatal mouse, hepatocarcinoma, mutation, gpt delta mouse
Animals treated with chemical carcinogens during the perinatal period typically experience higher tumor incidence and shorter latency of tumor emergence (Anderson et al., 2000; Rice, 1981). A well-documented example is the induction of hepatocellular carcinomas (HCC) by aflatoxin B1 (AFB1) in mice, which strongly varies with age of exposure. Brief exposures to large doses of aflatoxin during the neonatal period result in a high incidence of HCC in adulthood, whereas adult mice exposed to the same doses fail to develop HCC at any age (Vesselinovitch et al., 1972).
Despite an abundance of experimental data, the mechanistic basis for the high sensitivity of infant animals to AFB1 and other genotoxic carcinogens is not fully understood. A related unanswered question is why exposure during infancy induces higher incidence of HCC in males than in females in adulthood. Treatment of newborn B6C3F1 mice with single or multiple doses of AFB1 induced HCC by 82 weeks of age in >90% in males compared with <10% of similarly treated females (Vesselinovitch et al., 1972). Similar experiments showed that female mice treated with diethylnitrosamine (DEN) develop HCC much less frequently than males (Nakatani et al., 2001). Two possible explanations are: (1) in female mice, carcinogen activation may be less effective or inactivation and DNA repair more effective, resulting in less genetic damage and thus fewer tumor-initiating mutations than male mice, or (2) similar amounts of genetic damage may occur in both sexes and subsequent hormonal and/or other environmental factors differentially modulate HCC development. Prior studies showed that hormonal status had a profound effect on tumor development in animals treated during the neonatal period. For example, orchidectomy of neonatal male mice dosed with DEN delayed tumor onset and reduced tumor yield (Vesselinovitch, 1990). Estrogen-mediated inhibition of inflammatory responses in the liver following DEN treatment has also been shown to attenuate the postinitiation development of HCC in female mice (Naugler et al., 2007). Little information exists, however, on the relative importance of genotoxic damage in neonates as compared with postinitiation events that determine differences in HCC incidence between males and females later in life.
In mice, AFB1 is metabolized by cytochromes P450 1A2 and 3A4 to the 8,9-epoxide, which reacts with cellular DNA, producing the predominant AFB1-N7-guanine adduct (Eaton and Gallagher, 1994). DNA adduct formation by the AFB1-8,9-epoxide can be diminished by formation of AFB1-glutathione conjugates, mediated by alpha class glutathione-S-transferases (GSTs) (Hayes et al., 1992). Increased expression of GSTs during the postnatal period is believed to be largely responsible for the diminished sensitivity of older mice (Shupe and Sell, 2004). Furthermore, in the rat, it has been shown that chemoprotective chemicals such as dithiolethiones, that induced GSTs and inhibited formation of AFB1-DNA adducts also reduced the number of hepatic preneoplastic lesions and prevented tumor development (Roebuck et al., 2003). Importantly, epidemiological studies have validated aflatoxin-DNA adducts as biomarkers of risk of HCC from aflatoxin exposure (Groopman et al., 2002, 2008). An additional molecular connection between AFB1 exposure and human HCC was established by the observation that G:C to T:A transversions characteristic of those induced by AFB1-DNA adducts were found at high frequencies in codon 249 of the p53 gene in HCC of patients residing in geographical areas in which AFB1 exposure is an established risk factor (Hussain et al., 2007).
The overall objectives of our study were to quantify aflatoxin adduct levels, characterize the frequency and spectrum of mutations induced in the liver of male and female gpt delta B6C3F1 mice, and assess relationships of these parameters to the known sensitivity of this strain of mice to AFB1-induced HCC. The mutagenic potency of AFB1 is well established in bacteria and mammalian cells, and G:C to T:A transversions are the most frequent base substitution mutation induced by AFB1-DNA adducts. A prior study with similar objectives carried out in Big Blue lacI transgenic mice showed that AFB1 is a potent liver mutagen in neonatal animals, but much less potent in the adult (Chen et al., 2010), in which fewer AFB1-DNA adducts were previously found (Shupe and Sell, 2004). We carried out the study in the B6C3F1 mouse, which is extensively used as a bioassay animal for testing chemicals for carcinogenic activity and for which a large carcinogenesis literature exists. This work is an early stage of a continuing effort to define biochemical markers related to age, gender, and strain capable of identifying key biochemical processes that underlie sensitivity and resistance to carcinogens.
MATERIALS AND METHODS
Caution.
Aflatoxin B1 is toxic, mutagenic and carcinogenic. This compound should be handled using appropriate precautions.
Animals.
C57BL/6 gpt delta transgenic mice were obtained from Takehiko Nohmi (Nohmi et al., 1996). The gpt delta B6C3F1 mice used in our experiments were generated by breeding female gpt delta C57BL/6J mice, which harbor an estimated 80 copies of the gpt gene on chromosome 17 (Nohmi et al., 1996), with male C3H/HeJ mice purchased from the Jackson Laboratories (Bar Harbor, ME). All experiments were conducted in accordance with protocols approved by the MIT Committee on Animal Care.
AFB1 treatment for adduct analysis.
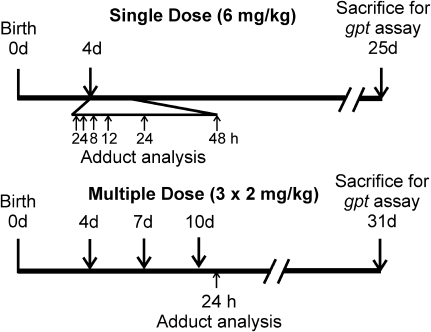
In the time course study of adduct formation, male and female transgenic B6C3F1 mice were injected ip on postnatal day 4 with a single dose of 6 mg/kg AFB1 in 10 μl of dimethyl sulfoxide (DMSO), both obtained from Sigma-Aldrich (St Louis, MO). Mice were euthanized and livers were collected 2, 4, 8, 12, 24, and 48 h after treatment; livers were collected from a minimum of three male and three female animals at each time point. To assess adduct formation induced by a multiple dose regimen, male and female mice were injected ip on postnatal days 4, 7, and 10 with a dose of 2 mg/kg AFB1 in 10 μl DMSO. Twenty-four hours after the last dose, animals were euthanized and livers were collected from 13 mice of each sex.
AFB1 treatment for gpt mutation assay.
Parallel experiments were conducted in which the single- or multiple-dose AFB1 regimens were employed. In the former protocol, as described above male and female mice were injected ip on postnatal day 4 with a single dose of 6 mg/kg AFB1 in 10 μl DMSO or 10 μl DMSO alone. Animals were euthanized 21 days after the single dose was administered and livers were collected from a minimum of four male and four female mice. In the second treatment schedule, male and female mice were injected ip on postnatal days 4, 7, and 10 with either 2 mg/kg AFB1 in 10 μl DMSO or DMSO alone. These animals were euthanized 21 days after the last dose and livers were collected from a minimum of four male and four female mice.
Isolation of liver DNA and hydrolysis of AFB1-DNA adducts.
DNA was isolated from livers of AFB1-treated mice and DNA isolated using previously described procedures (Groopman et al., 1980; Kensler et al., 1986). For adduct analysis, AFB1-DNA adducts were released by hydrolysis in 1.0 N HCl at 95°C for 15 min (Groopman et al., 1981). Internal 15N5-guanine-derived standards for both AFB1-N7-guanine and AFB1-FAPY were added after hydrolysis of AFB1-DNA adducts to permit quantitative analysis by isotope dilution mass spectrometry.
DNA adduct analysis.
Ultra-high performance liquid chromatography (UPLC) was used to separate AFB1-DNA adducts prior to measurement by isotope dilution mass spectrometry (MS) as previously described (Egner et al., 2006). UPLC was carried out on an Acuity C18 1.7 μm 1.0 × 150 mm column, and the composition of the initial mobile phase was 14% methanol, 1% acetonitrile, 0.1% formic acid, and 85% water. An 8-min linear gradient was employed, reaching a final mobile phase consisting of 37% methanol, 2% acetonitrile, 0.1% formic acid, and 61% water. Flow rate was 120 μl/min. The hydrolyzed DNA solution was diluted into the initial UPLC mobile phase and then injected for MS/MS analysis of AFB1-DNA adduct levels. The protonated parent ion of the AFB1-N7-guanine adduct (m/z 480.1) was selected and subjected to collision-induced fragmentation producing a m/z 152 product ion that was monitored to quantify adduct levels. The AFB1-FAPY adduct was quantified by selection of the m/z 498 parent ion and monitoring the collision-induced product ion m/z 452.
Gpt mutation assay and sequencing analysis.
The liver of each animal was pulverized in liquid nitrogen and divided into aliquots of ∼25 mg. Genomic DNA was extracted from 25 mg liver tissue using RecoverEase DNA Isolation Kit (Agilent Technologies, Santa Clara, CA); subsequently, λ–EG10 phages were packaged in vitro from the genomic DNA using Transpack Packaging Extract (Agilent Technologies) following the manufacturer’s instructions. The 6-thioguanine (6-TG) selection assay was performed as previously described (Nohmi et al., 1996). Briefly, Escherichia coli YG6020 expressing Cre recombinase was infected with λ–EG10 phages rescued from murine genomic DNA and incubated on selective media containing either chloramphenicol (Cm) at 25 μg/ml or Cm (25 μg/ml) plus 6-TG (25 μg/ml) for 72 h until the appearance of colonies. Confirmation of the recovered 6-TG-resistant phenotype was achieved by restreaking mutant colonies on selective media containing Cm plus 6-TG. DNA was isolated from confirmed 6-TG-resistant mutants using a Miniprep Kit (Qiagen) according to the manufacturer’s instructions. Sequencing of the gpt gene was performed at the Biopolymers Facility at Harvard Medical School (Boston, MA) using AMPure beads (Agencourt) and a 3730xL DNA Analyzer (Applied Biosystems) using the forward primer: 5′-TCTCGCGCAACCTATTTTCCC-3′. Sequences were aligned with the E. coli gpt gene (GenBank M13422.1) using NCBI Nucleotide Blast. Duplicate identical mutations from the same tissue sample were excluded from calculation of mutation frequency (MF) to avoid bias attributable to clonal expansion of sibling mutations. The MF was calculated by first taking the number of confirmed mutants and multiplying by the ratio of independent mutants to the total number of analyzed (sequenced) mutants and then dividing this product by the total number of colonies on the Cm only (control) plate. Samples from 33 mice were sequenced. Mutation frequencies and spectra were subjected to statistical analysis.
Statistical Analysis.
Student’s two-tailed t tests using PRIZM (Graphpad Software, Inc.) were used to determine the significance of differences in DNA adduct levels and mutation frequencies between experimental groups. Mutational spectra were compared using the Adams-Skopek test (Adams and Skopek, 1987; Cariello et al., 1994).
RESULTS
Time Course of AFB1 DNA Adducts in Neonatal Male and Female Mice
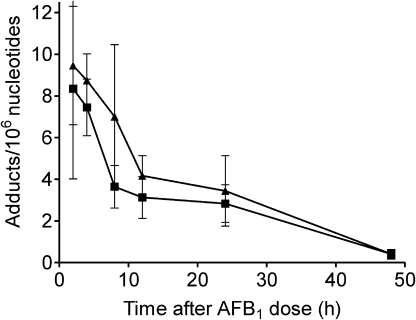
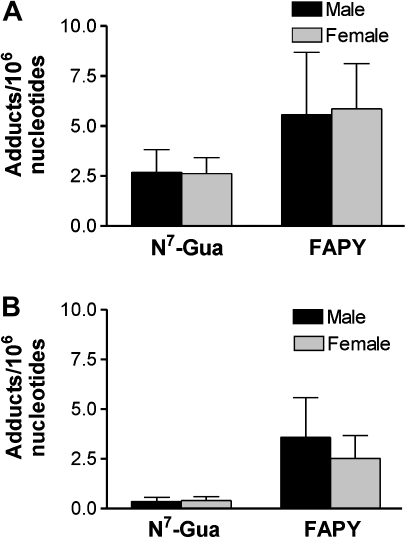
Newborn gpt delta B6C3F1 mice were administered AFB1 using two regimens adapted from those previously shown to induce a high incidence of HCC (Fig. 1; Vesselinovitch et al., 1972). A time-course analysis of levels of AFB1-N7-guanine in liver DNA was first performed in 4-day-old animals after administration of a single 6 mg/kg ip dose of AFB1. Figure 2 shows the levels of AFB1-N7-guanine in male and female mice at intervals up to 48 h after dosing. The average levels of AFB1-N7-guanine were uniformly lower in females than in males, but due to interindividual variation the differences were not statistically significant. The highest levels of AFB1-N7-guanine in liver DNA of both sexes (male, 9.4 ± 2.9; female 8.3 ± 4.3 adducts/106 nucleotides) were found at the earliest time point (2 h) after dosing. As previously observed in other species, adduct levels rapidly declined between 2 and 10–12 h. A second phase of slower adduct removal occurred between 10 and 48 h, with ∼10% of the peak level of AFB1-N7-guanine attained at 2 h remaining at 48 h. This decline in AFB1-N7-guanine could have resulted from its conversion to AFB1-FAPY (the imidazole ring-opened form of AFB1-N7-guanine), depurination, or enzymatic removal by repair enzymes. These results showed that there were no major gender differences in metabolic activation of AFB1 by P450s or other biochemical process that determine the fate of the AFB1-N7-guanine adduct in neonatal liver DNA. Further experiments were designed to examine whether levels of DNA adducts induced by the single-or multiple-dose regimens resulted in a gender difference in initial mutations that tracked with eventual tumor burden. To compare adduct levels, liver DNA was isolated from mice euthanized 24 h after administration of either treatment regimen (Fig. 1) and analyzed for amounts of both AFB1-N7-guanine and AFB1-FAPY adducts (Fig. 3). A single dose of 6 mg/kg AFB1 administered on postnatal day 4 produced similar levels of AFB1-N7-guanine in males and females as observed at 24 h in the kinetic study described above (Fig. 3A); male 2.7 ± 1; female 2.6 ± 0.8 adducts/106 nucleotides. Levels of the highly mutagenic AFB1-FAPY were twice as high as those of AFB1-N7-guanine in both sexes; male 5.5 ± 3 and female 5.8 ± 2 adducts/106 nucleotides.
FIG. 1.
Experimental scheme for treatment of newborn gpt delta B6C3F1 mice with AFB1, analysis of AFB1-DNA adducts and mutations in the gpt gene in liver.
FIG. 2.
Amounts of AFB1-N7-guanine in liver DNA of 4-day old male (-▴-) and female (-▪-) gpt delta B6C3F1 mice after administration of 6 mg/kg AFB1. Points = mean ± SD.
FIG. 3.
AFB1 DNA adducts in liver of gpt delta B6C3F1 mice 24 h after a single 6 mg/kg dose (A) or 3 × 2 mg/kg administered every third day (B). Plotted data = mean ± SD.
The multiple dose regimen of 3 × 2 mg/kg AFB1 given on days 4, 7, and 10 resulted in lower levels of adducts measured 24 h after the final dose compared with the single 6 mg/kg dose. As shown in Figure 3B, the levels of AFB1-N7-guanine were 6- to 7-fold lower; male 0.36 ± 0.2 adducts/106 nucleotides, female 0.40 ± 0.2 adducts/106 nucleotides. Levels of AFB1-FAPY adducts were also lower in the multiple dose animals, but only by 1.5- to 2-fold compared with the single dose; male 3.6 ± 2 adducts/106 nucleotides, female 2.5 ± 1 adducts/106 nucleotides. This pattern is consistent with the persistence and accumulation of FAPY adducts during chronic administration of AFB1 that has been observed in other animal models (Croy and Wogan, 1981). Importantly, however, levels of AFB1-N7-guanine or AFB1-FAPY induced by either treatment protocol showed no evidence of gender-related differences.
Mutation Frequencies in the gpt Gene of AFB1-Treated Neonatal Mice
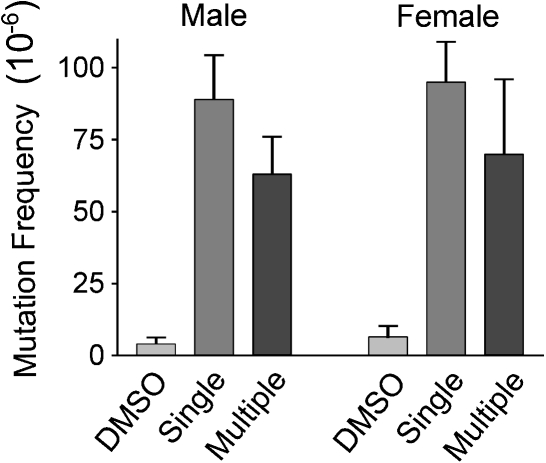
The early-stage genotoxicity and mutagenesis of AFB1 adducts in liver DNA were assessed in the gpt transgene following selection for 6-TG resistance. A minimum of four mice of each sex were treated with the single- or multiple-dose regimens as described above. Based on previous studies (Thybaud et al., 2003), for mutation analysis, livers were collected from animals euthanized 21 days after the final dosing. Figure 4 shows significant increases in MF in livers of mice treated with AFB1 administered by either the single- or multiple-dose regimens. In DMSO-treated mice, the average MF was 3 × 10−6, which is approximately half the spontaneous MF reported for gpt delta C57BL/6J mice (Masumura et al., 1999). The MF induced by the single-dose AFB1 regimen in males and females combined was 92 ± 14 × 10−6. A lower MF was observed in mice treated with the multiple-dose regimen; 66 ± 19 × 10−6 (p = 0.01, single vs. multiple dose). This is consonant with the lower amounts of mutagenic AFB1 adducts found at 24 h following the multiple dose protocol. There was no significant difference in the mutation frequencies observed in males as compared with females treated with either regimen, indicating that liver cells of both sexes were similarly susceptible to mutagenesis by AFB1.
FIG. 4.
MF in neonatal gpt delta B6C3F1 mice treated with AFB1 or DMSO vehicle. Mice were administered a single 6 mg/kg dose of AFB1 (single) or treated with 3 × 2 mg/kg doses of AFB1 (multiple). At 25 (single) or 31 (multiple) days of age DNA was isolated from liver and the number of 6-TG-resistant colonies determined. Plotted data = mean ± SD.
Mutation Types in the gpt Gene
DNA sequence analyses were performed on 6-TG-resistant mutants isolated from AFB1- and DMSO-treated mice. Although fewer 6-TG-resistant mutants from the DMSO-treated controls than from AFB1-treated mice were available for sequencing, the types of mutations present in both were very similar. Tables 1 and 2 summarize the types of mutations in livers of males and females treated with the two regimens. A total of 264 6-TG-resistant colonies from 17 AFB1-treated mice were analyzed by DNA sequencing to identify mutations present in the 459 bp gpt gene. Mutations found included base substitutions (248/264, 94%), insertions (4/264, 2%), and small deletions (12/264, 5%). Both transitions and transversions were present at G:C and A:T base pairs. The most frequent type of mutation seen in all the AFB1-treated groups was the G:C to T:A transversion (169/264, 64%) followed by G:C to A:T transition (44/264, 17%). Single base pair deletions occurred primarily at G:C base pairs (9/11, 81%). Overall, the types of mutations were similar in male and female mice treated with either a single dose (6 mg/kg) or multiple doses (3 × 2 mg/kg) of AFB1.
TABLE 1.
Summary of Mutations in the gpt Gene of Male and Female gpt Delta B6C3F1 Neonates 21 Days After Treatment with a Single 6 mg/kg Dose of AFB1
| Mutation types | Control |
AFB1 |
||
| Male | Female | Male | Female | |
| Transition | ||||
| G:C to A:T | 4 (29) | 6 (32) | 11 (15) | 12 (18) |
| A:T to G:C | 0 (0) | 1 (5) | 2 (3) | 0 (0) |
| Transversion | ||||
| G:C to T:A | 4 (29) | 4 (21) | 48 (65) | 45 (68) |
| G:C to C:G | 1 (7) | 1 (5) | 6 (8) | 5 (8) |
| A:T to T:A | 1 (7) | 0 (0) | 3 (4) | 0 (0) |
| A:T to C:G | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Deletion (bp) | ||||
| 1 | 1 (7) | 5 (26) | 1 (1) | 3 (5) |
| >1 | 1 (7) | 0 (0) | 0 (0) | 0 (0) |
| Insertion | 2 (14) | 2 (11) | 2 (3) | 1 (2) |
| Total number of mutants | 14 (100) | 19 (100) | 74 (100) | 66 (100) |
Note. Percentage of each type of mutation is given in parentheses.
TABLE 2.
Summary of Mutations in the gpt Gene of Male and Female B6C3F1 Neonates 21 Days After Treatment with Multiple Doses (3 × 2 mg/kg) of AFB1
| Mutation types | Control |
AFB1 |
||
| Male | Female | Male | Female | |
| Transition | ||||
| G:C to A:T | 9 (38) | 3 (60) | 8 (17) | 13 (17) |
| A:T to G:C | 1 (4) | 0 (0) | 0 (0) | 1 (1) |
| Transversion | ||||
| G:C to T:A | 4 (17) | 1 (20) | 31 (66) | 45 (58) |
| G:C to C:G | 1 (4) | 0 (0) | 1 (2) | 13 (17) |
| A:T to T:A | 1 (4) | 0 (0) | 0 (0) | 2 (3) |
| A:T to C:G | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Deletion (bp) | ||||
| 1 | 4 (17) | 0 (0) | 6 (13) | 2 (3) |
| >1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Insertion | 4 (17) | 1 (20) | 0 (0) | 1(1) |
| Total number of mutants | 24 (100) | 5 (100) | 47 (100) | 77 (100) |
Note. Percentage of each type of mutation is given in parentheses.
Sequence analysis of 62 mutants obtained from 16 DMSO-treated mice (combined data from both the single- and multiple-dose groups) revealed that G:C to A:T transitions were the predominant mutation (22/62, 35%) followed by G:C to T:A transversions (13/62, 21%). Single base pair deletions at G:C base pairs were found at the same frequency as in AFB1-treated animals (7/9, 78%). As described below, distribution of these mutations within the gpt gene differs significantly from that of AFB1-treated mice.
Comparison of Mutational Spectra
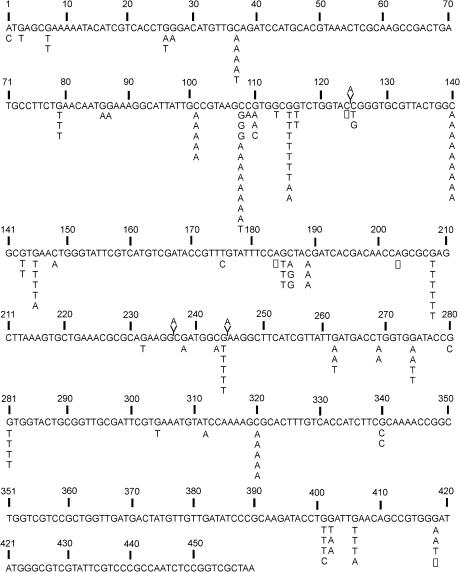
Differences in the distribution of mutations with regard to base number in the gpt gene among treatment groups were tested using Monte Carlo analysis (Adams and Skopek, 1987). Mutation spectra were significantly different between AFB1-treated and DMSO control groups (multiple dose vs. DMSO, p < 0.01; single dose vs. DMSO, p < 0.01). For illustration, Figure 5 shows the mutation spectrum in the gpt gene in liver of mice treated with a single (6 mg/kg)AFB1 dose. (See supplemental data for mutant spectra from the multiple dose and control animals.) Several mutational hot spots for base substitution mutations were observed in AFB1-treated mice. G:C to T:A transversions occurred as independent mutations in ≥ 5 mice at nucleotides 101 (5′-GCC-3′), 108 (5′-AGC-3′), 115 (5′-GGT-3′), 140 (5′-GCG-3′), and 208 (5′-GAG-3′). In DMSO-treated mice, G:C to A:T transitions occurred most frequently at nucleotides 64 (5′-CGA-3′), 110 (5′-CGT-3′), and 115 (5′-GGT-3′). These CpG sites have previously been identified as gpt mutational hot spots in untreated animals (Masumura et al., 2000).
FIG. 5.
Nucleotide sequence of the gpt gene indicating the position and type of base substitutions, deletions (□) and insertions (V) induced by a single 6 mg/kg AFB1 dose administered to 4-day-old gpt delta B6C3F1 mice. Mutations were assayed 21 days after dosing.
When the distributions of mutations within the gpt gene were compared between AFB1-treated groups, there was no significant difference in the types or positions of mutations (multiple dose vs. single dose, p = 0.214; 95% confidence level 0.199–0.229). Thus, the AFB1 mutational spectra in the gpt sequence were not dependent on the exposure conditions in this experimental model. Further comparisons of mutational spectra between male and female animals showed that they were also indistinguishable.
DISCUSSION
This study examined relationships among levels of liver AFB1-DNA adducts and mutagenesis in liver cells of newborn transgenic gpt delta B6C3F1 mice treated with dosing protocols previously shown to be highly effective in inducing HCC in male, but not female, B6C3F1 mice (Vesselinovitch et al., 1972). We found that dosing with AFB1 created very similar levels of adducts in liver DNA of both sexes and induced nearly identical mutation frequencies and types of mutations in the gpt transgene in liver cells. These results demonstrate that during infancy, male and female B6C3F1 mice are at similar risks of genotoxic damage and mutagenesis resulting from AFB1 exposure.
Monitoring of AFB1-DNA adducts revealed that administration of a single 6 mg/kg dose produced a higher level of DNA adducts at 24 h compared with the multiple dose regimen. Levels of AFB1-N7-guanine decreased rapidly during the 24 h after treatment, which has been shown to be a result of its removal by DNA repair processes together with its chemical transformation into secondary lesions, including apurinic sites and two imidazole ring-opened AFB1-FAPY forms that are believed to be rotamers (Brown et al., 2006). The lower level of adducts in liver DNA induced by treatment with the multiple-dose regimen was reflected in a lower gpt MF observed in animals treated with multiple doses of AFB1.
Our results identified G:C to T:A transversions in the gpt gene as the most prevalent mutation induced by AFB1 in the liver of mice. Studies of similar design by other investigators produced similar findings, with AFB1 inducing primarily G:C to T:A (76%) mutations in the cII gene in the neonatal liver of Big Blue mice (Chen et al., 2010). Overall, the types of mutations found in the gpt gene of AFB1-treated mice were characteristic of those induced by it in other experimental systems. The G:C to T:A transversion is the predominant mutation induced by either the AFB1-N7-guanine or AFB1-FAPY adduct in in vitro experimental systems (Bailey et al., 1996; Foster et al., 1983; Smela et al., 2002). In E. coli, the AFB1-FAPY minor rotamer was found to be the most potent mutagen, producing G:C to T:A transversions about six times more frequently than AFB1-N7-guanine (Smela et al., 2002). The high mutagenic potency of the FAPY adduct together with its persistence in DNA suggests that it could be responsible for a major fraction of the mutations induced by AFB1 in the rapidly growing neonatal liver.
Several mutational hot spots for G:C to T:A transversions in the gpt gene were observed in livers of AFB1-treated mice, including nucleotide positions 101, 108, 115, 140, 208, 244, and 320. Such hot spots may result from structural features of the DNA sequence that increase the reactivity of guanine with the AFB1-8,9-oxide (Benasutti et al., 1988), actions of the repair and replication processes, or properties of the gpt gene product used to select 6-TG-resistant mutants. The selection procedure requires nearly complete inactivation of the E. coli gpt enzyme since toxic levels of 6-TG remain even in the presence of very low enzyme activity (Thilly et al., 1978). In this regard, it is noteworthy that the most frequently encountered hot spots, including positions 108, 115, 140, and 208 are located in codons for amino acids involved in the binding sites of guanine or phosphoribosylpyrophosphate, the enzyme’s two substrates (Vos et al., 1998). This observation provides a plausible explanation of the partial overlap observed between AFB1-induced mutational spectra and those of other agents. Furthermore, it suggests the possibility that unrecognized mutational hot spots may exist at nucleotides in the gpt sequence that result in amino acid changes that do not strongly affect gpt enzyme activity. If this were the case, MF values calculated from our data would represent minimal estimates.
In both experimental animals and humans, the incidence of HCC is much higher in males than in females. Our findings of similar levels of AFB1 adducts and mutation frequencies in infants of both sexes suggests the possibility that biochemical or other factors may be responsible for the observed sex difference in HCC incidence occurring in adult mice treated with AFB1 as infants. Current conceptual models of HCC pathogenesis are based on initiating mutations produced by genotoxic compounds followed by enhanced tumor development driven by factors such as liver cell regeneration and chronic inflammation. In humans, aflatoxin synergizes with hepatitis B virus (HBV) to greatly increase the risk for HCC (Wild and Montesano, 2009). Host responses to HBV infection include activation of the nuclear factor kappa-B signaling pathway that contributes to chronic inflammation and promotion of HCC (Sun and Karin, 2008). One explanation offered for the resistance of female mice to HCC is the observation that estrogenic hormones can suppress inflammation and reduce cancer risk during the promotion phase of hepatocarcinogenesis in mice (Nakatani et al., 2001; Naugler et al., 2007). Although mechanisms responsible for different sex-based incidence of HCC remain to be defined, our findings support the suggestion that postinitiation host responses are important modulators of HCC development.
Aflatoxin-DNA adducts are validated biomarkers of AFB1 exposure and have enabled estimation of risk for HCC in exposed human populations (Groopman et al., 2008). In experimental animals, the chemoprotective agent oltapraz has been shown to be effective in inhibiting formation of AFB1-DNA adducts and reducing the frequency of preneoplastic lesions as well as tumor formation in the livers of AFB1-treated rats (Roebuck et al., 2003). Insights gained from these experimental studies laid the foundation for clinical trials of chemopreventive strategies that are effective in reducing AFB1-DNA adducts in exposed populations (Kensler et al., 2005). Our results suggest that aflatoxin-induced genetic changes are important contributors to tumor initiation, but additional factors yet to be defined are also determinants of the ultimate development of HCC in mice. The ninefold higher incidence of HCC in men compared with women highlights the importance of elucidating the additional hormonal and environmental factors that regulate HCC development.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health [R01 ES016313, P30 ES002109, P01 ES006052 and P30 ES003819].
Supplementary Material
Acknowledgments
The authors would like to thank Alex Sheh for providing technical assistance with the gpt mutation assay.
References
- Adams WT, Skopek TR. Statistical test for the comparison of samples from mutational spectra. J. Mol. Biol. 1987;194:391–396. doi: 10.1016/0022-2836(87)90669-3. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ. Health Perspect. 2000;108(Suppl. 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EA, Iyer RS, Stone MP, Harris TM, Essigmann JM. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1535–1539. doi: 10.1073/pnas.93.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasutti M, Ejadi S, Whitlow MD, Loechler EL. Mapping the binding site of aflatoxin B1 in DNA: systematic analysis of the reactivity of aflatoxin B1 with guanines in different DNA sequences. Biochemistry. 1988;27:472–481. doi: 10.1021/bi00401a068. [DOI] [PubMed] [Google Scholar]
- Brown KL, Deng JZ, Iyer RS, Iyer LG, Voehler MW, Stone MP, Harris CM, Harris TM. Unraveling the aflatoxin-FAPY conundrum: structural basis for differential replicative processing of isomeric forms of the formamidopyrimidine-type DNA adduct of aflatoxin B1. J. Am. Chem. Soc. 2006;128:15188–15199. doi: 10.1021/ja063781y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariello NF, Piegorsch WW, Adams WT, Skopek TR. Computer program for the analysis of mutational spectra: application to p53 mutations. Carcinogenesis. 1994;15:2281–2285. doi: 10.1093/carcin/15.10.2281. [DOI] [PubMed] [Google Scholar]
- Chen T, Heflich RH, Moore MM, Mei N. Differential mutagenicity of aflatoxin B1 in the liver of neonatal and adult mice. Environ. Mol. Mutagen. 2010;51:156–163. doi: 10.1002/em.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy RG, Wogan GN. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981;41:197–203. [PubMed] [Google Scholar]
- Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- Egner PA, Groopman JD, Wang JS, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- Foster PL, Eisenstadt E, Miller JH. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc. Natl. Acad. Sci. U.S.A. 1983;80:2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman JD, Busby WF, Jr, Wogan GN. Nuclear distribution of aflatoxin B1 and its interaction with histones in rat liver in vivo. Cancer Res. 1980;40:4343–4351. [PubMed] [Google Scholar]
- Groopman JD, Croy RG, Wogan GN. In vitro reactions of aflatoxin B1-adducted DNA. Proc. Natl. Acad. Sci. U.S.A. 1981;78:5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman JD, Jackson PE, Turner P, Wild CP, Kensler TW. Progress in Nucleic Acid Research and Molecular Biology. Boca Raton, FL: CRC Press; 2002. Validation of exposure and risk biomarkers: aflatoxin as a case study; pp. 307–318. [Google Scholar]
- Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu. Rev. Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Judah DJ, Neal GE, Nguyen T. Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S-transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J. 1992;285(Pt 1):173–180. doi: 10.1042/bj2850173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14(Pt 1):2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Davidson NE, Roebuck BD, Pikul A, Groopman JD. Modulation of aflatoxin metabolism, aflatoxin-N7-guanine formation, and hepatic tumorigenesis in rats fed ethoxyquin: role of induction of glutathione S-transferases. Cancer Res. 1986;46:3924–3931. [PubMed] [Google Scholar]
- Masumura K, Matsui K, Yamada M, Horiguchi M, Ishida K, Watanabe M, Ueda O, Suzuki H, Kanke Y, Tindall KR, et al. Mutagenicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in the new gpt delta transgenic mouse. Cancer Lett. 1999;143:241–244. doi: 10.1016/s0304-3835(99)00132-9. [DOI] [PubMed] [Google Scholar]
- Masumura K, Matsui K, Yamada M, Horiguchi M, Ishida K, Watanabe M, Wakabayashi K, Nohmi T. Characterization of mutations induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in the colon of gpt delta transgenic mouse: novel G: C deletions beside runs of identical bases. Carcinogenesis. 2000;21:2049–2056. doi: 10.1093/carcin/21.11.2049. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn. J. Cancer Res. 2001;92:249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nohmi T, Katoh M, Suzuki H, Matsui M, Yamada M, Watanabe M, Suzuki M, Horiya N, Ueda O, Shibuya T, et al. A new transgenic mouse mutagenesis test system using Spi- and 6-thioguanine selections. Environ. Mol. Mutagen. 1996;28:465–470. doi: 10.1002/(SICI)1098-2280(1996)28:4<465::AID-EM24>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rice JM. Prenatal susceptibility to carcinogenesis by xenobiotic substances including vinyl chloride. Environ. Health Perspect. 1981;41:179–188. doi: 10.1289/ehp.8141179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck BD, Curphey TJ, Li Y, Baumgartner KJ, Bodreddigari S, Yan J, Gange SJ, Kensler TW, Sutter TR. Evaluation of the cancer chemopreventive potency of dithiolethione analogs of oltipraz. Carcinogenesis. 2003;24:1919–1928. doi: 10.1093/carcin/bgg173. [DOI] [PubMed] [Google Scholar]
- Shupe T, Sell S. Low hepatic glutathione S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol. Lett. 2004;148:1–9. doi: 10.1016/j.toxlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- Thilly WG, Deluca JG, Hoppe H, Penman BW. Phenotypic lag and mutation to 6-thioguanine resistance in diploid human lymphoblasts. Mutat. Res. 1978;50:137–144. doi: 10.1016/0027-5107(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Thybaud V, Dean S, Nohmi T, de BJ, Douglas GR, Glickman BW, Gorelick NJ, Heddle JA, Heflich RH, Lambert I, et al. In vivo transgenic mutation assays. Mutat. Res. 2003;540:141–151. doi: 10.1016/j.mrgentox.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD. Perinatal mouse liver carcinogenesis as a sensitive carcinogenesis model and the role of the sex hormonal environment in tumor development. Prog. Clin. Biol. Res. 1990;331:53–68. [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N, Wogan GN, Lombard LS, Rao KV. Aflatoxin B 1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- Vos S, Parry RJ, Burns MR, de JJ, Martin JL. Structures of free and complexed forms of Escherichia coli xanthine-guanine phosphoribosyltransferase. J. Mol. Biol. 1998;282:875–889. doi: 10.1006/jmbi.1998.2051. [DOI] [PubMed] [Google Scholar]
- Wild CP, Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009;286:22–28. doi: 10.1016/j.canlet.2009.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.