Abstract
Manganese toxicity can cause a neurodegenerative disorder affecting cortical and basal ganglia structures with a neurological presentation resembling features of Parkinson’s disease. Children are more sensitive to Mn-induced neurological dysfunction than adults, and recent studies from our laboratory revealed a marked sensitivity of male juvenile mice to neuroinflammatory injury from Mn, relative to females. To determine the role of estrogen (E2) in mediating sex-dependent vulnerability to Mn-induced neurotoxicity, we exposed transgenic mice expressing an NF-κB-driven enhanced green fluorescent protein (EGFP) reporter construct (NF-κB-EGFP mice) to Mn, postulating that supplementing male mice with E2 during juvenile development would attenuate neuroinflammatory changes associated with glial activation, including expression of inducible nitric oxide synthase (NOS2) and neuronal protein nitration. Juvenile NF-κB-EGFP mice were separated in groups composed of females, males, and males surgically implanted with Silastic capsules containing 25 μg of 17-β-estradiol (E2) or vehicle control. Mice were then treated with 0 or 100 mg/Kg MnCl2 by intragastric gavage from postnatal days 21–34. Manganese treatment caused alterations in levels of striatal dopamine, as well as increases in NF-κB reporter activity and NOS2 expression in both microglia and astrocytes that were prevented by supplementation with E2. E2 also decreased neuronal protein nitration in Mn-treated mice and inhibited apoptosis in striatal neurons cocultured with Mn-treated astrocytes in vitro. These data indicate that E2 protects against Mn-induced neuroinflammation in developing mice and that NF-κB is an important regulator of neuroinflammatory gene expression in glia associated with nitrosative stress in the basal ganglia during Mn exposure.
Keywords: manganese, astrocyte, microglia, neuroprotection, 17-β-estradiol, neuroinflammation, nitric oxide
Manganese (Mn) is an essential dietary element but exposure to high concentrations may result in neurological dysfunction with pronounced motor features resembling Parkinson’s disease (Yamada et al., 1986). Manganese-induced neurological injury, termed manganism, has primarily been studied in adults during occupational exposure; however, there is increasing interest in the effects of prolonged low-level exposure to Mn in children due to the widespread distribution of Mn in drinking water and elevated brain levels of Mn during chronic iron deficiency (Chua and Morgan, 1996). The developing brain is more sensitive to Mn than the adult brain due to enhanced absorption of the metal, relatively lower biliary excretion, and the continued development of synapses throughout childhood until adolescence, particularly in the prefrontal cortex, all of which increase the potential for neurological injury from excessive exposures (Aschner et al., 2005; Rapoport and Gogtay, 2008; Tsujimoto, 2008).
Information on the toxicity of Mn in orally exposed children is relatively scarce. Several reports indicate that neurological dysfunction occurs in children who drink water with high (>1000 μg/l) concentrations of Mn (Hafeman et al., 2007; Woolf et al., 2002) and a potential association has been reported between ingestion of elevated levels of Mn and learning deficits (Collipp et al., 1983; Pihl and Parkes, 1977). Epidemiological evidence also supports a relationship between elevated Mn levels in water and neurotoxic effects in children. A Chinese study conducted in the province of Shanxi found that children aged 11–13 whose drinking water was contaminated with elevated levels of Mn (241–346 μg/l) performed more poorly in school and on neurobehavioral exams than control students (He et al., 1994). Furthermore, studies conducted in Bangladesh (Wasserman et al., 2006) and in Quebec, Canada (Bouchard et al., 2007, 2011), also reported that children who consumed drinking water with high levels of Mn had reduced standardized test scores (Wasserman et al., 2006) and exhibited increased hyperactive behavior (Bouchard et al., 2007). More recently, studies in Mexico found that environmental exposure to Mn is inversely associated with intellectual function in young school-age children (Riojas-Rodriguez et al., 2010). Additionally, studies in a cohort of children in Canada also suggested a possible gender difference between Mn exposure and IQ scores, with girls displaying an apparent decrease in susceptibility to neuro-cognitive impairment (Bouchard et al., 2011).
Earlier studies from our laboratory demonstrated that Mn exposure in adult mice results in apoptotic loss of neurons in the striatum and globus pallidus that was associated with marked activation of astrocytes expressing high levels of inducible nitric oxide synthase (NOS2) (Liu et al., 2006). More recently, we reported that developing male mice are more sensitive than female mice to both neurobehavioral and neuropathological effects of Mn, consistent with hyperactivity (Moreno et al., 2009b) and with an associated increase in neuroinflammation and nitrosative stress throughout the basal ganglia (Moreno et al., 2009a). The molecular basis for this observed sex-dependent differential susceptibility is not clear but may be a result of anti-inflammatory effects of E2 in female mice. The neuroprotective efficacy of E2 has been demonstrated by studies in mice exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA), where the presence of E2 prevented glial activation and loss of dopaminergic neurons (Callier et al., 2001; Murray et al., 2003; Tripanichkul et al., 2006). Likewise, E2 supplementation in ovariectomized mice suppressed expression of NOS2 in astrocytes and prevented loss of dopaminergic neurons following MPTP treatment (Morale et al., 2006). However, the role of E2 in regulating NOS2 expression in glia during exposure to Mn has not been previously investigated.
Expression of NOS2 is principally regulated by the transcription factor NF-κB (Xie et al., 1994, 1993) and studies from our laboratory reported that Mn stimulates cGMP-dependent activation of NF-κB in astrocytes that potentiates the effects of inflammatory cytokines on expression of NOS2 (Moreno et al., 2008). To determine if E2 directly modulates NF-κB activity in developing mice during exposure to Mn, we employed transgenic mice expressing an NF-κB-enhanced green fluorescent protein (EGFP) reporter construct to determine whether E2 supplementation in male mice during late postnatal development alters NF-κB activity and expression of NOS2. We postulated that E2 supplementation in developing male mice would inhibit NF-κB activation in glia and prevent neuroinflammatory gene expression. The results of these studies indicated that female mice are resistant to Mn during this developmental period and that E2 supplementation in male mice suppressed expression of NOS2 in glia and prevented subsequent neuronal injury through an NF-κB-dependent mechanism.
MATERIALS AND METHODS
Reagents.
All chemical reagents were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated. NF-κB-EGFP mice (Magness et al., 2004) were kindly provided by Dr Christian Jobin, University of North Carolina at Chapel Hill. Primary antibodies for glial fibrillary acidic protein (GFAP) were from Dako Cytomation (Denmark) and Sigma (St Louis). Antibodies for ionizing Ca2+-binding adaptor molecule-1 (IBA-1) and inducible nitric oxide synthase (NOS2) were from Wako Chemicals Inc. (Osaka, Japan) and BD Biosciences (San Jose, CA), respectively. Primary antibodies to 3-nitrotyrosine (3-NTyr) were from Upstate (Charlottesville, VA) and major microtuble association protein 2 (MAP2) from Abcam (Cambridge, MA). AlexaFluor-555 and 647-labeled secondary antibodies were from Invitrogen (Eugene, OR). 4′,6-diamidino-2-phenylindole (DAPI) were from Vector Lab (Burlingame, CA).
Animal exposure model.
Male and female NF-κB-EGFP mice were housed separately in microisolator cages and kept on 12-h light/dark cycles with access to laboratory chow and water ad libitum. Littermates were split into three groups; females, control males, and E2-exposed males (n = 6). At day 19, each group had Silastic capsules (1.98 mm inner diameter × 3.17 mm outer diameter) implanted subcutaneously on their back to deliver continuous circulating E2. The size of the Silastic capsules altered unrestrained movement in open-field activity assessments, which prevented acquisition of accurate behavioral data. Female and control males received capsules containing 25-μl safflower oil while the males supplemented with E2 received 25 μg of 17-β-estradiol benzoate suspended in 25 μl of safflower oil (1 μg E2/μl), similar to methods described previously (Kudwa et al., 2007; Tripanichkul et al., 2006). All mice received 0.9% normal saline or 100 mg/Kg MnCl2 by gastric gavage daily from day 21–34 postnatal, approximately equivalent to a 2-year-old child during the initial day of the juvenile exposure up to young adulthood by the end of the exposure period on day 34 (Dobbing and Sands, 1979; Flurkey et al., 2007; Koop et al., 1986). We previously demonstrated that this age is highly sensitive to both the neurobehavioral and neuroinflammatory effects of Mn exposure (Moreno et al., 2009a, 2009b). Animals were weighed prior to each gavage, and the amount of Mn delivered was adjusted for the molar concentration in the tetrahydrate form to achieve a precise dose of 100 mg/Kg Mn. Given that ∼5% of Mn2+ is absorbed from the GI tract, the daily dose of Mn per animal was approximately 5 mg/Kg, which is up to 50-fold higher than recommended dietary levels in humans (Greger, 1998). This is a plausible exposure model considering that Mn in soy-based infant formula can contain up to 300 μg/l Mn, approximately 50 times that of breast milk (Cockell et al., 2004; Krachler et al., 2000; Lonnerdal, 1994), and long-term exposures to Mn are associated with adverse neurobehavioral and intellectual deficits in children from drinking water containing >300 μg/l Mn (He et al., 1994), a level found in 6% of US domestic well water (Wasserman et al., 2006). All procedures were performed under the supervision of the Animal Care and Use Committee at the Colorado State University and were approved prior to onset of the studies.
E2 serum level assay.
Trunk blood was collected into tubes on ice with 20 μl of heparin to prevent clotting. Blood was centrifuged at 10,000 rpm at 4°C for 10 min. Following the centrifuge, serum was transferred to fresh tubes and stored at −80°C until radioimmunoassay could be performed for serum estradiol levels (Colorado State University, Animal Reproduction and Biotechnology Laboratory core facility).
Immunofluorescence.
Mice were anesthetized by inhalation of isofluorane and perfused intracardially with 4% paraformaldehyde in 20mM cacodylate in PBS (pH 7.4). Subsequently, brains were collected and kept in 4% paraformaldehyde for 2 h, then transferred to 15% sucrose in 20mM cacodylate in PBS overnight followed by overnight incubation in 30% sucrose in 20mM cacodylate in PBS until frozen in OCT embedding medium. Brains were kept frozen at −80°C until coronal 10-μm serial sections were cut using a Microm HM 500 cyrostat. Coimmunofluorescence was utilized to examine protein expression in substantia nigra pars reticulata (SNpr) and striatum (ST). Primary antibodies to anti-GFAP (1:250) and anti-IBA-1 (1:100) were used in combination with anti-NOS2 (1:100) to examine gliosis. Anti-MAP-2 (1:500) antibody was utilized in combination with anti-3-NTyr (1:100) to detect levels of protein nitration in neurons. Specific protein epitopes were visualized with secondary antibodies conjugated with AlexaFluor dyes (555 nm or 647 nm emission maxima), and slides were mounted in media containing DAPI to identify cell nuclei. Images were acquired using either a Zeiss 20× or 40× air Plan Apochromatic objective with six microscopic fields examined per region from three serial sections, for a total of 18 microscopic fields that were averaged for each animal. Data from individual animals were averaged to yield three biological replicates (n = 3). Images were acquired as a z-series with 1-μm step size. Final images are extrapolated from a maximum projection of each image plane. Quantitation of NOS2 and NF-κB in astrocytes and microglia was performed blindly by manual cell counts. Briefly, we recorded the number of total astrocytes and microglia as well as the number of astrocytes or microglia expressing NOS2 or NF-κB in each field, and percent expression was calculated. For quantification of 3-NTyr in neurons, sum intensity of 3-NTyr fluorescence in areas expressing 3-NTyr and MAP2 were acquired per field. Manual cell counts were not applicable due to dendritic staining by MAP2.
Determination of catecholamines and monoamine neurotransmitters.
Brain samples were frozen in liquid nitrogen the day following treatment and stored at −80°C until sample preparation. Samples were prepared essentially as described previously (Moreno et al., 2009b). Briefly, 5–10 mg of striatal tissue was dissected and sonicated in 0.2M perchloric acid. Twenty microliters of sample was utilized for total protein concentration via BCA protein assay. Levels of dopamine, 2-(3,4-dihydroxyphenyl)acetic acid (DOPAC), homovanillic acid (HVA), and serotonin (5-HT) were determined by high performance liquid chromatography with electrochemical detection as described in Moreno et al. (2009b). Peak area was assessed in the samples and compared with those observed in the standards with values expressed as nanogram of neurotransmitter per milligram of total protein. Striatal hemispheres were processed from three separate animals from all treatment groups.
Primary cell cultures.
Primary astrocytes were isolated from cortices of 1-day-old transgenic NF-κB-GFP mice as previously described (Aschner et al., 1992; Liu et al., 2006). Cells were maintained in minimum essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum, 50 units/ml penicillin, 50 ng/ml streptomycin, and 100 ng/ml neomycin (PSN) at 37°C, 5% CO2 in a humidified atmosphere. Astrocytes were grown 18 days to maturity before use in experiments. Primary striatal neurons were isolated from 1-day-old transgenic NF-κB-GFP mice as described previously (Carbone et al., 2009) and grown on poly-D-lysine coated glass for 4–5 days prior to experimental use. Neurons were maintained in neurobasal media supplemented with B27, 2mM l-Glutamine and 1% PSN.
Nos2 mRNA expression.
Exposure parameters for E2 was selected based upon data by Lee et al., (2009a, 2009b) and optimized in our model. The astrocyte media was changed to charcoal-stripped serum to remove any residual estrogenic or other steroidal compounds. Then, the cells were exposed to either 20nM E2 or ethanol (vehicle control) for 32 h prior to a 4-h treatment of saline or 30μM MnCl2 + 10 pg/ml TNF-α and 1000 pg IFNγ. These treatment conditions were previously demonstrated to result in robust induction of Nos2 mRNA, where relatively low micromolar doses of Mn potentiate the effects of inflammatory cytokines on neuroinflammatory gene expression (Moreno et al., 2008). Following cell treatments, RNA was isolated using the Qiagen RNeasy Kit with on-column DNase digestion, and reverse transcribed using iScript (Biorad, Hercules, CA). Relative expression of Nos2 mRNA was measured by semiquantitative real-time PCR and was normalized to β-actin mRNA utilizing an iCycler thermocycler and SYBR green supermix (Biorad). The following primers from mouse Nos2 and β-actin coding sequences used: (Nos2) 5′-TCACGCTTGGGTCTTGTT-3′(forward), 5′-CAGGTCACTTTGGTAGGATTTG-3′(reverse) (NM_01927.3); (β-actin) 5′-GCTGTGCTATGTTGCTCTAG-3′ (forward), 5′-CGCTCGTTGCCAATAGTG-3′ (reverse) (NM_007393.3).
In vitro determination of NF-κB-EGFP activation.
Primary astrocytes were isolated from NF-κB-EGFP reporter mice and treated with Mn and inflammatory cytokines in the presence of E2 or vehicle as described above for mRNA measurements. NF-κB activity was determined by live-cell imaging using a Zeiss 20× air Plan Apochromatic objective using SlideBook v 5.0 (Intelligent Imaging Innovations, Inc., Denver, CO). The percent of cells expressing EGFP was calculated and represented as fold induction relative to control. Three microscopic fields were examined from three individual coverslips of cells per treatment group. Data were averaged from at least three independent experiments.
Astrocyte-neuron coculture.
Astrocytes were subcultured onto permeable inserts (pore size = 0.4μM) at 104/well for 3 days before coculture with neurons. Astrocytes were preincubated with 20nM E2 for 32 h prior to addition of 30μM MnCl2 + 10 pg/ml TNF-α and 1000 pg IFNγ for an additional 24 h. Following the 24 h of exposure to Mn and cytokines in the presence of E2 or vehicle, astrocytes were washed with PBS and coincubated with primary striatal neurons for an additional 6 h. Subsequently, astrocyte inserts were removed and the neurons were visualized for several indices of apoptosis using 100μM Rhodamine-110 bis-(L-aspartic acid amide) to detect caspase activity, 50nM Annexin V-Alexa Fluor 647 to detect phosphatidyl serine accumulation and 2μM Hoescht 33342 to visualize nuclear morphology. Images were acquired using a 20× air Plan Apochromatic objective, ten microscopic fields were examined per treatment group over three independent experiments. Cell counts were acquired with differential interference contrast and DAPI. The sum intensity for active caspase and Annexin V signals were normalized to the number of cells in each field using SlideBook v 5.0.
Adenoviral-mediated expression of dominant-negative IκBα in cocultured astrocytes.
In studies examining the role of NF-κB in astrocyte activation and NOS2 expression in vitro, NF-κB was inhibited by adenoviral-mediated overexpression of dominant-negative IκBα containing serine-to-alanine mutations at amino acids 32 and 36. Adenovirus was added at 1 × 106 viral particles per ml of culture media for 24 h (construct provided by Dr Christian Jobin, through the Vector Core at the University of North Carolina at Chapel Hill). Following incubation with virus for 24 h, media was removed, cells washed with PBS to remove viral particles, and charcoal-stripped serum astrocyte medium was applied for 24 h prior to treatment. After incubation with virus, astrocytes were treated with Mn and cytokines for 6 h, media was removed and inserts with astrocytes were coincubated for 6 h with neurons. Parallel control experiments were done utilizing the same adenoviral construct expressing GFP. Neuronal apoptosis was evaluated by live-cell imaging as described above.
Statistical analysis.
Comparison of two independent variables was performed by two-way ANOVA followed by a Bonferroni’s post hoc test for in vivo studies using Prism software (v4.0c, Graphpad Software, Inc., San Diego, CA). p value is denoted in the following manner *p < 0.05, **p < 0.01, ***p < 0.001. Two-group comparisons between gender groups were evaluated using Bonferroni’s post hoc test, with differences denoted by †, p < 0.05. One-way ANOVA followed by the Tukey-Kramer multiple comparison post hoc test was used for in vitro studies (Prism software, v4.0c, Graphpad Software, Inc.). For all experiments, p < 0.05 was considered significant.
Results
Serum E2 Levels and Brain Mn Concentrations in NF-κB-EGFP Mice
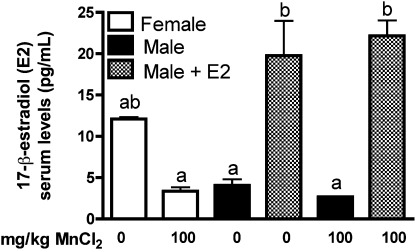
Serum from mice exposed to Mn for 2 weeks was analyzed for E2 levels by radioimmunoassay. Male mice supplemented with E2 had a significant increase in levels of serum E2 compared with unsupplemented males and to Mn-treated females implanted with control capsules (Fig. 1). E2 levels in saline-treated females were not significantly different from those in Mn-treated females, despite an apparent decreasing trend. Moreover, serum E2 levels in saline-treated females were not different from serum E2 levels in E2-supplemented males. Levels of Mn were significantly increased the substantia nigra and cortex in Mn-treated mice, indicating that there were no differences in uptake of Mn between treatment groups (Supplementary fig. 1). No changes were detected in iron or copper levels in any groups with or without Mn treatment (data not shown).
FIG. 1.
Administration of exogenous E2 to juvenile male mice increases serum E2 to physiologic levels observed in age-matched female mice. Female mice and control male mice had Silastic capsules with safflower oil (vehicle control) inserted subcutaneously at day 19 postnatal, whereas a subgroup of male mice received 25 μg of 17-β-estradiol. No statistical change was observed between female mice and unsupplemented male mice. Supplementation of E2 in male mice caused a significant increase in serum E2 levels compared with unsupplemented males and Mn-treated females. Differences between groups were analyzed by two-way ANOVA with Bonferroni’s post hoc test. Different lettering denotes statistical differences in mean value (p < 0.001, ±SEM).
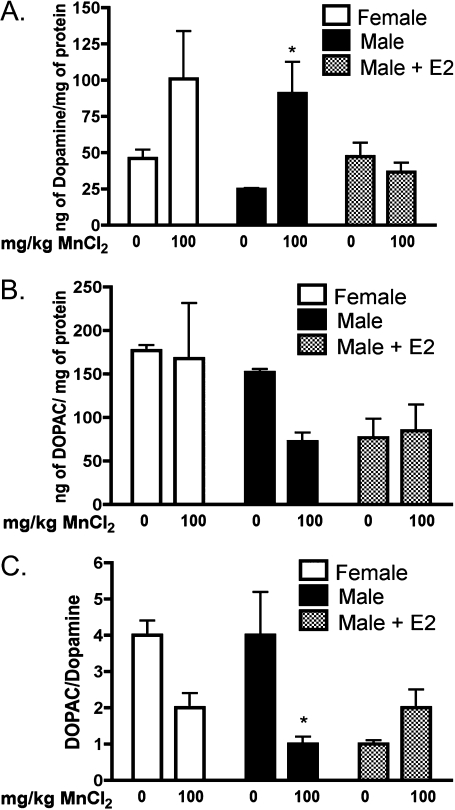
E2 Prevents Mn-Induced Changes in Striatal Dopamine
Striatal dopamine levels were significantly increased in Mn-treated male mice implanted with control capsules, whereas Mn-treated male mice supplemented with E2 had dopamine levels similar to the saline-treated male mice (Fig. 2A, p < 0.05). Levels of the dopamine metabolite, DOPAC, were unchanged in male mice following exposure to 100 mg/Kg Mn in the absence of E2, although a nonsignificant decreasing trend was noted (Fig. 2B). Mice supplemented with E2 had no change in DOPAC levels between saline and Mn-treated groups. The DOPAC/dopamine ratio was significantly reduced in male mice following exposure to 100 mg/Kg Mn in the absence of E2 (Fig. 2C, p < 0.05). There were no significant differences in dopamine or DOPAC levels between treatments in female mice (Figs. 2A–C), and no significant changes were detected in levels of serotonin or 5-hydroxyindoleacetic acid (5-HIAA) in the striatum in any treatment group (data not shown).
FIG. 2.
E2 protects against Mn-induced changes in striatal dopamine in juvenile male mice. (A) In the absence of E2, Mn-treated male mice had a significant increase in striatal dopamine, whereas male mice supplemented with E2 have no significant changes in dopamine levels. (B) The dopamine metabolite, DOPAC, was not significantly altered within any group, although a decreasing trend was noted in unsupplemented male mice. (C) The DOPAC/dopamine ratio was decreased in Mn-treated males in the absence of E2. Differences between groups were analyzed by two-way ANOVA with Bonferroni’s post hoc test. Statistical significance in mean value is denoted by * (p < 0.05, ±SEM).
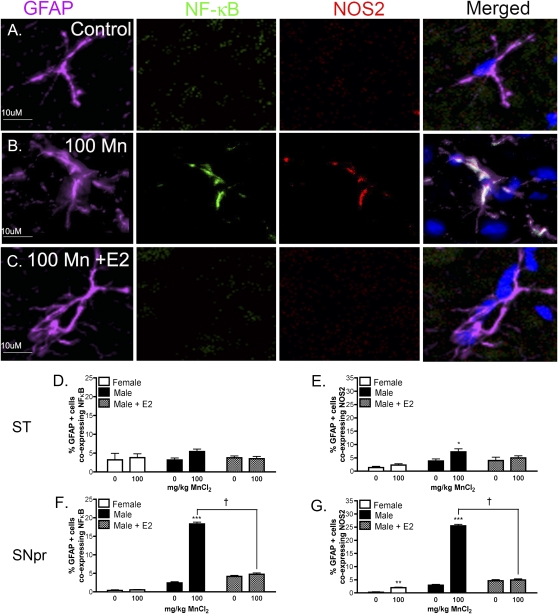
E2 Suppresses Glial Inflammatory Signaling
In order to determine the effect of E2 on Mn-induced neuroinflammatory activation of glia, intrinsic NF-κB activity and expression of NOS2 were assessed by coimmunofluorescence in both microglia and astrocytes in NF-κB-EGFP reporter mice. The SNpr and ST were the primary brain regions examined due to the known sensitivity of these nuclei to Mn in both humans and animal models (Olanow, 2004; Yamada et al., 1986). Representative images from the SNpr demonstrate that Mn increases expression of NOS2 and NF-κB-EGFP in GFAP-positive astrocytes in male mice, which is attenuated with E2 supplementation (Figs. 3A–C). Quantitative analysis of cell-specific expression of each neuroinflammatory marker indicated that expression of NF-κB-EGFP was not increased in striatal astrocytes in any treatment group (Fig. 3D), whereas expression of NOS2 was increased in striatal astrocytes in male mice exposed to 100 mg/Kg Mn that was prevented by supplementation with E2 (Fig. 3E). However, activation of NF-κB and expression of NOS2 was strongly increased in GFAP-positive astrocytes in the SNpr (***, p < 0.001) that was decreased to control levels by E2 (Figs. 3F and 3G). Direct post hoc comparison of the Mn-treated male groups indicates a significant decrease in expression of both inflammatory markers in the SNpr (†, p < 0.05) in the presence of E2 (Figs. 3F and 3G). Mn treatment in female mice resulted in a slight increase in the percentage of astrocytes expressing NOS2 in the SNpr compared with saline-treated females (Fig. 3G). No change in expression of the NF-κB-EGFP reporter was detected in astrocytes from female mice in either brain region.
FIG. 3.
E2 decreases Mn-induced activation of NF-κB and expression of NOS2 in astrocytes in transgenic NF-κB-EGFP reporter mice. Astrocyte-specific expression of the NF-κB-EGFP reporter and NOS2 was assessed via coimmunofluorescence in the ST and SNpr of mice exposed to Mn. Images of GFAP, NF-κB-EGFP, and NOS2 expression are shown in purple, green, and red channels, respectively, whereas cell nuclei are highlighted by staining with DAPI in blue. Representative images from the SNpr are presented from male control mice (A), those treated with 100 mg/Kg Mn (B), or 100 mg/Kg Mn with E2 (C). Merged images indicate colocalization of NF-κB-EGFP and NOS2 expression in GFAP-positive astroglia. Quantitative analysis reveals the percent of GFAP-positive cells expressing NF-κB-EGFP in the ST (D) and SNpr (F), and the percent of GFAP-positive cells expressing NOS2 in ST (E) and SNpr (G). For statistical analysis, two-way ANOVA was used with Bonferroni’s post hoc test to determine significance between treatment groups (*p < 0.05, ** p< 0.01, ***p < 0.001, ±SEM) and between Mn-treated male mice († p < 0.05, ±SEM). Images were acquired as a z-series using a 40× air Plan Apochromatic objective. Scale bar = 10 μm.
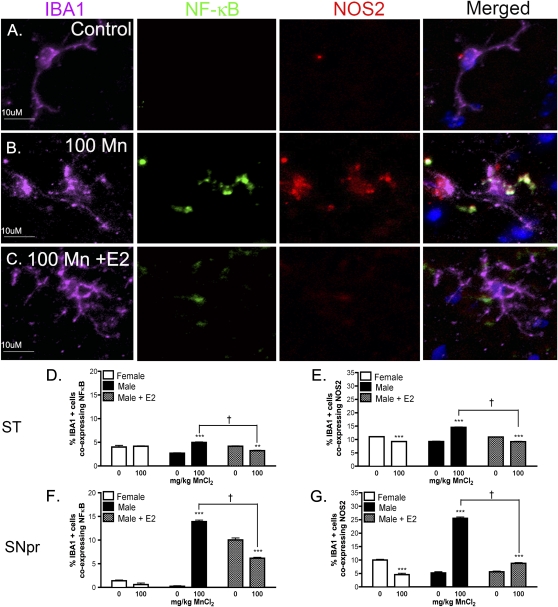
E2 also inhibited Mn-induced increases in expression of NOS2 and NF-κB-EGFP in microglia (Fig. 4). Representative images of IBA-1-positive microglia from the SNpr indicate that Mn treatment increased both NOS2 and NF-κB-EGFP expression (Figs. 4A–C), although colocalization of these markers was less robust than that observed in astrocytes, and the absolute number of detectable microglia was substantially lower than astrocytes. Quantitative analysis revealed that expression of NF-κB-EGFP was increased in striatal microglia in Mn-treated male mice (***, p < 0.001) that was prevented by E2 (Fig. 4E) (†, p < 0.05), as was expression of NOS2 (Fig. E). Likewise, Mn-induced expression of both NF-κB-EGFP and NOS2 in microglia within the SNpr was decreased by E2 (Figs. 4F and 4G). Interestingly, expression of the NF-κB-EGFP reporter also increased with E2 supplementation in control male mice in microglia within the SNpr, although the percentage of microglia expressing EGFP was still less in Mn-treated male mice supplemented with E2 than in those without E2.
FIG. 4.
E2 decreases Mn-induced activation of NF-κB and expression of NOS2 in microglia in transgenic NF-κB-EGFP reporter mice. Microglial-specific expression of the NF-κB-EGFP reporter and NOS2 was assessed via coimmunofluorescence in the ST and SNpr of mice exposed to Mn. Images of IBA-1, NF-κB-EGFP, and NOS2 expression are shown in purple, green, and red channels, respectively, and cell nuclei are highlighted by staining with DAPI in blue. Representative images of the SNpr are presented from male control mice (A), those treated with 100 mg/Kg Mn (B), or 100 mg/Kg Mn with E2 (C). Merged images indicate colocalization of NF-κB and NOS2 expression in IBA-1 positive microglia in mice exposed to Mn. Quantitative analysis reveals the percent of IBA-1 positive cells expressing NF-κB in ST (D) and SNpr (F), and the percent of IBA-1 positive cells expressing NOS2 in ST (E) and SNpr (G). For statistical analysis, two-way ANOVA was used with Bonferroni’s post hoc test (**p < 0.01, *** p < 0.001, ±SEM) and between Mn-treated male mice († p < 0.05, ±SEM). Images were acquired as a z-series using a 40× Plan Apochromatic objective. Scale bar = 10 μm.
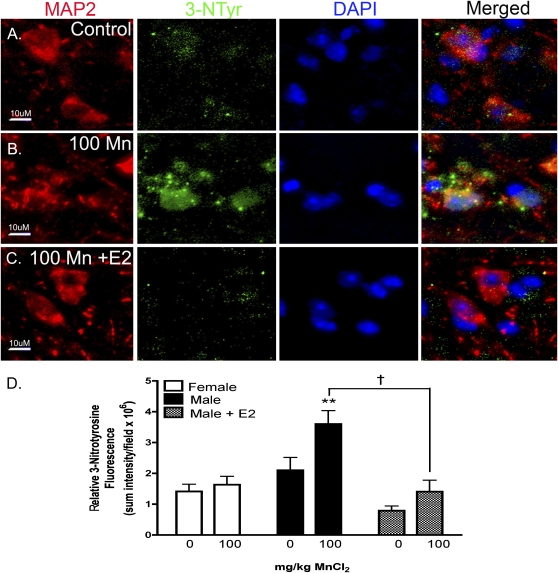
E2 Attenuates Mn-Induced Neuronal Protein Nitration
Neuronal protein nitration was examined in serial sections from the SNpr by coimmunofluorescence staining for MAP2, a general neuronal marker, and 3-nitrotyrosine (3-NTyr), a measure of NO/ONOO protein adduct formation (Fig. 5). Colocalization of 3-NTyr and MAP-2 is depicted in representative images from the SNpr of male mice exposed to Mn in the presence or absence of E2 (Figs. 5A–C). Low levels of 3-NTyr adducts were detected in control males (Fig. 5A), however exposure to 100 mg/Kg Mn (Fig. 5B) resulted in large increases in immunofluorescence staining for 3-NTyr protein adducts in MAP-2-positive neurons. Exposure to 100 mg/Kg Mn in males supplemented with E2 decreased neuronal 3-NTyr staining to control levels (Fig. 5C). Quantitation of 3-NTyr staining in SNpr neurons (Fig. 5D) indicated a significant increase in neuronal protein nitration in male mice treated with 100 mg/Kg Mn in the absence of E2 (**, p < 0.01) but not between treatments in females or males supplemented with E2 (†, p < 0.05).
FIG. 5.
Mn exposure increases levels of 3-nitrotyrosine (3-NTyr) protein adducts in SNpr neurons that are prevented by E2. To detect modification of neuronal proteins by peroxynitrite (ONOO−), an oxidative by-product from increased production of NO by activated glia, serial sections from the SNpr of juvenile mice were stained with antibodies against the neuronal marker MAP2 (green) and 3-NTyr (red) and were counterstained with DAPI to identify cell nuclei (blue). Representative images of the SNpr are presented from control mice (A), those treated with 100 mg/Kg Mn (B), and 100 mg/Kg Mn with E2 (C). Merged images indicate colocalization of 3-NTyr adducts with both neuronal soma and dendrites in the SNpr of Mn-treated mice. Scale bar = 10 μm. Quantitative analysis reveals the sum intensity of 3-NTyr fluorescence within MAP2-positive cells (D). Data were analyzed using two-way ANOVA with Bonferroni’s post hoc test (** p < 0.01), and a post hoc test was completed between gender groups († p < 0.05, ±SEM). Images were acquired as a z-series using a 63× Plan Apochromatic objective. Scale bar = 10 μm.
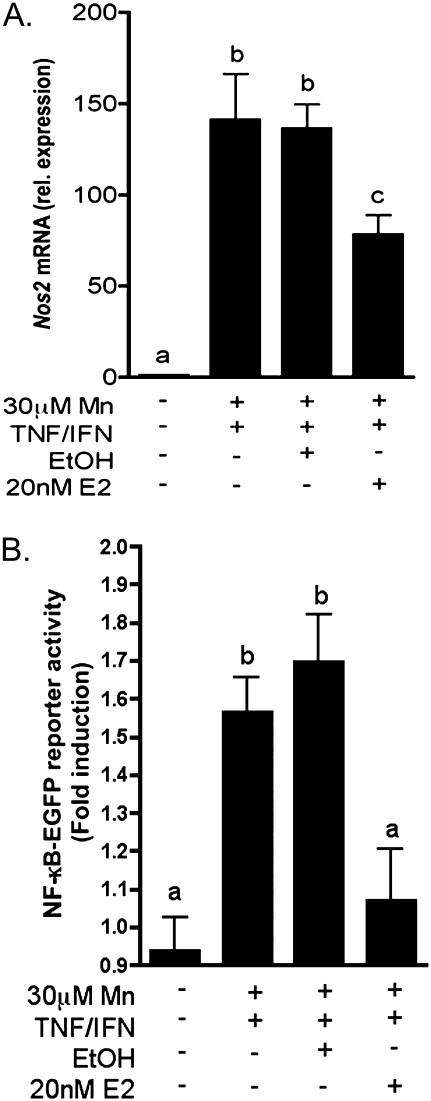
E2 Inhibits Expression of NOS2 in Primary Astrocytes In Vitro and Astrocyte-Dependent Apoptosis in Striatal Neurons
To further explore mechanisms underlying the neuroprotective efficacy of E2, we examined the capacity of E2 to modulate expression of NOS2 in primary cultured astrocytes. We previously reported that low micromolar levels of Mn were sufficient to potentiate expression of NOS2 induced by the inflammatory cytokines tumor necrosis factor alpha and interferon gamma (TNF-α/IFNγ) (Moreno et al., 2008). Accordingly, preincubation with 20nM E2 prior to treatment with Mn and TNF-α/IFNγ significantly suppressed expression of Nos2 mRNA but not completely to control levels (p < 0.05, Fig. 6A). E2 also prevented activation of NF-κB in primary astrocytes isolated from NF-κB-EGFP reporter mice (Fig. 6B), as determined by live-cell fluorescence imaging. Reporter activity was increased in transgenic astrocytes following 6 h exposure to 30μM MnCl2 + TNF-α/IFNγ. This expression was not prevented by cotreatment with vehicle (ethanol) but was reduced to control levels in astrocytes primed by pretreatment with 20nM E2 (p < 0.001).
FIG. 6.
E2 inhibits expression of Nos2 mRNA and activation of NF-κB in primary astrocytes exposed to Mn and inflammatory cytokines. (A) Nos2 mRNA expression was increased in astrocytes exposed to 30μM Mn + TNF-α/IFNγ but was reduced by pretreatment with 20nM E2. (B) Activation of NF-κB was determined by live-cell fluorescence imaging in astrocytes isolated from transgenic NF-κB-EGFP mice. NF-κB-EGFP reporter activity was significantly increased by treatment with Mn + TNF-α/IFNγ and reduced to control levels with pretreatment of 20nM E2. Differences between groups were determined using one-way ANOVA followed by the Tukey-Kramer multiple comparison post hoc test, significance is denoted by differing letters (p < 0.05, ±SEM).
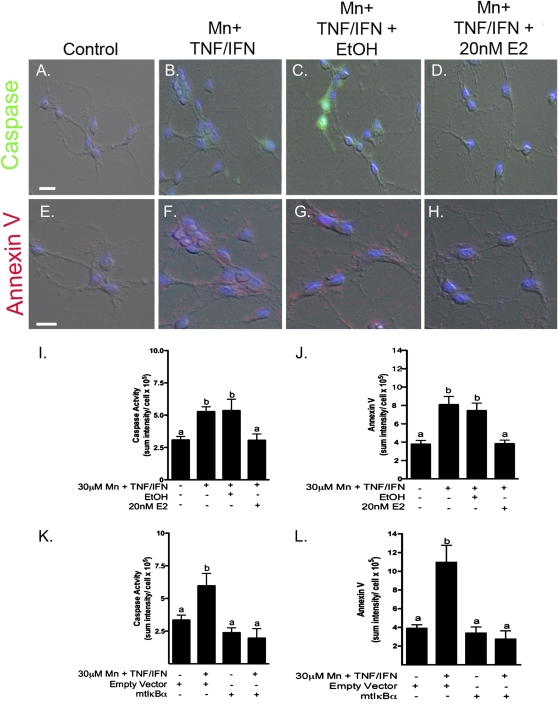
The capacity of E2 to inhibit Mn-induced neuronal apoptosis was determined in coculture studies (Fig. 7). Astrocytes cultured in permeable transwell inserts were primed by preexposure to 20nM E2 or vehicle and then exposed to saline or 30μM MnCl2 + TNF-α/IFNγ for 24 h. Following Mn exposure, astrocytes were incubated with primary striatal neurons for 6 h and apoptosis was evaluated by live-cell imaging for activation of caspases and binding of Annexin V. Astrocytes exposed to Mn and cytokines caused a significant increase in markers of apoptosis in striatal neurons that was inhibited by 20nM E2 but not by the ethanol vehicle control (Figs. 7A–J). To address the role of NF-κB activation in astrocytes as a mediator of neuronal injury during Mn exposure, coculture studies were also conducted with adenoviral-mediated overexpression of a dominant-negative mutant of IκBα, which inhibits activation of NF-κB and expression of NOS2 in astrocytes (Figs. 7K and 7L). Astrocytes exposed to Mn and cytokines in the presence of a control adenoviral vector induced activation of caspases (Fig. 7K) and increased Annexin V staining (Fig. 7J) in cocultured neurons that was prevented by overexpression of dominant-negative IκBα.
FIG. 7.
E2 protects cocultured striatal neurons from astrocyte-mediated apoptosis induced by Mn and cytokines. Caspase activation (green) and Annexin V binding (red) in primary striatal neurons cocultured with astrocytes is induced by exposure to Mn and TNF-α/IFNγ. Representative images indicate striatal neurons incubated with astrocytes treated with saline (A and E), 30μM Mn + TNF-α/IFNγ (B and F), Mn + TNF-α/IFNγ+ ethanol vehicle control (C and G), and Mn + TNF-α/IFNγ + 20nM E2 (D and H). Quantitative analysis reveals a significant increase in caspase activity and Annexin V with Mn + TNF-α/IFNγ and vehicle control, which was prevented by pretreatment with E2 (I and J). Overexpression of mutant IκBα (S32/36A) in astrocytes, a dominant-negative “super-repressor” of NF-κB, significantly decreased caspase activity and Annexin V in cocultured neurons (K and L). Differences between groups were determined using one-way ANOVA followed by the Tukey-Kramer multiple comparison post hoc test, significance is denoted by differing letters (p < 0.05, ±SEM). Images were acquired using a 40× Plan Apochromatic objective. Scale bar = 20 μm.
DISCUSSION
Activated microglia and astrocytes produce proinflammatory mediators such as NO, prostaglandins, TNF-α, and IL-1β that potentiate neuronal injury in multiple neurodegenerative disorders (Carreno-Muller et al., 2003; Hirsch and Hunot, 2000; Wu et al., 2002), including manganism (Aschner et al., 2009; Spranger et al., 1998). Studies of Mn neurotoxicity in rodent models have reported sex-dependent susceptibility to Mn (Miller et al., 1998) but the mechanisms by which E2 modulates neuroinflammatory gene expression in glia during developmental exposure are poorly characterized. In order to examine E2-dependent regulation of glial inflammatory signaling during Mn exposure in vivo, we utilized NF-κB-EGFP reporter mice to determine the effects of E2 on NF-κB activation and NOS2 expression in microglia and astrocytes. The data presented here indicate that E2 modulates Mn-induced glial activation and expression of NOS2 through the NF-κB pathway and subsequently reduces neuronal protein nitration.
Manganese levels in the basal ganglia of developing mice increased by 2–3 fold, comparable to results obtained in previous studies conducted in rodents from our group (Moreno et al., 2009b) and others (Kern et al., 2010), as well as results obtained in non-human primates exposed to Mn (Guilarte et al., 2006). The data in Figure 1 indicate that supplementing juvenile male mice with E2 raised serum levels comparably to those in age-matched female mice. Endogenous E2 levels showed a decreasing trend in female mice exposed to Mn, suggesting that Mn potentially alters neuroendocrine function in juvenile female mice. This finding is consistent with reported effects of Mn on the hypothalamic-pituitary axis in prepubertal female rats, where oral exposure to Mn altered patterns of luteinizing hormone-releasing hormone (LHRH) secretion (Lee et al., 2007).
Alterations in dopamine and the dopamine/DOPAC ratio in the striatum were prevented in estrogen-supplemented juvenile male mice exposed to Mn (Fig. 2), whereas female mice had no significant changes in dopamine or its metabolites. Dopamine levels increased in Mn-treated male mice in the absence of supplementation with E2, similar to earlier studies from our laboratory reporting both increased striatal dopamine levels and hyperactivity in juvenile mice exposed to Mn (Moreno et al., 2009b). A recent study by Kern et al. (2010) also indicated hyperactivity and behavioral disinhibition in open field activity assays in preweaning juvenile mice exposed to Mn that correlated with decreased striatal levels of the dopamine transporter and D1/D2 dopamine receptors. Other recent studies in cultured dopaminergic neuronal cells indicated that acute exposure to Mn increased the activity of tyrosine hydroxylase, suggesting a mechanism by which exposure to Mn could enhance dopamine production in the absence of any change in protein expression, at least for shorter duration exposures (Zhang et al., 2011). The present observations are also consistent with other reports demonstrating alterations in striatal dopamine following Mn exposure (Guilarte et al., 2006; Moreno et al., 2009b) and with the neuroprotective effect of E2 on catecholamine levels in models of 6-OHDA and MPTP neurotoxicity (D'Astous et al., 2003; Dluzen et al., 1996; Murray et al., 2003; Ramirez et al., 2003). The capacity of E2 to suppress neuroinflammation may therefore underlie its protective effect on striatal dopamine levels in Mn-treated mice, similar to earlier studies demonstrating that E2 prevented NOS2 expression in astrocytes and subsequent dopaminergic neurotoxicity in mice exposed to MPTP (Morale et al., 2006). Thus, inflammatory activation of glia during Mn exposure may be etiologically linked to alterations in dopamine levels and to ensuing behavioral disinhibition.
Inflammatory activation of microglia and astrocytes is linked to expression of NOS2 during Mn neurotoxicity both in vitro and in vivo (Liu et al., 2009, 2006; Moreno et al., 2008, 2009a) but the basis for the relative resistance of female mice to Mn-induced neuroinflammation has not been demonstrated. Using morphometric analysis and fluorescence colocalization studies, it was determined that Mn caused an increase in activation of NF-κB and expression of NOS2 in GFAP-positive astrocytes and IBA-1-positive microglia in the ST and SNpr of male NF-κB-EGFP mice, which was reversed by supplementation with E2 (Figs. 3 and 4). Expression of NOS2 in activated glia, particularly in astrocytes, causes neuronal protein nitration during MPTP neurotoxicity (Liberatore et al., 1999) that is antagonized by E2 (Morale et al., 2006). These data suggest a similar mechanism may occur during Mn intoxication because expression of NOS2 was detected much more robustly in astrocytes than in microglia within the basal ganglia, although this may be due in part to temporal differences in activation of each cell type. Microglia typically activate earlier than astrocytes and slowly return to a quiescent state (Liberatore et al., 1999), which may explain why imaging studies following 2 weeks exposure to Mn detected stronger colocalization of both the NF-κB-EGFP reporter and NOS2 in astrocytes compared with microglia (Figs. 3 and 4) and why greater variability was noted in the expression of each neuroinflammatory marker in microglia. The SNpr was notably more susceptible than the ST to the neuroinflammatory effects of Mn at this stage of development, based upon reactivity data for both microglia and astrocytes. It will be critical in future studies to conduct a more careful analysis of the regional and kinetic differences in activation of microglia and astrocytes to better understand the role of each cell type in the progression of the neuroinflammatory lesion caused by excess Mn.
The functional effect of NOS2 induction on neuronal pathology was determined by evaluating levels of 3-nitrotyrosine (3-NTyr) protein adducts in basal ganglia neurons following Mn exposure. Increases in nitrosative stress seen in the SNpr reflect the known sensitivity of this brain region to Mn-induced glial activation, recently reviewed by Aschner et al. (2009). Although control males had low levels of 3-NTyr adducts (Fig. 5A), exposure to Mn (Fig. 5B) resulted in large increases in immunofluorescence staining for 3-NTyr in the SNpr, whereas exposure to Mn + E2 reduced 3-NTyr adduct levels in MAP2-positive neurons to that of control animals (Fig. 5C). These findings are consistent with our previous observations that Mn increases neuronal protein nitration in interneurons of the basal ganglia in developing mice (Liu et al., 2006; Moreno et al., 2009a) and with other studies demonstrating that E2 administration reduced glial activation and 3-NTyr adduct formation in dopaminergic neurons (Tripanichkul et al., 2007, 2006). These findings further support the assertion that E2 may prevent neurochemical and neurobehavioral alterations in Mn-treated juvenile mice at least in part by inhibiting neuroinflammatory activation of glia and subsequent nitrosative stress in neurons within the basal ganglia.
The data in Figures 6 and 7 also support regulation of NOS2 as an important mechanism of E2-mediated neuroprotection because E2 reduced Mn-induced expression of Nos2 mRNA in astrocytes in vitro and protected striatal neurons from apoptosis during coculture with Mn-activated astrocytes through an NF-κB-dependent pathway (Figs. 6 and 7). Inhibition of NF-κB in cultured astrocytes using dominant-negative IκBα protected striatal neurons from Mn-dependent apoptosis (Figs. 7K and 7L), paralleling the in vivo findings in NF-κB reporter mice that E2 supplementation decreased Mn-induced expression of EGFP and NOS2 in astrocytes, as well as neuronal protein nitration. Related studies found that both E2 and tamoxifen reduced Mn-induced cytotoxicity in neurons and astrocytes (Lee et al., 2009b). These findings suggest that E2 may regulate the transcriptional activity of NF-κB but further studies are required to determine the requirement for the estrogen receptor (ER) or other transcriptional regulatory proteins in E2-mediated suppression of NOS2 and other NF-κB-regulated neuroinflammatory genes. Interestingly, it was reported that ERα can recruit nuclear corepressor proteins that inhibit NF-κB-mediated expression of NOS2 by preventing p65 binding (Ghisletti et al., 2009). Collectively, these studies suggest a plausible mechanism by which E2 could suppress Mn-induced expression of NOS2 in microglia and astrocytes in vivo by promoting interactions between ERα/β and nuclear corepressors that prevent NF-κB-dependent transcriptional activation.
In conclusion, these data indicate that NF-κB activation occurs in vivo in astrocytes and microglia during Mn exposure and this activity coincides with cell-specific increases in expression of NOS2 and increases in neuronal protein nitration in the basal ganglia. The capacity of E2 to prevent these neuroinflammatory changes in vivo and to protect co-cultured neurons from astrocyte-mediated apoptosis in vitro has several implications for understanding the mechanism of Mn neurotoxicity in developing animals. First, the presence of E2 may confer a higher basal level of resistance to the neuroinflammatory effects of Mn and may at least partly explain the notable sensitivity of developing mice to Mn-induced neurological dysfunction and neuroinflammatory activation of glia (Kern et al., 2010; Moreno et al., 2009a, 2009b). Second, these data strongly implicate NF-κB activation in glial cells as a mediator of nitrosative stress in neurons during Mn exposure. Finally, the identification of E2 as a modulatory factor of glial inflammatory activation provides insight into mechanisms underlying sex-dependent vulnerability to Mn neurotoxicity in children and suggests that therapeutic interventions targeting these neuroinflammatory changes could be useful approaches for treating the effects of Mn exposure.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (R01 ES012941 to R.B.T.).
Supplementary Material
Acknowledgments
The authors also gratefully acknowledge the advice of Dr Robert Handa regarding supplementation with estrogen.
References
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit. Rev. Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Hernandez EH, Tjalkens R. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Roels HA. Neuropsychiatric symptoms and past manganese exposure in a ferro-alloy plant. Neurotoxicology. 2007;28:290–297. doi: 10.1016/j.neuro.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41:131–138. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Popichak KA, Moreno JA, Safe S, Tjalkens RB. Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nitric-oxide synthase 2 expression in astrocytes by a novel diindolylmethane analog protects striatal neurons against apoptosis. Mol. Pharmacol. 2009;75:35–43. doi: 10.1124/mol.108.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno-Muller E, Herrera AJ, de Pablos RM, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Thrombin induces in vivo degeneration of nigral dopaminergic neurones along with the activation of microglia. J. Neurochem. 2003;84:1201–1214. doi: 10.1046/j.1471-4159.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace Elem. Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- Cockell KA, Bonacci G, Belonje B. Manganese content of soy or rice beverages is high in comparison to infant formulas. J. Am. Coll. Nutr. 2004;23:124–130. doi: 10.1080/07315724.2004.10719352. [DOI] [PubMed] [Google Scholar]
- Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann. Nutr. Metab. 1983;27:488–494. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- D'Astous M, Morissette M, Tanguay B, Callier S, Di Paolo T. Dehydroepiandrosterone (DHEA) such as 17beta-estradiol prevents MPTP-induced dopamine depletion in mice. Synapse. 2003;47:10–14. doi: 10.1002/syn.10145. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Liu B. Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J. Neurochem. 1996;66:658–666. doi: 10.1046/j.1471-4159.1996.66020658.x. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Flurkey KCJ, Harrison DE. The mouse in aging research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. American College Laboratory Animal Medicine, Burlington, MA; 2007. pp. 637–672. [Google Scholar]
- Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger JL. Dietary standards for manganese: overlap between nutritional and toxicological studies. J. Nutr. 1998;128:368S–371S. doi: 10.1093/jn/128.2.368S. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ. Health Perspect. 2007;115:1107–1112. doi: 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Liu DH, Zhang GQ. [Effects of high-level-manganese sewage irrigation on children's neurobehavior] Zhonghua Yu Fang Yi Xue Za Zhi. 1994;28:216–218. [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Nitric oxide, glial cells and neuronal degeneration in parkinsonism. Trends Pharmacol. Sci. 2000;21:163–165. doi: 10.1016/s0165-6147(00)01471-1. [DOI] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop M, Rilling G, Herrmann A, Kretschmann HJ. Volumetric development of the fetal telencephalon, cerebral cortex, diencephalon, and rhombencephalon including the cerebellum in man. Bibl. Anat. 1986;28:53–78. [PubMed] [Google Scholar]
- Krachler M, Prohaska T, Koellensperger G, Rossipal E, Stingeder G. Concentrations of selected trace elements in human milk and in infant formulas determined by magnetic sector field inductively coupled plasma-mass spectrometry. Biol. Trace Elem. Res. 2000;76:97–112. doi: 10.1385/BTER:76:2:97. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Effects of organisational oestradiol on adult immunoreactive oestrogen receptors (alpha and beta) in the male mouse brain. J. Neuroendocrinol. 2007;19:767–772. doi: 10.1111/j.1365-2826.2007.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. J. Physiol. 2007;578:765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J. Neurochem. 2009a;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol. Sci. 2009b;110:156–167. doi: 10.1093/toxsci/kfp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox. Res. 2009;16:42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol. Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- Miller DB, Ali SF, O'Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann. N Y Acad. Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Morale MC, Serra PA, L'Episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS, et al. Estrogen, neuroinflammation and neuroprotection in Parkinson's disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol. Sci. 2009a;112:405–415. doi: 10.1093/toxsci/kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J. Neurosci. Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol. Sci. 2009b;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, Gillies GE. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann. N Y Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Parkes M. Hair element content in learning disabled children. Science. 1977;198:204–206. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS. Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology. 2003;77:223–231. doi: 10.1159/000070277. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, Rodriguez-Agudelo Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. Manganese augments nitric oxide synthesis in murine astrocytes: a new pathogenetic mechanism in manganism? Exp. Neurol. 1998;149:277–283. doi: 10.1006/exnr.1997.6666. [DOI] [PubMed] [Google Scholar]
- Tripanichkul W, Sripanichkulchai K, Duce JA, Finkelstein DI. 17Beta-estradiol reduces nitrotyrosine immunoreactivity and increases SOD1 and SOD2 immunoreactivity in nigral neurons in male mice following MPTP insult. Brain Res. 2007;1164:24–31. doi: 10.1016/j.brainres.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Tripanichkul W, Sripanichkulchai K, Finkelstein DI. Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol (Berl) 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Anantharam V. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol. Appl. Pharmacol. 2011 doi: 10.1016/j.taap.2010.03.023. Advance Access published on February 15, 2011; doi:10.1016/j.taap.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.