Abstract
Intradialytic hypotension and hypertension are both independently associated with mortality among persons with end-stage renal disease on hemodialysis. Endothelial dysfunction and arterial stiffness are two possible mechanisms underlying these phenomena, but their association with hemodynamic instability during dialysis has not been evaluated. Thirty patients were recruited from chronic dialysis units at San Francisco General Hospital and San Francisco Veterans Affairs Medical Center. Endothelial dysfunction was assessed with flow-mediated dilation of the brachial artery after upper arm occlusion. Arterial stiffness was assessed using carotid-femoral pulse wave velocity measured by tonometry. Intradialytic hypotension and hypertension were defined as the average decrease in systolic blood pressure (SBP) over 1 week, as well as the frequency over 1 month of hypotension or hypertension. Every 5% decrease in flow-mediated dilation was associated with a 7.5mmHg decrease in SBP after adjustment for phosphorus, body mass index, atherosclerosis, and ultrafiltration (P=0.02). Every 5 m/s increase in pulse wave velocity was associated with an 8mmHg increase in SBP after adjustment for predialysis SBP and ultrafiltration (P=0.03). Over 1 month, every 5% lower flow-mediated dilation was associated with a 10% higher frequency of hypotension (P=0.09), and every 5 m/s increase in pulse wave velocity was associated with an 15% higher frequency of hypertension (P=0.02). In a cross-sectional analysis of 30 dialysis patients, endothelial dysfunction and arterial stiffness were independently associated with intradialytic hypotension and intradialytic hypertension, respectively. Elucidating these potential mechanisms of hemodynamic instability during dialysis may facilitate development of treatment strategies specific to this pathophysiology.
Keywords: Endothelial dysfunction, arterial stiffness, intradialytic hypotension, intradialytic hypertension, phosphorus
INTRODUCTION
KDOQI guidelines defines intradialytic hypotension as a drop in systolic blood pressure (SBP) of at least 20mmHg or a decrease in mean arterial pressure (MAP) of 10mmHg associated with symptoms such as muscle cramping.1 It is a common clinical problem, occurring with a frequency of approximately 25%.2 Episodes of hypotension frequently limit the amount of fluid that can be removed during dialysis and predispose the patient to volume overload. Empiric treatments include decreasing ultrafiltration, lowering dialyzate temperature, increasing dialyzate calcium, and administering midodrine,3 a vasopressor agent, but little is known about the long-term effects of these maneuvers. Intradialytic hypotension is independently associated with increased mortality. In a cohort of 1244 hemodialysis patients, a fall in SBP of ≥40mmHg was associated with increased overall 2-year mortality. For subjects with predialysis SBP<139, a fall in SBP≥40mmHg was associated with a 60% increased relative risk of death.4 Of note, this study used blood pressure only, without considering symptoms; survival studies for KDOQI-defined intradialytic hypotension are lacking.
Intradialytic hypertension, an increase of blood pressure during dialysis despite fluid removal, is also common (prevalence of 15%).5 However, intradialytic hypertension is less well studied because it typically does not present with clinical symptoms or limit dialysis sessions. In a retrospective analysis of 438 hemodialysis patients, every 10mmHg increase in SBP during dialysis was associated with an adjusted 22% increased odds of hospitalization or death at 6 months.5
The physiological mechanisms underlying hemodynamic instability during dialysis are incompletely understood. Recent investigations have shown that myocardial stunning is associated with intradialytic hypotension, as cause or effect.6 Impaired baroreflex sensitivity,7 removal of asymmetric dimethylarginine (ADMA), a naturally occurring nitric oxide synthase inhibitor,8 and inadequate vasopressin response9 are additional potential mechanisms. Putative mechanisms for intradialytic hypertension include volume overload, overactivity of sympathetic or renin–angiotensin systems and removal of antihypertensive medications during dialysis.10
Subjects with end-stage renal disease (ESRD) often have severe endothelial dysfunction and arterial stiffness, and these abnormalities are both independently associated with mortality.11–14 We hypothesized that both endothelial dysfunction and arterial stiffness would be associated with hemodynamic instability during dialysis.
METHODS
Subjects
We recruited patients from the San Francisco Veterans Affairs Medical Center (SFVAMC) and San Francisco General Hospital chronic dialysis units. To be included, patients had to be on dialysis. Subjects with the following criteria were excluded: current treatment for infection, major surgery within 1 month, newly diagnosed or metastatic cancer, current cocaine, intravenous drug use or chemotherapy, major debility or a language barrier limiting ability to give informed consent or to cooperate with the vascular tests. Four subjects could not have flow-mediated dilation (FMD), measured due to scarring of bilateral brachial arteries from previous access attempts. Four could not have pulse wave velocity (PWV) measured due to cardiac arrhythmia or obesity. Subjects gave written informed consent, and our protocol was approved by the UCSF Committee for Human Research and the SFVAMC Research and Development Committee.
Predictors
Brachial vasoreactivity was measured immediately before dialysis, before the second or third dialysis session of the week, as described previously.15–17 Subjects fasted and refrained from tobacco use for 12 hours before the test. An ultrasound image of the brachial artery was recorded before inflating a blood pressure cuff around the upper arm to 50mmHg above the patient’s SBP for 5 minutes. One minute after release of the blood pressure cuff, the brachial artery was imaged again to assess FMD. Pre-and posthyperemic brachial artery diameters were measured using Medical Imaging Applications Brachial Analyzer 5.6.12 (Coralville, IA, USA). Flow-mediated dilation was calculated as (hyperemia diameter − baseline diameter)/baseline diameter × 100%.
Pulse wave velocity was measured on a nondialysis day after the second or third dialysis session of the week using the Sphygmacor device (AtCor Medical, Itasca, IL, USA). Carotid to femoral PWV was measured by applanation tonometry. Distance was measured by adding the distances between carotid pulse, sternal notch, umbilicus, and femoral pulse. Pulse waves were analyzed at carotid and femoral sites, and velocity was calculated using Sphygmacor software (2000 version 7.1, AtCor Medical, Itasca, IL, USA). Pulse wave velocity was performed at least twice, and the measurement with the lowest standard deviation was used in the analysis. Mean and SD for the difference between blood pressure measured on the day of PWV and the average 1 month predialysis SBP were 7mmHg (± 20).
Outcomes
All patients underwent either 3 or 3.5 hours dialysis at standard temperature (37 °C), with the majority using 2.5 calcium dialyzate (2 had 3 mg calcium, 1 had 3.5 mg calcium). No subjects were prescribed midodrine. Intradialytic hypotension was calculated as the average difference between initial sitting SBP and lowest SBP during dialysis, averaged over the 3 dialysis sessions of the week of the vascular measurement. Intradialytic hypertension was calculated as the average difference between initial sitting SBP and highest SBP during dialysis, averaged over the 3 dialysis sessions of the week of the vascular measurement. Additional analyses were performed with hypotension defined as % of sessions over 1 month with drop in SBP≥ 20mmHg and hypertension defined as % of sessions over 1 month with increase in SBP≥ 20 mmHg.
Covariates
Age, gender, months on hemodialysis, history of atherosclerosis (myocardial infarction [MI], cerebral vascular accident [CVA], or peripheral artery disease [PAD]), history of diabetes, tobacco use, use of ACE-inhibitor, use of beta blocker, ultrafiltration, predialysis SBP, body mass index (BMI), single pool Kt/V, hemoglobin, calcium × phosphorus, PTH, and albumin were examined as potential covariates. Routine monthly dialysis laboratory tests were used in this analysis. Therefore, no more than 1 month elapsed between the labs and the study measures.
Statistical analysis
We tested univariate associations of FMD, PWV, and potential covariates with the outcomes intradialytic hypotension and hypertension. Multivariate analysis was performed for covariates that had associations with the outcomes with P values<0.2, with the following exceptions. Because of the likelihood that the association of age with hypertension may be mediated by arterial stiffness, we chose not to include these in the final model for PWV. Despite the fact that ultrafiltration was not associated with change in blood pressure during dialysis on univariate screen, we decided to adjust for it given the common clinical assumption that changes in blood pressure are related to ultrafiltration. Analyses were performed with STATA version 11 (StataCorp, College Station, TX, USA).
RESULTS
Participants’ characteristics
Baseline demographics are presented in Table 1. The mean age was 63 years ( ± 14), median time on hemodialysis 49 months (13, 66). Women comprised 17%, African Americans 43%, and Whites 23% of the cohort. Diabetes affected 60% and atherosclerotic disease (MI, CVA, or PAD) affected 47%. Flow-mediated dilation was feasible in 26 subjects (median, [IQR] 4.7% [0.85, 10.3]). Pulse wave velocity was feasible in 26 subjects (median, [IQR] 10.3 m/s [8.5, 13]). About 1/3 of subjects had an average drop in SBP > 40mmHg during dialysis, and another 1/3 had an average increase in SBP > 20mmHg during dialysis.
Table 1.
Patient characteristics
| Total N=30 | Mean (SD), N (%), | Median (IQ) |
|---|---|---|
| Age (y) | 63 ( ± 14) | |
| Women | 5 (17%) | |
| Ethnicity | White | 7 (23%) |
| African American | 13 (43%) | |
| Hispanic | 3 (10%) | |
| Philippino | 3 (10%) | |
| Other | 4 (13%) | |
| Months on hemodialysis | 49 (13, 66) | |
| Ultrafiltration (mL/dialysis session) | 1950 ( ± 1300) | |
| Predialysis SBP (mmHg) | 144 ( ± 15) | |
| Vascular access | 19 (65%) | |
| Kt/V | 1.5 ( ± 0.35) | |
| BMI (kg/m2) | 26 ( ± 6) | |
| Hx of atherosclerosis | 14 (47%) | |
| Hx of diabetes mellitus | 15 (60%) | |
| Hx of tobacco, past or present | 17 (57%) | |
| Etiology of ESRD=DM or HTN | 21 (70%) | |
| ACE-I | 15 (50%) | |
| Beta blocker | 23 (80%) | |
| Statin | 15 (50%) | |
| Ca × phos | 48 ( ± 14) | |
| FMD, % (N=26) | 4.7 (0.85, 10.3) | |
| PWV, m/s (N=26) | 10.3 (8.5, 13) | |
| % with decrease in BP > 20mmHg | 19 (63%) | |
| % with decrease in BP > 40mmHg | 9 (30%) | |
| % with increase in BP > 20mmHg | 9 (30%) |
ACE-I=ACE-inhibitor; BMI=body mass index; DM=diabetes; ESRD= end-stage renal disease; FMD=flow-mediated dilation; HTN=hypertension; Hx= history; IQ=interquartile; Ml=milliliter; PWV=pulse wave velocity; SD=standard deviation.
Univariate associations
On univariate analysis, the presence of diabetes was associated with impaired FMD and elevated PWV; diabetics had 4% lower FMD than nondiabetics (P=0.06), and 2.5m/s higher PWV than nondiabetics (P=0.03). Increased age was associated with higher PWV, with every ten years older associated with a 1m/s increase in PWV (P=0.03). There was a trend for atherosclerosis (history of MI, CVA, or PAD) to be associated with higher PWV and lower FMD, although these did not reach significance. Time on dialysis, gender, tobacco use, predialysis SBP, Kt/V, hemoglobin, calcium × phosphorus, and PTH were not associated with either vascular measure (Table 2).
Table 2.
Univariate associations of vascular dysfunction
| Total N=30 | FMD (%)
|
N=26
|
PWV (m/s)
|
N=26
|
|---|---|---|---|---|
| β co-efficient | P value | β co-efficient | P value | |
| Age (y) | −0.08 | 0.26 | 0.1 | 0.03 |
| Months on dialysis | −0.01 | 0.63 | −0.02 | 0.15 |
| History of atherosclerosisa | −2.3 | 0.27 | 1.7 | 0.12 |
| History of diabetes | −4.2 | 0.06 | 2.5 | 0.03 |
| ACE-I | −1.0 | 0.6 | 0.4 | 0.7 |
| Beta blocker | −1.1 | 0.7 | −0.6 | 0.7 |
| Statin | 1.8 | 0.4 | 2.5 | 0.03 |
| Ultrafiltration (mL) | −0.0005 | 0.6 | 0.0006 | 0.2 |
| Predialysis SBP (mmHg) | −0.04 | 0.4 | −0.004 | 1.0 |
| Calcium (mg/dL) | −0.47 | 0.8 | −0.1 | 0.9 |
| Phosphorus (mg/dL) | −0.09 | 1.0 | −0.4 | 0.3 |
| Ca × phos | −0.01 | 0.9 | −0.04 | 0.3 |
| PTH | 0.0004 | 0.9 | −0.002 | 0.3 |
| Albumin | 4.8 | 0.1 | −2.0 | 0.2 |
| FMD (%) | — | — | −0.27 | 0.03 |
| PWV (m/s) | −0.77 | 0.03 | — | — |
Atherosclerosis=history of myocardial infarction, cerebrovascular accident, or peripheral artery disease.
ACE-I=ace-inhibitor; FMD=flow-mediated dilation; PTH=parathyroid hormone; PWV=pulse wave velocity; SBP=systolic blood pressure.
Before adjustment, endothelial dysfunction was significantly associated with intradialytic hypotension, (P= 0.04), but not with hypertension. Every 1% decrease in FMD was associated with a 1.3 mmHg decrease in SBP during dialysis. Every 1 mg/dL higher phosphorus was associated with a 6mmHg decrease in blood pressure, averaged over 1 week of dialysis (P=0.006) and a 9% increased frequency of hypotension over 1 month (P=0.003). Increased arterial stiffness was associated with intradialytic hypertension, (P=0.04), but not with hypotension. Every 1 m/s increase in PWV was associated with a 1.5 mmHg increase in SBP during dialysis. Increased age was significantly associated with intradialytic hypertension (P=0.03). Time on dialysis, gender, tobacco use, use of ACE-inhibitors or beta-blocker, Kt/V, hemoglobin, and PTH were not associated with either hypotension or hypertension (Table 3).
Table 3.
Univariate associations of hemodynamic instability assessed over 1 wk
| Total N=30 | Decrease in SBP (mmHg)
|
Increase in SBP (mmHg)
|
||
|---|---|---|---|---|
| β coefficient | P value | β coefficient | P value | |
| Age (y) | −0.1 | 0.7 | 0.34 | 0.03 |
| Months on dialysis | −0.03 | 0.7 | −0.01 | 0.8 |
| BMI | 0.8 | 0.15 | −0.04 | 0.9 |
| History of atherosclerosisa | −10 | 0.15 | 2.8 | 0.5 |
| History of diabetes | −1.0 | 0.9 | 7.2 | 0.1 |
| ACE-I | −3.0 | 0.6 | 1.7 | 0.7 |
| Beta blocker | 6.0 | 0.4 | 0.5 | 0.9 |
| statin | 4.9 | 0.5 | 6 | 0.15 |
| Ultrafiltration (mL) | −0.002 | 0.5 | −0.001 | 0.5 |
| Pre-dialysis SBP (mmHg) | −0.11 | 0.5 | −0.3 | 0.07 |
| Calcium mg/dL | −8.1 | 0.19 | 1.5 | 0.7 |
| Phosphorus mg/dL | −6.0 | 0.006 | −1.4 | 0.3 |
| Ca × phos | −0.73 | 0.002 | −0.13 | 0.4 |
| PTH | −0.009 | 0.4 | 0.007 | 0.3 |
| Albumin | −6.8 | 0.5 | −4.0 | 0.5 |
| FMD (%) | 1.3 | 0.04 | 0.07 | 0.9 |
| PWV (m/s) | 2.5 | 0.3 | 1.5 | 0.04 |
Atherosclerosis=history of myocardial infarction, cerebrovascular accident, or peripheral artery disease
ACE-I=ace-inhibitor; BMI=body mass index; FMD=flow-mediated dilation; PTH=parathyroid hormone; PWV=pulse wave velocity;
SBP=systolic blood pressure.
Multivariate analysis
The association of FMD with intradialytic hypotension over 1 week was not significantly changed after adjustment for phosphorus, BMI, atherosclerosis, and ultrafiltration (β=1.5, P=0.02). After adjustment, every 5% decrease in FMD was associated with a 7.5 mmHg decrease in SBP (Figure 1). Adjustment for ultrafiltration and predialysis SBP did not significantly change the association of PWV with intradialytic hypertension over 1 week (β=1.6, P=0.03). Every 5 m/s increase in PWV was associated with an 8mmHg increase in SBP after adjustment for predialysis SBP and ultrafiltration. (Figure 2) These results were not significantly different when the three patients on higher calcium dialysis prescriptions were excluded from the analysis.
Figure 1.
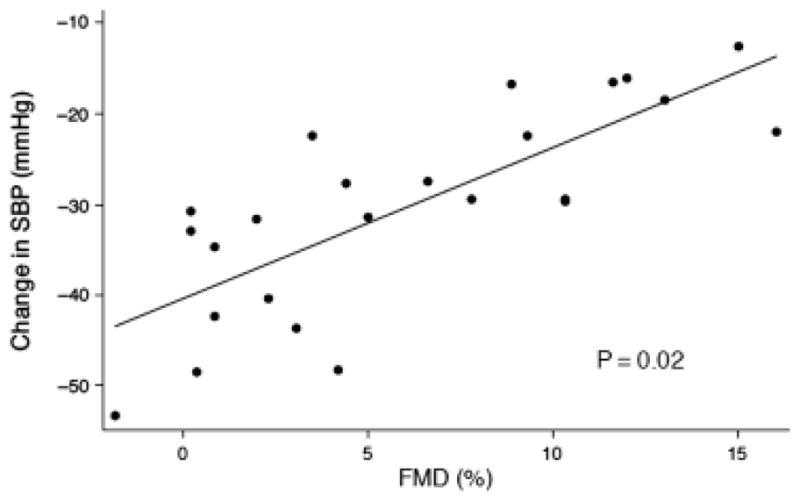
Association of flow-mediated dilation (FMD) with intradialytic hypotension adjusted for ultrafiltration, phosphorus, body mass index, and atherosclerosis.
Figure 2.
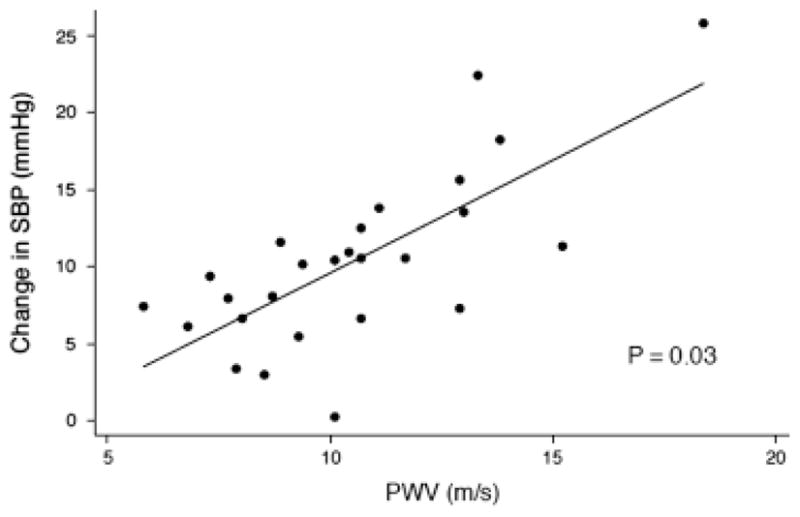
Association of pulse wave velocity (PWV) with intradialytic hypertension adjusted for predialysis systolic blood pressure and ultrafiltration.
Alternate analysis
Because the possibility that FMD and PWV may change over time, the primary outcome variable was defined as the average increase or decrease in SBP during 3 dialysis sessions during the week of the vascular test. To analyze frequency (rather than average) of hypotension or hypertension, we considered an alternative definition as % of dialysis sessions over 1 month (rather than 1 week) with drop in SBP≥20mmHg or increase in SBP≥20 mmHg. Every 5% lower FMD was associated with 10% higher frequency of hypotension, with a trend toward significance (univariate P=0.1; multivariate adjusted for ultrafiltration, predialysis SBP, phosphorus and albumin P=0.09). The association of PWV remained highly significant: every 5 m/s increase in PWV was associated with a 15% higher frequency of hypertension (univariate P=0.01, multivariate adjusted for ultrafiltration, P=0.02).
DISCUSSION
The novel findings of our study are: (1) endothelial dysfunction, as measured by flow-mediated, endothelium-dependent vasodilation, was associated with intradialytic hypotension, and (2) arterial stiffness, as measured by carotid-femoral artery PWV, was associated with intradialytic hypertension. These associations were independent of ultrafiltration or predialysis SBP. Although causality cannot be proved by our cross-sectional study, these results suggest that intradialytic hypotension and hypertension may be governed by distinct vascular pathologies.
Higher PWV or lower FMD independently predict cardiovascular mortality in the dialysis population.12–14 Higher PWV is associated with traditional factors (in particular, age, diabetes, high blood pressure) as well as risk factors related to uremia (volume overload, abnormal calcium, and phosphate metabolism).18 The endothelial dysfunction of patients with CKD is associated with increased age, higher blood pressure, and inflammation,13 and may be related to higher levels of ADMA, an inhibitor of endothelial nitric oxide synthase that is elevated in subjects with reduced kidney function.19 In concordance with prior studies, our subjects had low FMD and high PWV. The median FMD in this cohort was 4.7%, compared with typical upper arm FMD > 10% for healthy subjects without kidney disease reported from the laboratory of one of the authors.16,17 Median PWV was 10.3 m/s, compared with 9.3 m/s reported for controls of similar age.20 The associations of increased age with higher PWV, diabetes with both PWV and FMD, and the high correlation between PWV and FMD in our cohort were also in agreement with prior studies.
The novel finding that lower FMD is associated with intradialytic hypotension may be explained by the effect of dialysis on several serum mediators of endothelial function. ADMA is an inhibitor of endothelial nitric oxide synthase that is elevated in dialysis patients.21,22 Several groups have found that ADMA levels decrease during dialysis,23–25 which could reverse endothelial dysfunction leading to vasodilation and hypotension, particularly in those with the highest levels of ADMA and the worst endothelial function at the start of the dialysis session. Endothelin-1 (ET-1), a vasoconstrictor, produced by the endothelium, is another candidate mediator of the association between endothelial dysfunction and intradialytic hypotension. Increased ET-1 activity tonically suppresses endothelium-dependent dilation. ET-1 is increased in dialysis patients, and intradialytic hypotension is associated with a decrease in ET-1 during dialysis.26 It is also possible that subjects with impaired endothelial function have dysregulation of coronary blood flow, limiting the ability of the myocardium to increase cardiac output during hypotensive episodes during dialysis and leading to ischemia and stunning as previously described.6 As for the association between arterial stiffness and intradialytic hypertension, an interaction with sympathetic activity is a possible mechanism. It is plausible that fluid removal causes sympathetic activation, and in those with severe arterial stiffness, this produces an exaggerated pressor response.
Our results highlight a potential role for peripheral vascular disease in the cardiovascular mortality of subjects with ESRD. Low FMD and intradialytic hypotension are known risk factors for cardiovascular mortality in this population,4,13 but whether brachial FMD is simply a surrogate for coronary endothelial dysfunction, and whether intradialytic hypotension is mainly cardiac in origin, is unknown. While our study does not answer these questions, it suggests a possible mechanistic pathway whereby peripheral vascular dysfunction contributes to intradialytic hemodynamic instability, which in turn causes chronic cardiac ischemia and fibrosis, predisposing to cardiac arrhythmias and death. Alternatively, chronic hemodynamic instability may worsen vascular dysfunction. The direction of this association should be clarified by longitudinal studies.
We found a strong association between higher serum phosphate and intradialytic hypotension. This phenomenon has been observed previously by Tisler and colleagues in a cohort of 958 dialysis patients. Compared with patients with occasional hypotension, those with frequent hypotension had higher phosphorus (1.99 vs. 1.79 mmol, P<0.005).27 Whether this represents patient noncompliance (leading to higher intradialytic weight gain and hypotension when this extra fluid is removed), an effect of phosphorus-related medications, or represents a real cardiovascular effect of serum phosphate levels is unknown. In our cohort, this association became stronger and remained significant (10mmHg drop in SBP per mg/dL higher phosphorus, P=0.014) even when the 9 subjects with phosphorus > 6 mg/dL were dropped from the analysis. Moreover, ultrafiltration was not associated with hypotension in our cohort. Therefore, we doubt that noncompliance is the driving force behind this association. Our study was not structured to study possible effects of type or dose of phosphorus binders and calcimimetics. Recent studies suggest that binders and calcimetics may improve vascular structure and function, 28,29 which could explain our findings. We feel it is possible that phosphorus itself has a detrimental vascular effect. While phosphorus has been shown to predict arterial stiffness in dialysis patients,30,31 this was not true in our cohort. However, subjects with higher phosphate showed a trend for worse endothelial dysfunction: after adjustment for age and diabetes, every 1 mg/dL increase in phosphorus was associated with 1.7% lower FMD (P=0.054). In animals and in healthy human subjects, phosphorus loading has been shown to induce endothelial dysfunction,32 but to our knowledge a potential association between serum phosphate levels and endothelial function has not been studied in dialysis patients. Larger studies with adequate power to measure associations of phosphorus, PTH, and doses of binders and calcimimetics with vascular dysfunction are warranted.
In designing this study, we considered what effect the dialysis procedure may have on endothelial function and arterial stiffness. Studies are inconsistent in this area. An improvement in endothelial function after hemodialysis, possibly due to removal of ADMA,33 is plausible; however, in other studies hemodialysis had a detrimental34,35 or negligible effect36,37 on endothelial function. Likewise, it is plausible that fluid removal during dialysis results in temporary improvement in arterial stiffness, as shown by Di Iorio et al.,38 although other studies show worsening of arterial stiffness with dialysis.18 We assumed that dialysis would have an effect on vascular function testing. Therefore, we standardized our measurements in relation to the dialysis procedure. In order to avoid the volume overload associated with the first dialysis session of the week, we measured endothelial function directly before the second or third dialysis session of the week, and arterial stiffness on the day two following the second or third session. All FMD’s and all PWV’s were always performed at the same time in relation to dialysis for consistency of within-group comparisons. While the timing of the measures in relation to dialysis is unlikely to affect the cross-sectional associations we report, it may affect the comparison of our cohort with other dialysis patients with respect to values obtained for FMD and PWV measurements.
Another challenge in the design of this type of study is the lack of evidence-based standards for assessing intradialytic hypotension and hypertension. KDOQI guidelines define intradialytic hypotension as drop in SBP of at least 20mmHg or a decrease in MAP of 10mmHg associated with symptoms such as muscle cramping,39 while the main outcome study reporting outcomes related to intradialytic hypotension used blood pressure only in the definition.4 Symptomatic hypotension occurs in about 20–30% of dialysis sessions.2 In our cohort the rate of asymptomatic hypotension was higher, as would be expected: 63% had a drop of SBP > 20mmHg. The average drop in SBP of our cohort was 31mmHg ( ± 18), which is comparable to the average decrease of 28mmHg ( ± 23) in the cohort of 1244 patients studied by Shoji et al.4 On the basis of this limited comparison, our cohort likely has a typical rate of asymptomatic hypotension.
In assessing the generalizability of our results one must consider demographic characteristics that are pertinent to vascular dysfunction. With respect to age and diabetes status, our cohort is fairly representative of the United States dialysis population. In fact, the high percentage of diabetes as a cause of ESRD is typical for the United States, Taiwan, Japan, and Mexico, where diabetes accounts for > 40% of incident cases of ESRD.40,41 In addition, our group was 43% African American, compared with the United States dialysis population, which is 33% African American.40 In non-ESRD populations, Black race is associated with higher PWV42–44 and lower FMD;45 and therefore it is possible that vascular dysfunction was more prevalent in our cohort than in comparable studies. The most notable difference between our cohort and other dialysis populations was the predominance of male gender. In the Framingham cohort (with no ESRD subjects), male gender is associated with lower % FMD.46 Two studies have shown that gender is not a significant correlate of PWV.47,48 Thus, it is difficult to surmise how the predominance of male gender affected our results. Overall, while our sample size is modest and drawn from a Veteran population primarily of male gender, we think it is likely that our results would generalize fairly well to other dialysis patients. In addition, although the composition of this cohort could affect the FMD and PWV results, we have no reason to think that the observed associations of FMD and PWV with hemodynamic changes during dialysis would not be generalizable.
Additional limitations of this study that merit consideration include the possibility of type 2 error given the modest number of subjects. Initial power calculations for this pilot study were based on PWV because it has lower variability and higher expected effect size than FMD, which may account for the lower P values obtained for FMD associations. In addition, this study was cross sectional and therefore does not provide evidence of causality. Nevertheless, despite these limitations, we have found significant and independent associations between endothelial dysfunction and intradialytic hypotension, and between arterial stiffness and intradialytic hypertension. Further studies to elucidate these potential mechanisms of intradialytic hypotension and hypertension are on going.
Footnotes
Support and Financial Disclosure Declaration: This research is funded through the Clinical and Translational Science Institute by the Resource Allocation Program of the University of California, San Francisco. The authors have no conflicts of interest to declare.
References
- 1.KDOQI. NKF KDOQI Guidelines. KDOQI; 2002. Available at http://www.kidney.org/professionals/kdoqi/guidelines_cvd/intradialytic.htm. [Google Scholar]
- 2.Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol. 2008;19:8–11. doi: 10.1681/ASN.2007091006. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF. Can chronic volume overload be recognized and prevented in hemodialysis patients? Preventing intradialytic hypotension. Semin Dial. 2009;22:489–491. doi: 10.1111/j.1525-139X.2009.00643.x. [DOI] [PubMed] [Google Scholar]
- 4.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 5.Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55:580–589. doi: 10.1053/j.ajkd.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW. Categorization of the hemodynamic response to hemodialysis: The importance of baroreflex sensitivity. Hemodial Int. 2010;14:18–28. doi: 10.1111/j.1542-4758.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 8.Csiky B, Sulyok E, Lakatos O, Wittmann I, Martens-Lobenhoffer J, Bode-Boger SM. Response of asymmetric dimethylarginine to hemodialysis-associated hypotension in end-stage renal disease patients. Nephron Clin Pract. 2008;108:c127–34. doi: 10.1159/000114451. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AM, Oliver JA. Endogenous and exogenous vasopressin during hemodialysis. Semin Dial. 2009;22:472–475. doi: 10.1111/j.1525-139X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 10.Chazot C, Jean G. Intradialytic hypertension: It is time to act. Nephron Clin Pract. 2010;115:c182–c188. doi: 10.1159/000313031. [DOI] [PubMed] [Google Scholar]
- 11.Ghiadoni L, Cupisti A, Huang Y, et al. Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol. 2004;17:512–519. [PubMed] [Google Scholar]
- 12.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 13.London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65:700–704. doi: 10.1111/j.1523-1755.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 14.van Guldener C, Lambert J, Janssen MJ, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation and distensibility of large arteries in chronic haemodialysis patients. Nephrol Dial Transplant. 1997;12(Suppl 2):14–18. [PubMed] [Google Scholar]
- 15.Kinlay S, Behrendt D, Fang JC, et al. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol. 2004;43:629–634. doi: 10.1016/j.jacc.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman EH, Gerhard MD, Uehata A, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients<40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 17.Uehata A, Lieberman EH, Gerhard MD, et al. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vasc Med. 1997;2:87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 18.Covic A, Gusbeth-Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis. 2005;45:965–977. doi: 10.1053/j.ajkd.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 19.MacAllister RJ, Rambausek MH, Vallance P, Williams D, Hoffmann KH, Ritz E. Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure. Nephrol Dial Transplant. 1996;11:2449–2452. doi: 10.1093/oxfordjournals.ndt.a027213. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann Clin Biochem. 2010;47(Part 1):17–28. doi: 10.1258/acb.2009.009196. [DOI] [PubMed] [Google Scholar]
- 22.Mallamaci F, Zoccali C. Clinical implications of elevated asymmetric dimethylarginine in chronic kidney disease and end-stage renal disease. J Ren Nutr. 2009;19:25–28. doi: 10.1053/j.jrn.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Errakonda PR, Paladugu R, Bitla AR, et al. Effect of a single hemodialysis session on endothelial dysfunction. J Nephrol. 2011;24:83–90. doi: 10.5301/jn.2010.4926. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DL, Liu J, Liu S, Zhang Y, Liu WH. The differences of asymmetric dimethylarginine removal by different dialysis treatments. Ren Fail. 2010;32:935–940. doi: 10.3109/0886022X.2010.502281. [DOI] [PubMed] [Google Scholar]
- 25.Eiselt J, Rajdl D, Racek J, Siroka R, Trefil L, Opatrna S. Asymmetric dimethylarginine in hemodialysis, hemodiafiltration, and peritoneal dialysis. Artif Organs. 2010;34:420–425. doi: 10.1111/j.1525-1594.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 26.El-Shafey EM, El-Nagar GF, Selim MF, El-Sorogy HA, Sabry AA. Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis? Clin Exp Nephrol. 2008;12:370–375. doi: 10.1007/s10157-008-0065-2. [DOI] [PubMed] [Google Scholar]
- 27.Tisler A, Akocsi K, Harshegyi I, et al. Comparison of dialysis and clinical characteristics of patients with frequent and occasional hemodialysis-associated hypotension. Kidney Blood Press Res. 2002;25:97–102. doi: 10.1159/000063515. [DOI] [PubMed] [Google Scholar]
- 28.Marangon N, Lindholm B, Stenvinkel P. Nonphosphate-binding effects of sevelamer–are they of clinical relevance? Semin Dial. 2008;21:385–389. doi: 10.1111/j.1525-139X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 29.de Francisco AL, Pinera C, Palomar R, Arias M. Impact of treatment with calcimimetics on hyperparathyroidism and vascular mineralization. J Am Soc Nephrol. 2006;17(Suppl 3):S281–S285. doi: 10.1681/ASN.2006080927. [DOI] [PubMed] [Google Scholar]
- 30.Mitsnefes MM, Kimball TR, Kartal J, et al. Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: role of calcium-phosphorus metabolism. J Am Soc Nephrol. 2005;16:2796–2803. doi: 10.1681/ASN.2005030291. [DOI] [PubMed] [Google Scholar]
- 31.Level C, Lasseur C, Delmas Y, et al. Determinants of arterial compliance in patients treated by hemodialysis. Clin Nephrol. 2001;56:435–444. [PubMed] [Google Scholar]
- 32.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, MacAllister RJ. Dialysis improves endothelial function in humans. Nephrol Dial Transplant. 2001;16:1823–1829. doi: 10.1093/ndt/16.9.1823. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki H, Matsuoka H, Itabe H, et al. Hemodialysis impairs endothelial function via oxidative stress: effects of vitamin E-coated dialyzer. Circulation. 2000;101:1002–1006. doi: 10.1161/01.cir.101.9.1002. [DOI] [PubMed] [Google Scholar]
- 35.Lilien MR, Koomans HA, Schroder CH. Hemodialysis acutely impairs endothelial function in children. Pediatr Nephrol. 2005;20:200–204. doi: 10.1007/s00467-004-1718-3. [DOI] [PubMed] [Google Scholar]
- 36.Migliacci R, Falcinelli F, Imperiali P, Floridi A, Nenci GG, Gresele P. Endothelial dysfunction in patients with kidney failure and vascular risk factors: acute effects of hemodialysis. Ital Heart J. 2004;5:371–377. [PubMed] [Google Scholar]
- 37.Kosch M, Levers A, Barenbrock M, et al. Acute effects of haemodialysis on endothelial function and large artery elasticity. Nephrol Dial Transplant. 2001;16:1663–1668. doi: 10.1093/ndt/16.8.1663. [DOI] [PubMed] [Google Scholar]
- 38.Di Iorio B, Nazzaro P, Cucciniello E, Bellizzi V. Influence of haemodialysis on variability of pulse wave velocity in chronic haemodialysis patients. Nephrol Dial Transplant. 2010;25:1579–1583. doi: 10.1093/ndt/gfp662. [DOI] [PubMed] [Google Scholar]
- 39.K/DOQI. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;(Suppl 3):S1–S153. [PubMed] [Google Scholar]
- 40.USRDS . Incidence and Prevalence. Chapter 2. Minneapolis, MN, USA: USRDS; 2010. [Google Scholar]
- 41.USRDS. International Comparisons. Chapter 12. Minneapolis, MN, USA: USRDS; 2010. [Google Scholar]
- 42.Urbina EM, Srinivasan SR, Kieltyka RL, et al. Correlates of carotid artery stiffness in young adults: The Bogalusa Heart Study. Atherosclerosis. 2004;176:157–164. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: Evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004;17:304–313. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi N, Bulpitt CJ, Leggetter S, et al. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J Hypertens. 2004;22:1731–1737. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 47.Filipovsky J, Ticha M, Cifkova R, Lanska V, Stastna V, Roucka P. Large artery stiffness and pulse wave reflection: results of a population-based study. Blood Press. 2005;14:45–52. doi: 10.1080/08037050510008814. [DOI] [PubMed] [Google Scholar]
- 48.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51:1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]


