Abstract
Background
Stress has paradoxical effects on pain, causing stress-induced analgesia, but also exacerbating pain via poorly understood mechanisms. Adrenergic neurotransmission is integral in pathways that regulate the response to both pain and stress. Hyperalgesia is often associated with enhanced adrenergic sensitivity of primary afferents, but sympathetic nervous system outflow has not been demonstrated to exacerbate pain perception following stress.
Methods
Rats or C57/BL6 wild type mice treated with α-2 receptor antagonists or α-2A receptor knockout mice were exposed to ultrasonic noise stress or footshock stress and subsequently tested for hotplate paw withdrawal latencies. The sensory sensitivity of α-2A knockout mice to electrical and chemical stimuli was tested neurophysiologically and behaviorally. The effects of sympatholytic treatments were investigated.
Results
Noise and footshock stressors induced thermal hyperalgesia in rats pretreated systemically with α-2 antagonists. Wild type mice pretreated with α-2 antagonists and α-2A knockout mice also exhibited noise stress-induced thermal hyperalgesia. Local spinal or intraplantar injection of an α-2 antagonist counteracted stress-induced analgesia without causing hyperalgesia. α-2A knockout mice had decreased thresholds for peripheral sensitization with sulprostone and for windup of the dorsal horn neuronal response to repetitive electrical stimuli. Stress-induced hyperalgesia was abolished and the sensitization was attenuated by sympathectomy or systemic administration of an α-1-adrenergic antagonist.
Conclusions
Sympathetic postganglionic nerves can enhance pain sensation via a peripheral α-1-adrenoceptor mechanism when sympathetic outflow is disinhibited. The net effect of stress on pain sensation reflects a balance between descending spinal inhibition and sympathetic outflow that can shift towards pain facilitation when central and peripheral α-2-adrenoceptor inhibitory mechanisms are attenuated.
Introduction
Adrenergic neurotransmission is an integral part of the pain and stress response pathways. Studies have demonstrated that hyperalgesia is often associated with enhanced adrenergic sensitivity of primary afferents. Sensory nerves in animals become sensitive to sympathetic nerve stimulation or exogenous addition of the sympathetic adrenergic neurotransmitter, norepinephrine, following nerve injury or in the presence of inflammatory mediators.1–5 Potential mechanisms for the increased primary afferent adrenergic sensitivity include upregulation of α-2-adrenergic receptors6 and exacerbation of neurogenic inflammation by α-1-adrenergic receptors.7 Some of these mechanisms may occur in posttraumatic neuralgias, such as causalgia, which are sometimes diagnosed or treated with α-adrenergic blockade.8,9
Adrenergic mechanisms are also involved in the various ways that stress modulates the perception of pain. A classic effect of a variety of acute stressors in humans and in animal models is stress-induced analgesia, which is mediated by descending spinal pathways.10,11 Both opioid and nonopioid mechanisms, which differ with respect to naloxone sensitivity and cross-tolerance with morphine, have been described. Nonopioid descending inhibition of dorsal horn nociceptive transmission is in part mediated by α-2-adrenergic receptors.12 Conversely, pain disorders such as fibromyalgia, irritable bowel syndrome, and migraine headaches can be triggered or exacerbated by stress, particularly chronic stress, in ways that are not well understood. It was recently shown that chronic stress in rats results in increased primary afferent sensitivity to the sympathoadrenal adrenergic molecule, epinephrine, as a result of changes in β-2-adrenergic receptor signaling.13
The physiological response to stress is also mediated through sympathoneural outflow of norepinephrine. The sympathetic nervous system (SNS) is not thought to activate primary afferents in the absence of injury. Clinical studies in normal subjects have demonstrated that stimulation of sympathetic activity by physiological stimuli9 does not cause pain or increase the response of nociceptive sensory neurons to a painful stimulus. This is likely because norepinephrine release is under tight control by α-2A- and α-2C-adrenergic receptors. These α-2 receptors are located on postsynaptic sympathetic nerve endings,14 where they mediate feedback inhibition of norepinephrine release on peripheral terminals of sensory neurons.15 α-2 Receptors are also located in the spinal cord dorsal horn16 and in brain regions associated with pain modulation including the hypothalamus and amygdala.17–19
The α-2A knockout mice have been described previously with elevated SNS outflow during high frequency stimulation, due to the loss of feedback autoinhibition.14 Despite the absence of functional α-2A adrenoceptors in pathways that modulate pain transmission in mice expressing the D79N α-2A receptor mutation, the mice have normal sensitivity to acute noxious thermal stimuli in a temperature ramp hotplate test20 and in tail immersion tests.21,22 On the other hand, α-2A knockout mice have enhanced referred pain following capsaicin treatment,23 providing evidence of a role of α-2A-adrenergic receptors in attenuating the neuronal response to a sustained noxious stimulus.
Since SNS outflow is stimulated by stress, we hypothesized that loss of α-2A-adrenergic receptor activity, due to pharmacological blockade or genetic ablation, would reveal SNS facilitation of pain pathways following exposure to stress. Ultrasonic noise was selected as a noninvasive psychological stressor that involves activation of limbic pathways and the sympathoadrenal axis.24,25 Levine and colleagues have shown that 30 min of intermittent noise stress increases plasma corticosterone in rats. This stress response does not habituate and results in enhancement of bradykinin hyperalgesia over several days.25,26 Periods of acute continuous noise as short as 5-min durations have been demonstrated to result in analgesia that does not habituate and is opioid-independent.27
We find that rats and C57/BL6 wild type (WT) mice develop stress-induced hyperalgesia when they are pretreated systemically with α-2 receptor antagonists. Stress-induced hyperalgesia was also observed in α-2 antagonist–pretreated rats exposed to a classical physical stressor, footshock, under conditions that normally result in nonopioid analgesia.28 The C57/BL6 α-2A knockout mice, in the absence of receptor antagonists, also develop stress-induced hyperalgesia in response to noise stress. Further examination suggests that loss of peripheral α-2 receptor autoinhibition can counterbalance spinal stress-induced analgesia and, in the absence of sufficient descending inhibition, can lead to the development of stress-induced hyperalgesia. The pain facilitatory mechanism is dependent upon intact peripheral sympathetic innervation, and could potentially be due to an overflow of norepinephrine within the hyperalgesic location.
Materials and Methods
Animals
The α-2 knockout mice were generously provided by Dr. Brian Kobilka, Ph.D. (Professor, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Palo Alto, California). They were backcrossed onto a C57BL/6 background and bred from homozygous knockout mice breeding pairs. Age- and sex-matched WT C57BL/6 mice (Charles River, Wilmington, MA) were used as controls. For confirmatory purposes, additional animals were bred by heterozygote crossing to generate α-2A knockout mice and littermate controls. The hotplate paw withdrawal responses of these animals with and without stress were shown to be the same as those of the independently bred knockout mice and controls (data not shown). Mice were 3–5 months old and weighed 20–30g. Sprague-Dawley rats (Charles River) weighed 250–350g. Animals received food and water ad libitum and were kept on a 12-hour day-night cycle. All experiments were done in compliance with protocols approved by Allergan, Inc. (Irvine, CA) or the Johns Hopkins University (Baltimore, Maryland) Institutional Animal Care and Use Committee and are consistent with the National Institutes of Health Guide for the Use of Experimental Animals to minimize animal use and discomfort.
Drugs
Drug solutions were prepared as follows: Prazosin (Sigma, St. Louis, MO) and guanethidine (Sigma) were dissolved in saline, sulprostone (Cayman Chemical, Ann Arbor, MI) and N-methyl-D-aspartate (NMDA; Sigma) were dissolved in dimethyl sulfoxide, and stock solutions of rauwolscine (Sigma) and idazoxan (Sigma) were dissolved with dimethyl sulfoxide and subsequently diluted with saline (final concentration of dimethyl sulfoxide was <1%) for injection.
Intrathecal injections in mice were performed according to the method devised by Hylden and Wilcox.29 A sterile 30-gauge ½-inch needle attached to a microsyringe was inserted between the L5 and L6 vertebrae and a 5 µl volume slowly injected in the subarachnoid space. Intraperitoneal injections were administered in a volume of 1 ml/kg. Intraplantar rauwolscine was injected in a 25 µl volume.
Tactile allodynia assessment
Sensitivity to light touch was quantified according to methods published by Gil et al.30 Briefly, mice were acclimated to brushing of the hind flank with a paintbrush for the first 15 min following intraperitoneal injection of sulprostone or intrathecal injection of NMDA. Starting at 15-min postinjection, the behavioral response to light brushing of the hind flank was scored every 5 min for the time period 15–50 min postinjection. The behavioral response was scored as follows: animals showing aggressive escape responses along with squeaking and biting at the brush are given a score of 2; animals exhibiting mild squeaking with attempts to escape are given a score of 1; animals showing no response to the light stroking of the paintbrush are given a score of 0. The eight time point scores were summed for each animal to generate that animal’s cumulative pain score (maximum pain score of 16).30 An average of the individual cumulative pain scores was then determined for each treatment group.
Thermal latency assessment
To determine thermal withdrawal latencies (in seconds), rats or mice were individually placed on the hotplate surface preset to 50°C. A timer was started immediately and stopped when the animal first lifted and/or licked either hindpaw. The maximum withdrawal latency was 20 s, at which time the animals were removed in order to prevent tissue damage. In experiments with a unilateral intraplantar injection of rauwolscine, the withdrawal latency of each limb (contralateral and ipsilateral) was individually assessed in separate experiments. In chemical sensitization studies in mice, the withdrawal latencies were assessed 30 min following sulprostone or NMDA treatment.
Ultrasonic noise stress
The noise-induced stress experiments were conducted in an isolated room. The ultrasonic sound emitters (Ultrasonic Pest Repeller; Weitech, Wavre, Belgium) were attached to the top of each cage and confirmed to produce a 24–75 KHz, 100–105 dB noise at the relevant distance with an ultrasonic probe (UE Systems, Elmsford, NY). Twenty four hours prior to the initiation of a noise-induced stress experiment, animals were transferred into the test room. The animals were singly caged, and food, water, and 12-h light-dark cycle were unchanged. Intraperitoneal injections of rauwolscine, idazoxan, prazosin, or saline were administered 30 minutes prior to stress. Intraplantar and intrathecal injections were administered immediately prior to stress. The sound was initiated remotely for a 10-min period. Hotplate testing was performed in the same room within 5 min. Each animal was tested on the hotplate only once. The experimenter was blinded to the stress status, genotype, and drug treatment of animals for studies comparing rauwolscine and saline-treated rats (fig. 1), C57BL/6 and α-2A knockout mice (fig. 2A) and sympathectomized and sham rats.
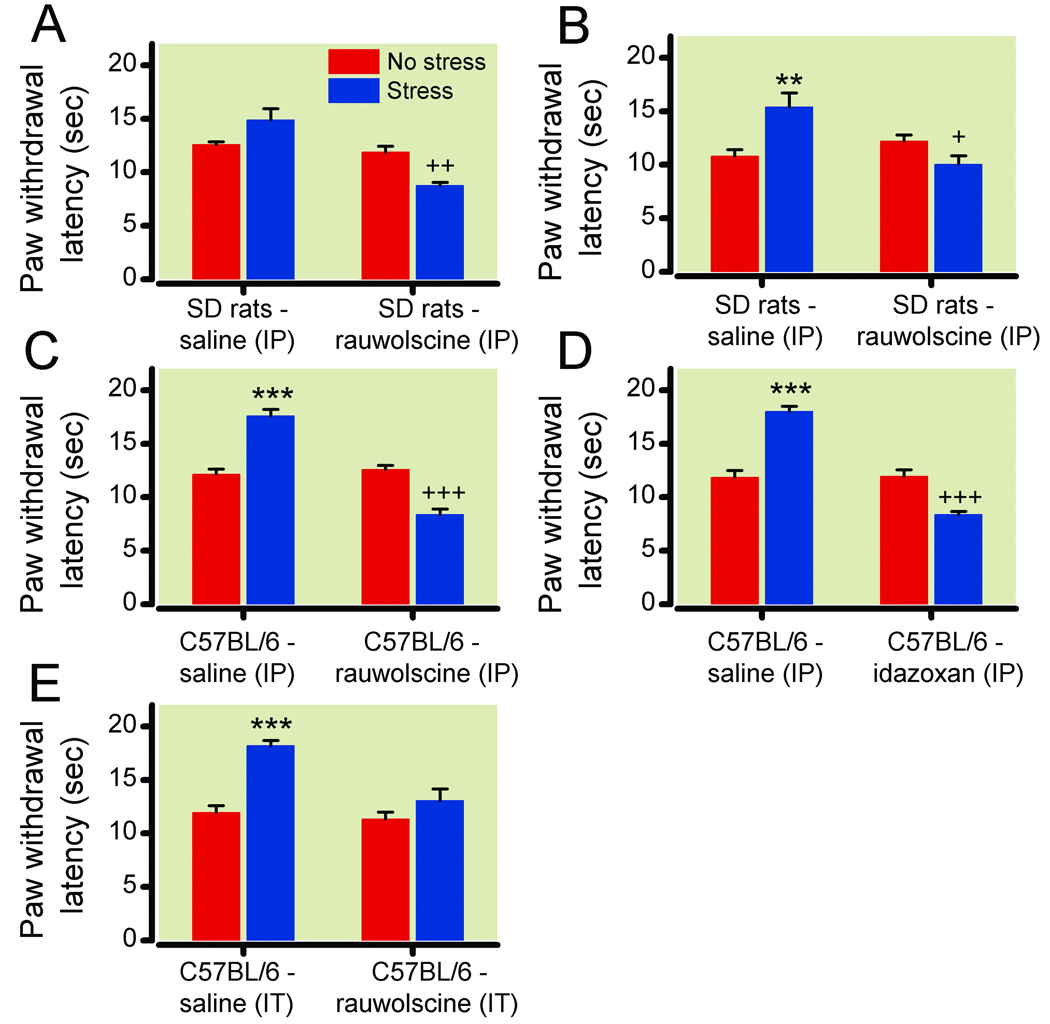
Figure 1. Stress-induced hyperalgesia resulting from systemic α-2-adrenergic blockade in rats and wild type (WT) mice.
(1A) Paw withdrawal latencies following no stress (red bars) or ultrasonic stress (blue bars) in Sprague-Dawley (SD) rats treated with intraperitoneal (IP) saline (n=6) or 0.1 mg kg−1 rauwolscine (n=6). (1B) Paw withdrawal latencies following no stress (red bars) or footshock stress (blue bars) in Sprague-Dawley rats treated with intraperitoneal saline (n=6) or 0.1 mg kg−1 rauwolscine (n=12). (1C, 1D) Paw withdrawal latencies following no stress (red bars) or ultrasonic stress (blue bars) in C57BL/6 WT mice treated with intraperitoneal saline (n=6) or the α-2-adrenergic receptor antagonists (1C) 0.1 mg kg−1 rauwolscine (n=6) or (1D) 0.03 mg kg−1 idazoxan (n=6). (1E) Paw withdrawal latencies following no stress (red bars) or ultrasonic stress (blue bars) in Sprague-Dawley rats treated with intrathecal (IT) saline (n=6) or 3 ug rauwolscine (n=6). The antagonists transform stress-induced analgesia into stress-induced hyperalgesia when dosed intraperitoneally and block stress-induced analgesia without inducing hyperalgesia when dosed intrathecally. **P<0.01; ***P<0.001, increase in latency vs no stress (analgesia); +P<0.05; ++P<0.01; +++P<0.001, decrease in latency vs no stress (hyperalgesia). Data are expressed as mean ± standard error of the mean.
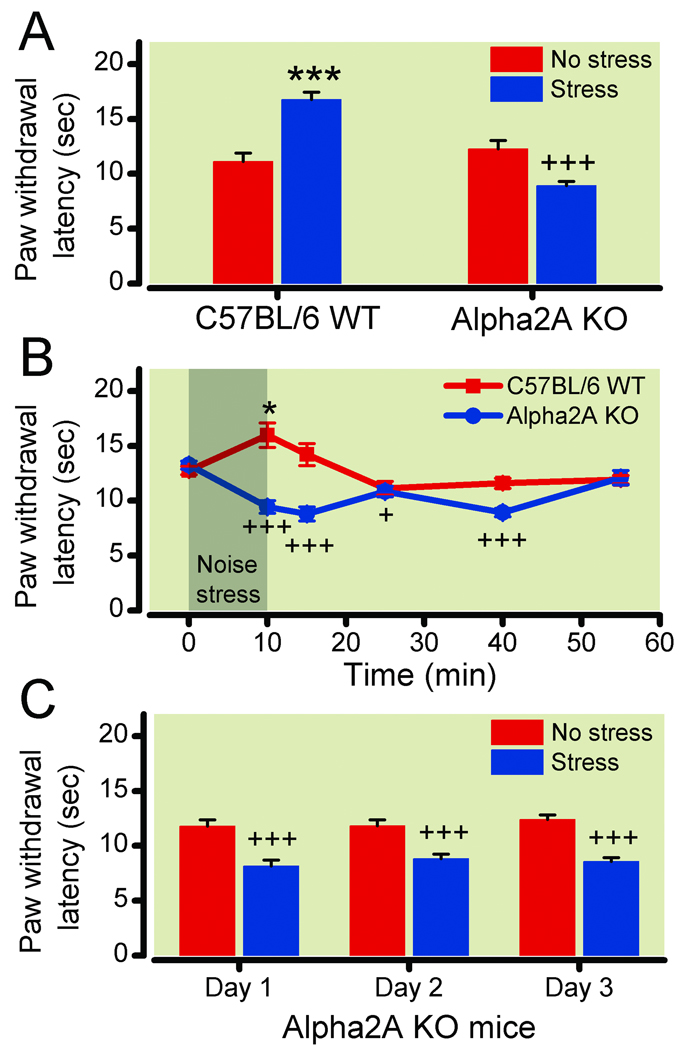
Figure 2. Stress-induced hyperalgesia in α-2A knockout mice.
(2A) Paw withdrawal latencies from a 50°C hotplate in wild type (WT) and α-2A knockout (KO) mice following no stress (red bars, n=6) or ultrasonic stress (blue bars, n=12). (2B) Paw withdrawal latencies in WT mice (n=11) and α-2A knockout mice (n=5) tested once on a hotplate at various times following the initiation of 10-minute ultrasonic stress. (2C) Paw withdrawal latencies in α-2A knockout mice following no stress (red bars, n=6) or ultrasonic stress (blue bars, n=6) on three consecutive days. The α-2A knockout mice exhibit a prolonged non-habituating hyperalgesia instead of analgesia in response to ultrasonic stress. *P<0.05; ***P<0.001, increase in latency vs no stress (analgesia); +P<0.05; +++P<0.001, decrease in latency vs no stress (hyperalgesia). Data are expressed as mean ± standard error of the mean.
Footshock stress
Rats were singly exposed to 3 min of continuous 60 Hz electric shock applied at an intensity of 3 mA through the floor of a Gemini Passive Avoidance apparatus (San Diego Instruments, San Diego, CA). Unstressed rats were placed in the apparatus without shock for 3 min. An experimenter, masked to stress condition and drug treatment, determined thermal latencies 7 min later.
Surgical sympathectomy
Lumbar sympathectomy was performed in male Sprague-Dawley rats under isoflurane anesthesia. The lumbar sympathetic chain was exposed by a ventrolateral, retroperitoneal approach. A shallow midline incision was made to separate the skin from the muscle beginning at the level of L2 and terminating at the level of L6. A second midline incision was made through the muscle, ensuring no damage to the liver and intestines. The superficial muscle layers were then retracted, separating the psoas and the quadratus lumborum muscles from the vertebral column and the transverse processes. The sympathetic paravertebral ganglia L2–L6 were removed bilaterally, beginning from the level of the crus of the diaphragm to the most distal ganglion approachable. Pseudo-sham sympathectomy was identical to lumbar sympathectomy except for removal of the sympathetic paravertebral ganglia. The sympathectomy was functionally confirmed by demonstrating a lack of cold-induced vasoconstriction in the hind paws (data not shown).
Assay of plasma corticosterone
Plasma samples were taken from mice following 10 min of ultrasonic stress and plasma corticosterone level was measured using an immunoassay kit (R&D Systems, Minneapolis, MN).
Electrophysiological study
Surgery
Surgical preparation for neurophysiological recording was conducted as described previously.31 Urethane was used (1.5–2.0 g kg−1 intraperitoneally) to ensure deep anesthesia in mice during surgery and recording,32,33 as the potential involvement of α-2A adrenoceptors in isoflurane-induced analgesia may compromise the analgesia and data interpretation in α-2A knockout mice.34,35 Areflexia to sensory stimuli and electrocardiogram was monitored to ensure sufficient depth of anesthesia. Mice were paralyzed with pancuronium bromide (0.15 mg kg−1 intraperitoneally; Elkins-Sinn Inc., Cherry Hill, NJ) during neurophysiological recording. A laminectomy was performed at vertebral levels T12–L1 to expose lumbar enlargement (L3–L6 spinal segments). The recording segments were continually bathed in a pool of warm saline (37°C) after the dura mater was incised and retracted. Core body temperature was maintained at 36.0–37.0°C with a circulating hot-water pad. A small animal ventilator (Model 683; Harvard Apparatus, South Natick, MA) provided mechanical ventilation (120–140 cycles min−1, stroke volume 0.2–0.3 ml). Mice were euthanized at the conclusion of each experiment by an overdose of sodium pentobarbital (300 mg kg−1 intraperitoneally).
Dorsal horn neuron recording
Extracellular recordings of single neuronal activity in the lumbar dorsal horn (segment L4–L5) were obtained by using fine-tip (<1.0 µm) tungsten micro-electrodes (8 mΩ at 1 kHz; Frederick Haer & Co., Brunswick, ME) that were advanced using an electronic micropositioner. A realtime computer-based data acquisition and processing system (DAPSYS 6; Brian Turnquist, B.S., Ph.D., Johns Hopkins University, Baltimore, Maryland) provided window discriminators for real-time sorting of the amplified action potential (AP) waveforms. Mechanical stimuli were used to search for dorsal horn neurons with cutaneous receptive fields located in the central plantar area of the hind paw. Deep wide dynamic range (WDR) cells (300–700 µm) that respond to both innocuous and noxious mechanical stimuli were defined as described previously.31,36,37 Electrical stimulus was applied through a pair of fine needles inserted subcutaneously across the central plantar area of the hind paw at 0.3–0.4 cm apart. Based on the axon conduction velocities, WDR neurons showed both A-fiber (0–40 ms) and C-fiber (40–250 ms)–mediated response to single intracutaneous electrical stimulus at intensity above the C-fiber activation threshold.31,38 The stimulus intensity-response functions of A- and C-fiber–mediated responses were determined by application of graded intracutaneous electrical stimuli (0.05 to 5.0 mA, 2.0 ms). The intensity that evoked at least 1 spike within the A-fiber and C-fiber latency range was considered the A-fiber and C-fiber threshold, respectively. The responses evoked by A- and C-fibers were quantified by separating the AP firing on a latency basis. WDR neurons were further assessed for their ability to show AP windup response to repeated intracutaneous electrical stimuli that are suprathreshold for C-fiber activation. Three stimulus trains (16 pulses, 3.0 mA, 2.0 ms) were applied at 0.2 Hz, 0.5 Hz, and 1.0 Hz, and with a 10-min interval between each trial. The experimenter was blinded to the treatment of mice in the prazosin studies.
Statistical analysis
Data are presented as mean ± standard error of mean. A P-value <0.05 was considered significant in all studies. In behavioral studies, single comparisons between stressed and unstressed groups were done by independent samples two-tailed Student’s t-test using Microsoft Excel 2007. GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA) was used for multiple comparisons. In the time course study for figure 2B, the responses at each time point were compared to baseline using a one-way analysis of variance (ANOVA) with a Dunnett’s multiple comparison test. In the repeated stress study for figure 2C, the responses of stressed and unstressed groups on each day were compared by a two-way ANOVA with a post-hoc Bonferroni test. For sulprostone and NMDA dose-response curves, one-way ANOVA with a Dunnett’s multiple comparison test was used to compare each concentration to vehicle and two-way ANOVA with a post-hoc Bonferroni test was used to compare the same drug concentration between genotypes. In electrophysiology studies, STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. The number of APs evoked by graded electrical stimuli was compared between two genotypes, using a two-way mixed-model ANOVA with Tukey honestly significant difference post-hoc test. An independent samples two-tailed Student’s t-test was used to compare the recording depth, latency, and threshold for activation of A-fiber and C-fiber–mediated responses, respectively, between the two groups. For analysis of windup, the primary parameter studied was the number of APs in the C-component response evoked by each stimulus in a train of repetitive electrical stimulation. Since the number of APs in the C-component varies among WDR neurons, the raw data for each cell were normalized, with 100% representing the response to the first stimulation in each trial (input). The normalized responses among cells were then averaged. Windup graphs were created by plotting the normalized values against the stimulation number in a train of 16 stimuli. A two-way mixed-model ANOVA (Tukey honestly significant difference post-hoc test) was used to compare windup functions and the averaged C-components for the stimuli 7–16 of the trial, which reflects the plateau level of windup in its maintenance phase, at each frequency tested between the two genotypes.31 A two-way repeated-measures ANOVA (Tukey honestly significant difference post-hoc test) was used to compare electrophysiology data between pre- and postprazosin conditions.
Results
Effect of pharmacological blockade of α-2 receptors on modulation of pain by stress
Intraperitoneal α-2-adrenergic antagonists transform stress-induced analgesia to hyperalgesia
To test if α-2 receptors influence stress-modulated pain behavior, rats were systemically treated with vehicle or the α-2 antagonist rauwolscine (0.1 mg kg−1 intraperitoneally). Thirty minutes post-injection, animals were exposed to either 10 min of normal noise (nonstress group) or ultrasonic noise (stress group). Paw withdrawal thermal latencies to a 50°C hotplate were immediately assessed by an observer masked to treatment condition (fig. 1A). Stressed vehicle-treated rats exhibited increased paw withdrawal latencies (analgesia), compared to unstressed animals (14.9 ± 1.5 s stressed vs. 12.6 ± 0.3 s unstressed; P = 0.15, n = 6/group). Paw withdrawal latencies of unstressed rauwolscine-treated rats were similar to unstressed vehicle-treated animals, suggesting that rauwolscine did not alter normal pain perception. Interestingly, stressed rauwolscine-treated animals had significantly decreased paw withdrawal latencies (hyperalgesia) versus unstressed rauwolscine-treated rats (8.8 ± 0.4 s immediately following ultrasonic stress vs. 11.9 ± 0.5 seconds without stress; P = 0.0016, n = 6/group).
In order to test whether the α-2 antagonist transformation of stress-induced analgesia into stress-induced hyperalgesia is generalizable to other stressors, we tested the effect in rats of systemic rauwolscine treatment (0.1 mg kg−1 intraperitoneally) on the thermal pain sensitivity following footshock. Electric footshock is an extensively studied non-habituating physical stressor.11,28 Depending on the intensity and duration, the footshock results in stress-induced analgesia that is either opioid-dependent or independent. A 3-minute, 3 mA footshock results in opioid-independent analgesia.28 Results similar to ultrasonic noise stress were obtained using this paradigm in a masked study (fig. 1B). Stressed vehicle-treated rats had increased paw withdrawal latencies (analgesia) compared to unstressed animals (15.4 ± 1.3 s stressed vs. 10.8 ± 0.6 seconds unstressed; P = 0.0087, n = 6/group). In contrast, stressed rauwolscine-treated rats had significantly decreased paw withdrawal latencies (hyperalgesia) compared to unstressed rauwolscine-treated rats (10.1 ± 0.8 s immediately following footshock stress vs. 12.2 ± 0.6 seconds without stress; P = 0.0442, n = 12/group).
The effect of ultrasonic stress was extended to mice to enable the use of genetic α-2A knockout mice for further mechanistic studies. Exposure of WT mice to a 10-min ultrasonic stress increased plasma corticosterone levels 3-fold (from 39.2 ± 06.7 to 151.0 ± 30.0 ng ml−1; P = 0.0051, n = 6/group) and produced stress-induced analgesia, consistent with previous studies using noise.27 In a separate experiment, systemic pretreatment (30 min) with α-2 antagonists, either intraperitoneal 0.1 mg kg−1 rauwolscine (fig. 1C) or 0.03 mg kg−1 idazoxan (fig. 1D), decreased paw withdrawal latencies in stressed mice compared to unstressed mice (rauwolscine: 8.4 ± 0.5 s stressed vs. 12.6 ± 0.4 s without stress, P = 0.0004, n = 6/group; idazoxan: 8.4±0.3 s stressed and 12.0 ± 0.6 s without stress, P = 0.0007, n = 6/group). Without stress, paw withdrawal latencies of antagonist-treated mice were indistinguishable from vehicle-treated mice. Vehicle-treated mice (n = 6/group) exhibited normal stress-induced analgesia. The antagonist effect was dose dependent. A 0.01 mg kg−1 intraperitoneal rauwolscine dose did not decrease stress-prolonged paw withdrawal latencies (17.4 ± 1.0 s with rauwolscine vs. 17.9 ± 0.9 seconds with vehicle, n = 6/group) while a 0.03 mg kg−1 intraperitoneal dose partially inhibited stress-induced analgesia (14.9 ± 0.8 s, n = 6). The 0.1 mg kg−1 intraperitoneal rauwolscine dose resulted in stress-induced hyperalgesia (8.4 ± 0.5 s). Thus, systemic treatment with α-2 antagonists, which presumably blocks receptor function both peripherally and centrally, causes a loss of stress-induced analgesia and a gain of behavioral hyperalgesia in rats and WT mice. It is unclear if these two behavioral alterations are distinct and separable mechanisms, or simply the net result of a loss of α-2-mediated spinal inhibition.
Intrathecal α-2-adrenergic antagonists block development of stress-induced analgesia
The role of α-2 receptors in descending inhibition is well established. To test whether the blockade of spinal α-2 receptors is sufficient to transform stress-induced analgesia into stress-induced hyperalgesia, mice were injected intrathecally with vehicle or 3 ug rauwolscine, which is an equivalent total body dose to 0.1 mg kg−1 intraperitoneal treatment in mice weighing approximately 30g (fig. 1E). Intrathecal vehicle-treated animals exhibited normal thermal paw withdrawal latencies and developed significant stress-induced analgesia (18.2 ± 0.5 s stressed vs. 12.0 ± 0.6 s without stress; P < 0.0001, n= 6/group). Paw withdrawal latencies of unstressed rauwolscine-treated mice were similar to unstressed vehicle-treated mice. However, paw withdrawal latencies of stressed mice treated with 3 µg intrathecal rauwolscine were not significantly different from those in the unstressed mice (13.1 ± 1.1 s stressed vs. 11.4 ± 0.6 s unstressed; P = 0.24, n = 6/group). Lower concentrations of intrathecal rauwolscine (0.3 and 1 µg) did not block stress-induced analgesia (data not shown). Intrathecal rauwolscine blocked the development of stress-induced analgesia, but did not result in the development of stress-induced hyperalgesia.
Loss of α-2A receptor activity in knockout mice results in stress-induced hyperalgesia
Rauwolscine is a highly selective α-2 receptor antagonist, but it does not exhibit selectivity among the α-2 receptors. Thus, to characterize the receptor subtype responsible for the loss of analgesia and gain of hyperalgesia, we studied the α-2A genetic knockout mouse. The WT and α-2A knockout mice were exposed to ultrasonic sound, and thermal latencies were determined by a masked observer at various times after ultrasonic exposure. As previously reported, thermal response latencies (unstressed) were similar between WT mice and α-2A knockout mice. The WT mice exhibited stress-induced analgesia immediately after stress (16.8 ± 0.7 s stressed vs. 11.1 ± 0.8 s unstressed; P = 0.0001, n = 6 unstressed, 12 stressed; fig. 2A). In contrast, paw withdrawal latencies of α-2A knockout mice were significantly decreased immediately following ultrasonic stress, compared with no stress (8.9 ± 0.4 s stressed vs. 12.3 ± 0.8 s unstressed; P = 0.0008, n = 6 unstressed, 12 stressed; fig. 2A). The analgesia in WT mice (n = 11) was only significant immediately following stress (fig. 2B), but the stress-induced hyperalgesia in the α-2A knockout mice (n = 5) lasted up to 30 min following stress (fig. 2B; F (5,29) = 9.6; P < 0.0001 for comparison to prestress baseline). In addition, the stress-induced hyperalgesia did not habituate when α-2A knockout mice were stressed on three consecutive days (fig. 2C). Two-way ANOVA demonstrated a significant group effect (stressed vs. unstressed α-2A knockout; F (1,20) = 53.96; P < 0.0001, n = 6/group), but no effect of time. The α-2A knockout mice exhibited a loss of stress-induced analgesia and a gain of stress-induced behavioral hyperalgesia, mirroring the finding in genetically unaltered rodents treated systemically with α-2 antagonists.
WDR neurons in α-2A knockout mice exhibit facilitation of windup response to repeated electrical stimuli
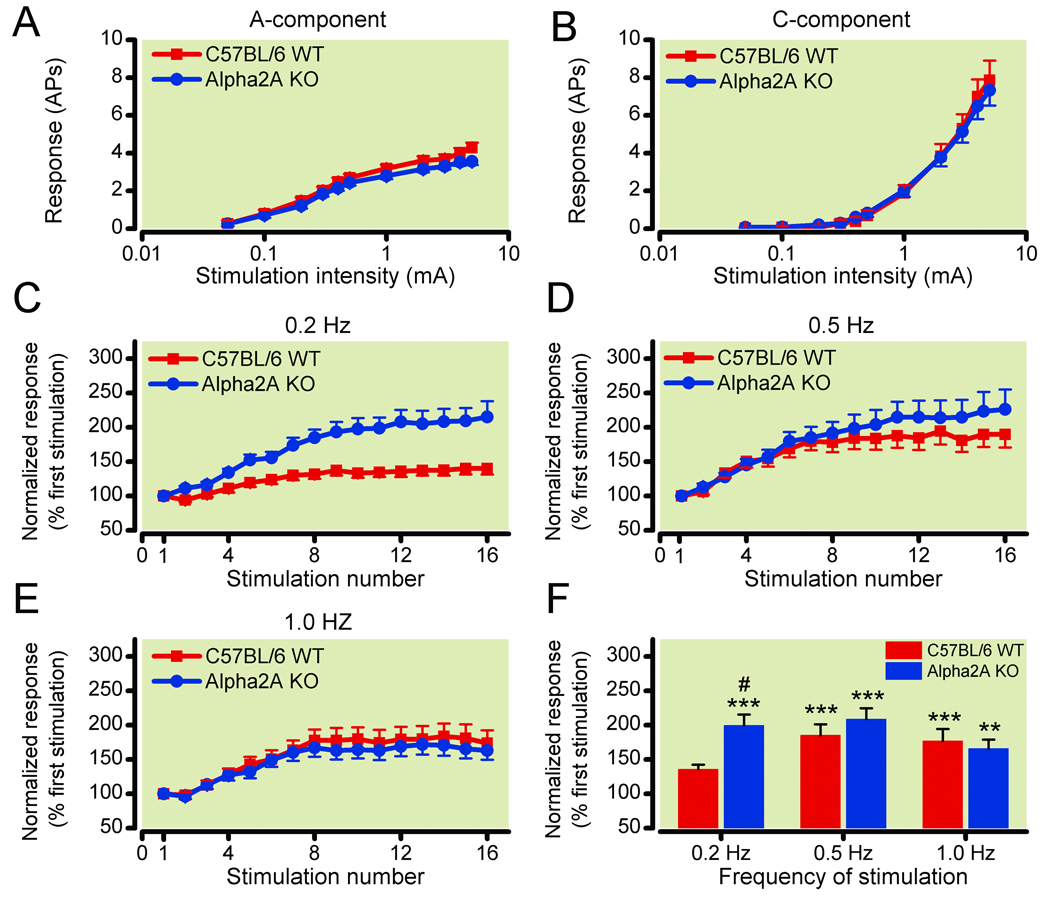
The hyperalgesia in α-2A knockout mice was further characterized using electrophysiological recordings from WDR neurons in the deep dorsal horn that receive converging inputs from afferent A- and C-fibers39,40 and exhibit increased excitability in response to repetitive noxious stimuli.31,41,42 Repeated electrophysiological stimuli in anesthetized rodents have many parallels with footshock and other behavioral stressors and enable a more mechanistic investigation of the α-2A knockout mice hyperalgesia.28,31,43,44 The α-2A knockout mice exhibit normal electrophysiological responses to single painful electrical stimuli, consistent with their normal thermal response latencies. There were no significant differences between the α-2A knockout (n = 62) and WT (n = 47) WDR neuronal response to graded intracutaneous electrical stimuli (0.05–5.0 mA, 2.0 ms) in the mean number of APs in the A-component and C-component (fig. 3A, B). The latency and threshold of the first C-fiber–mediated AP were not significantly different between the α-2A knockout (123.4 ± 3.9 ms, 1.67 ± 0.18 mA) and WT groups (129.5 ± 4.0 ms, 1.57 ± 0.17 mA). The latency and threshold of the first A-fiber–mediated AP were also comparable between the two genotypes (data not shown).
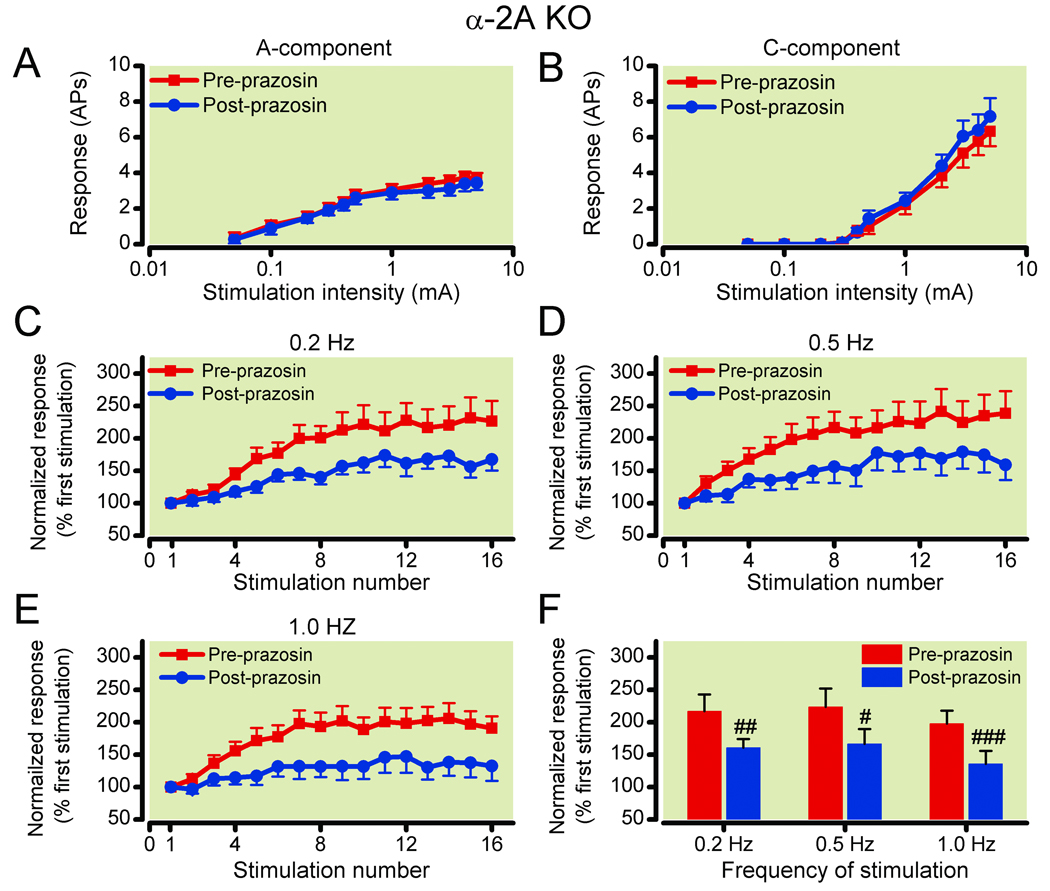
Figure 3. Deep wide dynamic range (WDR) neurons in α-2A knockout mice show enhanced windup response, as compared to that in wild type (WT) mice.
Deep WDR neurons in α-2A knockout (KO; n=62) and WT (n=47) mice showed similar stimulus intensity-response functions of (3A) A-fiber and (3B) C-fiber–mediated responses to graded intracutaneous electrical stimuli applied to the receptive field (0.05–5.0 mA, 2.0 ms). Data are expressed as mean ± standard error of the mean number of action potentials (AP). (3C–3E) C-component responses of deep WDR neurons in WT mice (n=47) and in α-2A knockout mice (n=62) to repetitive electrical stimulation (16 pulses, 3.0 mA, 2.0 ms) applied at frequencies of (3C) 0.2 Hz, (3D) 0.5 Hz, and (3E) 1.0 Hz, respectively, are plotted against the stimulation number of each trial. The WDR neurons in α-2A knockout mice, but not in the WT mice, displayed windup to 0.2 Hz stimulation in addition to 0.5 Hz and 1.0 Hz stimulation. (3F) The averaged C-fiber–mediated responses for stimulus 7–16 during the stimulation are plotted for various frequencies. Data are normalized by the response evoked by the first stimulation of each trial. **P<0.01 and ***P<0.001 vs the input value; #P<0.05 vs the WT group. Data are expressed as mean ± standard error of the mean.
In WT mice, many deep WDR neurons showed a windup response to repeated conditioning stimuli applied at frequencies of 0.5 Hz and 1.0 Hz, but rarely to 0.2 Hz stimulation (fig. 3C–E). The plateau level of windup measured as the averaged C-component responses during stimuli 7–16 of the trial were significantly increased at 0.5 Hz (185.3 ± 15.9%; P = 0.0004) and 1.0 Hz (177.1 ± 17.2%; P = 0.0008) stimulation, but not at 0.2 Hz (135.8 ± 6.8%; P = 0.34), as compared to the input (fig. 3F). In α-2A knockout mice, many WDR neurons exhibited strong windup to 0.2 Hz stimulation in addition to 0.5 and 1.0 Hz stimulation (fig. 3C–E). There were significant increases in the averaged C-component responses for stimuli 7–16 of the trial during all three frequencies tested (0.2 Hz: 199.4 ± 15.9%, P = 0.0003; 0.5 Hz: 208.6 ± 15.9%, P = 0.0003; 1.0 Hz: 165.8 ± 13.1%, P = 0.0011), as compared to the baseline input (fig. 3F). Importantly, the plateau level of windup to 0.2 Hz stimulation in α-2A knockout mice was significantly greater than that in WT mice (P = 0.018; fig. 3F). Thus, the α-2A knockout mice exhibit increased sensitivity to sustained activation of nociceptive afferents by electrical stimuli, revealing a facilitatory mechanism that is normally inhibited by the α-2A receptor.
Chemical-induced peripheral, but not central, sensitization enhanced in α-2A knockout mice
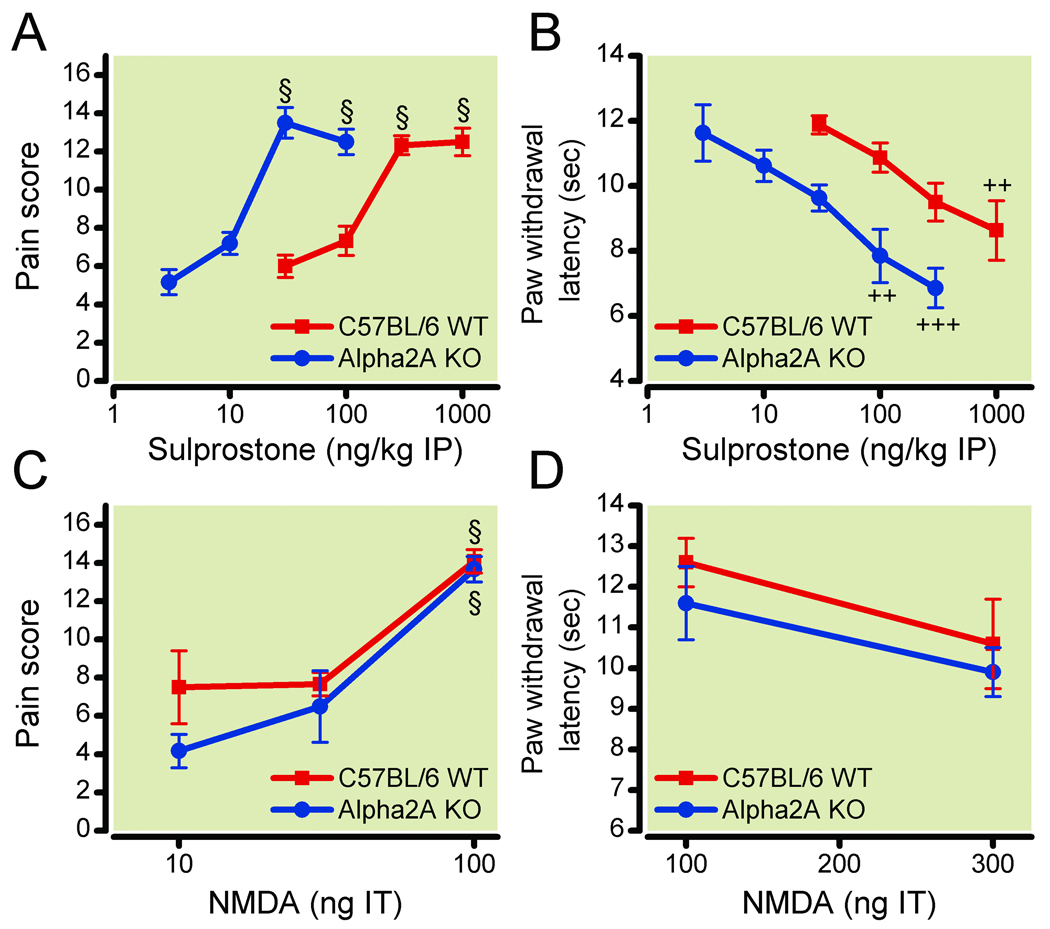
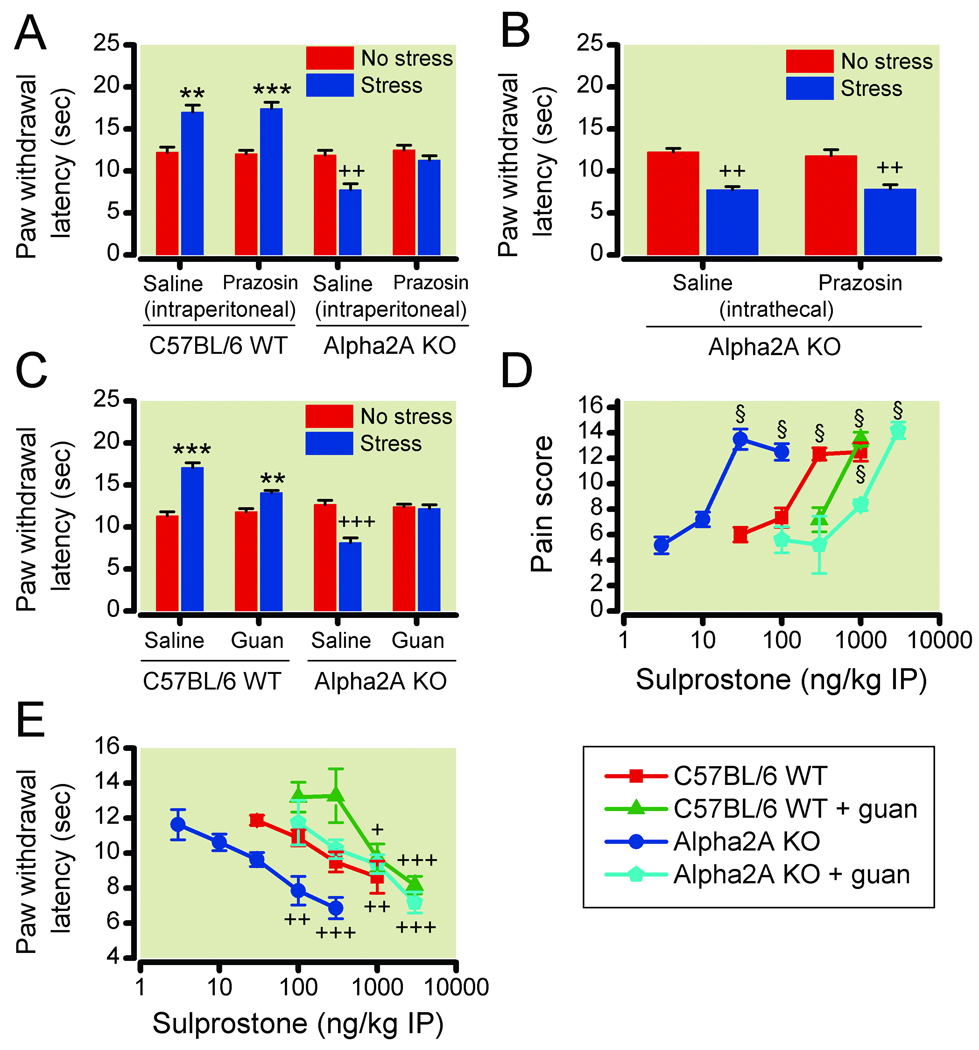
To further localize the facilitatory mechanism, we investigated the effect of distinct sensitizing agents on the response of the knockout mice to light stroking with a paint brush or to a hotplate.30 Peripheral sensitization was elicited with sulprostone, a selective non-inflammatory EP1/EP3 prostaglandin receptor agonist, and tactile allodynia and thermal hyperalgesia were measured. The dose response of sulprostone-induced sensitization to mechanical and thermal stimuli was shifted to the left 10-fold in the α-2A knockout mice (fig. 4A, B). Significant mechanical allodynia occurred at intraperitoneal sulprostone doses ≥300 ng kg−1 (n = 6/group) in WT mice and ≥30 ng kg−1 (n = 6/group) in α-2A knockout mice, and significant thermal hyperalgesia occurred at 1,000 ng kg−1 (n = 6) sulprostone in WT mice and at 100 ng kg−1 (n = 6) and 300 ng kg−1 (n = 7) sulprostone in α-2A knockout mice. The responses to mechanical and thermal stimuli were significantly different between genotypes at the 30 ng kg−1 and 100 ng kg−1 doses (mechanical, P < 0.001; thermal, P < 0.01). Thus, peripheral sensitization was facilitated in the α-2A knockout mice even in the absence of ultrasonic stress.
Figure 4. α-2A knockout mice show enhanced response to peripheral sensitizing agent.
Dose-responses in wild type (WT; red squares) and α-2A knockout (KO; blue circles) mice of intraperitoneal (IP) sulprostone effects on (4A) tactile pain scores (n=6, except n=5 in 10 ng kg−1 knockout group) and (4B) thermal paw withdrawal latencies (n=6, except n=5 in 300 ng kg−1 WT group, n=7 in 300 ng kg−1 knockout group, and n=6 data from two experiments were combined for 30 ng kg−1 WT and knockout groups). Dose-responses in WT (squares) and α-2A knockout (circles) mice of intrathecal (IT) N-methyl-D-aspartate (NMDA) effects on (4C) tactile pain scores (n=6, except n=6 data from two experiments were combined for 100 ng kg−1 WT group), and (4D) thermal paw withdrawal latencies (n=6). The α-2A knockout mice exhibit increased sensitivity to intraperitoneal sulprostone, but not intrathecal NMDA. §P<0.001, increase in pain score vs no drug (allodynia); ++P<0.01; +++P<0.001, decrease in latency vs no drug (hyperalgesia). Data are expressed as mean ± standard error of the mean.
To test whether the α-2 knockout mice also have an increase in susceptibility to central sensitizing stimuli, WT and α-2 knockout mice were injected intrathecally with varying concentrations of NMDA, which mimics the activation of dorsal horn neurons by the excitatory transmitter glutamate. Intrathecal NMDA induced a dose-dependent tactile allodynia (n = 6/group, except n = 6 data from two experiments combined for 100 ng kg−1 WT group) and thermal hyperalgesia (n = 6/group) with a similar dose-response in WT and knockout mice (fig. 4C, D). There were no significant between-genotype differences at any dose.
Systemic α-1-adrenergic receptor blockade prevents the development of stress-induced hyperalgesia and enhanced windup response in α-2A knockout mice
A previous study has demonstrated that in α-2A knockout mice the sympathetic nerves lose normal autoinhibition, resulting in increased sympathetic outflow during high-frequency stimulation, but autoinhibition of basal norepinephrine release is normal.14 Since stress can stimulate the SNS, we investigated whether the enhanced windup and stress-induced hyperalgesia in the α-2A knockout mice were potentially dependent upon elevated sympatho-neural outflow and subsequent α-1 receptor activation. The effect of post-synaptic blockade with the α-1 receptor antagonist prazosin was determined. Recordings from WDR neurons were conducted in the presence or absence of prazosin in a blinded manner. Treatment of the α-2A knockout mice with prazosin (100 ng kg−1 intraperitoneally45) did not affect the A-fiber and C-fiber– mediated response of WDR neurons (n = 18) to single electrical stimuli (fig. 5A, B), but suppressed windup to repetitive stimuli at frequencies of 0.2, 0.5, and 1.0 Hz (fig. 5C–F). The averaged C-component responses for the stimulus 7–16 of the trial were significantly reduced from the respective preprazosin levels (0.2 Hz: 217.1 ± 26.0% vs. 160.6 ± 13.6%, P = 0.0021; 0.5 Hz: 223.7 ± 28.4% vs. 166.6 ± 22.9%, P = 0.018; 1.0 Hz: 197.8 ± 20.2% vs. 135.9 ± 20.1%, P = 0.0007; fig. 5F). Systemic pretreatment (30 min) with 100 ng kg−1 intraperitoneal prazosin had no effect on the thermal latencies of unstressed WT or α-2A knockout mice (n = 6/group, fig. 6A). The antagonist did not decrease stress-induced analgesia in the WT mice. However, the antagonist prevented the development of the α-2A knockout mouse stress-induced hyperalgesic behavior, but did not restore stress-induced analgesia (n = 6/group, fig. 6A). Intrathecal injection of 10 ng prazosin, which is a 3-fold greater total body dose than 100 ng kg−1 intraperitoneally in mice weighing approximately 30g, did not prevent stress-induced hyperalgesia in α-2A knockout mice (n = 6/group, fig. 6B).
Figure 5. Systemic administration of prazosin attenuates windup levels without changing A-fiber and C-fiber–mediated responses of wide dynamic range (WDR) neurons to graded electrical stimulation in α-2A knockout mice.
(5A, 5B) In α-2A knockout (KO) mice, systemic administration of the α-1 receptor antagonist prazosin (0.1 µg kg−1 intraperitoneal) did not significantly change the (5A) A-fiber and (5B) C-fiber–mediated responses (number of action potentials, AP) of WDR neurons (n=18) to graded electrical stimulation. (5C–5E) Prazosin suppressed windup levels across (5C) 0.2 Hz, (5D) 0.5 Hz, and (5E) 1.0 Hz stimulation in α-2A knockout mice. (5F) The averaged C-component responses for the stimulus 7–16 of the trial were also significantly reduced during 0.2, 0.5, and 1.0 Hz stimulation after prazosin treatment, as compared to the pre-prazosin condition. Data are normalized by the response evoked by the first stimulation of each trial. #P<0.05; ##P<0.01; ###P<0.001 vs pre-prazosin condition. Data are expressed as mean ± standard error of the mean.
Figure 6. Inhibition of sympathetic outflow prevents stress-induced hyperalgesia in α-2A knockout mice.
(6A, 6B, 6C) Paw withdrawal latencies following no stress (red bars) or ultrasonic stress (blue bars) in wild type (WT) and α-2A knockout (KO) mice after pretreatment with a saline control (n=6) or the α-1 receptor antagonist prazosin, by (6A) intraperitoneal (0.1 µg kg−1; n=6) or (6B) intrathecal (10 ng; n=6) injection, or (6C) after guanethidine (guan) sympathectomy (n=8, except n=10 in guanethidine-treated knockout group). (6D) Tactile pain scores (n=6, except n=5 in 100, 300, 3000 ng kg−1 knockout groups) and (6E) paw withdrawal latencies (n=6, except n=4 in 100 ng kg−1 knockout group and n=7 in 3000 ng kg−1 knockout groups) in response to intraperitoneal (IP) sulprostone in WT and α-2A knockout mice with guanethidine sympathectomy (green, WT; light blue, KO) compared to data without guanethidine sympathectomy from Figure 4A, B (red, WT; blue, KO). Intraperitoneal prazosin and guanethidine sympathectomy prevent hyperalgesia in response to stress and sulprostone in α-2A knockout mice. **P<0.01; ***P<0.001, increase in latency vs no stress (analgesia); §P<0.001, increase in pain score vs no sulprostone (allodynia); +P<0.05; ++P<0.01; +++P<0.001, decrease in latency vs no stress or vs no sulprostone (hyperalgesia). Data are expressed as mean ± standard error of the mean.
Role of postganglionic sympathetic outflow in enhanced pain response
Chemical sympathectomy with guanethidine blocks stress-induced hyperalgesia and chemicalinduced peripheral sensitization in α-2A knockout mice
We investigated whether the postganglionic SNS was required for the stress-induced thermal hyperalgesia in the α-2A knockout mice. Chemical ablation of the mouse SNS with guanethidine (50 mg kg−1 intraperitoneally) one day prior to testing did not alter baseline thermal latencies. Compared with vehicle-treated WT mice (n = 8), the guanethidine-treated WT mice (n = 8) exhibited slightly reduced stress-induced analgesia. The vehicle-treated α-2A knockout mice (n = 8) developed stress-induced thermal hyperalgesia, but the guanethidine-treated α-2A knockout mice (n = 10) did not (fig. 6C), demonstrating that the stress-induced thermal hyperalgesia requires the SNS.
We also investigated whether the postganglionic SNS was required for the enhanced sensitivity to the peripheral sensitization by sulprostone. The WT and α-2A knockout mice were chemically sympathectomized by guanethidine (50 mg kg−1 intraperitoneally) treatment. Sulprostone sensitivity, both mechanical (fig. 6D) and thermal (fig. 6E), was decreased 30- to 100-fold in α-2A knockout mice one day after guanethidine sympathectomy. Guanethidine sympathectomy also decreased the sensitivity of WT mice to sulprostone, but to a lesser extent, so that the sympathectomized WT and α-2A knockout mice exhibited similar responsiveness. Significant mechanical allodynia was only observed at 1,000 ng kg−1 (n=6) in WT mice and 3,000 ng kg−1 (n = 5) in α-2A knockout mice with a significant between-genotype difference at 1,000 ng kg−1 (P < 0.001, fig. 6D). Significant thermal hyperalgesia occurred at ≥1,000 ng kg−1 (n = 6/group) in WT mice and 3,000 ng kg−1 (n = 7) in α-2A knockout mice with no between-genotype differences (fig. 6E).
Surgical sympathectomy: Postganglionic sympathetic nerves critical to the development of stress-induced hyperalgesia
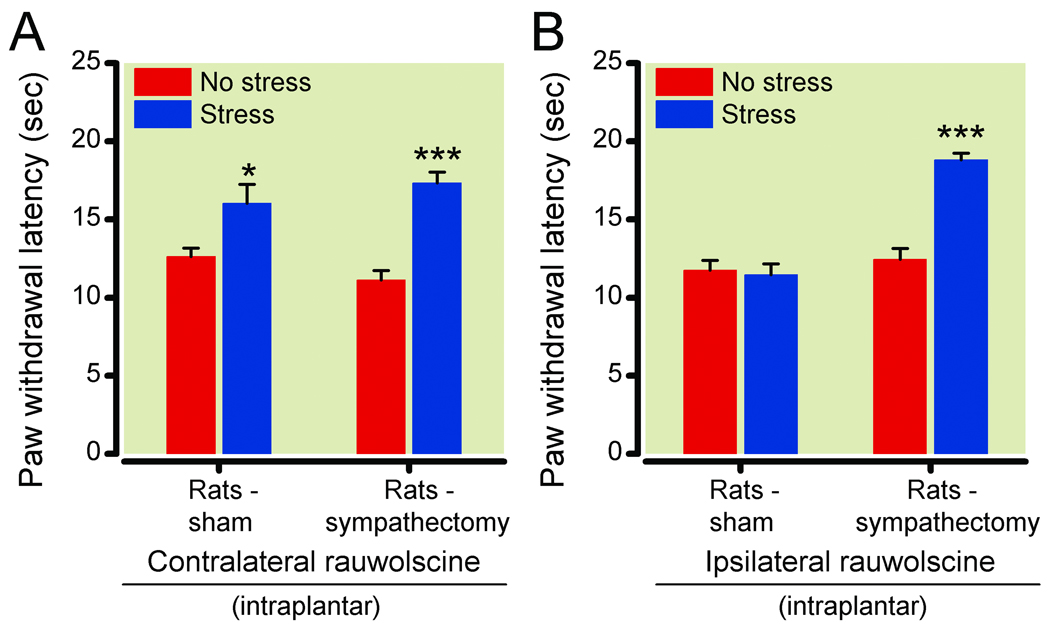
Guanethidine treatment could potentially have additional nonselective actions, and hence the effects of surgical sympathectomy were studied. In order to investigate the effect of surgical sympathectomy and better isolate peripheral drug effects, we conducted studies in the rat model rather than in mice. To determine if a loss of peripheral α-2 receptor function is sufficient to decrease paw withdrawal latencies, rats were unilaterally injected with intraplantar rauwolscine (1 µg) immediately before stress. The paw withdrawal latencies of the ipsilateral and contralateral paws were independently determined by a masked observer in separate experiments. Contralateral paw withdrawal latencies were significantly increased in stressed sham sympathectomy rats compared to unstressed controls (16.1 ± 1.2 s following stress and 12.6 ± 0.5 s without stress; P = 0.014, n = 12 unstressed, 11 stressed), demonstrating that in the stressed animals spinal-mediated inhibition and its behavioral manifestation (analgesia) are intact (fig. 7A). In contrast, ipsilateral paw withdrawal latencies in sham sympathectomy rats were not increased following stress (11.5 ± 0.7 s following stress and 11.8 ± 0.6 s without stress; P = 0.76, n = 12/group), demonstrating an effect of intraplantar rauwolscine that is limited to the injected paw (fig. 7B). The SNS dependence of the intraplantar rauwolscine-mediated prevention of increased paw withdrawal latencies was determined by surgical ablation of the L2–L5 postganglionic chain. Rats that had a surgical sympathectomy and were treated with intraplantar rauwolscine exhibited stress-induced analgesia in both paws, with ipsilateral paw withdrawal latencies of 18.8 ± 0.4 s following stress and 12.5 ± 0.7 s without stress (P < 0.0001, n = 13 unstressed, 12 stressed; fig. 7B) and contralateral paw withdrawal latencies of 17.3 ± 0.7 s following stress and 11.1 ± 0.6 s without stress (P < 0.0001, n = 11 unstressed, 12 stressed; fig. 7A). Hence, pain facilitation due to local blockade of α-2 receptors on postganglionic sympathetic nerves can counterbalance spinal inhibition and negate its behavioral manifestation (analgesia).
Figure 7. Stress-induced hyperalgesic response in rats ablated by sympathectomy.
(7A) Paw withdrawal latencies of paws contralateral to 1 µg rauwolscine-injected paws following no stress (red bars) or ultrasonic stress (blue bars) in sham (n=12 unstressed, 11 stressed) or surgically sympathectomized (n=11 unstressed, 12 stressed) Sprague-Dawley rats. Stress-induced analgesia was unaffected by contralateral intraplantar rauwolscine injection or sympathectomy. (7B) Paw withdrawal latencies of 1 µg rauwolscine-injected paw following no stress (red bars) or ultrasonic stress (blue bars) in sham (n=12) or surgically sympathectomized (n=13 unstressed, 12 stressed) Sprague-Dawley rats. Intraplantar rauwolscine blocked and sympathectomy restored a stress-induced analgesia response in the ipsilateral rauwolscine-treated paw. *P<0.05; ***P<0.001 vs no stress (analgesia). Data are expressed as mean ± standard error of the mean.
Discussion
α-2-Adrenergic receptor regulation is integral at many levels of the stress pathway. The results of the described studies demonstrate a balance between pain inhibitory/analgesic and pain facilitatory/hyperalgesic mechanisms activated by stress that is shifted towards analgesia by α-2 receptor activity. α-2A- and α-2C-adrenergic receptors are located in the spinal cord dorsal horn.16 Previous studies with α-2 knockout mice have demonstrated that the α-2A receptor is necessary for spinal analgesia with α-2 agonists20–22 and that the α-2C receptor interacts with the opioid system in the spinal cord.46 The lack of stress-induced analgesia following treatment with two different α-2 antagonists (fig. 1) and in the α-2A knockout mice (fig. 2) demonstrates a physiological role for the spinal α-2A receptors and is consistent with pharmacological studies using spinal idazoxan and yohimbine that demonstrated that descending inhibition of dorsal horn nociceptive inputs is mediated by α-2-adrenergic receptors.12
The unmasking of stress-induced hyperalgesia following α-2 antagonist treatment or in the α-2A knockout mice reveals an underlying pain facilitatory mechanism activated by acute stress that is normally attenuated by α-2 receptors. The hyperalgesia can be induced by both a psychological stressor (ultrasonic noise) and a physical stressor (noxious footshock), suggesting that it may involve a common pathway activated by different types of stressors (fig. 1A, B). Despite the differences between the two stressors, both induce a non-habituating, opioid-independent analgesia when the α-2A receptor is functional and activate the hypothalamic-pituitary-adrenal axis and SNS.24,25,47
Multiple lines of evidence demonstrate that the observed stress-induced hyperalgesia is mechanistically distinct from stress-induced analgesia, and not just due to the loss of spinally mediated analgesia. For example, 3 ug intrathecal rauwolscine treatment blocked stress-induced analgesia without the development of hyperalgesia. In contrast, an equivalent total body dose administered systemically (intraperitoneal, 0.1 mg kg−1) blocked analgesia and produced hyperalgesia (fig. 1C, E). The opposite result was also true. A 100 ng kg−1 intraperitoneal dose, but not a similar intrathecal total body dose (10 ng), of the α-1 antagonist prazosin (fig. 6A, B) or guanethidine treatment (fig. 6C) blocked the development of stress-induced hyperalgesia in α-2A knockout mice, but did not produce analgesia. These findings illustrate that the loss of analgesia and gain of hyperalgesia (or vice versa) are distinct mechanisms involving different sites of action.
Repeated nociceptive electrical stimuli applied to the footpads to induce windup share obvious common features with footshock stress, including activation of primary afferents, midbrain noradrenergic pathways, the SNS and spinal and supraspinal descending inhibitory circuits.28,31,43,44 The same frequency of electrical stimulation (0.5 Hz) that activates noradrenergic neurons in the locus coeruleus and induces descending pain inhibitory mechanisms also induces windup of dorsal horn WDR neurons.31,43,44 Because of these similarities to standard stressors, electrophysiological assessment of the deep dorsal horn WDR neuron response to repetitive noxious electrical stimuli was used to dissect the hyperalgesia mechanism.
The pain facilitatory/hyperalgesic mechanism is detectable in the α-2A knockout mice as a decreased frequency threshold for developing short-term neuronal sensitization to repeated electrical stimuli. The dorsal horn WDR neuron responses to acute intracutaneous electrical stimuli were similar between the α-2A knockout and WT mice (fig. 3A, B), demonstrating normal nociception. However, WDR neuronal responses differed with repetitive electrical stimuli that cause progressive increase in dorsal horn neuronal excitability called windup (fig. 3C–E). WDR neurons in normal rodents rarely exhibit windup to conditioning electrical stimulation at a frequency less than 0.3 Hz.31,48 In line with this observation, 0.2 Hz stimulation did not induce significant windup in WT mice in the current study. However, there was windup to the 0.2 Hz stimulus in α-2A knockout mice, demonstrating a decreased frequency threshold for the induction of windup. Interestingly, this pattern of facilitated windup is similar to that previously observed in mu-opioid receptor knockout mice.31
To further localize the pain facilitation mechanism, sensitizing agents were applied at various points in the pain pathway. The α-2A knockout mice exhibited a 10-fold decrease in the concentration of the noninflammatory prostaglandin analog sulprostone that caused dynamic tactile allodynia in response to a paint brush, and there was a 3- to 10-fold decrease in the concentration of sulprostone that reduced paw withdrawal latency to a thermal stimulus (fig. 4A, B). The increased sensitivity to both tactile and thermal stimuli suggests that both A- and C-fibers may be affected. There was no increase in the pain behavior to spinal NMDA in α-2A knockout mice (fig. 4C, D), consistent with a peripheral, and not postsynaptic spinal, location of the facilitation mechanism.
The decreased sensitization thresholds to sustained chemical and electrical stimuli in the α-2A knockout mice and the decreased thermal threshold following exposure to noise and footshock stress suggests that the α-2 receptor normally attenuates a mechanism that facilitates the pain response to sustained stimuli. Multiple lines of evidence presented in this paper point to a previously unrecognized role of the post-ganglionic SNS and sympathetic overflow in uninjured animals for the facilitated pain responses. The increased sensitivity of the α-2A knockout mice was reversed by various sympatholytic treatments. Since post-synaptic α-1-adrenergic receptors mediate many of the excitatory actions of the SNS, the role of α-1 receptor activation was tested by co-administration with the α-1 antagonist prazosin. Systemic prazosin did not alter normal thermal sensitivity, but completely blocked the development of stress-induced thermal hyperalgesia in the α-2A knockout mice (fig. 6A). Spinal administration of 10 ng prazosin was ineffective, despite being a greater total body dose than the effective 100 ng kg−1 intraperitoneal dose, consistent with a peripheral site of action (fig. 6B). Intraperitoneal prazosin treatment also suppressed windup to repetitive electrical stimuli at all frequencies in the α-2A knockout mice (fig. 5C–F). This result indicates that activation of peripheral α-1 receptors in the absence of α-2A receptors may lower the frequency threshold for the induction of windup, and also enhance the windup response at higher frequencies of stimulation. The finding is consistent with previous reports that α-1 receptor activation enhances neurogenic inflammation7 and opposes α-2 receptor-mediated analgesia.45
Guanethidine treatment depletes norepinephrine from postsynaptic SNS nerve endings, resulting in a transient chemical sympathectomy.49 As with prazosin, guanethidine pretreatment did not affect thermal response in the absence of stress, but eliminated hyperalgesia caused by ultrasonic stress in the α-2A knockout mice (fig. 6C). Guanethidine sympathectomy also dramatically increased the concentration threshold for sulprostone sensitization 30- to 100-fold in the α-2A knockout mice and increased, to a smaller extent, the sensitization threshold in WT mice (fig. 6D, E). Thus, a SNS dependent decrease of sensory thresholds can also occur in WT mice, but it is more pronounced in α-2A knockout mice.
The potential for SNS postganglionic nerves to mediate development of stress-induced pain facilitation in normal, uninjured animals was also demonstrated in the rats with rauwolscine blockade of α-2 receptors. Intraplantar injection of rauwolscine prevented increased paw withdrawal latency of the injected paw following stress (fig. 7B, sham) even though stress-induced analgesia was still apparent in the prolonged withdrawal response of the contralateral paw (fig. 7A, sham), demonstrating that rauwolscine was acting locally on peripheral α-2 receptors. The rauwolscine effect was prevented by surgical removal of lumbar sympathetic ganglia (fig. 7B, sympathectomy). Hence, the facilitatory mechanism that is normally attenuated by α-2 receptor activation requires postganglionic sympathetic fibers. This peripheral mechanism likely involves SNS overflow due to decreased α-2 receptor-mediated feedback inhibition of neurotransmitter release from SNS postganglionic nerve endings. Previous studies of hyperalgesia evoked by immune mediators50 have implicated sympathoadrenal enhancement of primary afferent sensitivity developing over several days. Since the effect of stress in this study is acute and the preganglionic innervation of the adrenal medulla was unaffected by the lumbar sympathectomy, this study does not involve a sympathoadrenal mechanism, but demonstrates sympathoneuronal enhancement of primary afferent responses.
The behavioral outcome of the effects of stress on pain sensation appears to reflect a balance between descending spinal pain inhibitory mechanisms and SNS-dependent pain facilitation. This was demonstrated by the different effect of intraperitoneal (fig. 1A) and intraplantar injection of rauwolscine (fig. 7B). The stress-induced reduction in thermal response latency following local intraplantar injection was less than that seen following systemic intraperitoneal rauwolscine injection, presumably because descending inhibition was not disrupted by the intraplantar injection. While stress-induced analgesia is distinct from the stress-induced hyperalgesia mechanism, a reduction of descending inhibition is needed to reveal the stress-induced hyperalgesia. The increased thermal response latencies of contralateral paws (fig. 7A) compared to ipsilateral paws (fig. 7B) following intraplantar rauwolscine injection further demonstrate that analgesia is only manifested when peripheral pain facilitation is attenuated. Interestingly, evidence for physiological antagonism between spinal inhibition and the SNS was shown in early studies characterizing footshock-induced analgesia. Watkins et al showed that inhibition of norepinephrine outflow from SNS postganglionic fibers with bretylium tosylate enhanced stress-induced analgesia.47
Previous findings demonstrate that the SNS can interact with pain fibers without prior injury or inflammation, resulting in a decreased response threshold. It has been shown in rabbits that sympathetic stimulation can affect the electrical properties of unmyelinated sensory neurons without activating them,51 and intradermal injection of norepinephrine and other α agonists resulted in a decreased thermal pain threshold in normal subjects.52 This study demonstrates that when SNS outflow is disinhibited and descending inhibition is diminished, as occurs in the α-2A knockout mice or after treatment with systemic rauwolscine, the SNS effect on primary afferents can be amplified, unmasking stress-induced hyperalgesia (see model in fig. 8). Activation of nociceptive afferents can elicit preganglionic sympathetic neuron activation via spinal and supraspinal pathways.50 When SNS outflow is increased and descending inhibition is diminished, the effects of stress, sensitizing and nociceptive stimuli could be expected to result in a positive feedback loop that triggers enhanced sensation of pain.
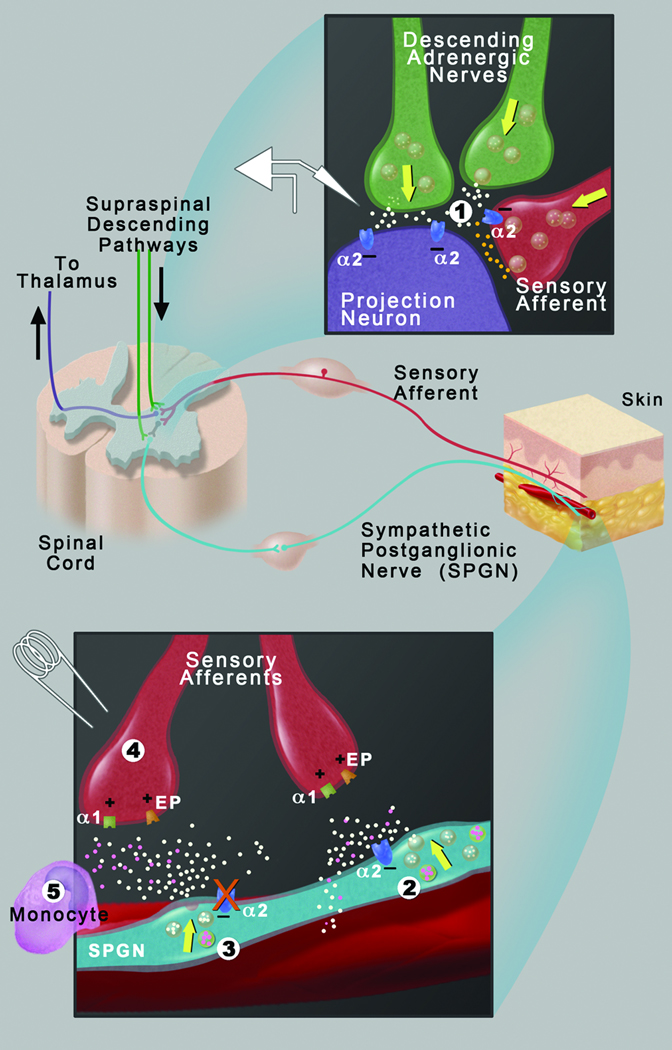
Figure 8. Model of stress-induced hyperalgesia.
(1) Spinal α-2-adrenergic receptors on central terminals of sensory afferents and dorsal horn projection neurons mediate descending adrenergic inhibition, resulting in stress-induced analgesia. (2) Peripheral α-2 receptors on sympathetic postganglionic nerves (SPGNs) mediate feedback inhibition of norepinephrine release in response to stress. (3) In α-2A knockout mice or following treatment with α-2 antagonists (intraperitoneal or intraplantar), α-2 feedback inhibition is reduced, resulting in dysregulated (enhanced) norepinephrine release from SPGNs and sensitization of sensory afferents (4), which leads to stress-induced hyperalgesia when descending adrenergic inhibition is reduced. Role of α-1-adrenergic activation (4) in stress-induced hyperalgesia and decreased sensory afferent thresholds to repeated transcutaneous electrical stimuli was demonstrated by prazosin block. Role of SPGNs in stress-induced hyperalgesia and enhanced sensitivity of sensory afferents to prostaglandin E2 receptor (EP) activation (4) was demonstrated by guanethidine depletion of norepinephrine and surgical sympathectomy. For simplicity, the α-1-adrenergic receptor is drawn on the sensory afferent, but sensitization could also be indirect via another cell type such as a monocyte (5). Dysregulated sympathetic outflow (3) can result in a positive feedback loop between sensory afferents and sympathetic nerves via spinal and supraspinal pathways when descending inhibition is reduced.
Physiologically, disinhibition of SNS outflow and reduced descending inhibition could result from α-2 receptor desensitization or depletion of norepinephrine during chronic stress. It has been reported that administration of a high dose of the α-2 receptor agonist clonidine to rats results in a delayed thermal and tactile hypersensitivity associated with changes in spinal cord excitatory neurotransmission.53 It is possible that this result is due to α-2 receptor desensitization and mimics the effects of sustained norepinephrine outflow during chronic stress. While the experiments reported here have focused on pain responses to acute stressors, the potential role of dysregulated SNS outflow and descending inhibition in chronic pain disorders is highlighted by our findings that the hyperalgesia, unlike the analgesia, persisted substantially longer than the stress (fig. 2B) and that the hyperalgesia did not habituate to repeated stress (fig. 2C). This mechanism could potentially contribute to episodes of heightened pain sensitivity in conditions such as irritable bowel syndrome and fibromyalgia that have been associated with autonomic dysfunction and excess sympathetic activity.54–56
MS #200912133 - Final Boxed Summary Statement.
What we already know about this topic
Stress exacerbates chronic pain in patients, however, the mechanisms for exacerbation of pain by stress are not understood
In most preclinical animal models of pain, stress produces analgesia not hyperalgesia
What this article tells us that is new
When the activity of the sympathetic nervous system was enhanced, stressors worsened pain behaviors through a peripheral α-1 adrenergic receptor.
Acknowledgements
We thank Brian Kobilka, Ph.D. (Professor, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Palo Alto, California) for originally supplying the α-2 receptor knockout mouse lines; Lauren Luhrs, Ph.D. (Senior Scientist, Biological Sciences, Allergan, Inc., Irvine, California) and Veena Viswanath, Ph.D. (Senior Scientist, Biological Sciences, Allergan, Inc., Irvine, California) for comments on the manuscript; Larry Wheeler, Ph.D. (Senior Vice President, Biological Sciences, Allergan, Inc., Irvine, California) for his support of the project; and Lisa Rubin, B.A. (Executive Secretary, Biological Sciences, Allergan, Inc., Irvine, Calfornia) for editorial assistance. Sushma Soni, B.Sc., B.Com. (Senior Medical Writer, Wolters Kluwer, Yardley, Pennsylvania) also provided editorial assistance (copyediting and formatting).
Source of financial support: Portions of this research were conducted at Allergan, Inc. (Irvine, California). Srinivasa N. Raja has received research grant support from the National Institutes of Health (Bethesda, Maryland; NINDS-NS26363) and Allergan, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Department and institution to which the work should be attributed: Department of Biological Sciences, Allergan, Inc., Irvine, California
Meetings at which the work has been presented: Portions of this work were presented at the Second International Congress on Neuropathic Pain, June 7–10, 2007, Berlin, Germany; Neuroscience 2007 (2007 Annual Meeting of the Society for Neuroscience), November 3–7, 2007, San Diego, California; and the 12th World Congress of the International Association for the Study of Pain, August 17–22, 2008, Glasgow, United Kingdom.
Contributor Information
John E. Donello, Department of Biological Sciences, Allergan, Inc., Irvine, California.
Yun Guan, Department of Anesthesiology/CCM, Johns Hopkins University, Baltimore, Maryland.
Mingting Tian, Department of Biological Sciences, Allergan, Inc., Irvine, California.
Cynthia V. Cheevers, Department of Biological Sciences, Allergan, Inc., Irvine, California.
Miguel Alcantara, Department of Biological Sciences, Allergan, Inc., Irvine, California.
Sara Cabrera, Department of Biological Sciences, Allergan, Inc., Irvine, California.
Srinivasa N. Raja, Department of Anesthesiology/CCM, Johns Hopkins University, Baltimore, Maryland.
Daniel W. Gil, BioScience, Department of Biological Sciences, Allergan, Inc., Irvine, California.
References
- 1.Levine JD, Taiwo YO, Collins SD, Tam JK. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postganglionic neurone terminals rather than activation of primary afferent nociceptors. Nature. 1986;323:158–160. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- 2.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci Lett. 1987;82:35–40. doi: 10.1016/0304-3940(87)90167-4. [DOI] [PubMed] [Google Scholar]
- 3.Hu SJ, Zhu J. Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain. 1989;38:85–90. doi: 10.1016/0304-3959(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 4.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 5.Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M. The α2A adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain. 2000;85:345–358. doi: 10.1016/S0304-3959(99)00286-9. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA, Perl ER. Expression of α2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol. 1999;515(Pt 2):533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Q, Zou X, Fang L, Willis WD. Sympathetic modulation of acute cutaneous flare induced by intradermal injection of capsaicin in anesthetized rats. J Neurophysiol. 2003;89:853–861. doi: 10.1152/jn.00568.2002. [DOI] [PubMed] [Google Scholar]
- 8.Raja SN, Treede RD, Davis KD, Campbell JN. Systemic α-adrenergic blockade with phentolamine: A diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698. doi: 10.1097/00000542-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Baron R, Wasner G, Borgstedt R, Hastedt E, Schulte H, Binder A, Kopper F, Rowbotham M, Levine JD, Fields HL. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52:923–932. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 11.Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 12.Budai D, Harasawa I, Fields HL. Midbrain periaqueductal gray (PAG) inhibits nociceptive inputs to sacral dorsal horn nociceptive neurons through α2-adrenergic receptors. J Neurophysiol. 1998;80:2244–2254. doi: 10.1152/jn.1998.80.5.2244. [DOI] [PubMed] [Google Scholar]
- 13.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 15.Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 16.Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz JP, Close LN, Heinricher MM, Selden NR. α2-Noradrenergic antagonist administration into the central nucleus of the amygdala blocks stress-induced hypoalgesia in awake behaving rats. Neuroscience. 2008;157:223–228. doi: 10.1016/j.neuroscience.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant α2a-adrenergic receptor via "hit and run" gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, Secchi R, Sutton J, Eglen RM. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansikka H, Lahdesmaki J, Scheinin M, Pertovaara A. α2A Adrenoceptors contribute to feedback inhibition of capsaicin-induced hyperalgesia. Anesthesiology. 2004;101:185–190. doi: 10.1097/00000542-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 25.Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- 26.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 27.Szikszay M, Benedek G, Hideg J. Non-opiate analgesia following stressful acoustic stimulation. Physiol Behav. 1985;35:135–138. doi: 10.1016/0031-9384(85)90185-4. [DOI] [PubMed] [Google Scholar]
- 28.Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: Activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- 29.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 30.Gil DW, Cheevers CV, Donello JE. Transient allodynia pain models in mice for early assessment of analgesic activity. Br J Pharmacol. 2008;153:769–774. doi: 10.1038/sj.bjp.0707412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal μ-opioid mechanisms. J Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–H1624. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 33.Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 34.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Davies MF, Clark JD, Kobilka BK, Maze M. Isoflurane and nociception: spinal α2A adrenoceptors mediate antinociception while supraspinal α1 adrenoceptors mediate pronociception. Anesthesiology. 2002;96:367–374. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Seeman P, Kapur S. Anesthetics inhibit high-affinity states of dopamine D2 and other G-linked receptors. Synapse. 2003;50:35–40. doi: 10.1002/syn.10221. [DOI] [PubMed] [Google Scholar]
- 36.Martin WJ, Malmberg AB, Basbaum AI. PKCγ contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng HR, Mansikka H, Winchurch R, Raja SN, Dougherty PM. Sensory processing in the deep spinal dorsal horn of neurokinin-1 receptor knockout mice. Anesthesiology. 2001;94:1105–1112. doi: 10.1097/00000542-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki R, Hunt SP, Dickenson AH. The coding of noxious mechanical and thermal stimuli of deep dorsal horn neurones is attenuated in NK1 knockout mice. Neuropharmacology. 2003;45:1093–1100. doi: 10.1016/s0028-3908(03)00281-8. [DOI] [PubMed] [Google Scholar]
- 39.Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr, Oliveras JL. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Res. 1986;374:385–388. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- 40.Willis WD., Jr Dorsal horn neurophysiology of pain. Ann N Y Acad Sci. 1988;531:76–89. doi: 10.1111/j.1749-6632.1988.tb31815.x. [DOI] [PubMed] [Google Scholar]
- 41.Schouenborg J, Sjolund BH. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983;50:1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 43.Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Hirata H, Aston-Jones G. A novel long-latency response of locus coeruleus neurons to noxious stimuli: Mediation by peripheral C-fibers. J Neurophysiol. 1994;71:1752–1761. doi: 10.1152/jn.1994.71.5.1752. [DOI] [PubMed] [Google Scholar]
- 45.Gil DW, Cheevers CV, Kedzie KM, Manlapaz CA, Rao S, Tang E, Donello JE. α-1-Adrenergic receptor agonist activity of clinical α-adrenergic receptor agonists interferes with α-2-mediated analgesia. Anesthesiology. 2009;110:401–407. doi: 10.1097/ALN.0b013e3181943226. [DOI] [PubMed] [Google Scholar]
- 46.Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. α2C-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- 47.Watkins LR, Cobelli DA, Newsome HH, Mayer DJ. Footshock induced analgesia is dependent neither on pituitary nor sympathetic activation. Brain Res. 1982;245:81–96. doi: 10.1016/0006-8993(82)90341-9. [DOI] [PubMed] [Google Scholar]
- 48.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 49.Malmberg AB, Hedley LR, Jasper JR, Hunter JC, Basbaum AI. Contribution of α2 receptor subtypes to nerve injury-induced pain and its regulation by dexmedetomidine. Br J Pharmacol. 2001;132:1827–1836. doi: 10.1038/sj.bjp.0704032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khasar SG, Miao FJ, Janig W, Levine JD. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. J Neurosci. 1998;18:3043–3049. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shyu BC, Olausson B, Andersson SA. Sympathetic and noradrenaline effects on C-fibre transmission: single-unit analysis. Acta Physiol Scand. 1989;137:85–91. doi: 10.1111/j.1748-1716.1989.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs PN, Meyer RA, Raja SN. Heat, but not mechanical hyperalgesia, following adrenergic injections in normal human skin. Pain. 2001;90:15–23. doi: 10.1016/s0304-3959(00)00381-x. [DOI] [PubMed] [Google Scholar]
- 53.Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Ossipov MH, Lai J, Porreca F, Malan TP., Jr Production of paradoxical sensory hypersensitivity by α2-adrenoreceptor agonists. Anesthesiology. 2004;100:1538–1544. doi: 10.1097/00000542-200406000-00029. [DOI] [PubMed] [Google Scholar]
- 54.Bharucha AE, Camilleri M, Low PA, Zinsmeister AR. Autonomic dysfunction in gastrointestinal motility disorders. Gut. 1993;34:397–401. doi: 10.1136/gut.34.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waring WS, Chui M, Japp A, Nicol EF, Ford MJ. Autonomic cardiovascular responses are impaired in women with irritable bowel syndrome. J Clin Gastroenterol. 2004;38:658–663. doi: 10.1097/01.mcg.0000135362.35665.49. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia (Letter) Arthritis Res Ther. 2007;9:216. doi: 10.1186/ar2146. [DOI] [PMC free article] [PubMed] [Google Scholar]