Abstract
Context
The axon guidance cues netrin-1 is a secreted protein overexpressed in many different cancer tissues.
Objectives
To determine whether plasma netrin-1 can be used as a diagnostic biomarker of human cancer.
Materials and Methods
A total of 300 cancer plasma samples from breast, renal, prostate, liver, meningioma, pituitary adenoma, glioblastoma, lung, pancreatic and colon cancer patients were compared against 138 control plasma samples. Netrin-1 levels were quantified by ELISA and immunohistochemistry.
Results
Plasma netrin-1 levels were significantly increased in breast, renal, prostate, liver, meningioma, pituitary adenoma, and glioblastoma cancers as compared to control samples.
Discussion and Conclusion
Our results suggest that plasma netrin-1 can be used as a diagnostic biomarker for many human cancers.
Introduction
The number of patients who are diagnosed with cancer is increasing and it is estimated that there will be 16 million new cases every year by 2020. Cancer accounts for 13% of global mortality and is projected to continue to rise with an estimated 9 million people dying from cancer in 2015 and 11.4 million dying in 2030 (6,7,11,13). Biomarkers represent powerful tools to determine cancer status, course of disease and gauging the efficacy and safety of novel therapeutic agents. Biomarkers can have tremendous therapeutic impact in clinical oncology, especially if the biomarker is detected before clinical symptoms or enables real-time monitoring of drug response. Currently there are very few biomarkers with diagnostic capabilities for cancer that are used in clinics (6,28). Even these lack sensitivity and specificity, and most of them cannot be used for early diagnosis of cancer. Moreover, most are specific for one type of cancer or are raised in a few cancers. In addition, some of these biomarkers are raised nonspecifically in the circulation in many other diseases (28). Therefore, there is a critical need for expedited development of biomarkers and their use to improve diagnosis and treatment for cancer. Malignant transformation involves alterations in protein expression with subsequent clonal proliferation of the altered cells. These altered proteins are often secreted from tumors and released into plasma, which can be monitored both qualitatively and quantitatively. Changes in the levels of these altered proteins from cancer provide valuable information that may be an aid to more effective diagnosis, prognosis, and response to therapy.
Netrin-1 is a diffusible, laminin-related protein identified as neuronal guidance cues during development of the nervous system. Netrin-1 mediates its biological effects through binding to two families of receptors: deleted in colorectal cancer (DCC) and uncoordinated-5-homolog (UNC5H), a group of the four UNC5H1, UNC5H2, UNC5H3, and UNC5H4 receptors (1,2,4). Recent studies have shown that netrin-1 is expressed outside the nervous system and contributes to the patterning of developing epithelial tissues such as mammary gland, pancreas, and lung by regulating diverse processes including adhesion, motility, proliferation, and differentiation of cells (17–20,26,29). Moreover, netrin-1 is known to regulate inflammation, angiogenesis, and apoptosis (16,18,21,22,29,30). The expression of netrin-1 in epithelial cell is induced after injury and these may serve as a biomarker of organ injury or disease (5,25).
Netrin-1 is overexpressed in many cancer tissues (3,8,12) and either inhibition of receptor or downregulation of netrin-1 expression induced apoptosis in tumor cells, leading to tumor regression. However, whether netrin-1 is secreted into the circulation by the tumor and whether circulating netrin-1 can be used as a diagnostic biomarker of cancer is unknown. Therefore we investigated whether netrin-1 is overexpressed in all types of cancer and whether levels of netrin-1 in plasma can be used as a biomarker of cancer diagnosis. Our results show that netrin-1 levels in the circulation is significantly increased in renal, liver, prostate, meningioma of brain, pituitary adenoma, glioblastoma and breast cancer but not in colon, pancreatic and lung adenocarcinoma. Therefore we conclude that netrin-1 can be used as a biomarker of many human cancers and may be useful as a prognostic tool as well.
Material and Methods
Patient population
Specimens from adult patients were collected by the Penn State Cancer Institute Tissue Bank in accordance with protocols submitted to and approved by the Institutional Review Boards of Penn State College of Medicine and Penn State Cancer Institute. The number of patients was based on our preliminary statistical analysis, and sample size was increased based on statistical power. All patients had metastatic tumors of different organs with pathologic confirmation of diagnosis. Tumor diagnoses included invasive ductal carcinoma of breast (IDC) (n = 40), prostate adenocarcinoma (n = 40), lung adenocarcinoma (n = 20), pancreatic adenocarcinoma (n = 20), renal cell carcinoma (n = 40), liver adenocarcinoma (n = 40), brain tumors including meningioma, pituitary adenoma, and glioblastoma (n = 80), and colon carcinoma (n = 20). All samples were from adult patients (age 22–90 years). Except for 3 patients, all patients had not received any chemotherapy before tumor resection. Plasma samples were collected just before surgery. The control patients were noncancerous age- and sex-matched volunteers who may have had different diseases. No control subjects had any known histories of tumors. Collected plasma samples were stored at −80°C, then analyzed for netrin-1 by ELISA (kit from USCN Life Sciences Inc.).
Quantification of plasma netrin-1 by ELISA
De-identified samples were blinded for netrin-1 analysis. Twenty microliters of plasma was used for the netrin-1 assay, which used an ELISA kit (catalogue no. E1827h; USCN Life Science Inc., Wuhan, China). Briefly, netrin-1 standard and plasma samples were added to antibody-coated 96-well plates and incubated for 2 hours at room temperature, followed by addition of biotin-conjugated polyclonal antibody specific for netrin-1 and incubation for an additional 1 hour. Plates were then washed and incubated with avidin conjugated to horseradish peroxidase for 1 hour. Color was developed using tetramethylbenzidine substrate, and reaction was arrested by adding sulfuric acid. The color change was measured using a plate reader (Labsystems) at a wavelength of 450 nm. The concentration of netrin-1 in the samples was determined by comparing the OD of the samples to the standard curve, with a minimal limit of detection of 7.8 pg/ml. All measurements were made in duplicate. Plasma netrin-1 concentration was expressed as picograms per milliliter of plasma. The intraassay coefficient of variation for plasma netrin-1 was 8.1%. The accuracy of the assay was tested by spiking plasma samples with known concentrations of netrin-1. The recovery was 103 ± 10 percent. Dilution linearity was determined by spiking different concentrations of netrin-1 in plasma. The assay was linear from 10 to 2000 pg.
Immunohistochemical localization of netrin-1
Stage I–III renal cell carcinoma and normal tissue section (Tissue Array) was obtained from Biomax to immunolocalize netrin-1, as described previously (29). Briefly, tissue sections were dewaxed and rehydrated with graded ethanol (100%, 90%, 70% and 30%) and then washed with PBS. Antigen retrieval was carried out using citrate buffer and steamer. The tissue section was permeabilized with 0.2% Triton X-100 in PBS, and washed and blocked with PBS containing 5% donkey serum and 1% BSA. Primary antibodies included a chicken anti-netrin-1 polyclonal antibody (Neuromics cat # CH23002). Primary antibodies were detected using secondary antibodies conjugated with biotin, which was followed by incubation with streptavidin-horseradish peroxidase (Pierce). Slides were mounted in permount and photographed using an Olympus microscope attached to a CCD camera.
Statistical analysis
Individual cancer types were compared with organ-matched controls for differences in plasma netrin-1 levels. Due to the skewness of the netrin-1 levels, the log values were used in the analysis. We also compared the individual tumors with combined controls. Receiver operating characteristic (ROC) curve analysis was done to calculate the area under the curve for the independent predictors and to identify threshold values (i.e., cutoff points) that provide the optimal tradeoff between sensitivity of specificity. Area under the curve (AUC) values were used to summarize diagnostic performance of the netrin-1 biomarker. A logistic regression was used to evaluate the effectiveness of netrin-1 as a predictor of cancer while allowing age and sex to be appropriate controls as covariates. P-values corresponding to netrin-1 as a predictor for cancer in the logistic regression are reported in Table 3. Statistical analysis was conducted using R version 2.11.1 (http://www.r-project.org/).
Table 3.
The effects of netrin-1 levels on different tumor types compared against the combined controls in a logistic regression model after adjusting for age and sex. P-values corresponding to the age, sex, and netrin-1 variables of the logistic regression model are provided.
| Tumor Type | Age | Sex | Netrin-1 | |
|---|---|---|---|---|
| 1 | Renal | 0.1842 | 0.0009 | 0.0102 |
| 2 | Breast | 0.0405 | 0.0029 | 0.0083 |
| 3 | Liver | 0.0563 | 0.9859 | 0.0578 |
| 4 | Brain (combined) | 0.0353 | 0.1188 | 0.001 |
| 5 | Pituitary adenoma | 0.2120 | 0.0253 | 0.0140 |
| 6 | Meningioma | 0.3456 | 0.1149 | 0.0662 |
| 7 | Glioblastoma | 0.0087 | 0.4066 | 0.001 |
| 8 | Prostate | 0.8576 | 0.9905 | 0.0002 |
| 9 | Colon | 0.0046 | 0.3063 | 0.6728 |
| 10 | Lung | 0.0001 | 0.4381 | 0.8586 |
| 11 | Pancreas | 0.0018 | 0.1851 | 0.6547 |
Results
Patient characteristics
The average age of the combined controls is 51.7 years, and the average age of the combined cancer patients is 59.8 years; the difference is statistically different (p<.001). The controls consisted of 71% female patients, and the cases consisted of 47% female patients; the difference is statistically different (p<0.001). Only 3.1% of the patients had chemotherapy prior to collection of the samples.
Netrin-1 protein levels in plasma collected from non-cancerous control patients who may have different diseases
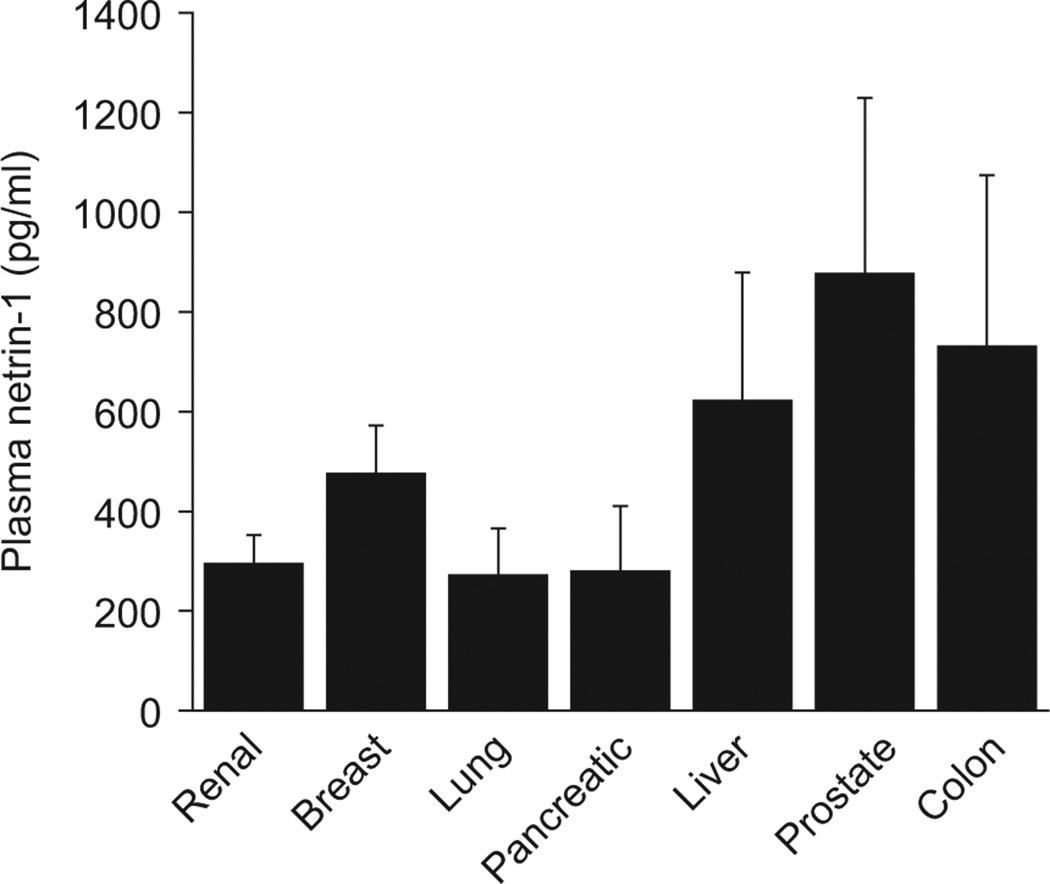
Control patient characteristics and their diagnose are listed in Table 1. In the plasma samples examined by ELISA, no significant difference in the levels of netrin-1 was found among various control patients for different organs (Figure 1). The average plasma netrin-1 levels in a combined control population was 479 ± 74 pg/ml.
Table 1.
Diagnostic description of the control patients for the different tumor types are shown.
| Tissue type | Tissue Type |
|---|---|
| Renal | Pancreas |
| Adenoma | Microcystic serous adenoma |
| Cystadenoma | Mucinous cystadenoma |
| Cystic nephroma | Chronic pancreatits |
| Mesenteric cyst | Fibrosis |
| Adenoma | Serous oligocystic adenoma |
| Benign cyst | No malignancy found |
| Atrophy | Atrophy |
| Nodular hyperplasia | Microcystic serous |
| Ganglioneuroma | cyst adenoma |
| Cortical adenoma | Adenoma |
| Mucinous cystic neoplasm | |
| Cystadenoma | |
| Ulcer | |
| Tubular adenoma | |
| Cystic neoplasm | |
| Colon | Mucinous cystadenoma |
| Adenoma | Serous cystadenoma |
| Fibrosis | |
| Diverticulosis | |
| Fibrosis | |
| Dysplasia | |
| Chronic inflammation | |
| No pathological diagnosis | |
| Breast | Prostate |
| Breast Reduction | Negative for tumor |
| No pathological Diagnosis | Reactive lymphoid Hyperplasia |
| Fibrosis | Negative for malignancy |
| Intraductal Papilloma | |
| Lung | Liver |
| Nodules (non-cancerous) | Hemangioma |
| Caseating granuloma | Calcified granuloma |
| Granulomatous inflammation | Fibrosis |
| Fungal Bronchropneumonia | Nodular Hyperplasia |
| Fibrosis | Bile duct hamartoma |
| Emphysema | Inflammation |
| No malignancy found | Endometrosis with hyperplasia |
| Interstitial lung disease | Fatty Infiltration |
| No tumor identified | Mucinous cystadenoma |
| Caseating granuloma | Cavernous hemangioma |
| Hyperplasia | Focal hyperplasia |
| Interstitial fibrosis | Negative for tumor |
| Hamartoma | Hepatocellular adenoma |
| Granduloma | Solitary cyst |
| Cystadenoma | |
| Bile duct proliferation |
Figure 1.
Plasma netrin-1 concentration in control patients. Plasma netrin-1 was measured as described in Materials and Methods. There is no significant difference in plasma netrin-1 concentration among controls for different types of tumors.
Netrin-1 protein levels in plasma collected from cancer patients is significantly elevated as compared to control patients
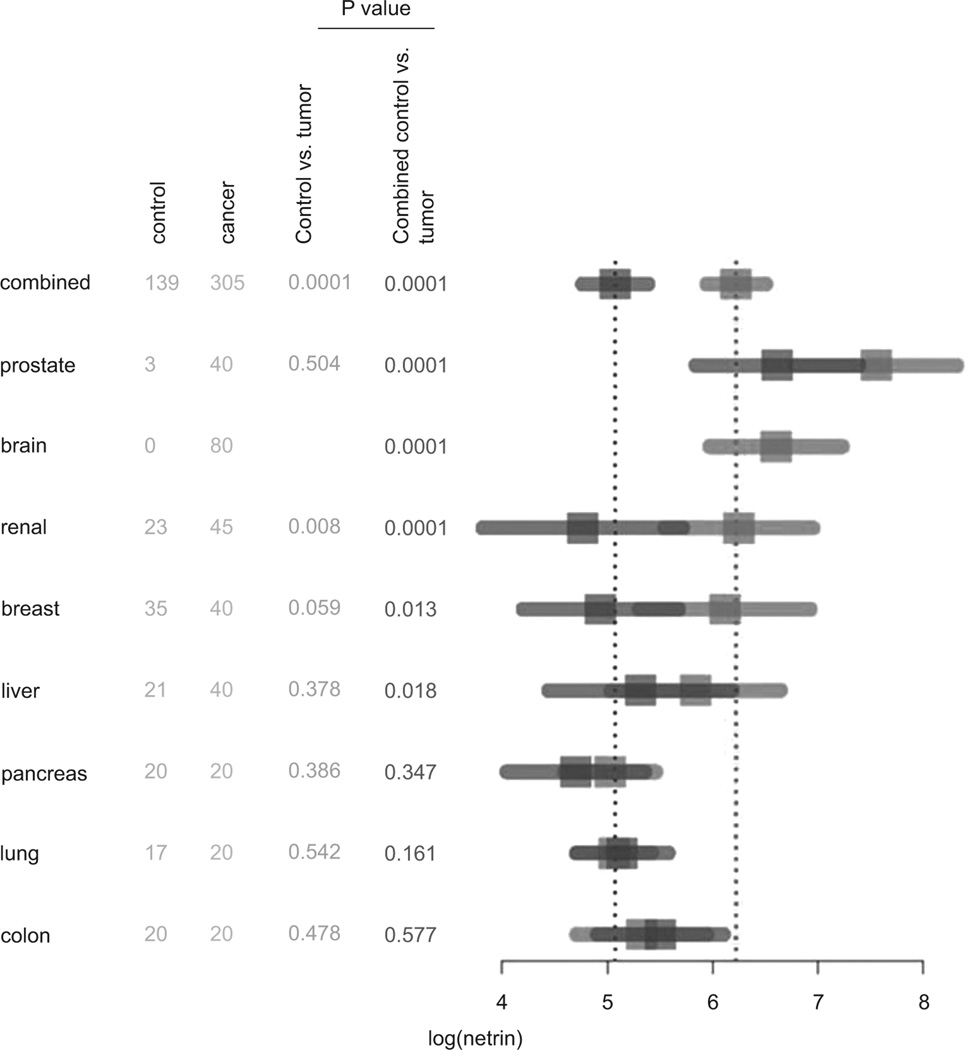
It has been shown that netrin-1 mRNA is overexpressed in many different cancers including breast, pancreatic, colon and brain (8,12,23). However, none of these studies had determined the quantity of netrin-1 protein expression and whether it is secreted into plasma. Moreover, whether netrin-1 is universally overexpressed in all cancers or specific to certain types of cancer is not known. Therefore we have determined whether netrin-1 is elevated in plasma from cancer patients, and if elevated, whether it is specific for one type of cancer or is a general cancer biomarker. The analysis was done in two ways. 1. Levels of plasma netrin-1 in each cancer type was compared with its own tissue control (no cancer but may have other disease of the organ). For this comparison, we did not have any control for brain tumors. 2. The second comparison was made with combining all tissue controls and then comparing with individual tumors types. As shown in Figure 2, plasma levels of netrin-1 are significantly elevated in adenocarcinoma of breast, prostate, liver, renal cell carcinoma, and brain tumors (pituitary adenoma, meningioma, glioblastoma) as compared to their corresponding control patient plasma (Figure 2) or combined control plasma (Figure 2). However, the netrin-1 level is not elevated in plasma from adenocarcinoma of lung, pancreas and colon as compared to their corresponding controls or combined controls.
Figure 2.
Summary of netrin-1 measurements and depiction with a forest plot. For the different cancer types, sample sizes, p-values, and 95% confidence intervals for the mean netrin-1 level are provided. The nonparametric Mann-Whitney test was used to compare the cases to controls or combined controls yielding the reported p-values. The graphic demonstrates the increased netrin-1 levels over the combined controls in the prostate, brain, renal, breast, and liver cancers. P value less than 0.05 was considered significant.
Netrin-1 is a diagnostic biomarker of many cancers
Since netrin-1 was found to be elevated in many cancer types, we determined whether plasma netrin-1 can be used as a diagnostic biomarker of most cancers.
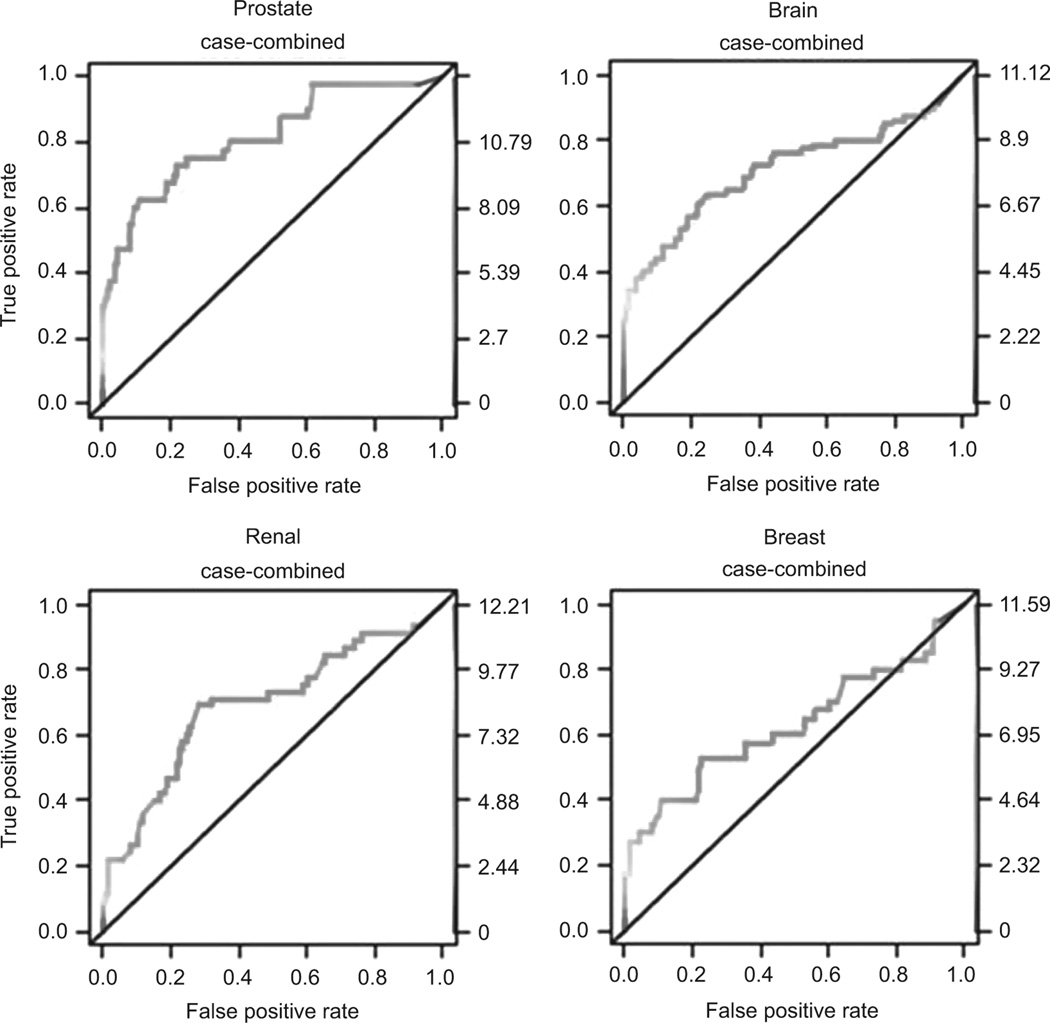
Conventional ROC curves for cancer versus no cancer were generated for plasma netrin-1. The AUC of the different tissue cancer ROC curves are presented in Table 2. Figure 3 displays the unadjusted ROC curve for plasma netrin-1 for different organ cancers. The sensitivities and specificities at log 6 plasma netrin-1 concentrations are listed in Table 2, which corresponds to optimal sensitivity and specificity. The lowest sensitivity was seen for lung and highest sensitivity was seen for prostate adenocarcinoma.
Table 2.
Diagnostic measures of plasma netrin-1 for different tumor types. Sensitivity. and specificity measurements are provided based on a fixed netrin-1 threshold of 6 on the log scale and 95% confidence interval. Area under curve (AUC) values are also provided.
| Tumor | AUC | Sensitivity (low, high) | Specificity (low, high) | |
|---|---|---|---|---|
| 1 | Prostate | 0.813 | 0.75 (0.588, 0.873) | 0.719 (0.637, 0.792) |
| 2 | Glioblastoma | 0.783 | 0.60 (0.361, 0.809) | 0.719 (0.637, 0.792) |
| 3 | Brain (combined) | 0.712 | 0.637 (0.522, 0.742) | 0.719 (0.637, 0.792) |
| 4 | Renal | 0.693 | 0.689 (0.534, 0.818) | 0.719 (0.637, 0.792) |
| 5 | Pituitary adenoma | 0.665 | 0.60 (0.421, 0.761) | 0.719 (0.637, 0.792) |
| 6 | Meningioma | 0.661 | 0.65 (0.408, 0.846) | 0.719 (0.637, 0.792) |
| 7 | Breast | 0.629 | 0.525 (0.361, 0.685) | 0.719 (0.637, 0.792) |
| 8 | Liver | 0.623 | 0.475 (0.315, 0.639) | 0.719 (0.637, 0.792) |
| 9 | Colon | 0.461 | 0.25 (0.087, 0.491) | 0.719 (0.637, 0.792) |
| 10 | Pancreas | 0.435 | 0.10 (0.012, 0.317) | 0.719 (0.637, 0.792) |
| 11 | Lung | 0.403 | 0.05 (0.001, 0.249) | 0.719 (0.637, 0.792) |
Figure 3.
Receiver operating characteristic curve (ROC) analysis for plasma netrin-1 at 6 log for different tumors compared with combined controls.
Univariable logistic regression identified female gender (p = 0.02) (for breast and renal) and higher netrin-1 concentration (p = 0.02) is significantly associated with higher odds of cancer (Table 3).
Associations of netrin-1 with patient characteristics
To determine whether netrin-1 levels in plasma are independently associated with patient characteristics, Pearson’s product-moment correlation coefficient of netrin-1 with different clinical characteristics was calculated. Netrin-1 did not have any significant association with patient’s gender, age, tumor stage, or tumor size.
Netrin-1 is overexpressed in renal cell carcinoma as determined by immunohistochemistry
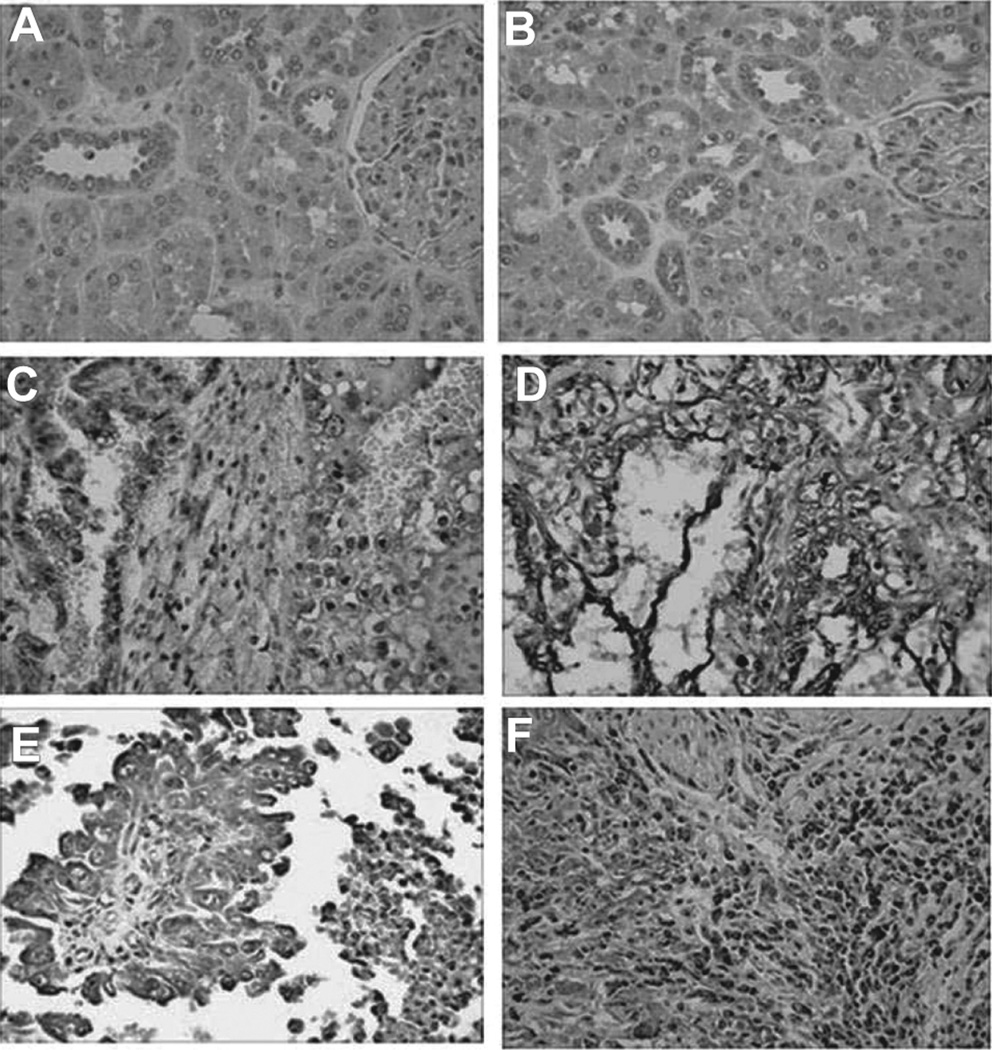
To determine whether elevated levels of plasma netrin-1 are associated with increased expression of netrin-1 in human renal carcinoma tissues, immunolocalization of netrin-1 was carried out from paraffin section (Tumor tissue array from US Biomax, Inc.,). A total of 10 patient tissues were used for immunohistochemistry. All 10 patients had malignant renal cell carcinoma (Stage I–III). There were 7 stage III patients and 1 patient each in stage I and stage II. 5 of 7 patients in stage III and all patients (1 in each) in stage I and II were positive for netrin-1 staining. We were not able to correlate tissue staining and plasma netrin-1 from the same patient as these two samples are came from totally unrelated patients. As shown in Figure 4, normal kidney tissue adjacent to tumor did not show any staining for netrin-1 (Figure 4B). However, increased netrin-1 staining was seen in all stages of renal cell carcinoma malignancies (Figure 4C–F). Secondary antibody control did not show any staining (Figure 4A). In addition, netrin-1 is also overexpressed in non-malignant renal cell carcinoma tissue (not shown). These results suggest that netrin-1 expression is up-regulated and may play a role in tumor growth and invasion.
Figure 4.
Immunohistochemical localization of netrin-1 in renal cell carcinoma (RCC) tissues. A. Secondary antibody control showing no staining. B. Normal adjacent tissues do not show any staining for netrin-1. C. Stage I RCC shows staining for netrin-1. D. Stage II RCC shows staining for netrin-1. E&F. Stage III RCC shows staining for netrin-1.
Discussion
We provide here the first demonstration that a large fraction of plasma samples from different cancer patients showed elevated levels of netrin-1, the ligand of the dependence receptors DCC and UNC5H. This expression was also confirmed by immunohistochemistry. Moreover, we determined whether netrin-1 in plasma could be used as a diagnostic biomarker of cancer. Our results showed that plasma netrin-1 can be used as a global cancer surveillance tool and measurement of netrin-1 can be used for diagnosing cancer of different organs. Earlier studies have documented that netrin-1 is overexpressed in breast (12), colorectal cancer (23), lung cancer (9), melanoma (14), pancreatic cancer (10), and brain tumors (glioblastoma) (8). However, whether overexpressed netrin-1 from tumors is secreted into the circulation and the levels of circulating netrin-1 were unknown. Moreover, whether netrin-1 levels in plasma can be used as a diagnostic tool for cancer was unknown.
The role of netrin-1 and netrin-1 receptors in cancer development and survival is well documented. Increased expression of netrin-1 or loss of netrin-1 receptor expression have been shown to play an important role in the development and progression of cancer. Moreover, the apoptosis-mediated regulatory function of netrin-1 receptor in the adult is involved in breast and intestinal tumor initiation and progression (3,12). Inhibition of apoptosis occurs by either a decrease in the levels of the receptors [e.g., DCC and UNC5H expression is lost in the majority of colorectal cancers] (3,12,19,23) or overexpression of netrin-1. Recent studies have shown that overexpression of netrin-1 confers a selective advantage for tumor cell survival in metastatic breast cancer (12), and Link et al. (15) have observed that netrin-1 overexpression is associated with worse outcome in poorly differentiated pancreatic adenocarcinomas.
Our study shows that netrin-1 is not only overexpressed in tumor tissues but is also secreted into the circulation. Elevated levels of netrin-1 in plasma suggests that plasma netrin-1 is a novel universal biomarker for human cancer. However, netrin-1 is not a specific biomarker for one type of cancer but rather is overexpressed and elevated in the plasma of many cancers as compared to most of the biomarkers currently available. In addition, netrin-1 does not show stage-specific expression and is elevated in all stages of tumors. Therefore, it could be used as an early diagnostic biomarker of cancer (Table. 2). The diagnostic sensitivity of plasma netrin-1 is comparable or better than currently validated biomarkers for cancer (6). Plasma netrin-1 levels do not significantly correlate with gender, stages of tumor, and size of the tumor. However, plasma netrin-1 has a strong correlation with the presence or absence of tumors suggesting that netrin-1 is an independent predictor of the presence of a tumor. Our results indicate that netrin-1 is a powerful diagnostic biomarker of human cancer. The magnitude of raise supports the notion that netrin-1 is a highly discriminatory biomarker with a wide dynamic range and cutoff values that allow for risk stratification.
Plasma netrin-1 likely originates from tumor cells because it is present in low levels in noncancerous patient plasma and high expression by tumor tissues. Moreover, all plasma samples were collected from patients before surgery and before treatment with any chemotherapy. However, the reason as to why netrin-1 levels were not increased in plasma from lung adenocarcinoma, pancreatic adenocarcinoma, and colon adenocarcinoma are not clear. It is possible that these tumors may secret netrin-1 outward into the gut and lung effusion. In this study we did not examine the prognostic value of netrin-1 for any cancer. However, our xenograft animal model showed that netrin-1 level decreases as tumors regress suggesting that netrin-1 may be a useful prognostic biomarker as well (Ramesh et al., Unpublished). This view was also supported by studies in a brain tumor (glioblastoma) xenograft model (8).
Previous studies had shown that breast tumors and a large fraction of lung tumors overexpress netrin-1 but not in colorectal tumors where most of them lose the receptors (only 7% of colorectal cancers show an increase in netrin-1 expression) (3,9,12). Consistent with these earlier observation, we see a larger fraction of colorectal tumor plasma samples do not show high levels of netrin-1. A possible explanation is that netrin-1 expression not only confers a gain in survival on the lung tumor cells but may also lead to enhancement of nonapoptotic signaling by netrin-1 receptors. The role of netrin-1 in nonapoptotic signaling in the adult is not known with certainty. Netrin-1 was shown to bind a complex that includes some integrins (27,32), and thus it is conceivable that netrin-1 overexpression triggers the activation of α6β4/α3 β1 integrins expressed by lung epithelial cells. These integrins regulate cell migration and invasiveness and therefore may have a role in cancer progression (24). It is also possible that netrin-1 is an angiogenic factor that promotes tumor vasculature, since netrin-1 was recently proposed to play a role in embryonic angiogenesis, although this hypothesis is controversial (21,22,30,31). Alternatively, because endogenous netrin-1 expression inhibits proapoptotic signaling by all dependence receptors, it may have more potent effects on survival than the loss of one or two netrin-1 receptors.
The mechanism of netrin-1 up-regulation in tumors is unknown. Hypoxia is known to induce netrin-1 in epithelial cells (25,26,29). Our in vitro studies suggested that netrin-1 is regulated at translational levels (Ramesh et al, unpublished) in renal epithelial cells. However, the mechanism as to how netrin-1 is rapidly translated is not known.
Our study has strengths. First, we retrospectively analysed a relatively homogeneous cohort of cancer patient samples in whom the only obvious etiology for elevated levels of netrin-1 is cancer. Second, in all subjects the plasma samples were collected just before surgery for tumor resection. Third, we eliminated the false rise of netrin-1 due to tumor lysis after chemotherapy since these patients did not receive any chemotherapy before surgery.
The current study also has limitations. First, it is a single-center pilot study of adult subjects who have already been diagnosed with cancer. Thus, these results will need to be validated in a larger population, in a prospective study, to determine the usefulness of netrin-1 as an early diagnostic biomarker of cancer. Second, a large number of our controls are from hospital patients with a different organ disease without cancer. Therefore, it will be important to have non-hospitalized subjects as controls to determine normal blood levels of netrin-1. Third, in addition to netrin-1, simultaneous examination of other plasma biomarkers as potential predictors of cancer may provide additional information. Recent studies have uncovered other cancer biomarkers such as alpha feto protein, carcinoembryonic antigen, prostate specific antigen, cancer antigen and Cyfra2 1-1(6). However, none of these are universal cancer biomarkers or are useful for early diagnosis and screening a healthy population. All biomarkers have individual strengths and weaknesses.
Conclusion
Netrin-1 is overexpressed in many tumors and secreted into the circulation. Elevated levels of netrin-1 in plasma was seen in several tumors at all stages of tumor progression as compared to control patients. Our results suggest that plasma netrin-1 can be used as diagnostic biomarker of many human cancers.
Acknowledgments
This work was supported by grants to Ganesan Ramesh from American Society of Nephrology (Carl S. Gottschalk Research Scholar Grant) and R01 grant (1R01DK083379 - 01A2) from NIH-NIDDK.
Footnotes
Declaration of interest
The authors have no financial conflicts of interest. The authors have no conflict of interest.
Reference
- 1.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 2.Barallobre MJ, Pascual M, Del Rí,o JA, Soriano E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY, Mehlen P. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernet A, Fitamant J. Netrin-1 and its receptors in tumour growth promotion. Expert Opin Ther Targets. 2008;12:995–1007. doi: 10.1517/14728222.12.8.995. [DOI] [PubMed] [Google Scholar]
- 5.Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F731–F738. doi: 10.1152/ajprenal.00507.2007. [DOI] [PubMed] [Google Scholar]
- 6.Cho WC. Contribution of oncoproteomics to cancer biomarker discovery. Mol Cancer. 2007;6:25. doi: 10.1186/1476-4598-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. Jama. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 8.Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin MA, Stupack D, Nakagawara A, Rousseau R, Combaret V, Puisieux A, Valteau-Couanet D, Bénard J, Bernet A, Mehlen P. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833–847. doi: 10.1084/jem.20082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delloye-Bourgeois C, Brambilla E, Coissieux MM, Guenebeaud C, Pedeux R, Firlej V, Cabon F, Brambilla C, Mehlen P, Bernet A. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J Natl Cancer Inst. 2009;101:237–247. doi: 10.1093/jnci/djn491. [DOI] [PubMed] [Google Scholar]
- 10.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK, Bikfalvi A, Hagedorn M. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595–1606. doi: 10.1053/j.gastro.2009.12.061. 1606.e1. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 12.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, Bachelot T, Bernet A, Mehlen P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann S, Kuphal S, Schubert T, Bosserhoff AK. Functional implication of Netrin expression in malignant melanoma. Cell Oncol. 2009;31:415–422. doi: 10.3233/CLO-2009-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Link BC, Reichelt U, Schreiber M, Kaifi JT, Wachowiak R, Bogoevski D, Bubenheim M, Cataldegirmen G, Gawad KA, Issa R, Koops S, Izbicki JR, Yekebas EF. Prognostic implications of netrin-1 expression and its receptors in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 2007;14:2591–2599. doi: 10.1245/s10434-007-9469-6. [DOI] [PubMed] [Google Scholar]
- 16.Llambi F, Lourenço FC, Gozuacik D, Guix C, Pays L, Del Rio G, Kimchi A, Mehlen P. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. Embo J. 2005;24:1192–1201. doi: 10.1038/sj.emboj.7600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. Embo J. 2001;20:2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol. 2010;22:46–54. doi: 10.1097/CCO.0b013e328333dcd1. [DOI] [PubMed] [Google Scholar]
- 20.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Köhler D, Rosenberger P. Netrin-1 dampens Pulmonary Inflammation during Acute Lung Injury. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.200905-0717OC. 200905–0717OC. [DOI] [PubMed] [Google Scholar]
- 21.Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, Zhao J, Park KW, Wythe JD, Thomas KR, Chien CB, Li DY. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–667. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradisi A, Maisse C, Coissieux MM, Gadot N, Lépinasse F, Delloye-Bourgeois C, Delcros JG, Svrcek M, Neufert C, Fléjou JF, Scoazec JY, Mehlen P. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA. 2009;106:17146–17151. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 27.Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci USA. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturgeon CM, Lai LC, Duffy MJ. Serum tumour markers: how to order and interpret them. Bmj. 2009;339:b3527. doi: 10.1136/bmj.b3527. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294:F739–F747. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Zou L, Wang Y, Xu KS, Zhang JX, Zhang JH. Axon Guidance Cue Netrin-1 has Dual Function in Angiogenesis. Cancer Biol Ther. 2007;6:743–748. doi: 10.4161/cbt.6.5.3976. [DOI] [PubMed] [Google Scholar]
- 32.Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]