Abstract
Men’s hunting has dominated the discourse on energy capture and flow in the past decade or so. We turn to women’s roles as critical to household formation, pair bonding and intergenerational bonds. Their pivotal contributions in food processing and distribution likely promoted kinship, both genetic and affinal, and appear to be the foundation from which households evolved. With conscious recognition of household social units, variable cultural constructions of human kinship systems could emerge that were sensitive to environmental and technological conditions. Kinship dramatically altered the organization of resource access for our species creating what we term “kinship ecologies.” We present simple mathematical models to show how hunting leads to dependence on women’s contributions, bonds men to women and generations together. Kinship, as it organized transfers of food and labor energy centered on women, also became integrated with the biological evolution of human reproduction and life history.
Keywords: Household formation, Kinship ecologies, Women’s food processing, Pair-bonds, Maternal energy deficits, Energy transfers, Life history
Introduction—Households and Kinship Ecologies
Resources and Kinship
Humans depend greatly on others for access to resources, and kinship is basic to this access. Understanding this adaptive role of kinship in human societies must employ an evolutionary perspective that recognizes kinship behaviors and ideologies as cultural responses to ecological (resource) opportunities and constraints present in humans, given environmental and technological conditions. As kinship systems become culturally constructed, inherited, and elaborated they also become, as concepts and norms, part of the ecological structure within which social resource flow takes place. For this reason, we refer to them as kinship ecologies: culturally constructed social environments that regulate resource control and access via kinship ties and their associated norms of rights and obligations. In kinship systems, both genetic ties and affinal (plus fictive) ties are subject to variable cultural identification, definition and interpretation. Kinship creates social ecological conditions, beyond those of kin selection (Hamilton 1964), that have the potential to act as evolutionary forces on human behavior and biology (e.g., Heyer et al. 2005). This change represents a major transition in human evolution in that energy flow became not just increased but organized by culture.1 Boyd and Richerson (1985) urged readers to explore the relationship between ecological and cultural evolutionary processes. In the case of kinship, we focus on the intersection between access to biologically critical resources via kinship ties and the cultural evolution of kinship. Households are at the heart of this intersection as the main building blocks of kinship ecologies.
At a basic level, households are defined as groupings for the domestic purposes of food gathering, processing, distribution and consumption to support members of both sexes and all ages. Households are often the locus of mating, learning and other activities. They are variably constituted from consanguineous, affinal and fictive ties and their membership and organization can change with new conditions (Mattison 2010), probably underlying shifts in higher levels of kinship organization (e.g., Holden and Mace 2003). Their form can become culturally expected where conditions are stable. Importantly, they foster cultural consciousness with their recognition as social units supported by cultural norms of food and labor sharing.2 Here, we specifically seek to explore the behavioral ecology underlying the emergence and stability of households. Then in the discussion we suggest how, through food and labor sharing, household formation may have deeply affected human life history. Although there has been intense interest among evolutionary anthropologists in human mating, pair-bonds, bi-parental care, and division of labor (e.g., Borgerhoff Mulder 1990; Marlowe 2003, 2007; Quinlan and Quinlan 2008), little attention has been paid to the evolution of household formation and stability, although households are often assumed. What ecological and behavioral constraints originally drove humans within a band to gather into these smaller sub-groupings? We regard domestic food gathering, processing and allocation by women as the engine that makes household formation possible. We present simple mathematical models to demonstrate 1) the stability of dual-sex, age-inclusive households, and 2) the factors driving men to join households for extended periods. Our models focus on the contributions of women through gathering and (especially) processing resources to provide reliable daily sustenance for household members. We then argue that women’s household functions and their relationship to pair-bonding created energy pooling and distribution effects that appear to have had important impacts on life history characteristics via effects on growth and reproduction. Ultimately, we argue that the evolution of households brought together activities of sustenance and reproduction in a way that allowed complex kinship systems to evolve and promoted integration of biological and cultural evolution.
Kinship Processes
Kinship relations in households are culturally defined and manipulated for energetic advantage, previously seen as the productive functions of households by Goody (1976) and Harrell (1997). Cultural designation of relationships and roles with respect to rights and obligations implies that kinship acts as a powerful but complex mechanism for coordination, cooperation, and conflict resolution among humans. Clutton-Brock (2009) recently advocated for more study of mutualism and manipulation, rather than reciprocity and genetic relatedness, as ultimate mechanisms for cooperation. This additional perspective may be relevant with regard to human households and kinship systems, which are characterized by inclusion of unrelated individuals and asymmetries in power and resource access, and seem ripe for manipulation. With households and kinship, cooperation in labor and food sharing may rest on embedded rewards and punishments, such that once started they became self reinforcing as ties of dependency among group members emerged. The choices became stark, as without cooperation from members, the systems, along with participants, would fail. As individuals became less self sufficient, compelling constraints on behavior emerged, selecting for prosocial and “other regarding” behavior (Boyd and Richerson 2009), with cultural evolution of kinship as its vehicle.
Cultural ideologies reinforce kinship norms and may prevent human kinship systems from eroding to interactions predicted from kin selection alone (Hamilton 1964). Cultural variation can increase the efficiency of accessing resources by permitting a variety of kinship systems appropriate to various environments and technologies. Kinship also solves coordination problems of resource access by assorting individuals and designating priorities of access that incorporate to some extent but also supersede r (genetic relatedness) and reduce potential conflicting claims (Alvard 2003, Cronk and Gerkey 2007, Nolin this issue, van den Berghe 1979).
Dependency and Manipulation
Among humans, individuals rarely live as separate, self-bounded energetic units after weaning (Kramer and Ellison 2010). Dependency is basic to kinship relations and is not entirely benign. For example, we tend to look at mother-child relationships as loving and indulgent, which of course is not always the case (Hrdy 1999). Dependency can be the foundation of manipulation. By making others dependent on them, individuals can improve their position and influence with respect to energy flow. Conversely, dependency involves manipulation of others to respond to an individual’s needs (Hrdy 2009). Consequently dependency can induce selection pressures that can modify appearance, biological development and life history stages. We must consider all directions of dependency when comprehending human kinship relations: younger generations on elder generations (and vice versa), mates on each other, and among relatives of the same generation. Dependency is likely a defining characteristic of human life history, implying that resource transfers are central to the human adaptive complex (Hawkes et al. 1998, Hrdy 2005, Kaplan 1996,R.D. Lee 2003). Thus watching social network energy flow is essential to understanding kinship as an adaptation. As Kaplan (1994) shows in graphs of production and consumption over the life span, individuals produce less than they require in youth and excess amounts at mature and older ages. Resultant transfers of food and labor fundamentally reconstruct the human energetic unit from the individual level to larger aggregations, with far-reaching energetic consequences. The household is a fundamental unit of resource transfers.
Household Cooperation—Pooling of Labor and Food
Households are usually the basic organizational level of energy concentration (foraging and processing), distribution and consumption. Kramer and Ellison (2010) posit pooling of energy (both food and labor) as critical to human reproduction. In ancestral groups of hunter-gatherers, pooling of energy probably occurred at the level of the band (such as large packages of meat), as well as the household (small packages of meat and gathered foods) and between households (e.g., Nolin 2010). The dynamics of energy pooling, however, need to be more rigorously specified in terms of both flow and storage. Pooling would not be as effective without storage mechanisms to create strategic reserves. Reproductive-age women appear to be a major target for pooling of energy; body fat deposition (greater in women than men) emerges as a possible mechanism of energy storage. Among most other mammals, the extra energy required for reproduction must be supplied by the female’s own efforts and is a major determinant of the slow pace of fertility among other apes (e.g., Charnov and Berrigan 1993) compared to the fast pace of human fertility (Hawkes et al. 1998). Pregnant and lactating human females draw on energy reserves stored in glutealfemoral fat deposits, unlike non-human primates who lack these deposits (McFarland 1992; Zihlman and McFarland 2000). By what mechanisms are these effects brought about? Household formation and energy pooling that allows shared resources to be captured and somatically stored by reproductive females is one possible answer. Pooling also supports immediate compensation for reduced labor of and increased consumption by pregnant and lactating women (Hawkes et al. 1997, Hurtado et al. 1992). Contributions by males peak around middle age, falling off with old age (Kaplan et al. 2000). Contributions by pre-reproductive-age males are small as they compete with hunting skills training and growth (Hill and Hurtado 2009). Pre-reproductive and post-reproductive-age female contributions have been documented to some extent (Bock 2002, Hawkes et al. 1997, Kaplan et al. 2000, Kramer 2005, Leonetti et al. 2005, Neill et al. 2005). Furthermore, girls show interest in productivity and work harder at earlier ages than do boys (Neill et al. 2005, Bock 2002).
Food sharing is rare among non-human primates but does occur among chimpanzees and more commonly among callitrichids (Rose 1997). Food sharing practices are ubiquitous among foragers and appear to differ by sex. Foraged products also tend to vary by sex creating complementary work roles and food sources, unlike other species which make food transfers of like kind (e.g., bats, wolves). Males tend to focus on larger game that requires greater peak strength to take (Kaplan et al. 2000). Both sexes tend to capture smaller game (fish, shellfish, goannas) that requires less strength to take. Females focus on plant foods more than do males (Bleige Bird et al. 2009; Marlowe 2007). Among African foragers females tend to collect a majority of the kilocalories eaten with reliable daily productivity (Marlowe 2010). Animal and plant foods require many and differing skills and much knowledge to exploit (Kaplan 1996; Kaplan et al. 2000). The foods each sex procures also have differing sharing potential. Males are subject to more sharing demands by those outside their households than are females because large packages of highly valued nutrients are publicly visible and desired by others, such that the cost of defending the meat is greater than its value to others (Blurton Jones 1987). Male hunting, however, entails issues other than supplying nutrients. Costly signaling via hunting prowess and reputation leads to higher status, reproductive opportunities and success (Hawkes and Bliege Bird 2002, Smith 2004), benefitting both the hunter and his audience who are the recipients of meat. Foods gathered by females, although of less nutritional value than meat (Schoeninger et al. 2001), provide micronutrients from vegetation and energy in the form of carbohydrates. Gathered foods are more reliably obtainable than hunted foods but often require intensive effort to gather (searching, digging, picking, transporting) and process (pounding, grinding, cracking, cooking). The smaller packages and more predictable availability of gathered foods also allow for more regular, smaller-scale collection intended mainly for immediate household consumption, with some but more limited and controlled sharing outside the household (Draper 1975).
Women’s Roles
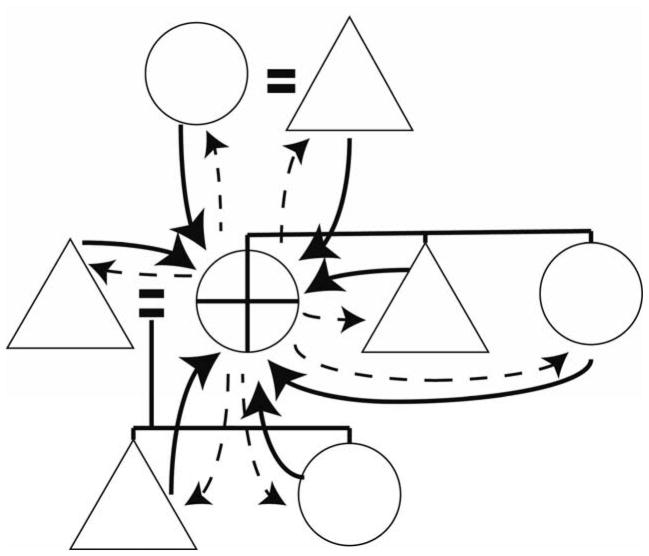
The role of meat sharing by men has dominated the discourse on food sharing (e.g., Hawkes and Bliege Bird 2002, Kaplan et al. 2000, Marlowe 2003, Smith 2004, Wood 2006), with some discussion of grandmother provisioning (Hawkes et al. 1997) and labors (Gibson and Mace 2003, Leonetti et al. 2005) and contributions by offspring (Kramer 2005). The focus on sharing of meat by men to advance their various goals (status, mating, provisioning) has eclipsed concentrated thought regarding food-sharing by women. The contributions made by women in gathering vegetal foods have been documented in terms of their caloric value or by weight (e.g., Bliege Bird et al. 2009, Hawkes et al. 1997, Kaplan et al. 2000, Marlowe 2010). Elston and Zeanah (2002) also conclude it is the complementary array of foods (diet breadth) foraged by men and women that allows human populations to persist. Yet the importance of gathered foods, particularly given their intensive processing requirements, to the evolution of social organization and cultural kinship has been mostly overlooked. We support the idea that it was food sharing by women, including food gathering, processing and allocation that created the bonds of household, underlay the bonding of mates, and created life-long intergenerational bonds that enable and enhance intergenerational cultural transmission. An important component of food sharing by women is the requirement to process many vegetal foods before consumption is possible. The cooking, chopping, grinding, pounding and mixing entailed are usually done by women. In many cultures, women also keep the fire burning (Marlowe 2010, Wrangham 2009). Apportionment also usually falls to the mother or grandmother after processing and this can represent a key role for women in the power dynamics of the group (Fig. 1). Little systematic research has been done on women’s roles in apportionment but a good example is found in Wood and Marlowe (2009) and various ethnographic sources that refer to this role (e.g., Carsten 1995). As Gurven et al. (2000:187) state, tracking food into individual mouths is an almost impossible task. The energetic compulsion to gather around this processed source, however, is seen to underlie household formation. Importantly, men feed on the foods gathered and processed by unrelated women.
Fig. 1.
Diagram of energy pooling (solid lines) and storage with reallocation (dashed lines) centered on reproductive-aged woman (circle with cross inside). Different pathways of pooling and reallocation can be emphasized by differing kinship systems.
Why do women feed men?
The nutritional value of meat is high (women are known to encourage men to hunt) but high variance in hunting success is basic to this question (Hawkes et al. 2001,R.B. Lee 1979:257). Men were able to become hunters, apparently dependent on women to provision them during periods of low hunting success with foods reliably obtained and differing from those mainly pursued by men. Marlowe’s (2003) observations of foods consumed by Hadza, for example, show that men are subsidized by women, eating tubers in large amounts. Men’s regular presence benefits women with protection (Smuts 1992; Wrangham 2009) of their gathered and processed foods. Women then also become protected from sexual harassment from other males, from infanticide, and they are unmolested when meat comes their way from the camp hunters. Plus, males offer small game, honey and other foods.
We posit food processing and allocation as centralizing forces, mostly performed by females, as fundamental to household formation and stability. To start the process of household formation, conditions must have originally developed to select for food-sharing behavior by women as it is little evident in other closely related species, including sharing with offspring. A major impetus to food-sharing probably first arose to provision children in a savanna environment. Access to daily foods is essential to weaned children who would not be capable of extracting the main available foods (roots and tubers) themselves. Collecting tubers and roots is difficult and productivity is age-based. Maximum productivity requires strength and skill associated with full maturity and extends into post-reproductive life (Hawkes et al. 1997, Kaplan 1994, Kaplan et al. 2000). Thus, kin-selected food sharing with children likely occurred first, including the contributions of grandmothers past reproductive age.
Given the long time to productive maturity among humans (Kaplan et al. 2000), provisioning was probably extended considerably to older ages of offspring. Sons who benefitted from food sharing would have continued to expect contributions from females as they grew older and devoted their time to unreliable hunting. From which females did males expect contributions? Their mothers would do so up to a point but also encourage some donations from their sons’ foraging activities, priming them to be contributors to a household. With puberty comes a new interest in mating, so another focus of young males might be young reproductive-age females who would attract their attention. Such females, however, would have been unlikely to produce surplus quantities of gathered foods and were possibly dependent on their own mothers for some food. Hence, the role of the woman’s mother may have been critical in the food pipeline. This situation may underlie the probably ancient practice of bride service to in-laws by the young hunter. We are also familiar with “arrangements” for marriage carried out by elders for their children in many cultures. The woman’s parents would then enjoy some control over the quality of the male (the value of his contributions) who has sexual access to their daughter (Leonetti et al. 2007). Thus, intergenerational households emerged with cultural recognition and reinforcement, with potential fitness benefits for all generations.
Pair-bonds and Dependency
In human kinship systems, households are the building blocks for networks of ties that set up larger kinship ecologies. Ties between households are usually built on marital links. Without marital links and recognition of paternity, these structures could not be as culturally expansive. Chapais (2008) makes the argument that pair bonding sets up the necessary conditions for primeval kinship formation through consistent paternity recognition. It is true that recognition of both maternal and paternal kin expands the set of possible kinship relations and allows the complexity seen in human kinship systems. Chapais’ proposition, however, begs the question of why pair-bonds would form. What motivates human males into long-term relationships with a particular female? Consort relationships among non-human primates rarely endure very long (Manson 1997). Food sharing occurs among chimpanzees to a small extent but it is fleeting and often begrudged. New evidence points to long-term sharing of meat by male chimpanzees for sex with females but households do not form (Gomes and Boesch 2009). Humans on the other hand enter into the long-term sharing arrangements of households, the stability of which is remarkable. We suggest mating acts as reinforcement within the complex relationship involving mutual dependency and resource transfers that emerges between human males and females in households. Since “love” between spouses is not considered to be a prerequisite to marriage in many societies and is even often discouraged by relatives of the partners (e.g., by mothers-in-law in India: Leonetti et al. 2007, Roy 1972; African American matrifocal kin: Stack 1974), we do not see it as the sustaining force behind households. Prolonged and repeated exposure between social partners via energetic dependency would, however, facilitate long-term bonding responses and their basis in shifting hormonal balances (Curtis and Wang 2003, Gray et al. 2002, Neumann 2009).
Bonding is thought to entail an emotional element that has been recognized since Bowlby’s (1969) evolutionary perspective on attachment of children to their mothers. His premise was that security (from predation, falls from trees) depended on attachment to the primate mother. This need set up emotional/motivational seeking of that comfort. Reaction to parental absence has been shown hormonally to be associated with high cortisol levels and anxiety in monkeys and humans (Levine and Weiner 1988, Flinn 2006). Once food sharing became established, the need and motivation for security became further expanded with this new dependency on others for food that would become life-long. Extensions of attachment theory to adult bonds and developmental trajectories have been developed (Belsky et al. 1991, Ellis et al. 2009, Chisholm 1996, Chisholm et al. 2005). The bond between mated pairs can vary in intensity and duration but a mechanism based on emotional attachment derived from security, alleviating anxiety with respect to food access could support it. Need and anxiety can also motivate mutual manipulation and become a selection force for cultural inventions of kinship identities, recognized signs of commitment (Frank 2001), and, importantly, norms to manage relations of labor and food transfers. Due to mutual dependency, generating intense focus on one another, and mating activity providing proximate mechanisms such as hormonal reinforcement (e.g., oxytocin and vasopressin, Young and Wang, 2004) variably enduring bonds could form. Even studies of populations with high mate turnover (e.g., Ache) show that males usually settle into long-term pair-bonds (Kaplan et al. 2010, Winking et al. 2007).
Models to Explain Household Composition and Stability
In Boxes 1 and 2 we explore stylistically how two factors fairly ubiquitous among hunter-gatherers (diet breadth and sexual division of labor) make the role of food processing by females key to maintaining household stability. The model in Box 1 assumes diet breadth (both plant and animal foods) based on anthropological knowledge of forager diets world-wide (Cordain et al. 2000). Furthermore, current nutritional sciences recognize that variety in dietary components, including macro and micronutrients, provides the best basis for optimal growth, development and health (Rolfes et al. 2009). Lean meat alone, for example, cannot sustain the human body (e.g., rabbit starvation, Cordain et al. 2000).
Box 1. How is household stability driven by female food processing effort?
To answer the question posed in this box, we first ask how female food processing effort in hunter-gatherer societies drives household instability. That is, we examine how deficits in food production (gathering and processing) effort by pregnant and lactating females threaten household resource security. Then, we show that to achieve household resource security other household members (i.e., juvenile and post-reproductive females, males) will compensate for these deficits if doing so maximizes their potential inclusive fitness. Our model captures two important aspects of households in hunter-gatherer societies: diet breadth and sexual division of labor. Diet breadth could be the result of an optimal foraging strategy (solved off-model) or the requirement to balance micro- and macronutrient content in the diet. We are agnostic as to whether the sexual division of labor results from cooperation, conflict, or both between males and females. We do, however, assume that members of a household are able to cooperate in order to fulfill diet breadth. These characteristics of household dynamics and human subsistence appear to be present in varying degrees among most hunter-gatherer societies that behave in a way remotely similar to what is believed of early Homo sapiens.
For simplicity, imagine there are two basic resource types: one that requires intensive food processing effort but minimal peak effort during foraging (which we call G); and one that requires minimal food processing effort but intensive peak effort during foraging (H). Diet breadth is defined by BG and BH, the minimum household effort that must be devoted to foraging and processing of G and H in order for a household’s resource base to be stable. Define FG and FH = 1 − FG, MG and MH = 1 − MG as the fraction of total individual effort TF and TM devoted to G and H by females and males, respectively. The sexual division of labor is defined by f, the minimal proportion of effort females must or will devote to G, and m for males with regard to H, where f ≫ 1 − m, 1 − f and m ≫ 1 − m,1 − f(females mainly pursue G while males mainly pursue H). The stability of a household comprised of Nf productive females and Nm productive males requires that aggregate foraging effort must satisfy or exceed BG and BH, or:
where FG ≥ f and MG ≤ 1 − m to satisfy the division of labor requirement. Assume that these requirements are met if no females in the household are pregnant or lactating (not a likely long-term scenario in a hunter-gatherer society). If any females are pregnant or lactating, female effort falls below some threshold τ and the diet breadth requirement is not satisfied. Female effort toward G suffers the most since f ≫ 1 − f. What type of female effort toward G will suffer the most: foraging or processing? Let pG ≫ pH ≈ 0 be the proportion of total effort devoted to processing of G and H, respectively. If pG>1 − pG, then female processing effort of G suffers the most. Thus, the discrepancy in diet breadth from the exogenously imposed requirement is:
is mostly due to losses in processing effort by pregnant and lactating females.
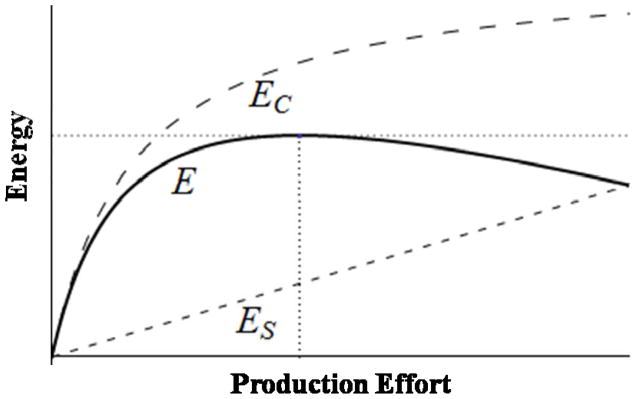
How will females suffer losses in production effort focused on processing? To answer this question, we query the state-dependent (e.g., pregnant, lactating or neither) optimal level of effort for females. Assume that females’ direct reproductive value is an increasing function of energy balance E = EC − ES, where EC and ES refer to energy consumed and spent, respectively. Assume also that EC and ES are monotonically increasing in TF, but d2EC/d2TF< 0 (i.e., diminishing returns), and ES ≤ EC between TF = 0 and some TF =tF(i.e., d2EC/d2TF< d2ES/d2TF). Thus, a females’ optimal effort satisfies, where.
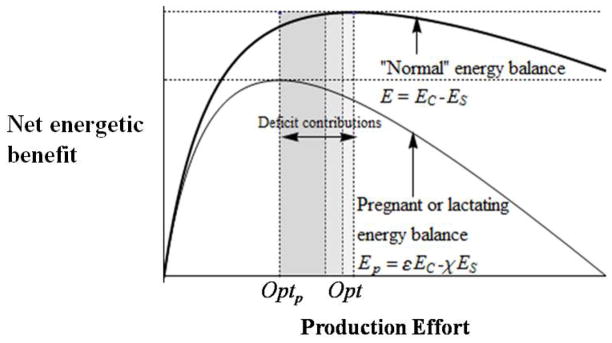
Figure 2 provides a graphical example of energy balance optimization of production effort. We define and χ>1 as the proportional effects of pregnancy or lactation on EC and ES respectively (assuming that effects on pregnancy and lactation are roughly equivalent). Thus, the energy balance function of a pregnant or lactating female is Ep = εEC − χES leading to a new level of optimal effort Optp. Because Ep<E, OptpOpt. The deficit contribution of a pregnant or lactating female is thus Df = Opt − Optp. Assuming pregnancy and lactation affect processing and foraging effort in roughly equivalent ways, the minimal proportion of a pregnant or lactating female’s deficit contribution due to losses in processing effort is fpGDf. The total household deficit against the diet breadth requirement, given Np pregnant or lactating females, is NpDf, and fpG NpDf the proportion of the total deficit due to losses in processing effort. These are conservative estimates given that they assume pregnancy and lactation affect processing and foraging effort of G in similar ways. Given the intensive processing requirement of G, the effect of processing effort on energy balance is at least that of foraging. Figure 3 graphically summarizes this argument. In the graph, the darkest shaded portion of the female deficit contributions polygon is the deficit due to processing G, the next darkest due to foraging for G, and the lightest due to producing H. Understand that the width of the shaded regions in Fig. 3 is a stylistic depiction flowing from our assumptions about sexual division of labor, and does not emerge from real data. One of our aims is to encourage researchers to obtain requisite data on processing versus foraging effort and energy balance to see if the model bears out empirically.
If for any individual i, his or her inclusive fitness is negatively affected by discrepancies between actual household production effort and diet breadth, then he or she is potentially motivated to compensate for at least some subset of pregnant and lactating females’ deficit contributions. Note that, because OptpOpt, compensation effort by pregnant or lactating females must be less than that of pre- and non-reproductive females. If the deficit is large relative to the aggregate compensation possible for pregnant or lactating females, then pre- and non-reproductive females will be the main contributors to compensation. In addition, the required effort toward H, BH, depends more on male effort than female effort (d BH/d TM ≫ d BH/d TF) because m ≫ 1 − f, 1 − m. As already shown, deficits arise mainly from diminished processing effort by females. Therefore, diet breadth and labor division constrains male more than female compensation efforts, and females must often compensate more than males. Female compensation will mainly entail increased processing effort because G requires intensive processing. These final remarks are not statements of additional assumptions in our model, but of logical conclusions that flow from our three assumptions, previously described, that there is a diet breadth that must be satisfied, there is a sexual division of labor, and females suffer diminished productivity due to pregnancy and lactation.
Box 2. Why would males bias compensation effort against processing?
(Refer to Box 1 for explanation of notation.)
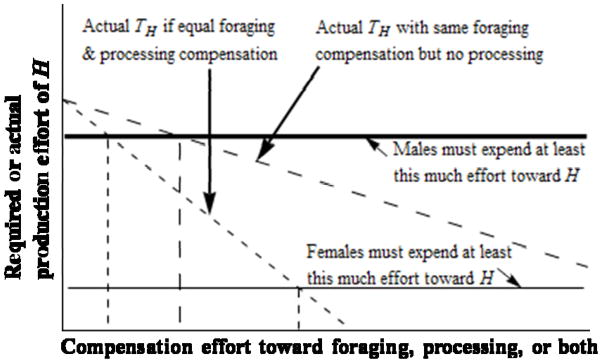
Males and females might bias their compensation effort toward processing or foraging for G. Assume that the production of H requires insubstantial processing effort (pH≈ 0), foraging for G is compatible with foraging for H, but processing G is less compatible with foraging for H. We justify these assumptions in a previous section in the main text. The baseline division of labor is stark, as before, so that most compensation effort is toward G, and males are mainly responsible for H. If individuals compensate, then their foraging effort t toward H(assume they perform roughly equivalent effort), is t = T + stfor + xstproc, where T is effort allocated toward H in the absence of compensation, − 1s< 0 represents the diminished time allocated to H due to processing effort, x>1 (−1<xs< 0) the increased incompatibility with H associated with processing G, and tfor(tproc) the time allocated toward foraging for G(processing G). For a household to be stable, t ≥ BH. Because d t/d tproc< d t/d tfor(because xs<s), the diet breadth is potentially threatened more by processing than foraging compensation. Because m ≫ 1 − f, the sexual division of labor, in combination with BH, constrains aggregate male more than aggregate female processing compensation. Thus, males must (in aggregate) compensate less in processing than foraging for G if T is very close to mBH ≈ BH. Figure 4 graphically summarizes this logic. If we dropped the assumption that H does not entail some intensive processing, the preceding argument could also explain in general a male preference against processing compensations.
In sum, we need not hold any assumptions about why the sexual division of labor arises, nor about the relative merits of women’s versus men’s work, to conclude that the stability of hunter-gatherer households is driven strongly by the processing effort of females. If there is a stark sexual division of labor and women’s production effort includes necessary processing, then hunter-gatherer households are held together through compensation of women’s production effort, biased toward processing effort by girls and non-reproductive women.
Energy deficits from pregnancy and lactation are critical to the model. These have been described as periods of lower female productivity (e.g. among the Ache, Hiwi, and Hadza: Hurtado et al. 1992, Marlowe 2003). Although energy requirements of pregnancy and lactation are not identical (lactation is more costly, Sellen 2007), and also change over the span of a reproductive bout, to simplify the model we assume they are similar and together represent the energy demands of the reproductive condition. Thus both an increase in energy needs along with a decrease in production occurs. This deficit must be compensated by help from others whose inclusive fitness is at stake. Increased food transfers from males to females during lactation have been documented (Marlowe 2003) and high productive efforts of grandmothers were first shown to affect child growth by Hawkes et al. (1997). We argue that males are less likely (willing), however, to compensate for food processing deficits than for gathering deficits, as the former is less compatible with hunting activities. Processing also requires a skill set that men are less likely to master given their keen interest in learning the very different skill set of hunting. Both require detailed knowledge and motor skills that differ. Increased proficiency with age for each has been documented by Kaplan et al. (2000) and Bock (2002), although challenged by Blurton Jones and Marlowe (2002) and Bleige Bird and Bird (2002). Processing is also a requirement prior to ingestion of many important food sources (roots, nut, seeds) so it must be carried out daily for survival, especially of small children. Thus, while both gathering and processing decrease, men will tend to compensate with gathering efforts as more compatible with hunting (also given priority by status-seeking males) and processing efforts are more likely to be performed by non-reproductive females (who will also compensate by gathering). It has been argued that females prefer gathering and processing as being more compatible with pregnancy and lactation than hunting (Hurtado et al. 1992, Meehan 2009, Panter-Brick 2002). While true, distinguishing between gathering and processing with respect to variable compensatory efforts by others reveals more effectively the interdependence (underlying household formation) between the sexes and generations than would appear considering gathering deficits alone. Consideration of processing requirements also identifies a role beyond gathering for senior generation females in households in a compelling less substitutable (by males) way, suggesting even greater selection for post-reproductive life. The necessity of processing foods appears to keep households inclusive and together once formed.
In Box 3, we explore the constraints on male hunters that drive them to join households. For the hunter, a steady source of food when meat was not available from large, widely-shared kills or their own bad luck would depend on having a regular relationship with a food gathering/processing woman. This long-term relationship would be preferable to repeated begging or stealing from women with food. Even where households are relatively unstable, men associate themselves regularly with one household for domestic purposes (usually that of their current mate or mother/sister). This positioning would also set up conditions for status building by males, by obscuring this resource base as ordinary and expected (given the low quality of the food shared compared to meat) and not public, but within household units. In contrast, begging would be highly visible (especially with vocal rebuffs), and set men in inferior positions to women, an intolerable state that would undermine male status building. By attaching to one household (a woman and her mother, or his mother and his attached mate), men could also reduce aggressive competition among themselves as household position would be determined by women and not be readily challenged by other males given the endowment effect 3 (Thayer 1985). Once a woman and man paired up, other men would tend to look for other opportunities that had lower access costs, and paired men would act to protect their endowment. Why would he stay with a particular woman when his hunting production is shared among the larger group and he might have multiple opportunities to mate? First, he could best secure his provisioning source by staying around and offering services such as protection or small meat packages or other high value foods. Once the relationship was acknowledged by others, his constant presence would not be necessary. Even cercopithecoids are able to remember relationships between others and act accordingly as shown by Silk (2007). With language development gossip could keep general vigilance high (Smith 2010). With cultural evolution of the cognitive identification of households as societal units, the achievement of being a central household member (breeding male or female) also conveyed status as an adult. Males in their status quests could leverage this position for public standing by representing their household to other males.
Box 3. Why does processing by females help dual-sex households form in the first place?
In Boxes 1 and 2, we presented arguments for why intensive food processing by women holds households together. Here, we use a similar framework to understand why households comprised of men and women initially emerge. We provide two explanations. First, males often need to subsidize their biased pursuit of H with G to satisfy an optimal diet breadth, and can most easily obtain G by attaching themselves to a particular female’s processing station. Second, attached males may provide females protection against coercive or surreptitious theft of G by other males.
Assume that individuals have an optimal diet requirement, measured now in terms of energy capture rather than production effort. Here, AG is the minimal amount of energy from G that an individual must consume and AH is the same for H. This formulation makes sense if individuals will be unable to consume enough energy without broadening the diet, if they must broaden the diet to avoid nutrient deficits, or both.
In hunter-gatherer societies, food is shared. Some types of food are shared more than others. Here, w and zε (0,1) are the proportional losses in personal energy capture due to sharing H and G, respectively. Assuming that H is shared outside the household much more than G(for whatever reason), w ≫ z ≈ 0. So, an individual’s actual energy capture is defined as K ≈ KG + wKH + h(w), where Kk is the capture of resource type k before sharing takes place and h(w) is the amount of H received from sharing. If the sexual division of labor exists prior to the formation of households, then males will pursue H more heavily than G. So, KG≪KH. If sharing of H does not threaten the optimal diet requirement, then the sexual division of labor still might. Because G is not shared as widely as H, a male with insufficient AG can only obtain it by begging or stealing from a female, or by exchanging with her. Because, as Boxes 1 and 2 suggest, females spend much of their time processing, he would likely find a female (and any co-resident kin) at the station where she processes G.
The male would prefer not to beg for his food if begging negatively affects his reputation. He could steal the food, but this could lead potentially to a diminished reputation, as well. If a male prefers exchanging with at least one female over begging and thievery, then he contributes what he can to whatever outstanding deficits she may have against the optimal diet, which primarily involve AH due to the division of labor. We assume that males are motivated enough by reputational concerns to avoid begging or stealing unless there exists no female accepting of their presence. We also assume that males are motivated also by mating concerns, and expect some mating access in return for services rendered. Yet females will not accept the services of a male with insufficient skill to make contributions toward A or compensations for D. Under these assumptions, there will be a number of destitute males whose only recourse to fulfill AG is begging, theft, or both.
Females may receive a good deal of H because it is shared widely. Thus a specific female is potentially worth more to the male than a specific male to the female in terms of resource exchange. If this is true, the female can potentially extract additional contributions beyond H during times when the condition above holds. For instance, she could demand he partially compensate for any deficits she and her co-resident kin have toward the diet breadth requirement (see Box 1). In addition, she could extract efforts toward vigilance and retaliation against theft (or unwanted begging) by destitute males.
If our argument holds weight, then processing by women in a society marked by sexual labor division leads indirectly to dual-sex household formation because men will find the resources they lack at women’s processing stations. Women will remain focused on processing effort, and men on foraging effort, given the conditions described in Box 2.
Discussion—Process, Men’s Psychology, Sexual/Intergenerational Conflict
Efficiencies, Dispersal and Attachment
From the process described and modeled, it appears that households are inherently female-centered units, even in patrilocal societies. Thus food and labor sharing with respect to fitness optimization for members can be strategically influenced by women both as managers of and targets for energy flow. Consequently, households enjoy gains in efficiencies of production, distribution, and consumption (Gurven et al. 2009) over what could be possible for individuals alone or for larger entities such as bands, according with economists’ views of households (Becker 1981) and marriage (Lundberg and Pollock 1996).
These efficiencies are likely worked out as individuals situate themselves in households within larger groups by dispersing to mate or by staying with natal kin (and receiving mates) to form households. Individuals can even each stay with natal kin (sister-brother) in bilocal arrangements. Household membership is particularly important for young newly mated/bonded individuals as they often are not productive enough to support reproduction alone and need assistance from the older generation. Ecological and social constraints such as access to resources via territories, home ranges, or property are likely to guide this process of dispersal and household attachment (e.g., Emlen 1995, Strassmann and Clarke 1998). Although basically facultative, dispersal and attachment are often institutionalized (presumably when environmental conditions are stable), restricting individuals’ choices. This process can also generate large variance in household membership, both within and between cultures. Usually, however, membership includes both sexes, children, and often senior generations, depending on ecological constraints on productivity and emergent sharing norms. Households composed of single individuals can also result from this process, as some are in transition, have run out of options, or have opted out. Organization around dependable food gathering/processing women seems to anchor this complex of behaviors. Since past conditions have changed rapidly at times, facultative cultural adaptations such as kinship to maintain organized access to food have been critical (Richerson et al. 2005). Thus, our processual understanding of household formation allows both cross-cultural and intracultural variation to emerge and cultural evolution to be responsive to differing conditions.
Men’s Psychology
Beyond the focus in evolutionary psychology on males seeking out attractive women (Buss 2006), male psychology and behavior also conform to an attachment (dependency) scenario since in pair-bonds males are usually supported by females in the domestic sphere. Men are likely susceptible to this support, indeed expect and demand it, as the consequence of a lengthy developmental trajectory during childhood and associated dependence on maternal care and provisioning. Men benefit in many ways from marriage including lower mortality, which has been shown not to be just a selection effect of healthy men into marriage (Lillard and Panis 1996). Further, recent information on the lowering of testosterone levels in bonded human males adds to this picture and suggests pacification of males via pair bonding (Gray 2002). The saying “the way to a man’s heart is through his stomach” may offer folkloric evidence of this relationship. As long as women had free access to resources on their own as gatherers, their provisioning of men (hunters) may have given them some control over men because of this dependence.
Males may seek to control the household units on which they depend. Their success varies by environment and technological capacities. When men started to control the resource base via land ownership or domesticated livestock, the tables turned on women, enabling men to control women via resource access, while maintaining benefits from their wives’ domestic services (Kaplan et al. 2009). Cultural inventions regarding supernatural forces (Smuts 1995), warfare (or raids, gang rape) can also terrorize women into compliance, amplifying the protective roles of men (Smuts 1992). With male control of resources, men can constrain female activities extensively, even abusively (Smuts 1995). Yet, even where men control access to resources, women often develop controlling relationships with their young sons, via their dependency on their mothers, which can persist until their mother’s death. Japan and India are important cases examined by psychologist Roland (1988). Women benefit from men’s presence as shown by Leonetti (2004) for matrilineal, matrilocal Khasi of NE India. Khasi women are central in kinship and household arenas as well as being highly productive. They can get along without men. Men, however, still contribute and enhance women’s reproductive success, so women usually seek to remarry after spousal death or divorce. Where women can secure resources independently of men’s control, however, they are usually able to maintain their own households and even make up a good proportion of households in some societies (e.g., the Caribbean, Brody 1981; Khasi, Leonetti et al. 2004).
What about Conflict?
Conflict is basic to kinship systems and pair-bonds (Bird 1999, Borgerhoff Mulder and Rauch 2009). Kinship systems are likely to have evolved from points of conflict or tension regarding benefits from resource access and control between the sexes and generations. Kinship norms likely emerged from manipulations and negotiations over these issues. It may serve to view kinship systems as solutions to conflict. Conflict can contribute to breakdown in cooperation but may be minimized with organizational strategies such as kinship systems that predetermine priorities. Lineage systems, for example, emphasize one sex or the other and order allegiances (Alvard 2003). Timing strategies for marriage, determined by kinship systems, allowing it to speed up or delay (Tuljapurkar and Wiener, 2000), could reduce intergenerational and sibling conflict.
From animal studies, we have theories of conflict that address the exploitation and manipulation of one sex or generation by the other for fitness benefits and the counter responses of the exploited sex or generation (Holland and Rice 1998, Trivers 1974). One possibility of manipulation provided in households is feeding (and/or reduction of work load) of reproductive-age females by males (or by grandmothers or even by children) which could up-load the female energetic base for ovulation and accumulation of body fat for energy storage for gestation and lactation that quickens the pace of reproduction. Most members of the group can potentially benefit with respect to reproductive success or inclusive fitness whether a previous child, a parent, or a mate. Hadza male provisioning is positively correlated with fertility levels and lactation (Marlowe 2001, 2003). Quinlan and Quinlan (2008) also find cross-cultural evidence for male partner support of lactation. The effect would be to bring about reduction of lactational amenorrhea and early resumption of ovulation compared to chimpanzees. Conceptions could then be more frequent and still-dependent children would accumulate with early weaning. Sexual conflict would thus be centered on increased reproduction sponsored by males and reluctance in females to take on greater reproductive costs and its survival risk to offspring. It is common for men’s family size goals to be larger than women’s (e.g., Borgerhoff Mulder and Rauch 2009, Mason and Taj 1987, Voas 2003). One solution would be provisioning and direct care of weaned dependent offspring by grandmothers, older siblings (Kramer 2005, Hawkes et al. 1997), or fathers, enhancing the value of male care relative to desertion (Fromhage et al 2007). Paternity certainty in the latter case would then be critical, reinforcing household formation and maintenance (Anderson et al. 2007).
Mothers versus Daughters
Isbell (2004) argues that it is advantageous for primate mothers and daughters to stay together if food distribution and abundance is sufficient, as female dispersal is dangerous, increasing predation and infanticide risk. She also discusses possible reproductive suppression in daughters who stay with their mothers, citing gibbons as a possible example, along with marmosets and tamarins. With late age at first reproduction, we must consider this possibility in humans. Among humans, after offspring are weaned, their access to sustenance largely lies within the household and under the control of an adult female, suggesting competition over food access within and between generations. Reproductive overlap between generations is fairly rare in humans compared to other primates (Cant and Johnstone 2008). The prospects for reproduction by a human daughter could be quite constrained while her mother is in her prime reproductive years and requires more of the pooled resources of the household herself, including contributions by offspring (Kramer and Ellison 2010). Among Roma oldest daughters delay reproduction and stay with their mothers even after marriage (Bereczkei and Dunbar 2002). Among Khasi youngest daughters, who inherit and live with their mothers, marry at older ages than elder daughters (Leonetti et al. 2007).
Possible intergenerational competition could underlie the slow growth rate, delayed maturity and adolescent subfecundity of humans compared to non-human primates described by Bogin (1999). Also Gurven and Walker (2006) have suggested that energetic drain on the mother to support multiple dependent offspring leads to this slow growth. Kramer and Ellison (2010) argue that children’s labor and donations to the energy pool (controlled by their mother) slow down their growth. Boyette (2008) and Crittenden and Marlowe (2008) have noted adult coercion of children among foragers into work activities that benefit reproductive women. Biological responses including genomic imprinting to shift behaviors or growth and maturation timing could be evolved accommodations to food transfer systems (Haig 2010) based in households. Given female dispersal in other ape species, another suggestion to explain low intergenerational reproductive overlap has been reproductive conflict between husband’s mother and daughter-in-law (Cant and Johnstone 2008). Any effect of growth suppression and delayed maturation, however, must be located in the natal household due to timing of exposure, according to research on early life origins of developmental pathways (Kuzawa and Quinn 2009). Greater adult body size and reduced sexual dimorphism in Homo erectus (McHenry 1996) may have resulted from pressure on females to increase productivity and reproduction, consequently delaying age at first birth to provide time for this growth.
The rapid growth and development that takes place at puberty in humans could set up a major shift in priority of access to pooled household resources between mother and daughter, as offspring seek mates and reduce contributions to mothers. Daughters would now be able to generate and control greater resources than before, with larger body size and attraction of mates who also contribute. Their still-productive mothers could then move into a grandmother mode of provisioning, as the center of the energy pool shifts to the next generation and access to resources becomes insufficient for their own continued rapid reproduction. However, with costs of reproduction so high, grandmothers can contribute to their daughters and further their own inclusive fitness as their effect via contributed resources is multiplied by the rapid pace of daughter’s fertility. Grandmothers would also be at a disadvantage as help from mates would be less probable, given mortality risks among older males and preference for younger women by long-term bonding younger males. Relative reproductive value shifts, with a daughter at maturation having higher residual reproductive value than her mother. This situation does not apply to female chimpanzees who remain attractive to males throughout their lives as older chimp mothers produce and support more viable offspring. Chimpanzee males are not bonded and long-term reproductive value of a female is not a consideration (Muller et al. 2006). The decline of fertility in human females after age 35 is noticeable (Kaplan et al. 2010) compared to the persistent high levels of fertility until time of death among healthy chimps, up to age 45 with death soon after (Emery Thompson et al. 2007). The decline in human fertility has been shown by Holman et al. (2000) to be a function of early fetal death rates, rather than lower conception rates. Spontaneous abortion is known to be related to stressful experiences (Neugebauer et al. 1996) which could arise from intergenerational competition and decline in male support.
If we accept a pooled energy model of households and see it as underpinning the fast fertility pace in reproductive-age women, then we must take into account a female’s potential sources of energy and the associated probabilities of receiving it by age from each type of kin. Norms specifying these roles can be altered to fit different ecological conditions with the result that variation in households and kinship ecologies is expected.
Cultural Evolution
Kinship is culturally recognized in all societies, but with much variation that often confounds understanding of underlying principles of organization. Studying kinship across cultures, starting with ideology as pursued by cultural anthropologists, can lead to confusion over how to reconcile the effusion of explanations generated by cultural processes. This confrontation with variation probably led some cultural anthropologists to conclude that kinship could only be understood as cultural constructions free of biology and only within individual cultures (Yanagisako and Collier 1987). Human behavioral ecology offers another approach from the ground up, so to speak. Looking at energy access and flow can incorporate both cultural and biological dimensions of kinship. Importantly, resource transfers start with the practice of sharing complementary foods and labors that are foundational to the generation of interdependencies of kinship ecologies. Only in recent times have transfers grown to include productive property. Kinship and households are a recognized and enduring aspect of every society, even those without productive property transfers. They tie humans together and to resource access for daily sustenance. Cultural evolution entails development of concepts that aid in organizing and manipulating the world for communities of people. As dependency on food sharing emerged as an essential part of human adaptation, it became culturally organized with households at its base. The usefulness of households probably selected for mental/cultural constructs to portray and influence the shifting scene of food transfers, generating expectations of food and anxieties regarding its receipt. This development may underlie the origins of the human-sized social brain (Dunbar and Shultz 2007; Silk 2007) and language where relational terms (you, me) appear to be among the oldest (Pagel 2010). Often kinship recognition revolves around household consumption or food sharing. On Langwiki, Malaysia, the consumption of food in the household is what is believed to create kinship (Carsten 1995). South American groups offer cases of affines being absorbed into kinship by partaking in a “common substance” (Riviere 2004). Eating together has always been an important form of bonding among humans and exclusion from this activity a sign of separation. We can see that by focusing on basic principles of human behavioral ecology, by tracking social energy flow and addressing the effect of cultural constructions in creating kinship ecologies, we can begin to tie together biological and cultural evolution in an integrated way.
In Conclusion
We wish to encourage greater focus on women’s contributions to subsistence, particularly processing of food. The emphasis on foraging neglects the importance of processing which makes foods edible for humans, particularly children. We encourage testing the robustness of our assumptions regarding diet breadth and sexual division of labor on the optimal levels of processing and foraging effort of males and females of different ages. The importance of women’s roles in storage and distribution of food (energy) has also been ignored and yet this vital energy pathway can be readily manipulated with effects on child growth and adult reproductive function. The conceptualization of kinship systems as ecologies, as systems through which energy flows, can also add greatly to our understanding of conflict reduction between the sexes and generations via social norms of resource access, labor division, dependency and dominance. Eliciting the connections between cultural recognition of kinship relations (whether biological, affinal, or fictive), dependency attachment, and their neuroendocrinological underpinnings would also aid in understanding enduring human relationships on an evolutionary basis. Finally, we emphasize the importance to evolutionary social science of developing more rigorous theories of household formation and stability, which we believe will shed light on the emergence and evolution of human kinship systems.
Fig. 2.
Energy balance optimization of production effort for a female who is neither pregnant nor lactating. Optimal production effort (E) lies at point where lines (horizontal and vertical dotted lines) intersect, maximizing the difference between EC (energy consumed) and ES (energy spent).
Fig. 3.
Deficit contribution (D) of an individual female resulting from her greater consumption εEC and greater expenditure χES reducing her optimal production from Opt (see intersection of lines in Fig. 2)) to Optp. This deficit is decomposed into three components: (1) processing effort deficit toward G (darkest region between Opt and Optp); (2) foraging effort deficit toward G (second darkest region); and (3) foraging and processing effort deficit toward H.
Fig. 4.
Production effort toward H as a function of compensation effort, assuming a foraging/processing tradeoff. Solid horizontal lines represent sex-specific production requirements for H to satisfy the diet breadth requirement, given the sexual division of labor constraint. Diagonal dashed lines represent actual production effort toward H (here denoted TH), assuming for simplicity that there are no differences in production efficiency between males and females. A steeper negative slope indicates more rapid declines in productivity toward H for a given level of compensation effort, dependent on the amount of this effort allocated toward foraging versus processing. Where the diagonal dashed lines intersect with the solid horizontal lines, actual production effort has fallen below the sex-specific diet breadth requirement. Thus, aggregate production effort has fallen below the overall diet breadth requirement. First, note that when more compensation effort is allocated to processing (small-dashed line), production effort toward H falls below the diet breadth requirement at a lower level of overall compensation effort. Also note that production effort falls below the male production effort requirement at a lower level of compensation effort than for females. While the difference between maximum allowable compensation efforts with and without processing allocation is larger for females (indicated by the space between vertical dashed lines), the female tradeoff between foraging and processing compensation occurs at much higher levels of overall compensation effort requirements than for males.
Acknowledgments
Thanks to the National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) Program in Evolutionary Modeling at the University of Washington (UW) and Washington State University for training and the challenge, the Center for Studies in Demography and Ecology, UW, for support, and to three very helpful reviewers.
Biographies
Donna Leonetti is a professor of anthropology at the University of Washington. She is a human behavioral ecologist and has studied kinship and household ecology in NE India among Khasi and Bengali groups with support from the NICHD and the Indian government. She is also interested in migration and settlement processes, including household formation, among Japanese migrants to the US in the pre-World War II era.
Benjamin Chabot-Hanowell has a BA in Anthropology from California State University, Sacramento. Currently, he is a PhD student in Anthropology at the University of Washington (UW), and an NICHD-sponsored fellow at the Center for Studies in Demography and Ecology. He has also been a fellow of the IGERT Program in Evolutionary Modeling at UW and Washington State University. His current research interests include migrants’ remittance behavior, the rise and fall of ancient Maya polities, and the application of bargaining models to research in human social evolution.
Footnotes
As noted by one reviewer.
This definition builds on that of Netting et al. (1984).
The endowment effect suggests that what one person has is usually more valuable to that person than it is to another person (Thayer 1980).
Or even men in these roles.
References
- Alvard M. Kinship, lineage, and an evolutionary perspective on cooperative hunting groups in Indonesia. Human Nature. 2003;14:129–163. doi: 10.1007/s12110-003-1001-5. [DOI] [PubMed] [Google Scholar]
- Anderson K, Kaplan H, Lancaster J. Confidence of paternity, divorce, and investment in children by Albuquerque men. Evolution and Human Behavior. 2007;28:1–10. [Google Scholar]
- Becker G. Treatise on the family. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Bereczkei T, Dunbar RIM. Helping-at-the-nest and sex-biased parental investment in a Hungarian Gypsy population. Current Anthropology. 2002;43:804–809. [Google Scholar]
- Bird R. Cooperation and conflict. The behavioral ecology of the sexual division of labor. Evolutionary Anthropology. 1999;8:65–75. [Google Scholar]
- Bliege Bird R, Bird D. Constraints of knowing or constraints of growing? Fishing and collecting by the children of Mer. Human Nature. 2002;13:239–267. doi: 10.1007/s12110-002-1009-2. [DOI] [PubMed] [Google Scholar]
- Bleige Bird R, Codding BF, Bird D. What explains differences in men’s and women’s production? Determinants of gendered foraging inequalities among Martu. Human Nature. 2009;20:105–129. doi: 10.1007/s12110-009-9061-9. [DOI] [PubMed] [Google Scholar]
- Blurton Jones N. Tolerated theft, suggestions about the ecology and evolution of sharing, hoarding and scrounging. Social Science Information. 1987;26:31–54. [Google Scholar]
- Blurton Jones N, Marlowe F. Selection for delayed maturity. Does it take 20 years to learn to hunt and gather? Human Nature. 2001;13:199–238. doi: 10.1007/s12110-002-1008-3. [DOI] [PubMed] [Google Scholar]
- Borgerhoff Mulder M. Kipsigis women’s preferences for wealthy men: evidence for female choice in mammals? Behavioral Ecology and Sociobiology. 1990;27:255–264. doi: 10.1007/BF00164897. [DOI] [PubMed] [Google Scholar]
- Borgerhoff Mulder M, Rauch K. Sexual conflict in humans: Variation in conflicts and solutions. Evolutionary Anthropology. 2009;18:201–214. [Google Scholar]
- Bock J. Evolutionary demography and intrahousehold time allocation: School attendance and child labor among the Okavango Delta peoples of Botswana. American Journal of Physical Anthropology. 2002;14:206–221. doi: 10.1002/ajhb.10040. [DOI] [PubMed] [Google Scholar]
- Bogin B. Evolutionary perspective on human growth. Annual Review of Anthropology. 1999;28:109–153. doi: 10.1146/annurev.anthro.28.1.109. [DOI] [PubMed] [Google Scholar]
- Boyd R, Richerson P. Culture and the evolutionary process. Chicago: University of Chicago Press; 1985. [Google Scholar]
- Boyd R, Richerson P. Culture and the evolution of human cooperation. Philosophical Transactions of the Royal Society B. 2009;364:3281–3288. doi: 10.1098/rstb.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss. New York: Basic Books; 1969. [Google Scholar]
- Boyette A. Scaffolding for cooperative breeding among Aka forager. Paper presented American Anthropological Association; San Francisco. 2008. [Google Scholar]
- Brody E. Sex, contraception and motherhood in Jamaica. Cambridge: Harvard University Press; 1981. [Google Scholar]
- Buss D. Strategies of human mating. Psychological Topics. 2006;15:239–260. [Google Scholar]
- Cant M, Johnstone R. Reproductive conflict and the separation of reproductive generations in humans. Proceedings of the National Academy of Sciences. 2008;105:5332–5336. doi: 10.1073/pnas.0711911105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsten J. The substance of kinship and the heat of the hearth: Feeding, personhood, and relatedness among Malays in Pulau Langkawi. American Ethnologist. 1995;22:223–241. [Google Scholar]
- Chapais B. Primeval kinship: How pair-bonding gave birth to human society. Cambridge, MA: Harvard University Press; 2008. [Google Scholar]
- Charnov E, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evolutionary Anthropology. 1993;2:191–194. [Google Scholar]
- Chisholm J. The evolutionary ecology of attachment organization. Human Nature. 1996;7:1–38. doi: 10.1007/BF02733488. [DOI] [PubMed] [Google Scholar]
- Chisholm J, Quinliven J, Petersen R, Coall D. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Human Nature. 2005;16:233–265. doi: 10.1007/s12110-005-1009-0. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. Cooperation between non-kin in animal societies. Nature. 2009;462:51–57. doi: 10.1038/nature08366. [DOI] [PubMed] [Google Scholar]
- Cordain L, Brand Miller J, Eaton BS, Mann N, Holt S, Speth J. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. American Journal of Clinical Nutrition. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- Crittenden A, Marlowe F. Allomaternal care among the Hadza of Tanzania. Human Nature. 2008;19:249–262. doi: 10.1007/s12110-008-9043-3. [DOI] [PubMed] [Google Scholar]
- Cronk L, Gerkey D. Kinship and descent. In: Dunbar RIM, Barret L, editors. Oxford handbook of evolutionary psychology. New York: Oxford University Press; 2007. pp. 463–478. [Google Scholar]
- Curtis JT, Wang Z. The neurochemistry of pair bonding. Current Directions in Psychological Science. 2003;12:49–53. [Google Scholar]
- Draper P. Contrasts in sexual egalitarianism in foraging and sedentary contexts. In: Reiter R, editor. Toward and anthropology of women. New York and London: Monthly Review Press; 1975. pp. 70–112. [Google Scholar]
- Dunbar R, Shultz S. Evolution of the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Ellis B, Figueredo AJ, Brumback B, Schlomer G. Fundamental dimensions of environmental risk. The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Elston R, Zeanah D. Thinking outside the box: A new perspective on diet breadth and sexual division of labor in the Prearchaic Great Basin. World Archaeology. 2002;34:103–130. doi: 10.1080/00438240220134287. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Jones J, Pusey A, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Current Biology. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S. An evolutionary theory of the family. Proceedings of the National Academy of Sciences. 1995;92:8092–8099. doi: 10.1073/pnas.92.18.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn M. Evolution and ontogeny of stress response to social challenges in the human child. Developmental Review. 2006;26:138–174. [Google Scholar]
- Frank R. Cooperation through emotional commitment. In: Nesse R, editor. Evolution and the capcity for commitment. New York: Russell Sage Foundation; 2001. pp. 57–76. [Google Scholar]
- Fromhage L, Mcnamara J, Houston A. Stability and value of male care for offspring: Is it worth only half the trouble? Biology Letters. 2007;3:234–236. doi: 10.1098/rsbl.2006.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M, Mace R. Helpful grandmothers in rural Ethiopia: A study of the effect of kin on child survival and growth. Evolution and Human Behavior. 2005;26:469–482. [Google Scholar]
- Goody J. Production and reproduction. Cambridge, UK: Cambridge University Press; 1976. [Google Scholar]
- Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE. 2009;4:e5116. doi: 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P, Kahlenberg S, Barrett E, Lipson S, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23:193–201. [Google Scholar]
- Gurven M, Hill K, Kaplan H, Hurtado A, Lyles R. Food transfers among Hiwi foragers of Venezuela: Tests of reciprocity. Human Ecology. 2000;28:171–218. [Google Scholar]
- Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proceedings of the Royal Society B. 2006;273:835–841. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Winking J, Kaplan H, von Rueden C, McAllister L. A bioeconomic approach to marriage and the sexual division of labor. Human Nature. 2009;20:151–183. doi: 10.1007/s12110-009-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Transfers and transitions: Parent-offspring conflict, genomic imprinting, and the evolution of human life history. Proceedings of the National Academy of Sciences. 2010;107:1731–1735. doi: 10.1073/pnas.0904111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. The genetical theory of social behavior, I, II. Journal of Theoretical Biology. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Harrell S. Human families. Boulder, CO: Westview Press; 1997. [Google Scholar]
- Hawkes K, Bliege Bird R. Showing off, handicap signaling, and the evolution of men’s work. Evolutionary Anthropology. 2002;11:58–67. [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG. Hadza women’s time allocation, offspring provisioning, and the evolution of long post-menopausal life spans. Current Anthropology. 1997;38:551–577. [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG. Hunting and nuclear families. Some lessons from the Hadza about men’s work. Current Anthropology. 2001;42:681–708. [Google Scholar]
- Hawkes K, O’Connell, Blurton Jones N, Charnov E, Alvarez H. Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of Sciences. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E, Sibert A, Austerlitz F. Cultural transmission of fitness: Genes take the fast lane. TRENDS in Genetics. 2005;21:234–239. doi: 10.1016/j.tig.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hill K, Hurtado AM. Cooperative breeding in South American hunter-gatherers. Proceedings of the Royal Society B. 2009;276:3863–3870. doi: 10.1098/rspb.2009.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CJ, Mace R. Spread of cattle led to the loss of matrilineal descent in Africa: A coevolutionary analysis. Philosophical Transactions of the Royal Society of London B. 2003;270:2425–2433. doi: 10.1098/rspb.2003.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice WR. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Holman D, Wood J, Campbell K. Age-dependent decline of female fecundity is caused by early fetal loss. In: te Velde E, Broekmans F, Pearson P, editors. Female reproductive ageing. Studies in profertility Series. 9 . Camforth, UK: Parthenon Publishing Group; 2000. pp. 123–136. [Google Scholar]
- Hrdy SB. Mother nature. Maternal instincts and how they shape the human species. New York: Ballantine Books; 1999. [Google Scholar]
- Hrdy SB. Cooperative breeders with an ace in the hole. In: Voland E, Chasiotis A, Schiefenhoevel W, editors. Grandmotherhood—The evolutionary significance of the second half of female life. Picsataway, NJ: Rutgers U. Press; 2005. pp. 295–317. [Google Scholar]
- Hrdy SB. Mothers and others. The evolutionary origin of mutual understanding. Cambridge, MA: The Belknap Press of Harvard University Press; 2009. [Google Scholar]
- Hurtado AM, Hill K, Kaplan H, Hurtado I. Tradeoffs between female food acquisition and childcare among Hiwi and Ache foragers. Human Nature. 1992;3:185–216. doi: 10.1007/BF02692239. [DOI] [PubMed] [Google Scholar]
- Isbell L. Is there no place like home? Ecological bases of female dispersal and philopatry and their consequences for the formation of kin groups. In: Chapais B, Berman C, editors. Kinship and behavior in primates. New York: Oxford University Press; 2004. pp. 71–108. [Google Scholar]
- Kaplan H. Evolutionary wealth flows theories of fertility: Empirical tests and new models. Population and Development Review. 1994;20:753–791. [Google Scholar]
- Kaplan H. A theory of fertility and parental investment in traditional and modern human societies. Yearbook of Physical Anthropology. 1996;39:91–135. [Google Scholar]
- Kaplan H, Gurven M, Winking J, Hooper P, Stieglitz J. Learning, menopause, and the human adaptive complex. Annals of the New York Academy of Sciences. 2010;1204:30–42. doi: 10.1111/j.1749-6632.2010.05528.x. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology. 2000;9:156–185. [Google Scholar]
- Kaplan H, Hooper P, Gurven M. The evolutionary and ecological roots of human social organization. Philosophical Transactions of the Royal Society B. 2009;364:3289–3299. doi: 10.1098/rstb.2009.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K. Children’s help and the pace of reproduction: Cooperative breeding in humans. Evolutionary Anthropology. 2005;14:224–237. [Google Scholar]
- Kramer K, Ellison P. Pooled energy budgets: Resituating human life history trade-offs. Evolutionary Anthropology. 2010;19:136–147. [Google Scholar]
- Kuzawa C, Quinn E. Developmental origins of adult function and health: Evolutionary hypotheses. Annual Review of Anthropology. 2009;38:131–147. [Google Scholar]
- Lee RB. The !Kung San. Men, women and work in a foraging society. Cambridge: Cambridge University Press; 1979. [Google Scholar]
- Lee RD. Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proceedings of the National Academy of Sciences. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti D, Nath DC, Hemam NS. “In-law conflict.” Women’s reproductive lives and the roles of their mothers and husbands among the matrilineal Khasi. Current Anthropology. 2007;48:861–890. [Google Scholar]
- Leonetti DL, Nath DC, Hemam NS, Neill DB. Do women really need marital partners for support of their reproductive success? The case of the matrilineal Khasi of N.E. India. Special volume, Socioeconomic aspects of human behavioral ecology. In: Alvard Michael., editor. Research in Economic Anthropology. Vol. 23. 2004. pp. 151–174. [Google Scholar]
- Leonetti DL, Nath DC, Hemam NS, Neill DB. Kinship organization and grandmother’s impact on reproductive success among the matrilineal Khasi and patrilineal Bengali of N.E. India. In: Voland E, Chasiotis A, Schiefenhoevel W, editors. Grandmotherhood—The evolutionary significance of the second half of female life. Picsataway, NJ: Rutgers U. Press; 2005. pp. 194–214. [Google Scholar]
- Levine S, Wiener S. Psychoendocrine aspects of mother-infant relationship in nonhuman primates. Psychoneuroendocrinology. 1988;13:143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Lillard LA, Panis CWA. Marital status and mortality. The role of health. Demography. 1996;33:313–327. [PubMed] [Google Scholar]
- Lundberg S, Pollak R. Bargaining and distribution in marriage. Journal of Economic Perspectives. 1996;10:139–158. [Google Scholar]
- Manson J. Primate consortships. A critical review. Current Anthropology. 1997;38:353–374. [Google Scholar]
- Marlowe F. Male contributions to diet and female reproductive success among foragers. Current Anthropology. 2001;42:755–760. [Google Scholar]
- Marlowe F. A critical period for provisioning by Hadza men. Implication for pair bonding. Evolution and Human Behavior. 2003;24:217–229. [Google Scholar]
- Marlowe F. Hunting and gathering. The human sexual division of foraging labor. Cross-Cultural Research. 2007;41:170–195. [Google Scholar]
- Marlowe F. The Hadza. Hunter-gatherers of Tanzania. Berkeley: University of California Press; 2010. [Google Scholar]
- Mason K, Taj A. Differences between women’s and men’s reproductive goals in developing countries. Population and Development Review. 1987;13:611–638. [Google Scholar]
- Mattison S. The economic impacts of tourism and erosion of the visiting system among the Mosuo of Lugu Lake. The Asia Pacific Journal of Anthropology. 2010;11:157–174. [Google Scholar]
- McFarland R. PhD thesis. University of Washington; Seattle: 1992. Body composition and reproduction in female pigtail macaques. [Google Scholar]
- McHenry H. Sexual dimorphism in fossil hominids and its socioecological implications. In: Steel J, Shennan S, editors. The archaeology of human ancestry: Power, sex and tradition. New York: Routledge; 1996. pp. 91–109. [Google Scholar]
- Meehan C. Maternal time allocation in two cooperative childrearing societies. Human Nature. 2009;20:375–393. [Google Scholar]
- Muller M, Emery Thompson M, Wrangham R. Male chimpanzees prefer mating with old females. Current Biology. 2006;16:2234–2238. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Neill D, Leonetti D, Nath D, Hemam S. Presentation. Human Behavior and Evolution Society; New Brunswick, NJ: 2005. Offspring contributions to household economy by age and sex among the Khasi of Northeast India. [Google Scholar]
- Netting R, Wilk R, Arnould E. Introduction. In: Netting R, Wilk R, Arnould E, editors. Households. Comparative and historical studies of the domestic group. Berkeley: University of California Press; 1984. pp. xiii–xxxviii. [Google Scholar]
- Neugebauer I, Kline J, Stein Z, Shrout P, Warburton D, Susser M. Association of stressful life events with chromosomally normal spontaneous abortion. American Journal of Epidemiology. 1996;143:588–596. doi: 10.1093/oxfordjournals.aje.a008789. [DOI] [PubMed] [Google Scholar]
- Neumann I. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in Neuroendocrinology. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Nolin D. Food-sharing networks in Lamalera, Indonesia. Reciprocity, kinship and distance. Human Nature. 2010 doi: 10.1007/s12110-010-9091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. Presentation. Human Behavior and Evolution Society; Eugene, Oregon: 2010. The rise of the speaking machine. Explorations in human language evolution. [Google Scholar]
- Panter-Brick C. Sexual division of labor: Energetic and evolutionary scenarios. American Journal of Human Biology. 2002;14:627–640. doi: 10.1002/ajhb.10074. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Quinlan M. Human lactation, pair bonds, and alloparents: A cross-cultural analysis. Human Nature. 2008;19:87–102. doi: 10.1007/s12110-007-9026-9. [DOI] [PubMed] [Google Scholar]
- Richerson P, Bettinger R, Boyd R. Evolution on a restless planet: Were environmental variability and environmental change major drivers of human evolution? In: Wuketits F, Ayala F, editors. Handbook of evolution, Vol. 2. The evolution of living systems (including Hominids) Weinheim: Wiley-Verlag; 2005. pp. 223–242. [Google Scholar]
- Rivière P. Marriage. A reassessment. In: Needham R, editor. Rethinking marriage and kinship. New York: Routledge; 2004. pp. 57–70. [Google Scholar]
- Roland A. In search of self in India and Japan. Toward a cross-cultural psychology. Princeton: Princeton University Press; 1988. [Google Scholar]
- Rolfes S, Pinna K, Whitney E. Understanding normal and clinical nutrition. 8. Belmont, CA: Wadsworth Cengage Learning, Inc; 2009. [Google Scholar]
- Rose L. Vertebrate predation and food sharing in Cebus and Pan. International Journal of Primatology. 1997;18:727–765. [Google Scholar]
- Roy M. Bengali women. Chicago: The University of Chicago Press; 1972. [Google Scholar]
- Schoeninger M, Bunn H, Murray S, Marlett J. Composition of tubers used by Hadza foragers of Tanzania. Journal of Food composition and Analysis. 2001;14:15–25. [Google Scholar]
- Sellen D. Evolution of infant and young child feeding. Implications for contemporary public health. Annual Review of Nutrition. 2007;27:123–148. doi: 10.1146/annurev.nutr.25.050304.092557. [DOI] [PubMed] [Google Scholar]
- Silk J. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Smith EA. Why do good hunters have higher reproductive success? Human Nature. 2004;15:1–17. doi: 10.1007/s12110-004-1013-9. [DOI] [PubMed] [Google Scholar]
- Smith EA. Communication and collective action: Language and the evolution of human cooperation. Evolution and Human Behavior. 2010;31:231–245. [Google Scholar]
- Smuts B. Male aggression against women: An evolutionary perspective. Human Nature. 1992;3:1–44. doi: 10.1007/BF02692265. [DOI] [PubMed] [Google Scholar]
- Smuts B. The evolutionary origins of patriarchy. Human Nature. 1995;6:1–32. doi: 10.1007/BF02734133. [DOI] [PubMed] [Google Scholar]
- Stack C. All our kin. Strategies for survival in a Black community. New York: Harper & Row; 1974. [Google Scholar]
- Strassmann BI, Clarke AL. Ecological constraints on marriage in rural Ireland. Evolution and Human Behavior. 1998;19:33–55. [Google Scholar]
- Thayer R. Toward a positive theory of consumer choice. Journal of Economic Behavior and Organization. 1980;1:39–60. [Google Scholar]
- Trivers R. Parent-offspring conflict. American Zoologist. 1974;14:249–264. [Google Scholar]
- Tuljapurkar S, Wiener P. Escape in time: Stay young or age gracefully? Ecological Modelling. 2000;133:143–159. [Google Scholar]
- Voas D. A reason fertility tends to be too high or too low. Population and Development Review. 2003;29:627–646. [Google Scholar]
- van den Berghe P. An evolutionary view. New York: Elsevier; 1979. Human family systems. [Google Scholar]
- Winking J, Kaplan H, Gurven M, Rucas S. Why do men marry and why do they stray? Proceedings of the Royal Society B. 2007;274:1643–1649. doi: 10.1098/rspb.2006.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. Prestige or provisioning? A test of foraging goals among the Hadza. Current Anthropology. 2006;47:383–387. [Google Scholar]
- Wood B, Marlowe F. Hadza kinship and its role in residence patterns and food sharing. Paper presented American Anthropological Association meetings; Philadelphia. 2009. [Google Scholar]
- Wrangham R. Catching fire. How cooking made us human. New York: Basic Books; 2009. [Google Scholar]
- Yanagisako S, Collier J. Toward a unified analysis of gender and kinship. In: Collier J, Yanagisako S, editors. Gender and kinship: Essays toward a unified analysis. Stanford: Stanford University Press; 1987. pp. 14–50. [Google Scholar]
- Young L, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zihlman A, McFarland R. Body mass in lowland gorillas: A quantitative analysis. American Journal of Physical Anthropology. 2000;113:61–78. doi: 10.1002/1096-8644(200009)113:1<61::AID-AJPA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]