Abstract
Objective
To investigate interhemispheric differences on naming and fluency tasks for living versus nonliving things among patients with semantic dementia (SD).
Background
In SD, left-temporal involvement impairs language and word comprehension, and right-temporal involvement impairs facial recognition. There may be other interhemispheric differences, particularly in the animate-inanimate dichotomy.
Method
On the basis of magnetic resonance imaging (MRI) ratings of anterior temporal atrophy, 36 patients who met criteria for SD were divided into 21 with left-predominant and 11 with right-predominant involvement (4 others were too symmetric for analysis). The left and right-predominant groups were compared on naming, fluency, and facial recognition tests.
Results
Consistent with greater language impairment, the left-predominant patients had worse naming, especially inanimate and letter fluency, than the right-predominant patients. In contrast, difference in scores suggested selective impairment of animal naming, animal name fluency, and semantic knowledge for animate items among the right-predominant patients. Proportionally more right than left-predominant patients misnamed animal items and faces.
Conclusions
These findings support interhemispheric differences in animal knowledge. Whereas left-predominant SD equally affects animate and inanimate words from language involvement, right-predominant SD, with greater sparing of language, continues to impair other semantic aspects of animals. The right anterior temporal region seems to make a unique contribution to knowledge of living things.
Keywords: semantic dementia, animate-inanimate, prosopagnosia
One of the 3 major frontotemporal lobar degenerations (FTLD) is semantic dementia (SD). This disorder causes loss of conceptual knowledge, manifested as deficits in word comprehension, facial recognition, and category-specific knowledge.1,2 SD is consequent to atrophy in the anterior inferolateral temporal pole, a convergence zone that may integrate semantic information. 3–5 Although there is usually bilateral involvement, SD tends to be asymmetric at onset, with the left anterior temporal pole most significantly and consistently affected.6–8 The neuropathology cannot be conclusively predicted during life, but most SD patients have ubiquitin-positive intraneuronal inclusions containing the TDP-43 protein.6,9
The defining clinical aspect of SD is an impairment in concepts or semantic knowledge. Most commonly, SD patients present with impairments in naming and in word comprehension in the presence of fluent and grammatical speech output.10,11 There is early loss of low-frequency nouns from left anterior temporal involvement. As the neurodegeneration progresses, there is an inability to know the meaning of the noun beyond the word and regardless of modality.12 Early or eventual involvement of the right anterior temporal region produces prosopagnosia, or the inability to recognize familiar faces.13,14 With progression, both left and right-sided SD patients develop impairments of meaning or identity that go beyond naming difficulties or modality-dependent agnosia.15,16
Two of the most intriguing aspects of SD are the occurrence of category-specific deficits and the effects of hemispheric asymmetry. In addition to words and faces, reports indicate selective deficits in living things, fruits and vegetables, and possibly tools and other categories.17–19 Among the most salient category-specific deficits in SD are differences in knowledge of animate versus inanimate entities.20 Asymmetries in the involvement of the right and left anterior temporal lobes, often evident on initial neuroimaging, may correlate with person-specific impairments and general semantic impairments, respectively.20–22 The possibility of right temporal predominance in knowledge of animate entities and left temporal predominance in knowledge of inanimate entities has implications for the theories of how knowledge is organized in the brain.23
This study explored hemispheric differences in knowledge of animate versus inanimate entities evident early in the course of SD on routine clinical testing of naming and fluency as well as facial identification. By dividing patients with SD into left and right-anterior temporal predominant involvement on initial neuroimaging, this study was able to retrospectively analyze hemispheric differences in living versus nonliving things in a sufficient number of rarer right-predominant patients, compared with the more common left-predominant patients.
METHODS
Subjects
All participants in this study presented for evaluation to a university specialty clinic in dementing disorders. All of these patients had the insidious onset and progression of cognitive changes, including declines in word comprehension. The patients underwent a comprehensive neurobehavioral evaluation, laboratory assessment (hemogram, chemistries, and thyroid function tests), and required magnetic resonance imaging (MRI) of the brain. They were screened for chronic mental illness, head trauma, extrapyramidal disorders, vitamin deficiency, hypothyroidism, syphilis, and other medical conditions. Finally, every subject met Clinical Consensus Criteria for SD and “imaging-supported” criteria for SD based on the initial MRI scan (Table 1).1 In this manner, this study identified a total of 36 SD subjects who met these criteria. The participants for this study were part of an institutional review board approval for the retrospective use of clinically obtained information in this clinic.
TABLE 1.
Imaging-supported Semantic Dementia
Both “core features” must be present
|
Three of the other diagnostic features must be present
|
mBNT indicates mini-Boston Naming Test.
Adapted with permission from Gorno-Tempin ML, Hillis AE, Weintraub S, et al. Recommendations for the classification of primary progressive aphasia and its variants. Neurology (in preparation).
Procedures
The neurobehavioral evaluation involved cognitive and behavioral assessments. In addition to the Mini-Mental State Examination,24 the patients underwent an intake speech-language examination. This examination included letter (“F words”) and category (“animals”), fluency and screening for agrammatism and phrase length, motor speech (dysarthria and apraxia of speech), repetition (single word and sentence), auditory comprehension (single word and sentence), and reading (nonsense, irregular, and regular words). Additional cognitive tests were the verbal-learning test from the Consortium to Establish a Registry in Alzheimer’s Disease,25 delayed recall and recognition scores, and 3-dimensional constructions graded on a 4-point scale. Finally, from the history and evaluation, the investigators retrospectively determined the FTLD-modified Clinical Dementia Rating scale with supplemental items for language and behavior, comportment, and personality.26 The total of 8 domains were incorporated into a “Sum of Boxes” score for all 8 domains.26
As part of the language battery, the patients were administered the 15-item mini-Boston Naming Test (mBNT) with supplementary semantic and recognition questions.25,27 For analysis, the mBNT was divided into 10 inanimate and 5 animate items. Mythical and representative animate items (ie, unicorn, Sphinx) were classified as animate as was the single plant (ie, cactus). The animate and inanimate items were all 1 to 3 syllable words that did not significantly differ in word length. Semantic knowledge was tested by asking for both a definition and at least one defining specific characteristic of the item.27,28 Definitions were correct if they described a supraordinate membership, for example, “plant” for cactus,” and characteristics were correct if they described a descriptive feature or trait, for example, “has needles” or “in desert” for cactus. Recognition was tested by asking the patients to pick the correct name from the 4 choices provided for the pictured mBNT items, first by reading the items, and then from the examiner’s reading of the words aloud. The mBNT was scored as 15/15 for naming, 30/30 for semantic knowledge (definition and defining characteristic), and 30/30 for word recognition (written and verbal presentations).
These SD patients also had facial recognition testing consisted of 24 photographs of famous faces presented for naming or identification. The famous people were either politicians or entertainers well-known to most residents of Southern California, and presented in 2.5×2.5 cm2 portraits, complete with hair. The patients were asked to name the person, and then describe a major associated feature of the individual, for example, “movie actor,” “president,” or “world leader.” Four faces were repeats (ie, 2 distinct pictures of Ronald Reagan). The examiner also recorded whether the patients volunteered that a face was a repeat. Prior use of the Face Identification Test yielded a normal performance of 21.36 (SD=2.4) (originally from a range of normals and subsequently applied to 12 normals, aged 55 to 63y, with 14 to 15y education).29
The MRI scans were re-read by 2 independent and experienced raters, blind to the clinical history but not to the diagnosis of SD. These MRI scans were clinically obtained, and originated from a range of scanners using different techniques. Visual reinspection allowed for comparison across these different clinical scans. The characteristic atrophy pattern in the anterior temporal lobes was independently rated by the 2 imagers. On account of the variable origin of the scans, the best views with the most atrophy were used for measurement. The blinded visual reinspections graded the scans on a 0 to 4 point scale (0=absent, 1=mild-to-moderate, 2=moderate-to- severe, and 3=very severe) for the left anterior temporal and right anterior temporal regions (Fig. 1). For the 2 raters, the inter-rater reliability for this reinspection was rs=0.42 for 72 ratings (P<0.001). The combination of the rater scores determined whether the scans were right-temporal or left-temporal predominant based on a difference of 2 or more total points (sum of rater scores) between the 2 sides.
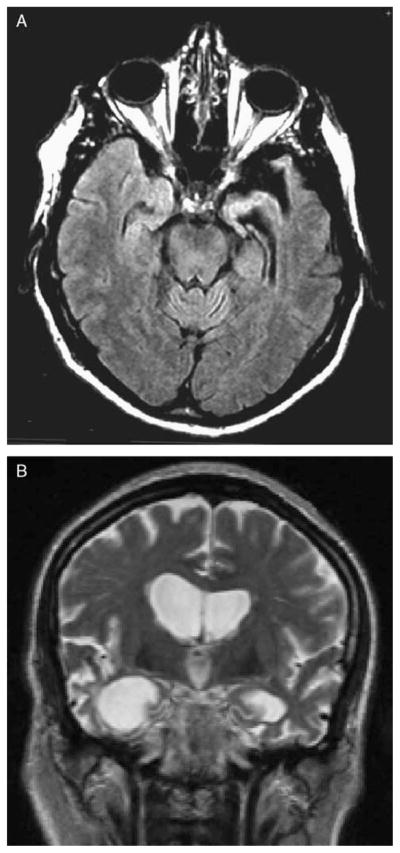
FIGURE 1.
Examples of magnetic resonance imaging and scoring. A, Axial view (fluid-attenuated inversion recovery) of SD patient with left-predominant anterior temporal atrophy and rating scores of 3 for left and 1 for right. B, Coronal view (T2) of SD patient with right-predominant anterior temporal atrophy and rating scores of 1 for left and 3 for right. SD indicates semantic dementia.
Data Analysis
Demographic and test characteristics were compared using 2-tailed t tests and χ2 analysis, as appropriate. Additional subgroup comparisons involved mBNT items matched for word frequency. Difference scores were calculated and compared for the animate-inanimate naming on the mBNT and the semantic knowledge-word recognition item differences on the mBNT.
RESULTS
There were 16 men and 20 women who met imaging-supported criteria for SD (Table 2). Most SD patients had asymmetric anterior temporal involvement. On MRI analysis, there were 21 with left-sided predominance and 11 with right-sided predominance. An additional 4 were not sufficiently asymmetric for this study, and are described but not compared with the left-sided and right-sided predominant groups. There were no differences between the males and females on the MRI rating scores. There were no correlations between years of education, Mini-Mental State Examination scores, age of onset or age of presentation, and the MRI rating scores.
TABLE 2.
Patient Characteristics
| Semantic Dementia Patients (n = 36)
| ||||
|---|---|---|---|---|
| Left n = 21 | Right n = 11 | Bilateral n = 4 | Left Versus Right | |
| Male/female | 9/12 | 5/6 | 2/2 | NS |
| Presenting age (y) | 62.1 (3.4) | 63.04 (4.1) | 62.4 (2.2) | NS |
| Age of onset (y) | 58.91 (3.2) | 59.71 (2.47) | 57.5 (3.11) | NS |
| Education (y) | 15.1 (1.87) | 14.91 (2.21) | 14.5 (1.91) | NS |
| MMSE | 23.1 (5.2) | 22.0 (6.1) | 22.8 (2.0) | NS |
| CERADdr | 1.57 (2.56) | 2.0 (2.91) | 0 (0) | NS |
| CERADrec | 15.76 (3.08) | 16.36 (3.14) | 14.0 (1.83) | NS |
| Constructions | 3.67 (0.66) | 3.64 (0.67) | 3.5 (0.58) | NS |
| FTLD-CDR | 6.33 (2.20) | 6.64 (2.20) | 9.0 (0.82) | NS |
| Face naming | 13.33 (6.62) | 2.36 (0.67) | 3.0 (0.82) | t = 5.39, P<0.001 |
| MRI anterior | L-R Dif: 2.9 (1.18) | L-R Dif: 2.64 (1.03) | L-R Dif: 0.5 (0.58) | NS |
| Temporal | Left: 2.33 (0.69) | Left: 0.86 (0.71) | Left: 2.25 (0.46) | t = 9.47, P<0.001 |
| Scores | Right: 0.88 (0.71) | Right: 2.18 (0.73) | Right: 2.50 (0.53) | t = −8.49, P<0.001 |
The t tests are 2-tailed with 30 degrees of freedom.
CERADdr indicates Consortium to Establish a Registry in Alzheimer’s Disease-Delayed Recall; CERADrec, Consortium to Establish a Registry in Alzheimer’s Disease-Recognition; FTLD-CDR, frontotemporal lobar degenerations-modified Clinical Dementia Rating Sum of Boxes scores; L-R Dif, left versus right-sided absolute difference scores (irrespective of valence); MMSE, Mini-Mental State Examination Score; MRI, magnetic resonance imaging; NS, nonsignificant.
In comparing the right and left-sided predominant patients, there were no group differences in age, sex, duration of illness, or activities of daily living on the FTLD-modified Clinical Dementia Rating (Table 2). All patients, regardless of temporal lobe asymmetry, had semantic anomia, but, on the face identification test, only 4 (19.0%) of the left-predominant patients had 3 or fewer correct facial identifications, compared with all 11 (100%) of the right-predominant patients (χ2=15.89, P<0.001).
The results of the naming tests also showed laterality differences. On the mBNT, the left-predominant patients were significantly more impaired in total scores and inanimate naming than the right-predominant patients (Table 3). In contrast, the right-predominant patients had lower mean animate naming scores, and significantly greater animate-inanimate differences, than the left-predominant patients, consistent with disproportionate difficulty with animate naming. Similarly, the left-predominant patients had worse letter, but not category (animal), fluency. Only 10 (47.6%) of the left-predominant patients missed all 5 of the “animate” items, compared with 8 (72.7%) of the right-predominant patients. When the single plant item (ie, cactus), was excluded, 13 (61.9%) of the left-predominant patients missed all 4 of the animal items, compared with all 11 (100%) of the right-predominant patients (χ2=3.74, P=0.05). Floor effects in animate naming were unlikely to explain the smaller animate-inanimate difference scores for the left-predominant patients as their animate naming was larger than for the right-predominant patients.
TABLE 3.
Screening Test Results
| Semantic Dementia Patients (n = 36)
| ||||
|---|---|---|---|---|
| Left n = 21 | Right n = 11 | Bilateral n = 4 | Left Versus Right | |
| mBNT total (n = 15) | 3.10 (1.67) | 4.73 (2.05) | 3.50 (2.89) | t = 2.19, P<0.05 |
| Animate (n = 5) | 0.52 (0.51) | 0.27 (0.47) | 0.50 (1.01) | NS |
| Inanimate (n = 10) | 2.57 (1.29) | 4.46 (1.75) | 3.02 (2.16) | t = 3.47, P<0.01 |
| Animate-inanimate % difference | 15.2 (9.80) | 40.0 (14.8) | 20.02 (1.83) | t = 5.69, P<0.001 |
| mBNT semantic knowledge (n = 30) | 13.67 (3.42) | 12.56 (5.07) | 13.12 (2.44) | NS |
| Animate (n = 10) | 6.60 (1.34) | 2.2 (3.35) | 4.66 (2.92) | t = 9.92, P<0.001 |
| Inanimate (n = 20) | 17.20 (2.74) | 17.76 (4.53) | 16.98 (2.13) | NS |
| mBNT word recognition (n = 30) | 10.20 (5.4) | 11.87 (3.28) | 10.81 (2.31) | NS |
| Animate (n = 10) | 4.61 (1.34) | 3.23 (1.64) | 3.33 (1.14) | t = 22.54, P<0.05 |
| Inanimate (n = 20) | 13.02 (5.35) | 15.95 (2.94) | 13.20 (2.45) | NS |
| Category fluency (animals/min) | 5.14 (3.23) | 5.27 (2.15) | 6.50 (4.95) | NS |
| Letter fluency (“F” words/min) | 5.64 (3.18) | 8.09 (2.51) | 5.50 (3.54) | t = 2.21, P<0.05 |
The t tests are 2-tailed with 30 degrees of freedom.
mBNT indicates mini-Boston Naming Test; NS, nonsignificant.
This study further compared the responses with semantic knowledge for items and the word recognition portion of the mBNT (Table 3). Semantic knowledge was significantly better than word recognition for the left-predominant group (t=2.49, P<0.05), but not for the right-predominant group. Within the left-predominant group, semantic knowledge for both animate and inanimate items was significantly better than word recognition (animate: t=4.84, P<0.001; inanimate: t=3.20, P<0.01). Within the right-predominant group, semantic knowledge was not significantly better than word recognition for either the animate or the inanimate items.
An evaluation of word frequency of the animate compared with the inanimate words indicated much greater frequency of the inanimate items (www.americancorpus.org). Consequently, the 2 patient groups were compared on a subgroup of words matched for raw word frequencies [beaver (1654) with comb (1725); unicorn (383) with stethoscope (417); and octopus (592) with hammock (771)]. This subgroup comparison found no significant differences between left and right-predominant groups on the 3 animate words [0.43 (0.81) vs. 0.18 (0.40)], respectively; however, the inanimate word difference persisted [0.62 (0.86) vs. 1.45 (1.13), t=2.34, P<0.05]. There were no animate-inanimate differences in the left-predominant group, but the right-predominant group had significantly worse animate compared with inanimate naming (t=3.51, P<0.01).
Individual responses to the misnamed animal items were compiled for the right-predominant SD patients. Examples of responses to the Octopus item were: “one of those funny animals,” “a bug on the beach,” “a ghost” (described by 2 patients), “not a dog,” and “a bunch of extra hands.” Examples of responses to the Beaver item were: “some kind of animal,” “something that can bite you,” “a rat,” “a little animal with a tail,” “a giraffe,” “a beetle,” and “a wild thing.” Examples of responses to the Unicorn item were: “a halloween horse,” “a dog,” “some sort of animal,” and “a cat.” Examples of responses to the Sphinx item were: “a person’s face” (described by 2 patients), “an animal with a human person,” and “a dog.” Among these right-predominant patients, one mistook stuffed animals for living animal, and another, a rancher, could not tell his ranch animals apart.
DISCUSSION
These findings were supportive of interhemispheric differences in category-specific semantic knowledge in SD. The left-predominant patients had the worst overall naming and fluency, especially inanimate and letter. The left-predominant patients were impaired on both the animate and inanimate items, consistent with language specialization in the left hemisphere. In contrast, the right-predominant patients were differentially impaired on the animate items, compared with inanimate naming and animal fluency compared with letter fluency, suggesting impaired semantic knowledge for living things. Together, these findings, along with the greater number of right than left-predominant patients who misnamed animals and faces (an animal characteristic), supports a right anterior temporal role for living things, and contributes to our understanding of the organization of semantic knowledge in the brain.
The findings of this study agree with the organization of semantic knowledge by categories. Category-specific areas include knowledge of letters, numbers, colors, manipulable or nonmanipulable objects, fruits and vegetables, or faces and bodies of people.30–33 These category-specific areas can be differentially impaired with focal brain injury. There is evidence that these acquired impairments constitute losses in semantic stores themselves. 34,35 There is progressive loss of the semantic features or attributes that enable discrimination between specific categories, such as size and aggressiveness of animals,36 and semantic errors reflect partial or degraded knowledge with superordinate substitutions for subordinate knowledge (eg, animal for beaver).37 In addition, losses in semantic categories can be impaired exclusively in modalities such as vision.38–40 For example, in herpes simplex encephalitis, experiments show that the deficit can specifically affect the ability to retrieve the perceptual shape features of the living stimuli while knowledge of their functional properties is preserved.19
Among category-specific deficits, evidence from brain damage particularly supports differential impairment for living objects.41–43 Investigators have described patients with herpes simplex encephalitis and bilateral inferior temporal lobe damage who have had significantly greater difficulty in recognizing and naming animals and food items than with inanimate objects.44–49 Differential impairment for living things has also occurred among head-injury patients and in SD.50 Furthermore, brain damage can specifically dissociate deficits in animate knowledge compared with inanimate knowledge.33,51
Do differences in hemispheric involvement correspond to differences in animate and inanimate knowledge? 20,22 Most, but not all, studies show that the right anterior temporal region is critical for knowledge of living things, and the left posterior middle temporal gyrus and adjacent regions are critical for tools and artifacts. 41,44,45,47–49,52–58 Brambati et al59 have evaluated cortical gray thickness and the relationship to naming living, nonliving, and manipulable objects in neurodegenerative patients, and report that naming living things correlates with increased gray thickness in the right temporal pole. During processing of “living” minus “nonliving” items, functional MRI signal changes have occurred in the right inferior frontal (Brodmann Area 47), middle temporal (Brodmann Area 21), and fusiform gyrus (Brodmann Area 19).56 Although other functional studies show activation for living things in the posterior visual association cortices, a pooling of positron emission tomography data shows activation for living objects in medial aspects of the anterior temporal poles bilaterally.60 Moreover, there is a role for the right anterotemporal lobe (including the amygdala) in recognizing human faces and their emotional message,13,61 and right-sided lesions result in person misidentification syndromes and prosopagnosia. 19,62,63 Other studies, especially with temporal lobectomy patients, emphasize a left anterior temporal role in naming people and living things, although usually accompanied by a general role in naming objects.54,64–68 In summary, animal naming is impaired after both right and left temporal lesions, but probably through different mechanisms. The right anterior temporal lobe may impair animal naming through perceptual or emotional semantics required for living things but not for nonliving things, such as tools. In contrast, the left anterior temporal lobe may impair animal naming through general language semantic processes, such as conveying semantic distinctions to the lexical system.69–71
There are several potential limitations of this study. First of all, there is the retrospective nature of the investigation, and the reliance on clinical testing. This methodology, however, allowed us to evaluate significant numbers of SD patients at the earliest asymmetric stages, when they could be characterized into left and right-predominant patients. Second, the actual clinical items, were not originally chosen to investigate the animate-inanimate distinction, were comprised of only 10 inanimate and 5 animate items, and included mythical items, such as a unicorn and Sphynx and a plant. Despite these limitations of the clinical test, significant findings emerged when the items were divided into animate and inanimate. Moreover, despite their unusual aspects, there is clearly an animate aspect to the mythical items. A separate localization for plants in the inferomesial parts of the left temporo-occipital area may justify the separate report of the patients missing only the animal items.54 Third, the items were not controlled for object familiarity, and this can affect SD performance.72 The separate analysis controlling for word frequency, however, continued to show that the right-predominant group was significantly worse on animate compared with inanimate naming. Finally, the MRI analysis was not quantitative because of the need to include patients evaluated on different scanners. There is valid support for an MRI visual rating scale in the evaluation of these patients.73
In conclusion, patients with SD show interhemispheric differences in knowledge of animate-inanimate entities. Whereas left-predominant SD equally affects animate and inanimate entities, right-predominant SD, which relatively spares language, continues to impair animate entities. The current results support the concept of a right-hemisphere region for person-specific semantic information.20–22 Further studies are needed to confirm that the right anterior temporal region makes a unique contribution to knowledge of living things.
Acknowledgments
This study was supported by grant #R01AG034499-02.
References
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127(Pt 4):860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- 3.Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Comput. 1989;1:123–132. [Google Scholar]
- 4.Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 5.Pobric G, Jefferies E, Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci U S A. 2007;104:20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies RR, Hodges JR, Kril JJ, et al. The pathological basis of semantic dementia. Brain. 2005;128(Pt 9):1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- 7.Desgranges B, Matuszewski V, Piolino P, et al. Anatomical and functional alterations in semantic dementia: a voxel-based MRI and PET study. Neurobiol Aging. 2007;28:1904–1913. doi: 10.1016/j.neurobiolaging.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 9.Josephs KA, Whitwell JL, Vemuri P, et al. The anatomic correlate of prosopagnosia in semantic dementia. Neurology. 2008;71:1628–1633. doi: 10.1212/01.wnl.0000334756.18558.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129(Pt 11):3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- 11.Hodges JR, Martinos M, Woollams AM, et al. Repeat and Point: differentiating semantic dementia from progressive non-fluent aphasia. Cortex. 2008;44:1265–1270. doi: 10.1016/j.cortex.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Bright P, Moss HE, Stamatakis EA, et al. Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia. 2008;46:2177–2188. doi: 10.1016/j.neuropsychologia.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Evans JJ, Heggs AJ, Antoun N, et al. Progressive prosopagnosia associated with selective right temporal lobe atrophy: a new syndrome? Brain. 1995;118(Pt 1):1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia. 2007;45:1591–1607. doi: 10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Gainotti G, Ferraccioli M, Quaranta D, et al. Cross-modal recognition disorders for persons and other unique entities in a patient with right fronto-temporal degeneration. Cortex. 2008;44:238–248. doi: 10.1016/j.cortex.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Rogers TT, Ivanoiu A, Patterson K, et al. Semantic memory in Alzheimer’s disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20:319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- 17.Cross K, Smith EE, Grossman M. Knowledge of natural kinds in semantic dementia and Alzheimer’s disease. Brain Lang. 2008;105:32–40. doi: 10.1016/j.bandl.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain the animate-inanimate distinction. J Cogn Neurosci. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- 19.De Renzi E, Lucchelli F. Are semantic systems separately represented in the brain? The case of living category impairment. Cortex. 1994;30:3–25. doi: 10.1016/s0010-9452(13)80322-x. [DOI] [PubMed] [Google Scholar]
- 20.Panza F, Solfrizzi V, D’Introno A, et al. Semantic dementia: neuropsychological and behavioral patterns in relation to hemispheric asymmetries. Am J Geriatr Psychiatry. 2003;11:695–696. doi: 10.1176/appi.ajgp.11.6.695. [DOI] [PubMed] [Google Scholar]
- 21.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SA, Graham KS, Williams G, et al. Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia. 2004;42:359–370. doi: 10.1016/j.neuropsychologia.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(Pt 11):2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balthazar ML, Cendes F, Damasceno BP. Semantic error patterns on the Boston Naming Test in normal aging, amnestic mild cognitive impairment, and mild Alzheimer’s disease: is there semantic disruption? Neuropsychology. 2008;22:703–709. doi: 10.1037/a0012919. [DOI] [PubMed] [Google Scholar]
- 28.Astell AJ, Harley TA. Accessing semantic knowledge in dementia: evidence from a word definition task. Brain Lang. 2002;82:312–326. doi: 10.1016/s0093-934x(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 29.Mendez MF, Ghajarnia M. Agnosia for familiar faces and odors in a patient with right temporal lobe dysfunction. Neurology. 2001;57:519–521. doi: 10.1212/wnl.57.3.519. [DOI] [PubMed] [Google Scholar]
- 30.Coslett HB, Saffran EM, Schwoebel J. Knowledge of the human body: a distinct semantic domain. Neurology. 2002;59:357–363. doi: 10.1212/wnl.59.3.357. [DOI] [PubMed] [Google Scholar]
- 31.Hart J, Jr, Berndt RS, Caramazza A. Category-specific naming deficit following cerebral infarction. Nature. 1985;316:439–440. doi: 10.1038/316439a0. [DOI] [PubMed] [Google Scholar]
- 32.Haslam C, Wills AJ, Haslam SA, et al. Does maintenance of colour categories rely on language? Evidence to the contrary from a case of semantic dementia. Brain Lang. 2007;103:251–263. doi: 10.1016/j.bandl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy RA, Warrington EK. Evidence for modality-specific meaning systems in the brain. Nature. 1988;334:428–430. doi: 10.1038/334428a0. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda M, Patterson K, Graham KS, et al. A horse of a different colour: do patients with semantic dementia recognise different versions of the same object as the same? Neuropsychologia. 2006;44:566–575. doi: 10.1016/j.neuropsychologia.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Laiacona M, Capitani E, Barbarotto R. Do living and nonliving categories need further fractionation? A study of picture naming in a pathological sample. Brain Cogn. 2000;43:291–296. [PubMed] [Google Scholar]
- 36.Warrington EK, Crutch SJ. A within-modality test of semantic knowledge: The Size/Weight Attribute Test. Neuropsychology. 2007;21:803–811. doi: 10.1037/0894-4105.21.6.803. [DOI] [PubMed] [Google Scholar]
- 37.Crutch SJ, Warrington EK. Contrasting patterns of comprehension for superordinate, basic-level, and subordinate names in semantic dementia and aphasic stroke patients. Cogn Neuropsychol. 2008;25:582–600. doi: 10.1080/02643290701862290. [DOI] [PubMed] [Google Scholar]
- 38.Carroll E, Garrard P. Knowledge of living, nonliving and “sensory quality” categories in semantic dementia. Neurocase. 2005;11:338–350. doi: 10.1080/13554790591006339. [DOI] [PubMed] [Google Scholar]
- 39.Thompson-Schill SL, Aguirre GK, D’Esposito M, et al. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37:671–676. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy RA, Warrington EK. Disorders of semantic memory. Philos Trans R Soc Lond B Biol Sci. 1994;346:89–96. doi: 10.1098/rstb.1994.0132. [DOI] [PubMed] [Google Scholar]
- 41.Kurbat MA. Can the recognition of living things really be selectively impaired? Neuropsychologia. 1997;35:813–827. doi: 10.1016/s0028-3932(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 42.Kurbat MA, Farah MJ. Is the category-specific deficit for living things spurious? J Cogn Neurosci. 1998;10:355–361. doi: 10.1162/089892998562780. [DOI] [PubMed] [Google Scholar]
- 43.Laws KR, Crawford JR, Gnoato F, et al. A predominance of category deficits for living things in Alzheimer’s disease and Lewy body dementia. J Int Neuropsychol Soc. 2007;13:401–409. doi: 10.1017/S1355617707070610. [DOI] [PubMed] [Google Scholar]
- 44.Barbarotto R, Capitani E, Laiacona M. Naming deficit in herpes simplex encephalitis. Acta Neurol Scand. 1996;93:272–280. doi: 10.1111/j.1600-0404.1996.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 45.Borgo F, Shallice T. When living things and other “sensory quality” categories behave in the same fashion: a novel category specificity effect. Neurocase. 2001;7:201–220. doi: 10.1093/neucas/7.3.201. [DOI] [PubMed] [Google Scholar]
- 46.Noppeney U, Patterson K, Tyler LK, et al. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130(Pt 4):1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- 47.Stewart F, Parkin AJ, Hunkin NM. Naming impairments following recovery from herpes simplex encephalitis: category-specific? Q J Exp Psychol A. 1992;44:261–284. doi: 10.1080/02724989243000037. [DOI] [PubMed] [Google Scholar]
- 48.Utley TF, Ogden JA, Gibb A, et al. The long-term neuropsychological outcome of herpes simplex encephalitis in a series of unselected survivors. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:180–189. [PubMed] [Google Scholar]
- 49.Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(Pt 3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- 50.Laiacona M, Barbarotto R, Capitani E. Perceptual and associative knowledge in category specific impairment of semantic memory: a study of two cases. Cortex. 1993;29:727–740. doi: 10.1016/s0010-9452(13)80293-6. [DOI] [PubMed] [Google Scholar]
- 51.Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126(Pt 4):792–803. doi: 10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- 52.Bunn EM, Tyler LK, Moss HE. Category-specific semantic deficits: the role of familiarity and property type reexamined. Neuropsychology. 1998;12:367–379. doi: 10.1037//0894-4105.12.3.367. [DOI] [PubMed] [Google Scholar]
- 53.Farah MJ, McMullen PA, Meyer MM. Can recognition of living things be selectively impaired? Neuropsychologia. 1991;29:185–193. doi: 10.1016/0028-3932(91)90020-9. [DOI] [PubMed] [Google Scholar]
- 54.Gainotti G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: a review. Cortex. 2000;36:539–559. doi: 10.1016/s0010-9452(08)70537-9. [DOI] [PubMed] [Google Scholar]
- 55.Ilmberger J, Rau S, Noachtar S, et al. Naming tools and animals: asymmetries observed during direct electrical cortical stimulation. Neuropsychologia. 2002;40:695–700. doi: 10.1016/s0028-3932(01)00194-4. [DOI] [PubMed] [Google Scholar]
- 56.Leube DT, Erb M, Grodd W, et al. Activation of right fronto-temporal cortex characterizes the “living” category in semantic processing. Brain Res Cogn Brain Res. 2001;12:425–430. doi: 10.1016/s0926-6410(01)00068-4. [DOI] [PubMed] [Google Scholar]
- 57.Moss HE, Tyler LK. A progressive category-specific semantic deficit for non-living things. Neuropsychologia. 2000;38:60–82. doi: 10.1016/s0028-3932(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 58.Mummery CJ, Patterson K, Hodges JR, et al. Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci. 1998;10:766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- 59.Brambati SM, Myers D, Wilson A, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- 60.Devlin JT, Moore CJ, Mummery CJ, et al. Anatomic constraints on cognitive theories of category specificity. Neuroimage. 2002;15:675–685. doi: 10.1006/nimg.2001.1002. [DOI] [PubMed] [Google Scholar]
- 61.Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- 62.Silva JA, Leong GB, Wine DB. Misidentification delusions, facial misrecognition, and right brain injury. Can J Psychiatry. 1993;38:239–241. doi: 10.1177/070674379303800401. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi N, Kawamura M, Hirayama K, et al. Prosopagnosia: a clinical and anatomical study of four patients. Cortex. 1995;31:317–329. doi: 10.1016/s0010-9452(13)80365-6. [DOI] [PubMed] [Google Scholar]
- 64.Damasio H, Grabowski TJ, Tranel D, et al. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 65.Gorno-Tempini ML, Price CJ, Josephs O, et al. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- 66.Luckhurst L, Lloyd-Jones TJ. A selective deficit for living things after temporal lobectomy for relief of epileptic seizures. Brain Lang. 2001;79:266–296. doi: 10.1006/brln.2001.2485. [DOI] [PubMed] [Google Scholar]
- 67.Strauss E, Semenza C, Hunter M, et al. Left anterior lobectomy and category-specific naming. Brain Cogn. 2000;43:403–406. [PubMed] [Google Scholar]
- 68.Tippett LJ, Glosser G, Farah MJ. A category-specific naming impairment after temporal lobectomy. Neuropsychologia. 1996;34:139–146. doi: 10.1016/0028-3932(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 69.Garrard P, Carroll E. Lost in semantic space: a multi-modal, non-verbal assessment of feature knowledge in semantic dementia. Brain. 2006;129(Pt 5):1152–1163. doi: 10.1093/brain/awl069. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz M, Kimberg DY, Walker GM, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reilly J, Peelle JE. Effects of semantic impairment on language processing in semantic dementia. Semin Speech Lang. 2008;29:32–43. doi: 10.1055/s-2008-1061623. [DOI] [PubMed] [Google Scholar]
- 72.Kipps CM, Davies RR, Mitchell J, et al. Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement Geriatr Cogn Disord. 2007;23:334–342. doi: 10.1159/000100973. [DOI] [PubMed] [Google Scholar]
- 73.Lambon Ralph MA, Graham KS, Ellis AW, et al. Naming in semantic dementia-what matters? Neuropsychologia. 1998;36:775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]