Abstract
Craniopharyngioma accounts for 2.5-4 percent of all intracranial tumors. The tumor is more observed in the chiasmatic region in adults and the intraventricular subtype is rare. We report an intraventricular craniopharyngioma in a 22-year-old woman presented with chronic headache. Magnetic Resonance Imaging showed hyperintense large mass on T1-weighted images and hypointense mass on T2-weighted images in third ventricle with pressure effect on both lateral ventricles and foramen of Monro. The diagnosis of craniopharyngioma was confirmed through histopathological examination of the resected tumor after surgery. After a follow-up period of nine months, neither tumor recurrence nor regrowth occurred. The early diagnosis of this relatively frequent tumor would help to prevent related sequelae.
Keywords: Craniopharyngioma, Headache, Histopathology
INTRODUCTION
Craniopharyngiomas are benign partly cystic epithelial tumors, which originate from the squamous epithelial remnants of Rathke's pouch in the subpial space.1–4 The location of the tumor is determined by embryological events of the suprasellar region.5 This kind of tumor accounts for 2.5 to 4 percent of all intracranial tumors.6 Twenty percent of these tumors are located in the sellar (chiasmatic) region in adult,1 while 5% of them are purely intrasellar.6 Suprasellar Craniopharyngiomas in 30% of cases extend to the anterior fossa, in 23% to the middle, and in 20% to the posterior fossa and/or retroclival region.6 Rare ectopic locations include: third ventricle, nasopharynx, pineal gland, sphenoid sinus, and clivus.6 The intra-ventricular craniopharyngiomas usually present at an older age.7
CASE REPORT
A 22-year-old right handed woman was admitted to our institution who presented with chronic headache lasting for 3 years. She had generalized non pulsatile headache accompanied by nausea and vomiting. She had also one episode of generalized tonic clonic seizure.
On the day of admission, she was alert and oriented and neurological examinations were completely normal, except for fundoscopy which revealed mild bilateral papilledema. Non- enhanced computed tomography (NECT) of the head revealed a round and homogeneous hyperdense mass (49 × 54 × 51 mm), below the lateral ventricles, in the third ventricle, which was accompanied by mild dilatation of the bitemporal horns of lateral ventricles due to pressure effect on the foramen of Monro. No calcification was identified in the lesion and posterior fossa and fourth ventricle were unremarkable (Figures 1–3).
Figure 1.
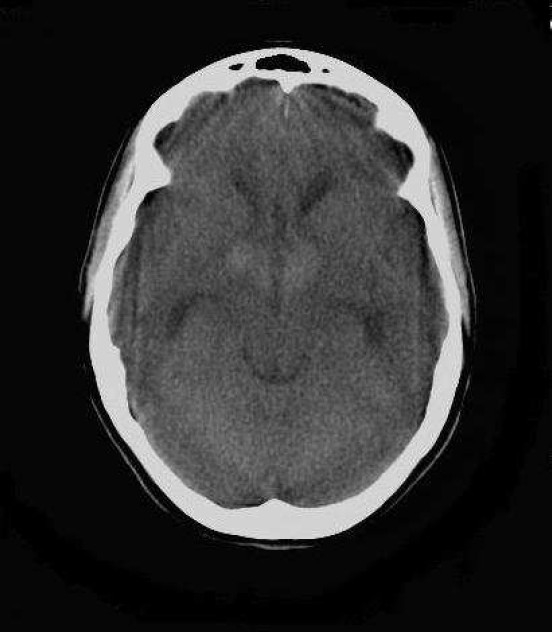
Axial non enhanced CT (NECT) scan of brain (KV: 120, MAS: 60) of a 22-year-old woman with intrinsic third ventricular craniopharyngioma
At the level of temporal horns revealed mid line, homogenous, hyperdense mass below the bifrontal horns with mild obstructive hydrocephaly due to pressure effect on foramen of Monroe.
Figure 3.
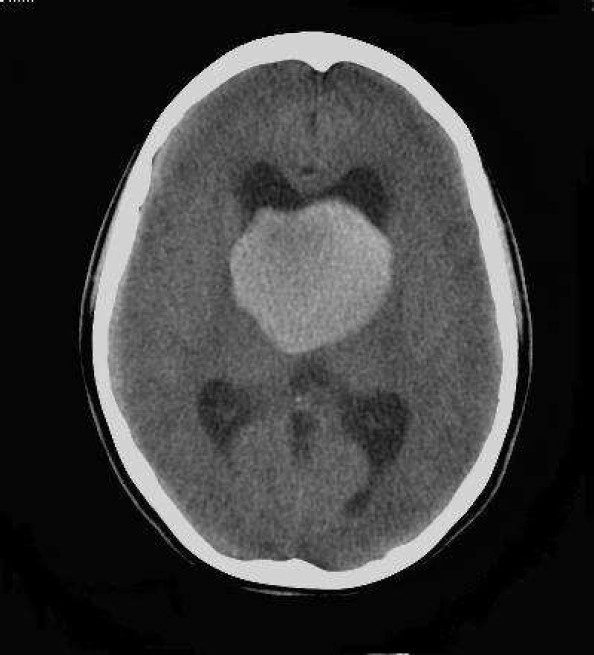
Axial non enhanced CT (NECT) scan of brain (KV: 120, MAS: 60) of a 22-year-old woman with intrinsic third ventricular craniopharyngiom
At the level of occipital horns revealed largest dimension of hyperdense mass in third ventricle associated with ventriculomegaly
Figure 2.
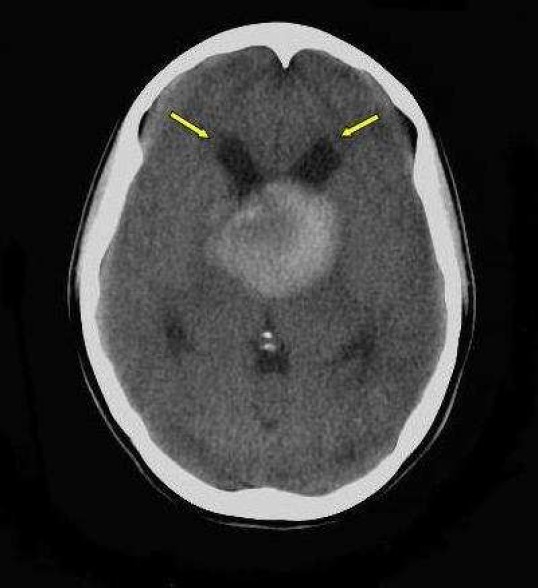
Axial Non enhanced CT (NECT) scan of brain (KV: 120, MAS: 60) of a 22-year-old woman male with intrinsic third ventricular craniopharyngioma
At the level of thalamus revealed midline relatively large homogenous, hyperdense mass in third ventricle with subtle periventricular hypodensity (arrow) due to interstitial edema. Dilatation of frontal horns is due to obstructive hydrocephaly
Magnetic resonance imaging without injection of contrast media (non enhanced MRI) showed a hyperintense large mass on T1-weighted and a hypointense lesion on T2-weighted and fluid attenuated inversion recovery (FLAIR) images in the third ventricle accompanied by pressure effect on both lateral ventricles and foramen of Monro with interstitial edema due to transependymal leakage of cerebrospinal fluid (CSF) as hyperintensely cloudy like area around the ventricles on FLAIR images (Figures 4–7).
Figure 4.
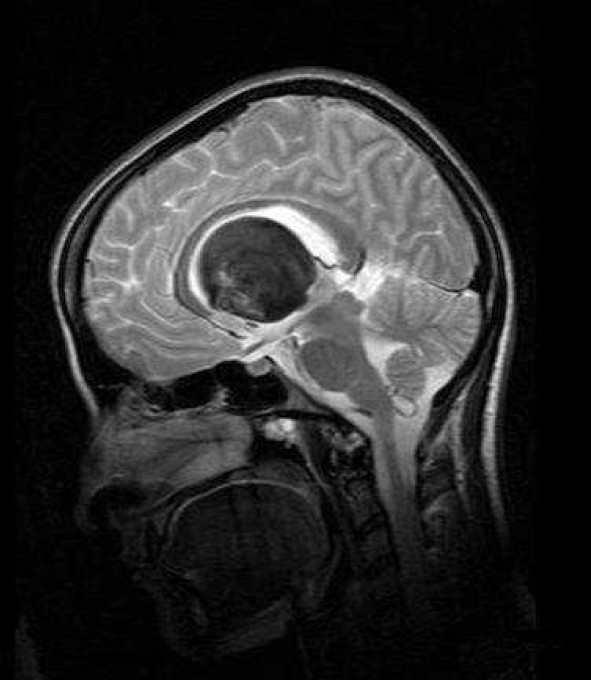
Mid sagital T2 weighted spin–echo (SE) magnetic resonance imaging (MRI) of a 22-year-old woman with intrinsic third ventricular craniopharyngioma
The MRI of brain (Philips intra 1.5 T, TE:100 msec, TR: 3646.8 msec) revealed large round, heterogeneous more prominent hypointense mass placed in third ventricle with obvious bowing of corpus callosum and non communicating hydrocephalus. No pressure effect on optic chiasm was seen
Figure 7.
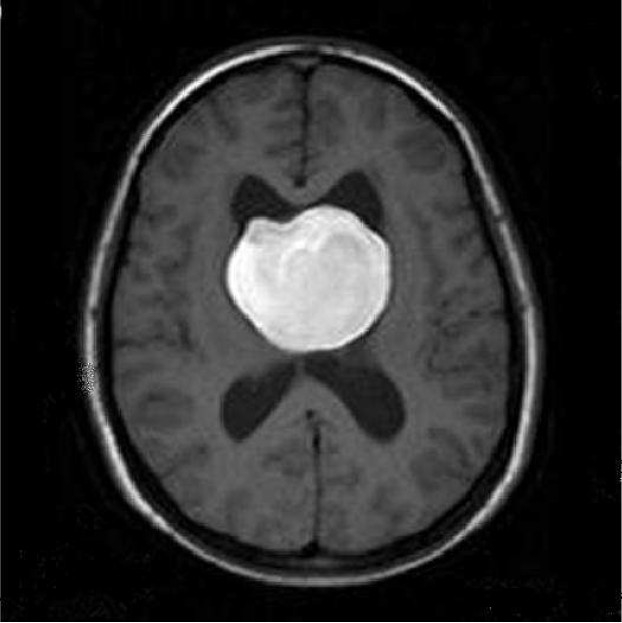
Axial unenhanced T1 weighted spin-echo (SE) Magnetic resonance imaging (MRI) of a 22-year-old woman with intrinsic third ventricular craniopharyngioma
The MRI of brain (Philips intra 1.5 T, TE:15 msec, TR: 486.1 msec) at the level of body lateral ventricles showed large midline, intra ventricular, hyperintense mass
Figure 5.
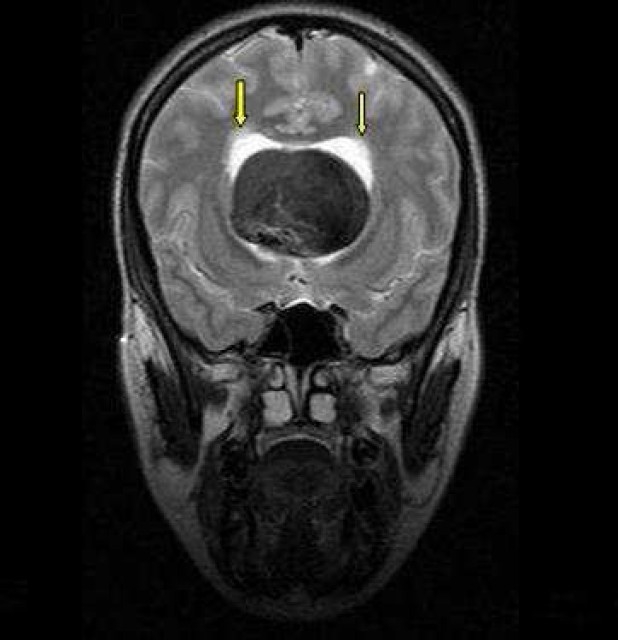
Coronal T2 weighted spin–echo (SE) magnetic resonance imaging (MRI) of a 22-year-old woman with intrinsic third ventricular craniopharyngioma
The MRI of brain (Philips intra 1.5 T, TE:100 msec, TR: 3641.6 msec) at the level of temporal lobes revealed large midline hypointense mass in third ventricle with dilatation of lateral ventricles and subtle hyperintensity around lateral ventricle due to hydrocephalus (arrow)
Figure 6.
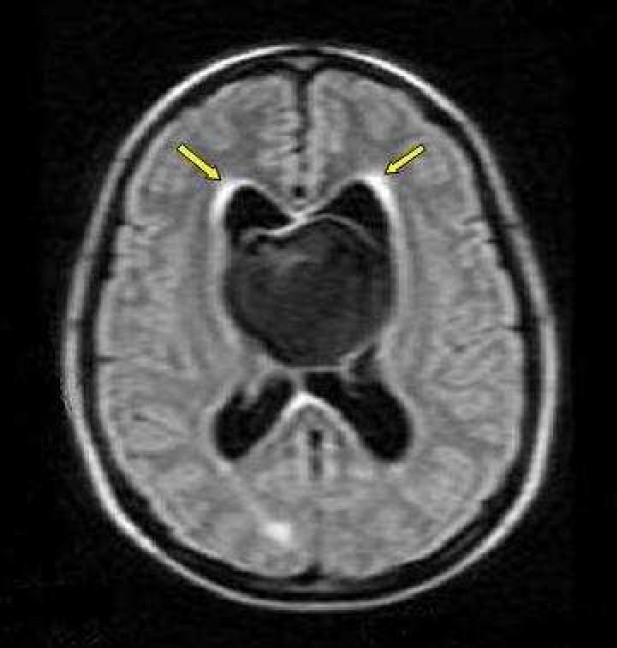
Axial FLAIR image spin-echo (SE) of magnetic resonance imaging (MRI) of a 22-year-old woman with intrinsic third ventricular craniopharyngioma
The MRI of brain (philips intra 1.5 T, TE:140 msec, TR: 11000.6 msec) at the level of body lateral ventricles showed large midline hypointense mass, with periventricular hyperintensity due to interestial edema (arrow)
The patient underwent right frontal–precentral–parasagittal craniotomy. The right lateral ventricle was approached through a transcallosal path. The tumor obstructing the foramen of Monro was completely resected. The histological examination revealed a mixed cystic and solid papillary type craniopharyngioma (figure 8,9). After nine months of follow-up, neither tumor recurrence nor regrowth occurred.
Figure 8.
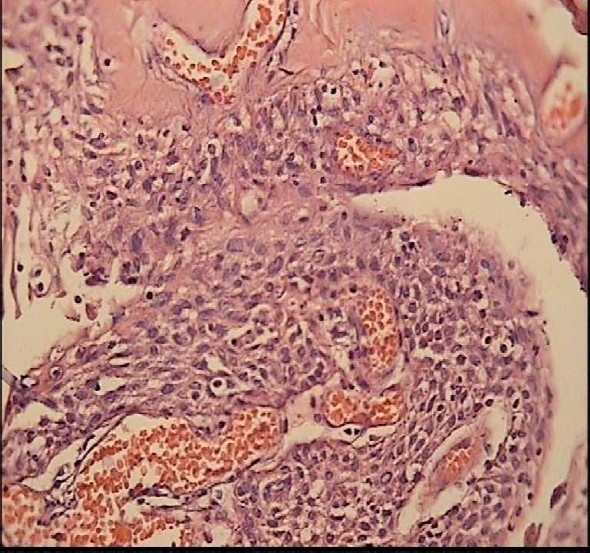
Papillary craniopharyngioma in a 22-year-old woman patient On H&E staining (Magnification: ×40) it is microscopically composed of solid, well differentiated, pseudopapillary squamous epithelium with separation and desquamation of the epithelium.
Figure 9.
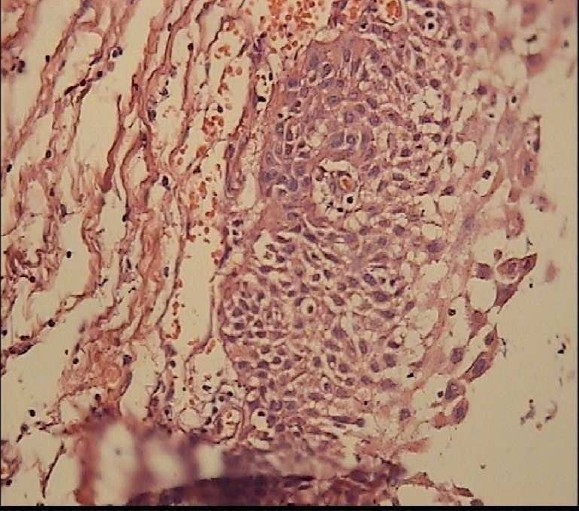
Papillary craniopharyngioma in a 22-year-old woman patient On H &E staining (Magnification: ×10) it is microscopically composed of solid, well differentiated, pseudopapillary squamous epithelium with separation and desquamation of the epithelium.
DISCUSSION
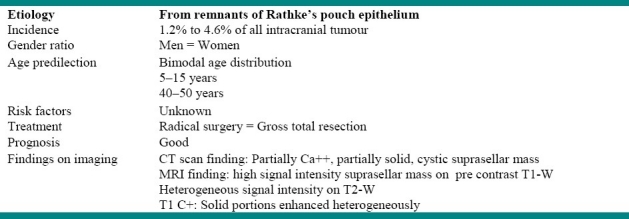
Craniopharyngiomas are benign partly cystic epithelial tumors, which originate from the squamous epithelial remnants of Rathke's pouch in the subpial space.1,4 The location of the tumor is determined by embryological events of the suprasellar region.5 This kind of tumor accounts for 2.5 to 4 percent of all intracranial tumors.6 Twenty percent of these tumors are located in the sellar (chiasmatic) region in adult,1 while 5% of them are purely intrasellar.6 Suprasellarcraniopharyngiomas in 30% of cases extend to the anterior fossa, in 23% to the middle, and in 20% to the posterior fossa and/or retroclival region.6 Rare ectopic locations include: third ventricle, nasopharynx, pineal gland, sphenoid sinus, and clivus.6 The intra-ventricular craniopharyngiomas usually present at an older age.7 Table 1 summarizes the features of craniopharyngioma.
Table 1.
Information about craniopharyngioma
Behari et al. reported six patients with purely intra-ventricular craniopharyngioma; including 4 patients with cystic lesions and 2 with solid lesions.8 All of them presented with manifestations of raised intracranial pressure, and papilledema. In all patients, the purely intra-ventricular nature of the craniopharyngioma was ascertained on the basis of preoperative MRI. In the above mentioned study, during follow-up period of 8 to 36 months, neither tumor recurrence nor regrowth was detected in any of patients. The symptom of raised intracranial pressure, such as papilledema and visual field defect, were resolved after surgery.8
The slow growth of craniopharyngiomas coupled with their location in the third ventricular lumen might delay the encroachment of vital structures and cerebrospinal fluid CSF pathway obstruction for a considerable amount of time. Therefore, they cannot be detected at an early stage, and this might be the reason for the older age at onset of this subtype of craniopharyngiomas. However, when the tumor becomes large enough to obstruct the CSF pathway, the patients present with headache and/or vomiting as the first symptoms.
Visual disturbances with endocrinological disorders which are the presenting symptoms in cases of suprasellar craniopharyngioma are very rare in intraventricular subtype.7 In addition, calcification which is common in suprasellar craniopharyngioma, is rare in intra-ventricular type tumor.8
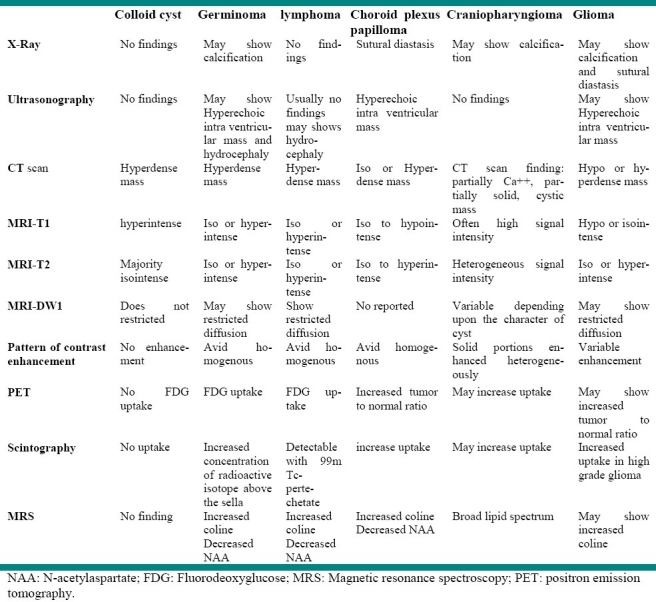
Colloid cyst, germinoma, lymphoma, choroid plexus papilloma and glioma are the main differential diagnoses of third ventricular craniopharyngioma. In computed tomography (CT) scan all aforementioned tumors could appear as a hyperdense mass, while craniopharyngioma usually appears as a partially solid cystic mass.1
Preventive related pointes
The tumor is present at birth, but it may not be symptomatic until childhood or adulthood.
The cause is not totally understood, although it is believed to be primarily a congenital illness.
Beta-catenin gene mutations have been identified to be important only in the adamantinomatous subtype.9,10
Duringthe fetal period, ultrasonography, later CT scan, and MRI are regarded to be the most effective tools for diagnosis.11
Imaging and differential diagnosis
MRI is the best imaging technique for precise anatomical localization of the intra-ventricular craniopharyngioma, however, no specific signal could be observed.9 Craniopharyngiomas usually appear as heterogeneous masses of variable intensity(often high signal intensity) on T1-weighted MRI images and hypointense to mildly hyperintense compared to gray matter On T2 Weighted images.12 Table 2 shows the differential diagnoses of craniopharyngioma and their patterns on different imaging modalities.
Table 2.
Differential diagnosis of third ventricular mass
CONCLUSION:
Craniopharyngioma accounts for 2.5 to 4 percent of all intracranial tumors, and is more frequently detected in the sellar region and the intra-ventricular subtype is rare. Cranio-pharyngiomas appear as heterogeneous masses of variable intensity on T1- and T2- weighted MRI images. The early diagnosis of this tumor would help to prevent related sequelae.
Footnotes
Conflict of interest statement: All authors declare that they have no conflict of interest.
Source of funding: None.
REFERENCES
- 1.Osborn A, Blaser S, Salzman K. AMIRSYS. 1st ed 2004. Diagnostic Imaging: Brain. [Google Scholar]
- 2.Bose B, Huang P, Myers D, Osterholm J. Intrinsic third ventricular craniopharyngioma: two case reports with review of the literature 1. Del Med J. 1985;57(6):389–94. [PubMed] [Google Scholar]
- 3.Kunishio K, Sunami N, Yamamoto Y, Asari S, Akagi T, Ohtsuki Y. [An autopsy study on the origin of craniopharyngioma confined to the third ventricle.Case report] 1. Neurol Med Chir (Tokyo) 1986;26(9):712–7. doi: 10.2176/nmc.26.712. [DOI] [PubMed] [Google Scholar]
- 4.Kunishio K, Yamamoto Y, Sunami N, Asari S, Akagi T, Ohtsuki Y. Craniopharyngioma in the third ventricle: necropsy findings and histogenesis. J Neurol Neurosurg Psychiatry. 1987;50(8):1053–6. doi: 10.1136/jnnp.50.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: trans-lamina terminalis approach. Neurosurgery. 2000;47(4):857–63. doi: 10.1097/00006123-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Haaga JR. CT and MRI of the Whole Body. In: Haaga JR, Dogra VS, Forsting M, Gilkeson RC, Ha HK, Sundaram M, editors. Mosby Elsevier; 2009. [Google Scholar]
- 7.Ikezaki K, Fujii K, Kishikawa T. Magnetic resonance imaging of an intraventricular craniopharyngioma. Neuroradiology. 1990;32(3):247–9. doi: 10.1007/BF00589123. [DOI] [PubMed] [Google Scholar]
- 8.Behari S, Banerji D, Mishra A, Sharma S, Sharma S, Chhabra DK, et al. Intrinsic third ventricular craniopharyngiomas: report on six cases and a review of the literature. Surg Neurol. 2003;60(3):245–52. doi: 10.1016/s0090-3019(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 9.Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, et al. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol. 2002;161(6):1997–2001. doi: 10.1016/s0002-9440(10)64477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrubl F, et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 2005;109(6):589–97. doi: 10.1007/s00401-005-1004-x. [DOI] [PubMed] [Google Scholar]
- 11.Joo JG, Rigo J, Jr, Sapi Z, Timar B. Foetal craniopharyngioma diagnosed by prenatal ultrasonography and confirmed by histopathological examination. Prenat Diagn. 2009;29(2):160–3. doi: 10.1002/pd.2202. [DOI] [PubMed] [Google Scholar]
- 12.Migliori A, Calzolari F, Marzola A, Ghadirpour R, Migliori M. 8 ed. 1992. Intrinsic third ventricle craniopharyngioma. [DOI] [PubMed] [Google Scholar]


