Abstract
The critical trace element zinc is essential for normal insulin production, and plays a central role in cellular protection against apoptosis and oxidative stress. The regulation of zinc within the pancreas and β-cells is controlled by the zinc transporter families ZnT and ZIP. Pancreatic islets display wide variability in the occurrence of these molecules. The zinc transporter, ZnT8 is an important target for autoimmunity in type 1 diabetes. Gene polymorphisms of this transporter confer sensitivity for immunosuppressive drugs used in islet transplantation. Understanding the biology of zinc transport within pancreatic islets will provide insight into the mechanisms of β-cell death, and may well reveal new pathways for improvement of diabetes therapy, including islet transplantation. This review discusses the possible roles of zinc in β-cell physiology with a special focus on islet transplantation.
Keywords: type 1 diabetes; pancreatic islet; beta-cell; zinc transporter; oxidative stress; apoptosis; ZnT; ZIP; insulin secretion; transplantation
Abbreviations: α-TC cell - simian virus 40 T glucagonoma cell; BBDR - Bio Breeding diabetes-resistant; Bcl-2 - B-cell lymphoma 2; CsA - cyclosporine A; Cu-Zn SOD - copper zinc superoxide dismutase; CXC - chemokine receptor family (mediate the biological activities of chemokines); FasL - Fas ligand (or CD95L, transmembrane protein belonging to the TNF family, binding with the Fas receptor induces apoptosis); GWAS - genome-wide association studies; IL-1 - interleukin 1 (inflammatory signaling cytokine); IL-8 - interleukin 8 (chemokine of the CXC family); INS-1E - rat insulinoma 1E cell line; Irt - iron-regulated transporter; mRNA - messenger ribonucleic acid; NF-κB - nuclear factor-kappa B; OH - hydroxide; PBMC - peripheral blood mononuclear cells; PCR - polymerase chain reaction; ROS - reactive oxygen species; SLC39A - soluble carrier 39A; SNP - single nucleotide polymorphism; SOD - superoxide dismutase; T1D - type 1 diabetes; T2D - type 2 diabetes; TNF-α - tumor necrose factor alpha; TRPM3 - transient receptor potential ion channel melastatin 3; ZIP - Zrt- and Irt-like protein; ZnT - zinc transporter; Zrt - zinc-regulated transporter
Introduction
Pancreatic islet cell transplantation is emerging as a promising treatment for selected patients with type 1 diabetes (T1D). In this therapy, cadaveric human donor pancreata are enzymatically digested to create islets for infusion into the T1D recipient’s liver under immunosuppressive drug regimen [1-3]. Shortly after isolation and within the first few days of transplantation, multiple factors, including apoptosis of transplanted islets, contribute by approximately 60% to the functional loss of β-cells. Half of this loss occurs within the first three days of transplantation [4]. One of the limitations to a wider application of islet transplantation for treatment of T1D patients is the requirement of more than one donor pancreas to obtain an adequate mass of donor islets for effective treatment. Long-term success of the therapy is compromised by the loss of islets because of a variety of processes, including islet apoptosis, instant blood-mediated inflammatory response, toxicity of drug therapy, and recurrence of autoimmunity. β-cell apoptosis, either during islet isolation or immediately after transplantation, and the susceptibility of the islets to immunosuppressive toxicity are important long-term causes of islet loss [5, 6]. Understanding the factors and causes that predispose β-cells to apoptosis or protect against it, is therefore an important issue with direct impact on the field of islet transplantation.
The trace element zinc is known to play an important role in pancreatic islets as a specific structural component of the insulin molecule and also in insulin secretion [7]. Zinc has been shown to possess both antioxidant and anti-apoptotic properties. The availability of zinc is controlled by two major families of transporters, the Zrt- and Irt-like protein (ZIP) family (responsible for zinc influx into cells) and the ZnT family (responsible for intracellular transport of zinc into organelles or zinc efflux from cells). Whether alteration of zinc transporters contributes to stress and cell death during islet cell transplantation is presently unknown. However, autoimmunity targeting zinc transporter proteins, in particular the ZnT family member ZnT8, has been identified as a new autoimmune pathway in T1D. Polymorphisms for the same zinc transporter also confer risk in type 2 diabetes (T2D). Studies focusing on the regulation of zinc transporters in islet transplantation are still lacking, but the connection between zinc, apoptosis, and autoimmunity makes zinc a relevant element for islet viability in transplantation regimens. This review describes the current state of knowledge of the role of zinc and zinc transporters in islet biology and the importance of zinc transporters in islet regulation.
Role of zinc in the pancreas
Zinc is important for a number of functions in the pancreas, including synthesis, secretion, and signaling of insulin, glucagon secretion, and pancreatic digestive enzyme secretion and activity. In the exocrine cells, zinc is abundant in the granules of acinar cells where digestive proenzymes are stored and released via exocytosis [8]. Under normal conditions, approximately 1-2 mg/day of zinc enter the pancreatic acinar cells through the digestive system [9]. In zinc deficiency states, pancreatic acinar cells undergo zinc depletion. During zinc excess, zinc alters acinar cell structure and reduces digestive enzyme secretion [8].
Recently, Wagner et al. investigated the TRPM3 channel, which regulates the influx of zinc into pancreatic β-cells [10]. TRPM3 is expressed endogenously in pancreatic β-cells, and is highly permeable to zinc ions. Zinc uptake is increased through TRPM3 and voltage-gated calcium channels, if extracellular TRPM3 is activated. This process replenishes the intracellular stores of zinc in the pancreatic islets.
The highest zinc content in the body has been detected in the islets [11]. Most of the intracellular zinc is stored with insulin in the insulin secretory vesicles in pancreatic β-cells as a zinc insulin complex. The concentration of zinc in these vesicles is very high, approximately 20mM [12]. However, zinc transporters are also found in pancreatic α-cells and are supposed to regulate glucagon secretion [13]. During insulin secretion, zinc is released together with insulin into the extracellular islet space, and is taken up by neighboring cells [13]. Within β-cell insulin granules, each hexameric insulin crystal contains two zinc ions [14]. Zinc-deficient rats have lower insulin secretion and glucose uptake compared to normal rats [15]. Faure and colleagues demonstrated that zinc depletion decreased insulin activity in rats [15]. Nutritional zinc supplementation improved fasting insulinemia and glycemia in rodents. The mechanism of action of zinc, whether it acts directly on insulin receptors and glucose transporters, or indirectly via intracellular pathways of insulin, is unknown [16].
During insulin exocytosis, insulin granules fuse with the β-cell plasma membrane, and release their content into the pancreatic micro-circulation [17]. The important role of zinc in pancreatic β-cell function requires that these cells are equipped with sophisticated mechanisms to take up zinc and incorporate it into their secretory granules [18].
Zinc transporter proteins
Zinc transporters control the influx and efflux of zinc within cells, and play an important role in maintaining cellular homeostasis between cell growth and disease prevention [19, 20]. There are two main families of zinc transporters, the ZIP and ZnT families. At present, 14 members of the ZIP family have been identified in humans, and 10 members of the ZnT family (or cation diffusion facilitator family). The latter are designated ZnT1-ZnT10 [18, 21-24].
Variation of zinc transporters in isolated pancreatic islets
Given the importance of zinc in insulin secretory function, the availability of zinc in donor islets is a potentially critical variable for good islet function. Critically ill patients in intensive care units are thought to have lower levels of serum zinc than healthy persons [25]. To verify this hypothesis we measured plasma zinc in 76 human pancreatic organ donors. The mean plasma zinc level was 6.48 mmol/l (±2.26), with a range in donors from 0.5-14.4 mmol/l. This finding indicates a wide variability in plasma zinc levels in organ donors, which potentially could influence post-isolation function. Donor zinc levels were lower than the reference range for healthy adults (9.0-21.0 mmol/l), indicating potential acute zinc deficiency in this population.
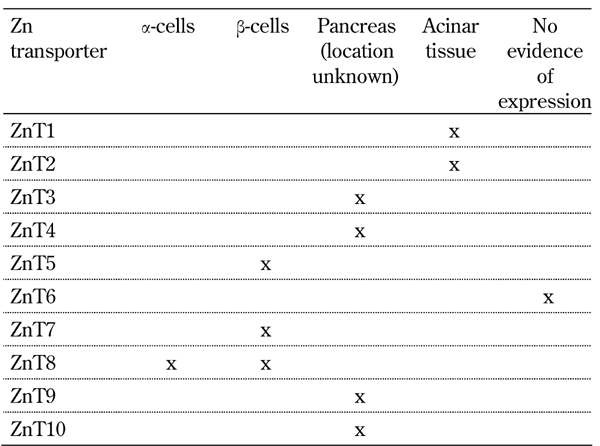
To investigate the expression of zinc transporters in human islet donors, isolated pancreatic islets were investigated using real time PCR. We identified 19 of the 24 known mammalian zinc transporters in isolated human islets, which contrasts with previously published findings for the pancreas (Tables 1 and 2). Those not expressed included two members of the ZnT family, ZnT3 and ZnT10, and three members of the Zip family, ZIP2, ZIP4, and ZIP12. The islet restricted zinc transporter ZnT8 was the most abundant ZnT family transcript within islets, expressed at a level 3 times greater than ZnT2 and ZnT9, the second and third most abundant transcripts, respectively. Within the ZIP family of transporters ZIP7 and ZIP14 were the most abundant transcripts. The expression of ZnT8 and ZIP14 was shown to be highly variable across multiple human islet isolations (n = 10) [26]. A possible explanation may be that the process of isolating islets can lead to dysregulation of these two transcripts and potentially lead to perturbations in islet function.
Table 1. ZIP family zinc transporter expression in the pancreas of organ donors.
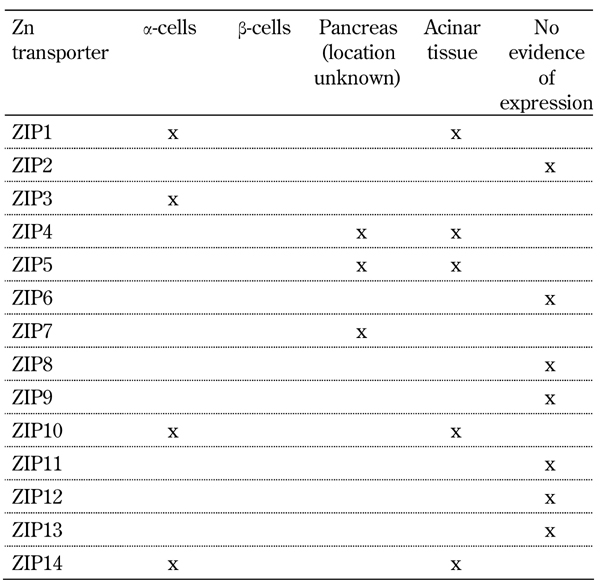
Table 2. ZnT family zinc transporter expression in the pancreas of organ donors.
Islet oxidative stress and zinc as an antioxidant
Oxidative stress
Islets have a significantly higher proportion of pancreatic blood supply compared with pancreatic acinar tissue, and are very sensitive to hypoxia and hypoxia-induced oxidative stress [27, 28]. The chronic hyperglycemia that occurs in diabetes also causes oxidative stress and promotes the formation of reactive oxygen species (ROS) [29], leading to mitochondrial dysfunction [30], endoplasmic reticulum stress [31-33], and ultimately to β-cell dysfunction [34]. Oxidative stress also plays an important role in decreasing islet cell viability during isolation and transplantation [35]. During islet transplantation procedures, islets undergo hypoxia and decreased oxygen consumption [28]. This initiates biochemical reactions leading to the production of ROS, and subsequent damage and injury to the islets [36, 37]. Increased oxidative stress in islets during this period is partly due to decreased expression of antioxidant enzymes, such as glutathione peroxidise, catalase, and xanthine oxidase [38].
Zinc as an antioxidant
The antioxidant properties of zinc in organs such as skin and lung have been thoroughly investigated. However, the role of zinc as antioxidant in the pancreas has not been extensively studied. Disturbances in zinc homeostasis, and in particular zinc depletion, in the pancreas have been associated with oxidative stress [39]. In some studies, zinc supplementation has been found to reduce the progression of diabetes by reducing oxidative stress and apoptosis [40-42]. Zinc plays a major role in the maintenance of the structural integrity of copper zinc superoxide dismutase (Cu-Zn SOD) [43]. Zinc supplementation increases superoxide dismutase activity in vitro. Correspondingly, SOD activity in zinc-deficient rats is decreased. Zinc supplementation of T2D patients can prevent decreased synthesis of the zinc-containing antioxidant enzymes superoxide dismutase and glutathione peroxidase, and thereby reduce albumin excretion in microalbuminuric T2D patients [44].
Another enzyme important in oxidative stress is xanthine oxidase, which catalyses the hydroxylation of xanthine to form superoxide radicals. Zinc inhibits xanthine oxidase activity in vitro, thereby reducing lipid peroxidation [45]. In humans, Roussel and colleagues demonstrated that 30 mg/day of zinc as supplementation reduced lipoperoxidation in the blood samples [46]. It was proposed that zinc metallothionein complex inhibits xanthine oxidase by interrupting the binding of iron in the Fenton reaction and subsequent redox reaction [47]. Zinc as a component of zinc metallothionein complexes in pancreatic islets provides protection against the inflammatory reaction induced by multiple low doses of streptozotocin [48] (Figure 1). Mechanistically, zinc-upregulated metallothionein inhibits OH generation by inhibiting the Fenton reaction through the binding of Fe2+. Zinc is involved in protecting sulfhydryl groups against oxidation and in inhibiting free radical production in the Haber Weiss cycle by competing with transition metals [48, 49]. By preventing proteins from oxidation, zinc contributes to sulphydryl SH stabilization [50] (Figure 1). In summary, zinc has antioxidant properties mediated through SOD and metallotheionein pathways protecting proteins from reactive oxygen species and free radical attacks. These pathways are summarized in Figure 1.
Figure 1. Role of zinc as an antioxidant.
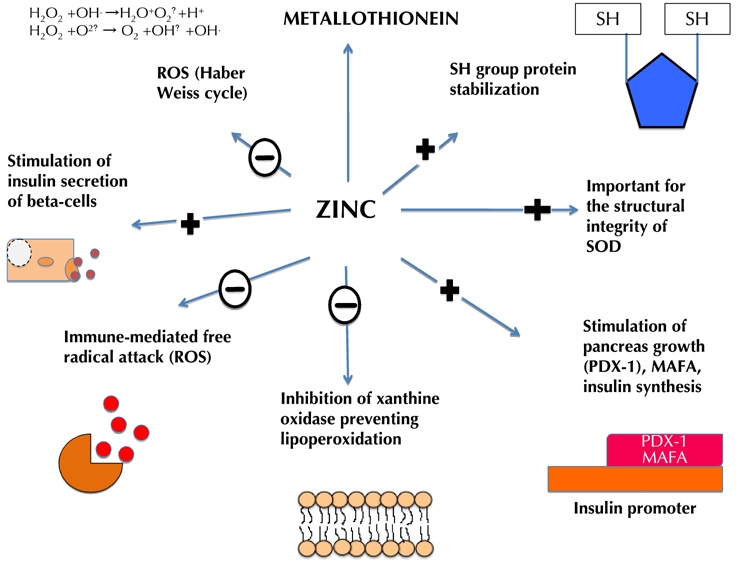
The inhibition of ROS by zinc reduces glucose toxicity. Zinc stimulates metallothionein transcription. Metallothionein itself has antioxidant effects. Zinc provides protection against immune-mediated free radical attack by protecting sulfhydryl (SH) groups against oxidation and participation in the inhibition of the free radical production in the Haber Weiss cycle by competing with transition metals. Zinc contributes to SH stabilization by protecting proteins from oxidation. It has also been shown to reduce directly .O2 and .OH- radicals, H2O2, and xanthine oxidase levels, thereby improving mitochondrial function. Decreasing these radicals decreases lipid peroxidation. Zinc also stimulates PDX-1 insulin promoter activity, and inhibits xanthine oxidase activity, thereby reducing lipid peroxidation. +: stimulation of pathway. -: inhibition of pathway.
Zinc is known to act as an antioxidant in many organs. However, the role of zinc as an antioxidant in the pancreas is limited and not extensively studied. Optimizing zinc contents in pancreatic islets may be an important factor in improving their survival during transplantation. Zinc may have protective effects against oxidative stress that occurs during the progression of diabetes or in islets prepared for transplantation purposes. Further research is necessary to verify this hypothesis.
Islet apoptosis and zinc as an anti-apoptotic factor
Inflammation and survival of islets after transplantation
After islet isolation and transplantation, it is estimated that up to 70% of β-cells are destroyed in the early post-transplant period. The major factor impacting the survival of islets within the liver is the inflammatory environment into which they are infused. This includes the inflammation-associated platelet activation, clot formation, and post-transplant lymphocyte recruitment. Another factor which contributes to the decrease in long-term islet cell mass is the toxic effect of immunosuppressive drugs on islet function [51-53].
Zinc and inflammatory cytokines
Zinc has a concentration-dependent effect on peripheral blood mononuclear cells (PBMC). It can suppress or stimulate the production of pro-inflammatory cytokines such as IL-1 and TNF-α [54]. Zinc supplementation of human PBMC leads to an increased mRNA production and release of the cytokines IL-6, IL-1β, and TNF-α. On the other hand, several reports indicate that zinc treatment suppresses the formation of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-8 [55]. Kee-Lung and colleagues showed that the effect of zinc is concentration-dependent [54]. Zinc administration of 100 µM stimulated cytokine production and expression of caspase-3 and pro-apoptotic genes, including Fas (FasL) and c-fos. Zinc concentrations above 100 µM decreased cytokine stimulation and the expression of the anti-apoptotic factors nuclear factor (NF) κB, Bcl-2, and Bcl-XL in PBMC from healthy subjects [54]. Zinc supplementation decreased TNFα-induced NF-κB activity in PBMC [56]. Zinc supplementation in cell lines upregulated anti-apoptotic protein zinc finger protein A20 in vitro [57]. A20 inhibits the activity of proinflammatory cytokines via TNF receptor-associated factors in cells [58, 59]. A20 is expressed in various cell types in response to a number of stimuli, such as TNF-α, IL1-β, Epstein-Barr virus latent membrane protein, and others [60]. A20 inhibits the activation of NF-κB by IL-1β and TNF-α gene expression in endothelial cells [61]. Cooper and colleagues suggested that A20 may play a role in regulating gene expression of IL-1β, IL-8, and TNF-α affected by zinc [60].
In summary, zinc exerts effects in a concentration-dependant manner in the body. It has direct effects on T cells and macrophage cytokine production, and indirect negative effects on the transcription of pro-apoptotic genes. Thus, zinc is an important mediator that may reduce apoptosis in the pancreas during diabetes progression.
Zinc efflux transporters in the pancreas (ZIP family)
The principal ZIP family zinc transporters identified in the pancreas are ZIP1, 3, 4, 5, 7, 10 and 14 (Table 1).
ZIP1 and ZIP3
In the literature, information regarding ZIP transporter expression in α- and β-cells is limited. Real-time quantitative PCR analysis for ZIP transcripts reveals that ZIP1 and ZIP3 expressions are present in a glucagon-producing α-cell line (α-TC cells) [13].
ZIP4 and ZIP5
ZIP4 is expressed in β-cells. It has been suggested that it plays a role in the uptake of zinc into β-cells [62], which is required for the correct packaging of insulin. ZIP4 is a major zinc transporter in the gastrointestinal tract responsible for adequate zinc homeostasis in humans [63, 64]. It has also been shown to play a central role in zinc homeostasis in the pancreas [65]. In zinc deficiency states, significant amounts of zinc are released from the pancreas into the intestinal tract by means of the intestinal pancreatic axis [66]. ZIP4 is highly expressed in pancreatic acinar cells [67].
ZIP5 is expressed in organs and tissues involved in zinc homeostasis, including intestine, visceral endoderm, and pancreas [62]. Under conditions of zinc deficiency, intestinal absorption of zinc is enhanced. ZIP5 is abundantly expressed on the basolateral surface of pancreatic acinar cells, and is downregulated in response to dietary zinc deficiency. These genes have also shown to be highly expressed in the murine pancreas. The function of ZIP5 is to take zinc from the blood and to transport it into pancreatic acinar cells [67-69].
ZIP7, 10, and 14
ZIP7 mRNA is expressed in the pancreas. However, its location and function have still not been characterized [70]. In mice, ZIP 10 and 14 transporter genes were found in glucagon-producing cells [13].
Zinc influx transporters in the pancreas (ZnT family)
The principal ZnT family zinc transporters identified in the pancreas are ZnT 1, 2, 3, 4, 5, 7, 8, 9 and 10 (Table 1).
ZnT1, 2, 3, and 4
ZnT1 and ZnT2 are expressed in pancreatic acinar cells. Dietary zinc intake regulates the expression of ZnT1 and ZnT2 in the pancreas. Zinc deficiency reduced zinc concentration in both the cytoplasm and zymogen granule compartments of acinar cells. Overexpression of ZnT2 resulted in more sequestered intracellular zinc with normal zinc efflux rate [71].
Clifford and colleagues used RT-PCR to estimate the quantity of mRNAs encoding various metal-complexing proteins in the pancreas of 3-day old animals and in islets from 10 days old and adult normal Sprague Dawley and diabetes-resistant (BBDR) rats [72]. ZnT1 was shown to be expressed at all ages tested. However, ZnT4 was not found in the pancreas of three days old animals, but was present in islets from 10 days through to adulthood. Genes encoding ZnT2 and ZnT3 were not expressed in the pancreas in either 3-day old or adult animals, but were present in islets of 10-day and 5-week old animals. The presence of ZnT2 and ZnT3 mRNA was found in islets of 10-day and 5-week old animals, a feature of rapidly developing islets [72]. This indicates that zinc transporters are differently expressed during the development period from infant to adulthood, according to zinc requirement.
ZnT3 is expressed in pancreatic β-cells. However, the sub-cellular location is still unknown [73]. Knockdown of ZnT3 in INS-1E cells showed that insulin secretion was significantly reduced. This indicates that ZnT3 plays a role in the secretion of insulin [74]. However, the mechanism of action is unknown.
ZnT5
ZnT5 is abundantly expressed in the secretory granules of β-cells and some acinar cells [18, 75]. Another study showed that ZnT5 protein is abundantly expressed in human pancreatic β-cells, but not in glucagons-secreting α-cells and most acinar cells [18].
ZnT7 and ZnT8
Huang and colleagues showed that ZnT7 is expressed in pancreatic islets of mice [76]. When co-stained with insulin, it is localized in the perinuclear region of β-cells, located close to the Golgi apparatus [77-79]. ZnT7 is involved in two transporting mechanisms. Firstly, it facilitates transportation of the cytoplasmic zinc into the Golgi apparatus of the cell for zinc storage. Secondly, it mediates incorporation of zinc into newly synthesized zinc transporter proteins [80]. ZnT7 overexpression in β-cells significantly increased total cellular insulin content and basal insulin secretion [76].
ZnT8 is highly expressed in β-cells [81, 82], and localized in the membranes of the secretory vesicles. ZnT8 localized with insulin-containing secretory vesicles in cultured rat INS-1 cells [12], a pancreatic β-cell line derived from rat insulinoma [83], and also in human pancreatic islets [82]. Furthermore, ZnT8 is also expressed in α-cells [13, 84], and subcutaneous fat tissue [84]. Chimienti and colleagues reported that over-expression of ZnT8 (SLC30A8) enhanced the insulin capacity of a β-cell line [82]. ZnT8 knockdown in the INS-1 β-cell line showed reduced insulin content and decreased insulin secretion in response to a hyperglycemic stimulus.
ZnT8 knockout mice also had fewer dense core vesicles in insulin producing β-cells (as determined by electron microscopy). ZnT8 gene deletion showed modest impairment in insulin secretion without affecting glucose metabolism [85]. Decreased secretion of insulin was due to decreased ZnT8 expression, which may be attributed to reduced transport of zinc into secretory vesicles. This may impair the packaging of insulin as hexamers around zinc cores, and thereby alter insulin secretion [86, 87]. Complete loss of ZnT8 expression in mice homozygous for null mutation of SLC39A8 leads to decreased zinc accumulation in islets [11, 88, 89].
ZnT8 is involved in the pathogenesis of type 1 and 2 diabetes [8, 69, 73, 88-93]. It is a major target of T1D, humoral autoimmunity [94-96]. Achenbach and colleagues were the first to report humoral autoimmune responses against ZnT8 (SLC30A8) genotype in a large cohort of children with a first-degree family history of T1D [69]. They also reported that the COOH terminal in ZnT8A was found to be an epitope for T1D rather than the NH2-terminal of this protein [95]. ZnT8A-COOH-positive children who carried homozygous SLC30A8 SNP rs13266634 genotypes progressed faster to diabetes than those who were heterozygous [69]. Another study investigated two major isoforms of ZnT8, ZnT8-arginine (ZnT8R) and ZnT8-tryptophan (ZnT8W) on type 1 diabetes patient cohorts with different age distributions at onset. Most of the type 1 diabetic patients tested positive for ZnT8Ab to both isoforms. However, ZnT8Ab titers were significantly higher in the younger age group (148 and 29 U/ml) [97].
In T2D, genetic polymorphisms for ZnT8 influence the level of expression and function of the protein [98, 99]. Population studies revealed a strong association of single nucleotide polymorphism (SNP) RS13266634, a nonsynonymous [92] arg325Trp (C>T) variant in SLC30A8 with type 2 diabetes [100-102]. The C allele variant was suggested to be the risk allele for type 2 diabetes [103-107]. Genome-wide association studies (GWAS) [108] identified that single nucleotide polymorphisms are associated with increased risk of T2D [100, 102]. Other studies reported that variations in SLC30A8 may effect zinc accumulation in insulin granules, which influence insulin stability and insulin trafficking [82, 106]. The R325W mutation in SLC30A8 is associated with T2D and decreased first-phase insulin secretion in non-diabetic subjects bearing at least one copy of the risk allele [108, 109].
The handling of immunosuppressive drugs necessary for islet transplantation is also influenced by ZnT8 polymorphisms. The calcineurin inhibitor cyclosporine did not suppress glucose-stimulated insulin secretion in islets with the ZnT8 W325 variant expressed in insulinoma cells. Whereas, cells expressing ZnT8 R325 were suppressed by these drugs. This indicates that the structure of the ZnT8 variant W325 is important for protection against post-transplant diabetes [90]. Kang and colleagues reported that a polymorphism in the zinc transporter gene SLC30A8 confers resistance against post-transplant diabetes in renal transplant recipients [110]. In addition, Kim and colleagues reported that the ZnT8 R325 expressed in the INS-1E cell line showed reduced insulin content and secretion when treated with cyclosporine A (CsA) [90]. When ZnT8 variant W325 expression was treated with CsA, insulin content and secretion was not affected. [91]. This indicates that by altering the ZnT8 structure, as in the W325 variant, cells are protected from immunosuppressive drug toxicity. Studies in islet transplantation have not yet been performed to examine the effect of these polymorphisms on islet function post transplantation. These studies would require multi-center collaborative effort.
ZnT9 and ZnT10
ZnT9 and ZnT10 mRNA is expressed in the pancreas. However, their functional roles are still unknown [73].
Conclusions
Zinc plays a fundamental role in the structural integrity of insulin. The availability of zinc is crucial for normal insulin formation and secretion. Zinc also stabilizes the enzymes that protect against apoptosis. It is thus an important antioxidant. Zinc transporters are also important proteins, which regulate the availability of zinc. They show a wide variation in isolated pancreatic islets. Understanding the role of these transporters in pancreatic islets may provide new pathways to improve islet survival and function after islet isolation and transplantation.
Disclosures (conflict of interests statement): Grant funding from Juvenile Diabetes Research Foundation (JDRF) and the Australian Federal Government Department of Health and Ageing.
Acknowledgments
The authors thank Clyde Milner for his time and effort in editing and proofreading the manuscript.
References
- 1.Leitao CB, Cure P, Tharavanij T, Baidal DA, Alejandro R. Current challenges in islet transplantation. Curr Diab Rep. 2008;8:324–331. doi: 10.1007/s11892-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 2.Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, Frank A, Markmann E, Palanjian M, Brayman K. et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237:741–749. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Nagata M, Mullen Y, Matsuo S, Herrera M, Clare-Salzler M. Destruction of islet isografts by severe nonspecific inflammation. Transplant Proc. 1990;22:855–856. [PubMed] [Google Scholar]
- 5.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Emamaullee J, Liston P, Korneluk RG, Shapiro AM, Elliott JF. XIAP overexpression in islet beta-cells enhances engraftment and minimizes hypoxia-reperfusion injury. Am J Transplant. 2005;5:1297–1305. doi: 10.1111/j.1600-6143.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 7.Ynsa MD, Ren MQ, Rajendran R, Sidhapuriwala JN, van Kan JA, Bhatia M, Watt F. Zinc mapping and density imaging of rabbit pancreas endocrine tissue sections using nuclear microscopy. Microsc Microanal. 2009;15:345–352. doi: 10.1017/S1431927609090795. [DOI] [PubMed] [Google Scholar]
- 8.Guo L, Lichten LA, Ryu MS, Liuzzi JP, Wang F, Cousins RJ. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci U S A. 2010;107:2818–2823. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr. 2010;91:1478S–1483S. doi: 10.3945/ajcn.2010.28674I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner TF, Drews A, Loch S, Mohr F, Philipp SE, Lambert S, Oberwinkler J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch. 2010;460:755–765. doi: 10.1007/s00424-010-0838-9. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA. et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 13.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283:10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 14.Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer - a review. BioMetals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 15.Faure P, Roussel AM, Martinie M, Osman M, Favier A, Halimi S. Insulin sensitivity in zinc-depleted rats: assessment with the euglycaemic hyperinsulinic clamp technique. Diabetes Metab. 1991;17:325–331. [PubMed] [Google Scholar]
- 16.Chen MD, Liou SJ, Lin PY, Yang VC, Alexander PS, Lin WH. Effects of zinc supplementation on the plasma glucose level and insulin activity in genetically obese (ob/ob) mice. Biol Trace Elem Res. 1998;61:303–311. doi: 10.1007/BF02789090. [DOI] [PubMed] [Google Scholar]
- 17.Qian WJ, Aspinwall CA, Battiste MA, Kennedy RT. Detection of secretion from single pancreatic beta-cells using extracellular fluorogenic reactions and confocal fluorescence microscopy. Anal Chem. 2000;72:711–717. doi: 10.1021/ac991085t. [DOI] [PubMed] [Google Scholar]
- 18.Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 19.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 20.MacDiarmid CW, Gaither LA, Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J Biol Chem. 2002;277:26389–26395. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- 22.Cragg RA, Christie GR, Phillips SR, Russi RM, Küry S, Mathers JC, Taylor PM, Ford D. A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J Biol Chem. 2002;277:22789–22797. doi: 10.1074/jbc.M200577200. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 24.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins TL, Murray M, Kett DH, Fulda G, Kramer KM, Gelmont D, Dedhia HV, Levy H, Teres D, Zaloga GP, Ko H, Thompson KA. Trace element homeostasis during continuous sedation with propofol containing EDTA versus other sedatives in critically ill patients. Intensive Care Med. 2000;26(Suppl 4):S413–S421. doi: 10.1007/pl00003785. [DOI] [PubMed] [Google Scholar]
- 26.Drogemuller C, Mohanasundaram DM, Murgia C, Mee C, Milner CR, Lang CJ, Zalewski PD, Hawthorne W, Loudovaris T, Russ GR, Coates PT. Transcriptome analysis of isolated human pancreatic islets: expression profiles of zinc transporter genes in particular ZnT8 and ZIP14, critical islet marker genes and glucose metabolic genes. Xenotranspantation. 2009;16:5. [Google Scholar]
- 27.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004;114:877–883. doi: 10.1172/JCI23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE 3rd, Wildey GM, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi H, Tran PO, LeRoy E, Harmon JS, Tanaka Y, Robertson RP. D-glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective beta cell function via non-mitochondrial pathways. J Biol Chem. 2004;279:37316–37323. doi: 10.1074/jbc.M403070200. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Brun T, Cnop M, Cunha DA, Eizirik DL, Maechler P. Transient oxidative stress damages mitochondrial machinery inducing persistent beta-cell dysfunction. J Biol Chem. 2009;284:23602–23612. doi: 10.1074/jbc.M109.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 32.Lupi R, Del Guerra S, Mancarella R, Novelli M, Valgimigli L, Pedulli GF, Paolini M, Soleti A, Filipponi F, Mosca F, Boggi U, Del Prato S, Masiello P, Marchetti P. Insulin secretion defects of human type 2 diabetic islets are corrected in vitro by a new reactive oxygen species scavenger. Diabetes Metab. 2007;33:340–345. doi: 10.1016/j.diabet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Tang C, Han P, Oprescu AI, Lee SC, Gyulkhandanyan AV, Chan GN, Wheeler MB, Giacca A. Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes. 2007;56:2722–2731. doi: 10.2337/db07-0279. [DOI] [PubMed] [Google Scholar]
- 35.Mohseni Salehi Monfared SS, Larijani B, Abdollahi M. Islet transplantation and antioxidant management: a comprehensive review. World J Gastroenterol. 2009;15:1153–1161. doi: 10.3748/wjg.15.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine MJ, Fan W. Islet cell transplantation as a cure for insulin dependent diabetes: current improvements in preserving islet cell mass and function. Hepatobiliary Pancreat Dis Int. 2003;2:170–179. [PubMed] [Google Scholar]
- 37.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 39.Blostein-Fujii A, DiSilvestro R, Frid D, Katz C, Malarkey W. Short-term zinc supplementation in women with non-insulin-dependent diabetes mellitus: effects on plasma 5’-nucleotidase activities, insulin-like growth factor I concentrations, and lipoprotein oxidation rates in vitro. Am J Clin Nutr. 1997;66:639–642. doi: 10.1093/ajcn/66.3.639. [DOI] [PubMed] [Google Scholar]
- 40.Zago MP, Oteiza PI. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med. 2001;31:266–274. doi: 10.1016/s0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 41.Bolkent S, Yanardag R, Mutlu O. The influence of zinc supplementation on the pancreas of streptozotocin-diabetic rats. Dig Dis Sci. 2009;54(12):2583–2587. doi: 10.1007/s10620-008-0675-2. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa Y, Ueda E, Kojima Y, Sakurai H. The action mechanism of zinc(II) complexes with insulinomimetic activity in rat adipocytes. Life Sci. 2004;75:741–751. doi: 10.1016/j.lfs.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Coudray C, Richard MJ, Laporte F, Faure P, Roussel AM, Favier A. Superoxide dismutase activity and zinc status: a study in animals and man. J Nutr Environ Med. 1992;3:13–26. [Google Scholar]
- 44.Parham M, Amini M, Aminorroaya A, Heidarian E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud. 2008;5(2):102–109. doi: 10.1900/RDS.2008.5.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You ZL, Shi DH, Zhu HL. The inhibition of xanthine oxidase by the schiff base zinc (II) complex. Inorg Chem Commun. 2006;9:642–644. [Google Scholar]
- 46.Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr. 2003;22:316–321. doi: 10.1080/07315724.2003.10719310. [DOI] [PubMed] [Google Scholar]
- 47.Ryan MJ, Jackson JR, Hao Y, Leonard SS, Alway SE. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2011.04.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–1030. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- 49.Bettger WJ. Zinc and selenium, site-specific versus general antioxidation. Can J Physiol Pharmacol. 1993;71:721–724. doi: 10.1139/y93-108. [DOI] [PubMed] [Google Scholar]
- 50.Verhaegh GW, Parat MO, Richard MJ, Hainaut P. Modulation of p53 protein conformation and DNA-binding activity by intracellular chelation of zinc. Mol Carcinog. 1998;21:205–214. doi: 10.1002/(sici)1098-2744(199803)21:3<205::aid-mc8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 51.Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes. 2006;55:1907–1914. doi: 10.2337/db05-1254. [DOI] [PubMed] [Google Scholar]
- 52.Hughes A, Jessup C, Drogemuller C, Mohanasundaram D, Milner C, Rojas D, Russ GR, Coates PT. Gene therapy to improve pancreatic islet transplantation for type 1 diabetes mellitus. Curr Diabetes Rev. 2010;6(5):274–284. doi: 10.2174/157339910793360897. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 54.Chang KL, Hung TC, Hsieh BS, Chen YH, Chen TF, Cheng HL. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22(5):465–474. doi: 10.1016/j.nut.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285:E1095–E1102. doi: 10.1152/ajpendo.00545.2002. [DOI] [PubMed] [Google Scholar]
- 56.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-kappaB. Nutrition. 2010 doi: 10.1016/j.nut.2010.08.010. In press. [DOI] [PubMed] [Google Scholar]
- 58.Tewari M, Wolf F, Seldin M, O'Shea K, Dixit V, Turka L. Lymphoid expression and regulation of A20, an inhibitor of programmed cell death. J Immunol. 1995;154:1699–1706. [PubMed] [Google Scholar]
- 59.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 60.Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- 61.Falchuk KH. The molecular basis for the role of zinc in developmental biology. Mol Cell Biochem. 1998;188:41–48. [PubMed] [Google Scholar]
- 62.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- 63.Moynahan EJ. Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. 1974;2:399–400. doi: 10.1016/s0140-6736(74)91772-3. [DOI] [PubMed] [Google Scholar]
- 64.Neldner KH, Hambidge KM. Zinc therapy of acrodermatitis enteropathica. N Engl J Med. 1975;292:879–882. doi: 10.1056/NEJM197504242921702. [DOI] [PubMed] [Google Scholar]
- 65.McClain CJ. The pancreas and zinc homeostasis. J Lab Clin Med. 1990;116:275–276. [PubMed] [Google Scholar]
- 66.Hambidge M, Krebs NF. Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu Rev Nutr. 2001;21:429–452. doi: 10.1146/annurev.nutr.21.1.429. [DOI] [PubMed] [Google Scholar]
- 67.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci U S A. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng D, Feeney GP, Kille P, Hogstrand C. Regulation of ZIP and ZnT zinc transporters in zebrafish gill: zinc repression of ZIP10 transcription by an intronic MRE cluster. Physiol Genomics. 2008;34:205–214. doi: 10.1152/physiolgenomics.90206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achenbach P, Lampasona V, Landherr U, Koczwara K, Krause S, Grallert H, Winkler C, Pfluger M, Illig T, Bonifacio E, Ziegler AG. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia. 2009;52:1881–1888. doi: 10.1007/s00125-009-1438-0. [DOI] [PubMed] [Google Scholar]
- 70.Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 71.Guo L, Cousins RJ. ZnT1 (SLC30A1) and ZnT2 (SLC30A2) regulate zinc efflux through two different pathways in pancreatic acinar cells. FASEB J. 2008;22:674. [Google Scholar]
- 72.Clifford KS, MacDonald MJ. Survey of mRNAs encoding zinc transporters and other metal complexing proteins in pancreatic islets of rats from birth to adulthood: similar patterns in the Sprague-Dawley and Wistar BB strains. Diabetes Res Clin Pract. 2000;49:77–85. doi: 10.1016/s0168-8227(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 73.Smidt K, Jessen N, Petersen AB, Larsen A, Magnusson N, Jeppesen JB, Stoltenberg M, Culvenor JG, Tsatsanis A, Brock B. et al. SLC30A3 responds to glucose and zinc variations in beta-cells and is critical for insulin production and in vivo glucose metabolism during beta-cell stress. PLoS One. 2009;4:e5684. doi: 10.1371/journal.pone.0005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen AB, Smidt K, Magnusson NE, Moore F, Egefjord L, Rungby J. siRNA-mediated knock-down of ZnT3 and ZnT8 affects production and secretion of insulin and apoptosis in INS-1E cells. APMIS. 2011 doi: 10.1111/j.1600-0463.2010.02698.x. In press. [DOI] [PubMed] [Google Scholar]
- 75.Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem. 2003;278:50142–50150. doi: 10.1074/jbc.M304163200. [DOI] [PubMed] [Google Scholar]
- 76.Huang L, Yan M, Kirschke CP. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp Cell Res. 2010;316:2630–2643. doi: 10.1016/j.yexcr.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Chi ZH, Ren H, Wang X, Rong M, Huang L, Wang ZY. The cellular and subcellular localization of zinc transporter 7 in the mouse spinal cord. Histol Histopathol. 2008;23:781–787. doi: 10.14670/HH-23.781. [DOI] [PubMed] [Google Scholar]
- 78.Chi ZH, Wang X, Wang ZY, Gao HL, Dahlstrom A, Huang L. Zinc transporter 7 is located in the cis-Golgi apparatus of mouse choroid epithelial cells. NeuroReport. 2006;17:1807–1811. doi: 10.1097/01.wnr.0000239968.06438.c5. [DOI] [PubMed] [Google Scholar]
- 79.Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the golgi apparatus. J Biol Chem. 2003;278:4096–4102. doi: 10.1074/jbc.M207644200. [DOI] [PubMed] [Google Scholar]
- 80.Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J Biol Chem. 2007;282:37053–37063. doi: 10.1074/jbc.M706631200. [DOI] [PubMed] [Google Scholar]
- 81.Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic beta-cell-specific zinc transporter. BioMetals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 82.Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 83.Asfari M, Janjic D, Meda P, Li G, Halban P, Wollheim C. Establishment of 2-mercaptoethanol-dependent differentiated insulin- secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 84.Smidt K, Pedersen SB, Brock B, Schmitz O, Fisker S, Bendix J, Wogensen L, Rungby J. Zinc-transporter genes in human visceral and subcutaneous adipocytes: Lean versus obese. Mol Cell Endocrinol. 2007;264:68–73. doi: 10.1016/j.mce.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Cauchi S, Del Guerra S, Choquet H, D'Aleo V, Groves CJ, Lupi R, McCarthy MI, Froguel P, Marchetti P. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 86.El Muayed M, Billings L, Raja M, Zhang X, Park P, Newman M, Kaufmen D, O'Halloran T, Lowe WL. Acute cytokine mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta cell function. J Endocrinol. 2010;206(2):159–169. doi: 10.1677/JOE-09-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta-cells. Structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–20423. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- 88.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K. et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pound LD, Sarkar SA, Benninger RKP, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O'Brien RM. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim I, Kang ES, Yim YS, Ko SJ, Jeong SH, Rim JH, Kim YS, Ahn CW, Cha BS, Lee HC, Kim CH. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.22. In press. [DOI] [PubMed] [Google Scholar]
- 91.Fu Y, Tian W, Pratt EB, Dirling LB, Shyng SL, Meshul CK, Cohen DM. Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PLoS One. 2009;4:e5679. doi: 10.1371/journal.pone.0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A Common Nonsynonymous Single Nucleotide Polymorphism in the SLC30A8 Gene Determines ZnT8 Autoantibody Specificity in Type 1 Diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wijesekara N, Dai F, Hardy A, Giglou P, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano H, Rutter G, Wheeler M. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenzlau JM, Moua O, Liu Y, Eisenbarth GS, Hutton JC, Davidson HW. Identification of a major humoral epitope in Slc30A8 (ZnT8) Ann N Y Acad Sci. 2008;1150:252–255. doi: 10.1196/annals.1447.028. [DOI] [PubMed] [Google Scholar]
- 95.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gohlke H, Ferrari U, Koczwara K, Bonifacio E, Illig T, Ziegler AG. SLC30A8 (ZnT8) Polymorphism is Associated with Young Age at Type 1 Diabetes Onset. Rev Diabet Stud. 2008;5:25–27. doi: 10.1900/RDS.2008.5.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaziri-Sani F, Oak S, Radtke J, Lernmark K, Lynch K, Agardh CD, Cilio CM, Lethagen AL, Ortqvist E, Landin-Olsson M, Torn C, Hampe CS. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity. 2010;43:598–606. doi: 10.3109/08916930903555927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan H, Yuan W, Velculescu VE, Vogelstein B, Kinzler KW. Allelic variation in human gene expression. Science. 2002;297:1143. doi: 10.1126/science.1072545. [DOI] [PubMed] [Google Scholar]
- 99.Lo HS, Wang Z, Hu Y, Yang HH, Gere S, Buetow KH, Lee MP. Allelic variation in gene expression is common in the human genome. Genome Res. 2003;13:1855–1862. doi: 10.1101/gr.1006603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 101.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ. et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 102.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU. et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S. et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 104.Staiger H, Machicao F, Stefan N, Tschritter O, Thamer C, Kantartzis K, Schäfer SA, Kirchhoff K, Fritsche A, Häring HU. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS One. 2007;2:e832. doi: 10.1371/journal.pone.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palmer ND, Goodarzi MO, Langefeld CD, Ziegler J, Norris JM, Haffner SM, Bryer-Ash M, Bergman RN, Wagenknecht LE, Taylor KD, Rotter JI, Bowden DW. Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the insulin resistance atherosclerosis family study. Diabetes. 2008;57:1093–1100. doi: 10.2337/db07-1169. [DOI] [PubMed] [Google Scholar]
- 106.Xiang J, Li XY, Xu M, Hong J, Huang Y, Tan JR, Lu X, Dai M, Yu B, Ning G. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. J Clin Endocrinol Metab. 2008;93:4107–4112. doi: 10.1210/jc.2008-0161. [DOI] [PubMed] [Google Scholar]
- 107.Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M. Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic beta-cell function. Diabetes. 2007;56:3101–3104. doi: 10.2337/db07-0634. [DOI] [PubMed] [Google Scholar]
- 108.Weijers R. Three-dimensional structure of beta-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol Metab Syndr. 2010;2:33. doi: 10.1186/1758-5996-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hertel J, Johansson S, Ræder H, Midthjell K, Lyssenko V, Groop L, Molven A, Njølstad P. Genetic analysis of recently identified type 2 diabetes loci in 1,638 unselected patients with type 2 diabetes and 1,858 control participants from a Norwegian population-based cohort (the HUNT study) Diabetologia. 2008;51:971–977. doi: 10.1007/s00125-008-0982-3. [DOI] [PubMed] [Google Scholar]
- 110.Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, Hur KY, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes. 2008;57:1043–1047. doi: 10.2337/db07-0761. [DOI] [PubMed] [Google Scholar]


