Abstract
Male and female germ cells follow distinct developmental paths with respect to germline stem cell (GSC) production and the types of differentiated progeny they produce (sperm versus egg). An essential aspect of germline development is how sexual identity is used to differentially regulate the male and female germ cell genomes to allow for these distinct outcomes. Here, we identify a gene, no child left behind (nclb), that plays very different roles in the male versus female germline in Drosophila. In particular, nclb is required for GSC maintenance in males, but not in females. Male GSCs mutant for nclb are rapidly lost from the niche, and begin to differentiate but cannot complete spermatogenesis. We further find that nclb encodes a member of a new family of conserved chromatin-associated proteins. NCLB interacts with chromatin in a specific manner and is associated with sites of active transcription. Thus, NCLB appears to be a novel chromatin regulator that exhibits very different effects on the male and female germ cell genomes.
Keywords: nclb, Germline stem cells, Germline sexual identity, Chromatin, Epigenetics, Drosophila
INTRODUCTION
An important aspect of germline development is how sexual identity is used to differentially regulate the male and female germ cell genomes to allow for sex-specific germ cell development. The Drosophila gonads have proven to be excellent systems for studying germ cell development and stem cell biology. Both males and females have germline stem cell populations that share many characteristics and are formed from a similar pool of primordial germ cells. However, they are also distinct cell types that can be distinguished based on gene expression and cell biological characteristics, as well as the behavior of their differentiating progeny (Dansereau and Lasko, 2008). To what extent male and female germline stem cells differ from one another and how germline sex determination leads to these differences are key issues in germ cell development.
In addition to the germline stem cells (GSCs), adult testes and ovaries contain somatic stem cells and, together, these stem cells produce progeny that differentiate to form spermatogenic or oogenic cysts (Fuller, 1993; Fuller and Spradling, 2007; Spradling, 1993). In the testis, the GSCs and somatic stem cells (cyst stem cells, CySCs) are found at the apical end of the testis in close association with a somatic structure known as the `hub'. The hub acts as a signaling center to regulate stem cell maintenance and division through both the JAK/STAT and TGFβ pathways (Kawase et al., 2004; Kiger et al., 2001; Schulz et al., 2004; Shivdasani and Ingham, 2003; Tulina and Matunis, 2001). The hub also physically anchors the stem cells and regulates the orientation of GSC division (Yamashita et al., 2003). As GSC progeny begin to differentiate into gonia, they associate with somatic cyst cells and divide to produce a cyst of 16 interconnected cells that undergo meiosis to form sperm. In the female, GSCs are found within each ovariole of the ovary. These cells lie adjacent to the cap cells and terminal filament cells, which play an analogous role to the hub to physically anchor the GSCs and signal through the JAK/STAT and TGFβ pathways (Decotto and Spradling, 2005; Song and Xie, 2002; Xie and Spradling, 1998). As GSC progeny enter differentiation, they first associate with escort cells but then associate with the follicle cells to create egg-forming units known as egg chambers. The follicle cells are produced from follicle stem cells located more distally in the first region of the ovariole (Decotto and Spradling, 2005; Nystul and Spradling, 2007). As in the male, the differentiating germ cells will divide to produce a cyst of interconnected cells, but only one will commit to meiosis and become the oocyte, while the others become nurse cells.
During development, the gonad initially forms as the germ cells associate with somatic gonadal precursors (SGPs) and coalesce into the embryonic gonads (Dansereau and Lasko, 2008). At the time of gonad formation, sex-specific gene expression is observed in the SGPs and the germ cells, indicating that sexual identity has been established in both of these cell types (Camara et al., 2008; Casper and Van Doren, 2006). In the male, the hub forms by the end of embryogenesis (24 hours AEL) (Le Bras and Van Doren, 2006), and a subset of germ cells takes on the characteristics of adult GSCs at this time (Sheng et al., 2009). Spermatogenesis begins by the first instar larval period (Aboïm, 1945), as evidenced by the expression of the germline differentiation marker Bag of Marbles and the formation of interconnected cysts (Sheng et al., 2009). In females, both the germ cells and the SGPs have sex-specific identity in the embryo (Casper and Van Doren, 2009), but morphogenesis of the ovary does not begin until the larval stages (King, 1970), and cells are not thought to take on GSC identity until the larval/pupal transition (5 days AEL) (Zhu and Xie, 2003). Little is known about how sex-specific germ cell development is regulated to create the differences in male versus female GSC development and behavior.
To identify genes important for germline sexual development, we conducted an in situ hybridization screen for genes expressed sex specifically in embryonic germ cells (Casper and Van Doren, 2009). Such genes may be involved in regulating germline sexual identity, or may reflect differences in the timing of male versus female germline development, such as in the establishment of GSCs. Here, we report the study of one of these genes, no child left behind (nclb). We find that nclb is required in males for both GSC maintenance and early stages of germ cell differentiation. By contrast, nclb is not required in female GSCs and only acts to regulate later aspects of germ cell differentiation. Interestingly, nclb encodes a highly conserved protein of previously unknown function, and we demonstrate that NCLB is a chromatin-associated factor that regulates transcription. Thus, NCLB is a putative epigenetic regulator of GSC maintenance in the testis and reveals important differences in how the male versus female GSCs are controlled.
MATERIALS AND METHODS
Fly stocks
Oregon-R and precise excision of nclbEY10712 (as wild types), P(Dfd-lacZ-HZ2.7) on X (W. McGinnis, UCSD, CA, USA), tubulin-GAL4-LL7, nanos-GAL4-VP16 (Van Doren et al., 1998), paired-GAL4, nclbEY10712, nclbEY15483, Df(2R)Exel6059, Df(2R)27 and UASp-nclb. Unspecified fly stocks are from Bloomington Stock Center. nclbEY10712 is inserted into the 5′UTR, 45 bp from the start of transcription. nclb1 and nclb2 excisions from nclbEY10712 were characterized by assaying lethality, PCR, sequencing and western blots (see Fig. S1 in the supplementary material). The nclb2 excision deletes an upstream gene CG30016, which does not cause the lethality or germ cell defects as CG30016f02466/nclb2 flies are both viable and fertile.
For creating mutant GSC clones, 0- to 2-day-old male and female flies were heatshocked twice for 45 minutes at 37°C with 1 hour of recovery at 25°C in between. Flies of the following genotypes were mated to create the clones hsFLP;FRT42B: armlacZ/cyo × FRT42B nclb2/cyo or FRT42B +/FRT42B+.
Antibodies, RNA and DNA probes
Antibodies used are as follows (dilution, source): mouse anti-EYA 10H6 (1:25, DSHB; N. Bonini, University of Pennsylvania, Philadelphia, PA, USA), rabbit anti-NCLB [1:1000 (gonads), 1:400 (polytenes), 1:10,000 (westerns), M.V.D.], guinea pig anti-NCLB [1:1000 (gonads), 1:10,000 (westerns), M.V.D.], Su(Hw) (1:200, V. Corces, Emory University, Atlanta, GA, USA), mouse anti-Pol IIoser2 H5 (1:50, Covance), mouse anti-Pol IIoser5 H14 (1:50, Covance), rat anti-CP190 (1:20,000, V. Corces), rat anti-BAM (1:100, D. McKearin, University of Texas Southwestern Medical Center, Dallas TX, USA), mouse anti-GFP B-2 (1:50, Santa Cruz), rabbit anti-GFP (1:2000, Torrey Pines Biolabs), rat anti-DN-cadherin Ex#8 (1:20, DSHB; T. Uemura, Kyoto University, Japan), chick anti-VASA (1:10,000, K. Howard, UCL, London, UK), rabbit anti-VASA (1:10,000, R. Lehmann, Skirball Institute, NY, USA), rabbit anti-STAT92E (1:1000, S. Hou, NCI, Frederick, MD, USA), mouse anti-β-GAL (1:10,000, Promega), rabbit anti-β-GAL (1:10,000, Cappel), mouse anti-Fasciclin 3 7G10 (1:30, DSHB; C. Goodman, UC Berkeley, CA, USA), mouse anti-α-spectrin 3A9 [1:2, DSHB; D. Branton (University of Minnesota, Minneapolis, MN, USA) and R. Dubreuil (University of Illinois, Chicago, IL, USA)], mouse anti-γ-TUBULIN (1:100, Sigma), rat anti-E-cadherin (1:4, DSHB), rabbit anti-ZFH-1 (1:1000, Ruth Lehmann), rabbit anti-H3pSer10 (1:1000 Upstate Biotechnologies), rabbit anti-H3 (1:1000, Upstate Biotechnologies) and AP-conjugated sheep anti-digoxigenin (1:2000, Roche). Fluorescently conjugated secondary antibodies were used at 1:500 (Molecular Probes, Rockland Scientific, Amersham Pharmacia Biotech) or 1:400 (Jackson Laboratories). HRP-conjugated secondary antibody (Jackson ImmunoResearch Labratories). DAPI (1 μg/ml, Sigma), Hoechst (1 μg/ml, Invitrogen) and OliGreen (1:10,000, Molecular Probes) were used to detect DNA.
The antisense RNA probes for CG6751 were synthesized by digesting clone LD21021 with SacI (NEB) and transcribed with T7 RNA polymerase (Promega) using digoxigenin-labeled UTP (Boehringer-Mannheim).
A DNA probe for Hsp70 was amplified from genomic DNA using the following pair of primers: Hsp70 IIIA (5′-TGGTGCTGACCAAGATGAAG-3′) and Hsp70 IIIB (5′-TAGTCTGCTTGCACGGAATG-3′).
In situ hybridization, immunostaining and western blots
Adult testes, ovaries and L3 gonads were dissected in PBS and fixed in 0.1% Triton-X and PBS with 4.5% formaldehyde for 30 minutes and immunostained as previously described (Gönczy et al., 1997). L2 posterior ends were pulled off before fixing and gonads dissected during mounting. Embryos were fixed, devitellinized and immunostained as described previously (Patel, 1994) with modifications (Le Bras and Van Doren, 2006). Whole-mount in situ hybridization on embryos were performed as previously described (Lehmann and Tautz, 1994). Salivary gland polytene chromosomes were dissected from wandering third instar larvae. For heat-shock experiments, larvae were heat-shocked at 37°C for 20 minutes and dissected within 5 minutes. DNA in situ hybridization on polytenes was preformed as described previously (Ivaldi et al., 2007). The glands for immunostaining were transferred to 45% acetic acid for 30 seconds, then transferred to a drop of 1:2:3 fixative (one volume of lactic acid, two volumes of water and three volumes glacial acetic acid) on a siliconized cover slip. The glands were fixed for 2 minutes in 45% acetic acid or dissected and only fixed for 2 minutes in 120 μl of paraformaldehyde (3.7%) and 120 μl of absolute acetic acid. Next, all were squashed and frozen in liquid nitrogen. All stainings were mounted in VectaShield (Vector Laboratories) or 2.5% DABCO (Sigma) in 70% glycerol. All were visualized by Zeiss Axioskop or Zeiss 510 LSM confocal microscope. Whole-larvae extracts were used for westerns conducted as described previously (Ivaldi et al., 2007).
RESULTS
no child left behind (nclb) is required for male GSC maintenance
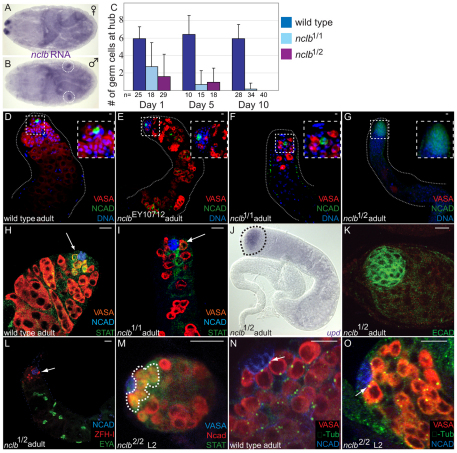
We identified CG6751 due to its male-specific expression in the embryonic gonad (Fig. 1A,B). Previous analysis indicated that CG6751 expression in the male gonad is germ cell specific and is controlled both autonomously by the chromosomal content of the germline and nonautonmously by sex-specific signals from the surrounding somatic cells (Casper and Van Doren, 2009). Our analysis of a P-element allele (CG6751EY10712) revealed that it was adult viable, but males were sterile and females exhibited only limited fertility (data not shown). CG6751EY10712 mutant adult testes exhibited a reduced number of germ cells and the absence of mature sperm (Fig. 1E). Owing to the defects in fertility and germline development associated with CG6751, we named this gene no child left behind (nclb).
Fig. 1.
nclb is required for male GSC maintenance. (A,B) In situ hybridization in stage 17 embryos reveals that nclb is expressed in male gonads and not female gonads. Staining in A is paternal X marker. Gonads are circled in B. (C) nclb mutants exhibit a reduced number of germline cells proximal to the hub compared with wild type (all wild type in this figure are a precise excision allele of EY10712). (D-G) Five-day-old adult testes immunostained for germ cells (VASA, red), hub cells (NCAD, green) and DNA (DAPI, blue). Note that nclb mutant testes exhibit germline loss relative to wild type that is more severe in stronger loss-of-function nclb mutants (e.g. G). (H,I) Newly eclosed adult testes immunostained for germ cells (VASA, red), hub cells (NCAD, blue) and STAT92E (anti-STAT92E, green). Germ cells adjacent to the hub (arrows) in nclb mutant testes do not exhibit anti-STAT 92E immunoreactivity. (J) Adult nclb mutant testis labeled by in situ hybridization for upd. Outline indicates apical end of testis. (K) nclb mutant adult testis immunostained for DE-cadherin (green). Note that the hub is also enlarged in nclb mutants. (L) nclb mutant adult testis immunolabeled for cyst stem cells (anti-ZFH-1, red, arrow), late somatic cyst cells (anti-EYA, green) and the hub (anti-NCAD, blue). Arrow indicates cyst stem cell. (M) Immunostaining of STAT92E (outline) is restricted to GSC in larval nclb mutant testis. (N,O) Centrosomes visualized with γ-tubulin antibody (green, arrows) are properly oriented in nclb mutant L2 larval testis (O), similar to wild-type adult (N) and larvae (not shown). Scale bars: 10 μm.
As the nclbEY10712 P element is inserted into the 5′UTR and did not behave as a null allele, new alleles were created through P-element excision. Two imprecise excision alleles, nclb1 and nclb2, exhibited stronger phenotypes than the original nclbEY10712 allele (Fig. 1F,G). nclb1 was found to contain an internal deletion in the P-element, and is adult viable and male and female sterile. We found that nclb2 contains a 2170 bp deletion that removes the entire nclb-coding region (see Fig. S1 in the supplementary material), and is homozygous lethal at the late larval stage. nclb2 homozygous mutant larvae were found to be smaller in size than heterozygous siblings and failed to enter pupariation; in rare cases, nclb2 mutant larvae lived to 9 or 10 days of age without forming pupae. Precise excisions of the EY10712 P-element were also generated as controls and animals homozygous for the precise excisions exhibited no defects in growth, gonad development or adult gonad function (e.g., Fig. 1D, Fig. 4A). In addition, we observed that the adult testis phenotype of nclb1/Df is similar to that of nclb1/2 (see Fig. S1C in the supplementary material). Therefore, we conclude that the mutant phenotype of our imprecise excision alleles are not due to other lesions on the parental chromosome.
Fig. 4.
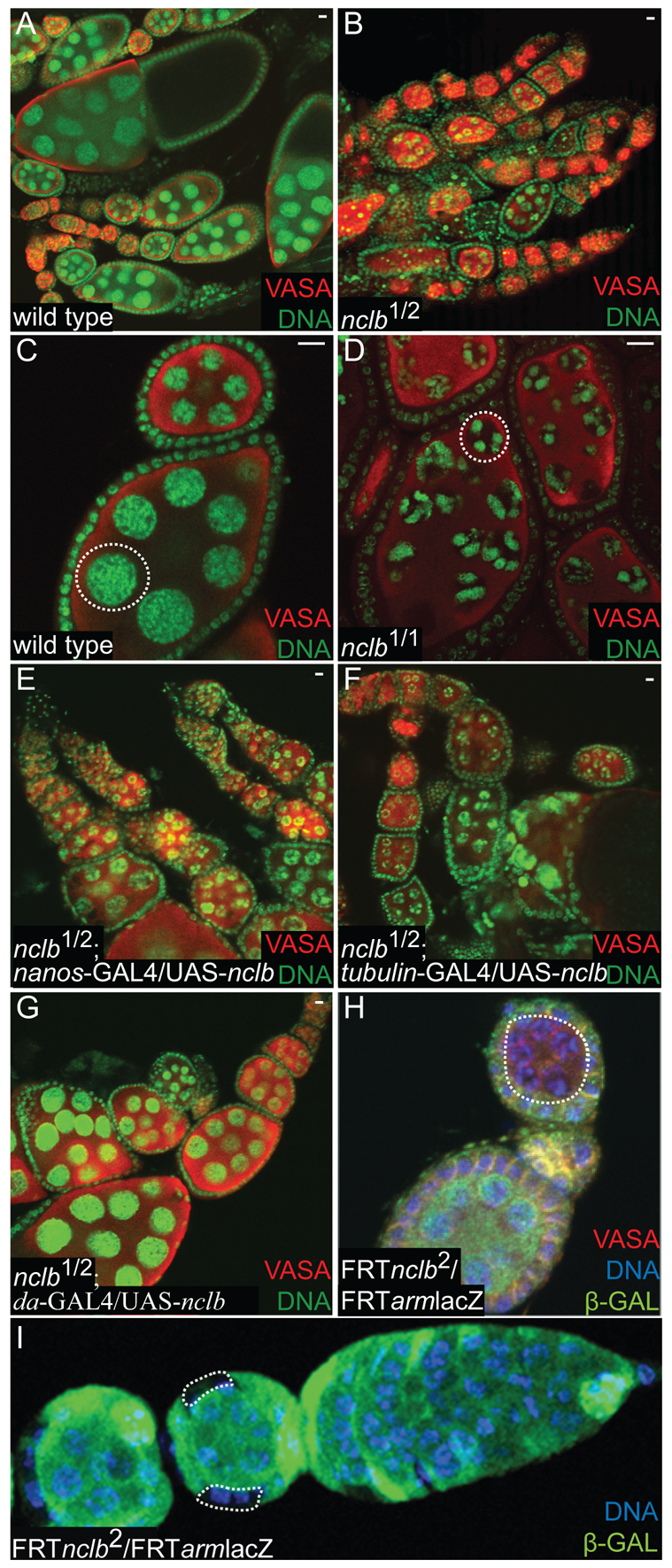
nclb affects only late stages of germ cell differentiation in females. (A-G) Five-day-old mated female ovaries immunostained for germ cells (anti-VASA, red) and DNA (green). Genotypes as indicated in individual panels. All wild type in this figure are a precise excision allele of EY10712. (A-D) nclb mutant ovaries show ample germline and normal early cyst and egg chamber development. nclb mutant egg chambers arrest with a `five blob' chromatin structure (dashed outline, D). (E-G) nclb mutants expressing UAS-nclb in combination with various Gal4 `drivers'. The egg chamber arrest phenotype is rescued only when nclb is expressed strongly in both the germline and soma (da-Gal4, G). (H,I) nclb homozygous null mutant cells generated using the FLP/FRT system. (H) nclb-null mutant germ cells (dashed outline) are able to form normal cysts and early egg chambers, but arrest with the same `five blob' chromatin phenotype as hypomorphic mutants. (I) nclb null mutant follicle cells contribute to the follicular epithelium (dashed outlines), but large clones of mutant follicle cells indicative of mutant follicle stem cells are not observed. Scale bars: 10 μm.
The phenotype of nclb mutant testes indicated that germ cells were failing to behave properly as GSCs and to properly differentiate into sperm. We first investigated the germ cells associated with the hub, which would normally act as GSCs in wild-type testes. Using viable nclb alleles, we found that testes from newly eclosed adults had fewer hub-proximal germ cells compared with wild type, and further reduction was seen at days 5 and 10 (Fig. 1C). Male GSCs receive a signal from the hub that activates the JAK/STAT pathway (Kiger et al., 2001; Tulina and Matunis, 2001) and leads to upregulation of STAT92E protein levels (Fig. 1H). Germ cells adjacent to the hub in young adult nclb mutant testes failed to exhibit STAT92E expression, indicating that they are not behaving properly as GSCs (Fig. 1I).
The loss of GSCs does not appear to be due to defects in the surrounding niche environment, as we still observed expression of the upd ligand for the JAK/STAT pathway in the hub (Fig. 1J). In addition, the adhesion molecules DE- and DN-cadherin, which are thought to be important for hub-GSC interaction, were also still seen in the hub (Fig. 1G,K). The hub often appeared expanded in nclb mutants (Fig. 1J,K), which is characteristic of the hub in the absence of proper hub-GSC interaction (Gönczy and DiNardo, 1996; Kitadate et al., 2007). Finally, the cyst stem cells that also associate with the hub and are essential for maintaining the GSCs (Leatherman and Dinardo, 2008) were still observed in nclb mutants (anti-ZFH1, Fig. 1L). These data indicate that the lack of STAT92E activation in the hub-proximal germ cells, and the failure of these cells to behave as GSCs, is not likely to be due to defects in the niche environment.
We next examined earlier stages in testis development. We have found that the hub forms by the end of embryogenesis (Le Bras and Van Doren, 2006), and the hub-proximal germ cells already behave as GSCs by the first instar larval period (Sheng et al., 2009). In nclb-null mutants, a subset of germ cells still associated with the hub during larval stages and exhibited STAT92E expression (Fig. 1M), characteristic of wild-type GSCs. Furthermore, these hub-proximal germ cells correctly localized one of their centrosomes to the hub-germ cell interface, again similar to wild-type GSCs (Fig. 1O) (Sheng et al., 2009; Yamashita et al., 2003). Finally, we found that nclb mutant germ cells could still exhibit the proper pattern of male-specific germ cell gene expression, based on a number of male-specific genes we have characterized (Casper and Van Doren, 2009) (see Fig. S2 in the supplementary material). We conclude that, in nclb-null mutants, germ cells still establish a male sexual identity and transition normally to become male GSCs. However, these GSCs are not properly maintained in the testis. The initially normal behavior of male germ cells in nclb-null mutants may indicate that nclb is not required for GSC establishment, or may be due to maternally contributed NCLB protein (Fig. 5C; see Fig. S1D in the supplementary material).
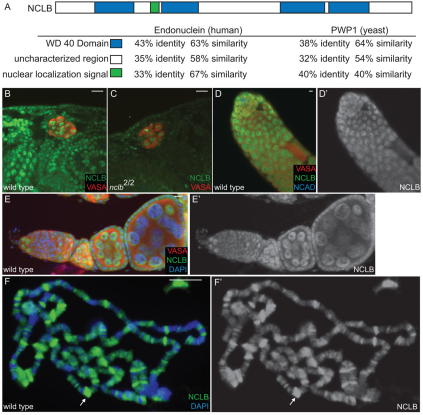
Fig. 5.
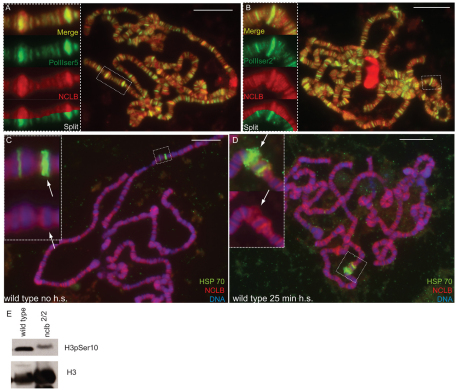
NCLB is a chromatin-associated protein. (A) The NCLB predicted protein contains WD40 repeats (blue) and a nuclear localization signal (green), and shows extensive homology to human (endonuclein) and yeast (PWP1). (B-F′) Immunostaining using a polyclonal anti-NCLB antibody (green). Genotypes and other antibodies used are as shown on individual panels. (B) NCLB immunoreactivity is observed in the nucleus of many embryonic cell types, including the germ cells (anti-VASA, red). (C) Anti-NCLB immunoreactivity is greatly reduced in nclb-null mutant embryos, indicating the specificity of the antisera. Residual staining may reflect maternal protein. (D-E′) Anti-NCLB immunoreactivity is observed in the germline of the adult testis (D,D′), and in both the germline and the soma in the ovary (E,E′). (F,F′) anti-NCLB immunostaining of third instar larval polytene chromosomes. Arrow indicates a `puff' or area of active transcription. Scale bars: 10 μm.
nclb mutant male germ cells begin to differentiate but cannot complete spermatogenesis
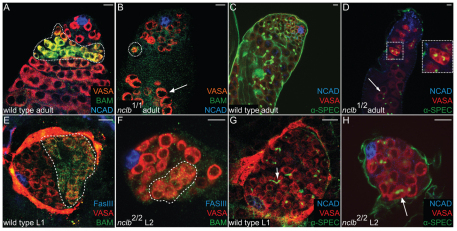
We failed to find mature sperm in nclb mutants, indicating that there is also a defect in spermatogenesis. To determine whether nclb mutant germ cells enter differentiation, we examined expression of Bag of Marbles (BAM), which is expressed in differentiating spermatogenic cysts (McKearin and Spradling, 1990) (Fig. 2A). In nclb mutant adult testis, we observed multicellular cysts (four to eight cells) that express BAM (Fig. 2B), but cysts beyond four to eight cells were not observed. We also examined fusome formation and observed multicellular cysts with branched fusomes between cells (Fig. 2D, inset). Fewer differentiating cysts were observed in nclb mutants than in wild type and, as older cysts were not observed, this indicates that cysts degenerate after the four- to eight-cell stage. However, we did not observe increased expression of markers of apoptosis (activated caspase) in nclb mutant testes (data not shown). A few large germ cells were observed further from the hub, but these contained only round fusome material that did not extend between germ cells, indicating that any germ cells that had progressed to this stage were no longer part of cysts and were developing abnormally (Fig. 2D, arrow). Somatic cyst cells do appear to be able to differentiate in nclb mutants, as EYA-positive cyst cells were still observed distal to the hub (Fig. 1L).
Fig. 2.
nclb mutant male germ cells initiate differentiation but do not progress in spermatogenesis. Genotypes and antibodies used are as indicated on panels; all wild type in this figure are a precise excision allele of EY10712. (A,B) Adult testes immunostained for the differentiation marker BAM (anti-BAM, green). Dotted outline indicates BAM expression in wild-type cysts (A) and in initial cysts in nclb mutants (B). (C,D) Adult testes immunostained to reveal the fusome (anti-spectrin, green). Note that small cysts in nclb mutants still produce branched fusomes characteristic of differentiating spermatogonia (dashed outline). Inset is a higher magnification of this region. Arrow in D indicates larger germ cells that do not have branched fusomes. (E,F) Larval testes immunostained with the differentiation marker BAM (anti-BAM, green). nclb-null mutant larval germ cells form initial cysts that express BAM similar to wild-type cysts (broken outlines). (G,H) Larval testes immunostained to reveal the fusome (anti-spectrin, green). Note that nclb-null larval testis form cysts with interconnected branched fusomes (arrow in H). Scale bars: 10 μm.
We also examined the onset of spermatogenesis during larval stages in nclb-null mutants. Again we saw that nclb mutants could form small cysts that expressed BAM (Fig. 2F) and contained branched fusomes (Fig. 2H), but cysts older than this stage were not observed. We conclude that in nclb mutant testes, germ cells can enter differentiation but spermatogenic cysts cannot develop normally.
nclb is required in the germline
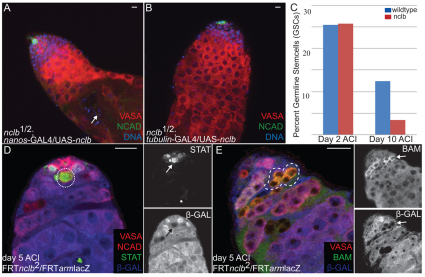
We next wanted to determine whether the function of nclb in the testis is autonomous to the germline. We first addressed this by driving UASp-nclb expression in a cell type-specific manner. Expression of nclb specifically in the germline (nanos-VP16-GAL4) was sufficient to rescue the germline loss phenotype observed in nclb1/2 mutants (Fig. 3A). Rescued testes had a wild-type appearance, with numerous GSCs associated with the hub and ample numbers of differentiating spermatogenic cysts. In addition, these rescued males had elongated spermatids and were completely fertile (Fig. 3A, arrow; see Table S1 in the supplementary material). Expression of nclb achieved using a general tubulin-Gal4 driver was also able to rescue the germline and fertility defects in nclb1/2 mutants (Fig. 3B; see Table S1 in the supplementary material), but was no more effective than germline-specific expression. These data indicate that the testis defect observed in nclb mutants is due to loss of nclb specifically in the germline. Expression of nclb using tubulin-Gal4 was also sufficient to rescue the lethality of nclb2/2-null mutants (see Table S1 in the supplementary material). Fertility was not rescued in these animals, presumably because tubulin-Gal4 is less active in the germline compared with the soma (A.L.C., K.B. and M.V.D., unpublished).
Fig. 3.
nclb is autonomously required in the germline. (A,B,D,E) Immunostaining of adult testes. Antibodies used and genotypes are indicated on each panel. (A,B) Five-day-old nclb mutant testes show complete rescue of germline defects when UAS-nclb is expressed specifically in the (A) germline (nanos-GAL4) or (B) broadly (tubulin-GAL4). Compare with Fig. 1G. Arrow in A indicates elongated sperm. (C) FLP/FRT induction of wild-type versus nclb homozygous mutant GSCs. Graph indicates the percentage of testes with labeled wild-type or nclb mutant GSCs at the indicated times ACI. nclb mutant GSCs are lost at a higher frequency than wild-type GSCs. (D,E) FLP/FRT induced nclb-null mutant germ cells, as indicated by loss of β-Galactosidase expression (anti-β-gal, blue). (D) nclb-null mutant GSCs (broken outline and arrow) still exhibit increased STAT immunoreactivity (green) similar to wild-type GSCs. (E) nclb-null mutant germline cysts (broken outline and arrows) still exhibit BAM expression characteristic of differentiating cysts. Scale bars: 10 μm.
We next conducted the reciprocal experiment where we removed nclb function from germ cells by making germline clones with our nclb2-null mutant allele using the FLP/FRT system (Golic and Lindquist, 1989). We found that nclb mutant GSCs were present in similar numbers to wild-type control clones 2 days after clone induction (ACI) [wild type, 25.5% (n=51); nclb, 25.8% (n=93), Fig. 3C]. However, by 10 days ACI, the number of testes with nclb mutant GSCs was dramatically reduced relative to wild-type control clones [wild type 12.5% (n=96); nclb, 3.4% (n=118)]. Unlike what we observed in nclb homozygous mutant adults (Fig. 1I), nclb-null mutant germ cells adjacent to the hub still exhibited upregulation of STAT92E (Fig. 3D), even 5 days ACI. However, we also observed that NCLB protein was still present in these clones, indicating a strong perdurance of the protein. Thus, those GSCs that are still present 5 days ACI may be those GSCs that retain sufficient NCLB and can still respond to STAT. Despite the perdurance of NCLB protein, the clear GSC loss phenotype leads us to conclude that nclb is required in GSCs for their maintenance.
We also examined the fate of nclb-null mutant germline clones further from the hub. We found that nclb mutant germ cells could form multicellular cysts that expressed BAM, but that these did not progress beyond the four- to eight-cell stage (Fig. 3E). Thus, nclb mutant germ cells are able to enter differentiation even many days after generating null mutant GSC clones. We conclude that nclb is not required for viability of male GSCs, but is required for proper GSC maintenance and for germ cell cysts to complete spermatogenesis once they have initiated differentiation.
nclb is required in females for germ cell differentiation but not for GSC maintenance
Next, we examined the ovaries of nclb mutants. Unlike what was observed in testes, ovaries from 5-day-old nclb1/nclb2 mutant adults contained ample germline and exhibited clear signs of egg chamber development (Fig. 4B); however, some germline loss was observed in 10-15 day old females. In newly eclosed females, a few mature eggs were observed, which probably account for the limited fertility of these females. However, most of the egg chambers were arrested around stage 5 of development (Fig. 4B). When nurse cells endoreplicate and become polyploid, the five major chromosome arms form individual territories in the nuclei that can be observed by DNA staining (the `5 blob' stage) (Dej and Spradling, 1999). Subsequently, the nurse cell chromatin becomes more uniformly distributed in the nucleus (Fig. 4C). In nclb mutants, the nurse cell chromatin remained in the `five blob' configuration (Fig. 4D). Thus, egg chamber development arrests approximately at stage 5, and this may represent defects in nurse cell chromatin organization as this phenotype has been previously observed for chromatin regulators [e.g. Su(Hw)] (Harrison et al., 1993).
To investigate whether nclb is required in the germline or the soma, we first attempted to rescue nclb mutant females with tissue-specific expression of UASp-nclb. Expression in the germline (nanos-Gal-VP16) failed to rescue the stage 5 egg chamber arrest or fertility defects (Fig. 4E; see Table S1 in the supplementary material). More general expression using tubulin-Gal4, which expresses more strongly in the soma than in the germline, also failed to completely rescue the five blob phenotype but did partially rescue fertility (Fig. 4F; see Table S1 in the supplementary material). Expression with daughterless-Gal4, which expresses strongly in both the germline and the soma (Cronmiller and Cline, 1987), was sufficient to rescue both the egg chamber arrest and fertility defects of nclb mutants (Fig. 4G; see Table S1 in the supplementary material). We next examined the phenotype of null nclb mutant germline clones. We did not observe any difference in the number of nclb mutant GSC clones compared with control clones even 15 days ACI (see Table S2 in the supplementary material). Unlike what was observed in males, we did not see significant perdurance of the NCLB protein in nclb mutant female GSC clones; NCLB immunoreactivity was dramatically reduced or absent in nclb mutant GSCs as early as 5 days ACI. Thus, nclb is not required in the germline for female GSC maintenance. In nclb mutant clones, we did observe the same `five blob' egg chamber arrest phenotype observed in nclb hypomorphic adult females (Fig. 4H). We conclude that nclb is required in the female germline for germ cell differentiation and egg chamber development, but not for GSC maintenance.
Last, we examined null nclb mutant follicle cells in the ovary. Two days ACI, we observed small groups of nclb-mutant follicle cells that appeared to contribute normally to the follicle cell epithelium (Fig. 4I). Mutant follicle cells were still observed in older egg chambers at later timepoints; however, the overall number of follicle cell clones decreased dramatically over time (see Table S2 in the supplementary material). In particular, egg chambers containing large regions of mutant follicle cells, which are indicative of mutant follicle stem cells, were not observed. We conclude that nclb is not required in differentiated follicle cells, but is required in follicle stem cells for their survival or maintenance.
NCLB is a member of a new family of chromatin proteins
nclb encodes a protein with a predicted nuclear localization signal and four WD40 repeats, and shows extensive homology to predicted proteins in other species (Fig. 5A). Little is known about this family of proteins, but the yeast homolog (PWP1) has been shown to associate with chromatin in a manner dependent on the N-terminal tail of Histone H4 (Suka et al., 2006). In addition, the human homolog, endonuclein, has been shown to localize to the nucleus (Honore et al., 2002). Using polyclonal antisera raised against the full-length protein, we observed NCLB immunoreactivity localized to the nucleus in a broad range of embryonic cell types, including in the germ cells (Fig. 5B). Immunoreactivity was greatly reduced in nclb2 mutant embryos (Fig. 5C; see Fig. S1B in the supplementary material). This allele lacks the entire nclb-coding region, and the residual staining is likely to be due to maternally contributed protein as it was observed with two independent anti-NCLB antibodies. NCLB immunoreactivity was observed in the germline of the adult testes (Fig. 5D,D′) and ovary (Fig. 5E,E′). NCLB was also observed in the somatic cells of the ovary (Fig. 5E,E′) and in ZFH-1-positive cyst cells in the testis, but is absent from differentiated EYA-positive cyst cells (see Fig. S3 in the supplementary material).
Given that PWP1 can associate with chromatin, we next wanted to determine whether NCLB is a chromatin-associated protein. We analyzed the larval salivary gland polytene chromosomes, where many copies of each individual chromosome are aligned allowing for direct visualization of proteins bound to chromatin. NCLB exhibited a highly specific pattern of localization, with regions of intense NCLB staining along with many more regions of weaker staining (Fig. 5F,F′). NCLB staining colocalized with regions of weak DAPI staining (`interbands'), indicating that it binds to regions of open chromatin, rather than to regions that are condensed. In addition, NCLB was observed labeling chromosomal `puffs' (e.g. arrow in Fig. 5F,F′), which are indicative of active transcription. We conclude that NCLB is a nuclear protein that can interact with chromatin in a specific manner.
NCLB is associated with sites of active transcription
During transcriptional initiation, RNA polymerase II (RNA Pol II) is first phosphorylated on the serine 5 position of its C-terminal domain and then becomes phosphorylated on serine 2 as it transitions to transcriptional elongation (Gilmour, 2009). We observed that NCLB immunoreactivity colocalized in many places with RNA Pol II phospho-serine 5 (Fig. 6A), and that the co-localization was even more extensive with RNA Pol II phospho-serine 2 (Fig. 6B). Although there were also sites of NCLB localization that did not correlate with activated forms of RNA Pol II, overall these data indicate that NCLB is localized at sites of active transcription. Consistent with this, we found that nclb is required for proper levels of active transcription. During the transition from transcriptional initiation to elongation, Histone H3 becomes phosphorylated on residue serine 10 (Ivaldi et al., 2007). Although Histone H3 is also phosphorylated on this residue during mitosis, the relatively small percentage of dividing cells in larvae suggests that this can be used as an indicator of active transcription. In nclb mutant larvae, we observed a dramatic decrease in serine 10 phosphorylated H3, relative to the total H3 (Fig. 6C). Thus, we conclude that the total amount of active transcription is decreased in nclb mutants.
Fig. 6.
NCLB colocalizes with active RNA polymerase and facilitates active transcription. (A,B) Third instar larval polytene chromosomes immunostained to reveal NCLB (anti-NCLB, red) and active forms of RNA polymerase II (green). There are many positions where NCLB colocalizes with RNA polymerase II phosphorylated at serine 5 (A) and RNA polymerase II phosphorylated at serine 2 (B). Insets show the boxed region at a higher magnification, and with the channels merged and split to reveal opposing sides of the polytene chromosome. (C,D) Third instar larval polytene chromosomes labeled by fluorescent in situ hybridization to reveal the Hsp70 loci (green) and anti-NCLB (red). Insets show a higher magnification of the boxed region, arrows indicate the Hsp70 87C locus. NCLB is not observed at the Hsp70 loci prior to heat shock (C), but is present at the puffed Hsp70 loci after heat shock (D). Scale bars: 10 μm. (E) Western blot of third instar larval protein extracts probed with antibodies that recognize all Histone H3, or Histone H3 phosphorylated on serine 10.
To examine the relationship between NCLB and active transcription, we took advantage of the heat shock response. After a brief heat shock, transcription is repressed throughout most of the genome, whereas it is rapidly activated at specific heat shock loci that produce stress response genes (Spradling et al., 1975). Prior to heat shock, we found that NCLB was not present at the hsp70 87C locus (Fig. 6C, arrow). The hsp70 87A locus exhibited a small amount of NCLB staining; however, this locus has previously been shown to stain for marks of active transcription prior to heat shock (Ivaldi et al., 2007), which is probably due to the activity of a nearby, non-heat shock gene. After heat shock, both the 87A and 87C loci exhibited robust NCLB staining, along with the characteristic `puff' morphology of the DNA that is indicative of active transcription (Fig. 6D). NCLB staining was also observed at other chromosomal locations, both before and after heat shock (Fig. 6C,D). Therefore, NCLB recruitment to the heat shock loci correlates with the active transcription of the heat shock genes, but NCLB was not lost from other regions of the genome that are repressed after heat shock.
DISCUSSION
nclb is essential for germline development
Our work indicates that nclb is essential for several different aspects of germ cell development. Within the testis, nclb is required autonomously in the germline for GSC maintenance and germ cell differentiation. nclb hypomorphic mutant adult testes show a progressive loss of GSCs and nclb-null mutant GSCs are lost from the niche at a much higher rate than wild-type GSCs. nclb mutant GSCs begin to differentiate, as they form multicellular cysts that have branched fusomes and express the differentiation marker BAM; however, differentiation is blocked soon after this stage. Germ cells that remain localized near the hub in nclb homozygous mutant adult testis do not respond appropriately to hub signals; no increase in STAT92E immunoreactivity is observed in these cells. However, we also do not observe precocious differentiation of these hub-associated germ cells, as premature BAM expression and branched fusome formation is not observed. Recently, it has been shown that JAK/STAT signaling in the GSCs is important for adhesion to the hub, but not for repression of differentiation (Leatherman and Dinardo, 2010). Instead, it is the association of the GSCs with neighboring cyst stem cells that maintains their undifferentiated state. Thus, nclb mutant germ cells may be lost from the niche due to decreased JAK/STAT response and therefore decreased adhesion to the hub. However, they must still be able to respond at least partly to signals from the cyst stem cells that inhibit differentiation.
Interestingly, we find that nclb is not required for GSC maintenance in females. In contrast to what we saw in males, we observed no difference in the maintenance of nclb-null mutant GSCs in the ovary, compared with wild-type GSCs. We did observe defects in a later step of female germ cell differentiation in nclb mutants, where egg chambers arrest prior to a reorganization of the nurse cell chromatin. This is consistent with NCLB playing a role in chromatin organization, and other chromatin factors exhibit a similar nurse cell chromatin phenotype (Harrison et al., 1993). Our data also reveal a stronger requirement for nclb in the somatic cells in the female, as germline expression was insufficient for rescuing mutant females and nclb is also required for follicle stem cell maintenance. Thus, although nclb is expressed in many cell types in the embryo and the gonad, different cell types have very different requirements for nclb function.
NCLB identifies a new family of chromatin factors
Our data indicate that NCLB is a chromatin-associated factor that binds predominantly to regions of the genome that are transcriptionally active. By examining the salivary gland polytene chromosomes, we find that NCLB colocalizes with active forms of RNA polymerase II. We also observed NCLB localized to polytene chromosome `puffs', which are sites of active transcription (Fig. 5F), and NCLB is recruited to the heat shock loci specifically upon their activation. Last, in nclb mutants, we observed a decrease in a phosphorylated form of histone H3 that is associated with active transcription. Thus, NCLB is associated with chromatin in a specific and dynamic manner that correlates with, and appears to be required for, active transcription.
Homology searches reveal single putative orthologs for NCLB in diverse eukaryotic species, including humans and yeast, with homology extending throughout the NCLB predicted protein sequence. Thus, it is likely that NCLB homologs in other species share a role as chromatin factors. This is supported by previous finding that yeast Pwp1p can associate with chromatin in a manner dependent on the N-terminal tail of Histone H4 (Suka et al., 2006). In that work, Pwp1p was found to associate with the rDNA loci; however, a global analysis of Pwp1p binding to chromatin was not conducted. Analogous with our work with NCLB, we propose that Pwp1p and other homologs are likely to more broadly regulate expression of a variety of targets, many of which will be RNA polymerase II targets.
The homology between NCLB/Pwp1 family members includes both the predicted WD40 repeats and intervening regions. WD40 repeats are protein-protein interaction domains commonly found in chromatin-associated proteins, and have been shown to interact directly with modified histones. For example, WD40 Repeat Domain Protein 5 (WDR5) interacts with Histone H3 methylated on lysine 4 and can promote gene expression (Wysocka et al., 2006). Thus, it is likely that NCLB is recruited to chromatin via its WD40 repeats, possibly by directly binding to modified histones. Alternatively, WD40 repeat proteins can also be recruited to chomatin by directly binding to modified DNA or non-coding RNAs (Scrima et al., 2008; Wang et al., 2011). Interestingly, WDR5 has also been implicated in a common pathway with the important chromatin remodeling complex NURF (Wysocka et al., 2006). Like NCLB, NURF has been shown to be important for regulation of both male GSC maintenance (Cherry and Matunis, 2010) and sperm differentiation (Kwon et al., 2009) in Drosophila. Another feature common to both nclb mutants and mutants for NURF complex components is a failure to pupate (this work) (Badenhorst et al., 2005), indicating a defect in ecdysone response. Thus, NCLB may act by influencing NURF complex activity. The molecular mechanism by which NCLB influences transcriptional activity in vivo, and what other aspects of gene expression and chromatin structure NCLB might regulate, are interesting directions for future research.
Epigenetic regulation of germ cell sexual development
The epigenetic regulation of germ cell development is of particular interest, as the germline must undergo many specific developmental transitions yet must ultimately retain totipotency. Given that NCLB appears to be an important chromatin regulator, the study of nclb can reveal important aspects of the epigenetic regulation of the germline. One important transition in the germline involves germ cell sex determination, where germ cells take on a male or female identity. Germ cell sexual identity is influenced both by factors acting autonomously in the germline, but also by influences from the surrounding soma (Casper and Van Doren, 2006; Hempel et al., 2008). We see clear differences between the sexes in the role of NCLB in the germline. In males, nclb is required for GSC maintenance, but this is not the case in females. This indicates that, even though these two types of stem cells are highly similar in many ways, they are regulated differently at the chromatin level. The male and female germline also exhibit different requirements for nclb during differentiation. In males, germline cysts arrest early in spermatogenesis, while female cysts form normal egg chambers that arrest only later in development. The early defect in male germline differentiation may be linked to the requirement for nclb in male GSCs, as the chromatin state of the GSC is also the starting point for any epigenetic changes that accompany differentiation. One difference between male and female GSCs is their requirement for signaling through the JAK/STAT pathway. The JAK/STAT pathway regulates male germline identity early in development (Wawersik et al., 2005). In the adult, JAK/STAT signaling is present in both the male and female GSCs niches, but this pathway is required only in GSCs in males (Kiger et al., 2001; Tulina and Matunis, 2001) and not in females (Decotto and Spradling, 2005). As already discussed, nclb mutant male GSCs are defective in their ability to respond to JAK/STAT signaling from the hub, which may contribute to why nclb is required in male GSCs but not in female GSCs.
The role of nclb emphasizes how even closely related stem cell types, such as male and female GSCs, are subject to very different epigenetic regulation. Much of the work on regulation of stem cells has focused on principles that stem cells have in common, such as the need to maintain an undifferentiated state (e.g. Pietersen and van Lohuizen, 2008). Indeed, the requirement for chromatin factors such as Imitation Switch is similar in male and female GSCs (Ables and Drummond-Barbosa, 2010; Cherry and Matunis, 2010; Xi and Xie, 2005), and it is possible to find epigenetic regulators that affect a variety of adult stem cell types (Buszczak et al., 2009). However, it is equally clear that adult stem cells in vivo have different potentials, and normally give rise to very different types of differentiated progeny. Although the male and female GSCs are closely related stem cells that share a common origin, the progeny to which they give rise, sperm versus egg and nurse cells, are clearly very different. Thus, the differences in epigenetic regulation of male and female GSCs may prove as interesting as their similarities.
Supplementary Material
Acknowledgments
We are grateful to numerous colleagues for supplying reagents, as indicated specifically in the Materials and methods. We also very much appreciate the contribution of essential repositories of reagents and information, including FlyBase, the Drosophila Genomic Resources Center, the Developmental Studies Hybridoma Bank and the Bloomington Drosophila Stock Center. We thank many colleagues for discussions and advice on the manuscript, including Soledad Ivaldi and members of the Van Doren lab. This work was supported by NIH grant GM084356 to M.V.D. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.067942/-/DC1
References
- Ables E. T., Drummond-Barbosa D. (2010). The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboïm A. N. (1945). Développement embryonnaire et post-embryonnaire des gonades normales et agamétiques de Drosophila melanogaster. Rev. Suisse Zool. 52, 53-154 [Google Scholar]
- Badenhorst P., Xiao H., Cherbas L., Kwon S. Y., Voas M., Rebay I., Cherbas P., Wu C. (2005). The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes Dev. 19, 2540-2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M., Paterno S., Spradling A. C. (2009). Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science 323, 248-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara N., Whitworth C., Van Doren M. (2008). The creation of sexual dimorphism in the Drosophila soma. Curr. Top. Dev. Biol. 83, 65-107 [DOI] [PubMed] [Google Scholar]
- Casper A., Van Doren M. (2006). The control of sexual identity in the Drosophila germline. Development 133, 2783-2791 [DOI] [PubMed] [Google Scholar]
- Casper A. L., Van Doren M. (2009). The establishment of sexual identity in the Drosophila germline. Development 136, 3821-3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry C. M., Matunis E. L. (2010). Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell 6, 557-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C., Cline T. W. (1987). The Drosophila sex determination gene daughterless has different functions in the germ line versus the soma. Cell 48, 479-487 [DOI] [PubMed] [Google Scholar]
- Dansereau D. A., Lasko P. (2008). The development of germline stem cells in Drosophila. Methods Mol. Biol. 450, 3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E., Spradling A. C. (2005). The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501-510 [DOI] [PubMed] [Google Scholar]
- Dej K. J., Spradling A. C. (1999). The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development 126, 293-303 [DOI] [PubMed] [Google Scholar]
- Fuller M. (1993). Spermatogenesis. In The Development of Drosophila melanogaster, Vol. 1 (ed. Bate M., Martinez Arias A.), pp. 71-147 Cold Spring Harbor: Cold Spring Harbor Press; [Google Scholar]
- Fuller M. T., Spradling A. C. (2007). Male and female Drosophila germline stem cells: two versions of immortality. Science 316, 402-404 [DOI] [PubMed] [Google Scholar]
- Gilmour D. S. (2009). Promoter proximal pausing on genes in metazoans. Chromosoma 118, 1-10 [DOI] [PubMed] [Google Scholar]
- Golic K., Lindquist S. (1989). The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499-509 [DOI] [PubMed] [Google Scholar]
- Gönczy P., DiNardo S. (1996). The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437-2447 [DOI] [PubMed] [Google Scholar]
- Gönczy P., Matunis E., DiNardo S. (1997). bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development 124, 4361-4371 [DOI] [PubMed] [Google Scholar]
- Harrison D. A., Gdula D. A., Coyne R. S., Corces V. G. (1993). A leucine zipper domain of the suppressor of Hairy-wing protein mediates its repressive effect on enhancer function. Genes Dev. 7, 1966-1978 [DOI] [PubMed] [Google Scholar]
- Hempel L. U., Kalamegham R., Smith J. E., 3rd, Oliver B. (2008). Drosophila germline sex determination: integration of germline autonomous cues and somatic signals. Curr. Top. Dev. Biol. 83, 109-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore B., Baandrup U., Nielsen S., Vorum H. (2002). Endonuclein is a cell cycle regulated WD-repeat protein that is up-regulated in adenocarcinoma of the pancreas. Oncogene 21, 1123-1129 [DOI] [PubMed] [Google Scholar]
- Ivaldi M. S., Karam C. S., Corces V. G. (2007). Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 21, 2818-2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E., Wong M. D., Ding B. C., Xie T. (2004). Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365-1375 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B., Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542-2545 [DOI] [PubMed] [Google Scholar]
- King R. C. (1970). Ovarian Development in Drosophila melanogaster. New York: Academic Press; [Google Scholar]
- Kitadate Y., Shigenobu S., Arita K., Kobayashi S. (2007). Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev. Cell 13, 151-159 [DOI] [PubMed] [Google Scholar]
- Kwon S. Y., Xiao H., Wu C., Badenhorst P. (2009). Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities. PLoS Genet. 5, e1000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S., Van Doren M. (2006). Development of the male germline stem cell niche in Drosophila. Dev. Biol. 294, 92-103 [DOI] [PubMed] [Google Scholar]
- Leatherman J. L., Dinardo S. (2008). Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3, 44-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman J. L., Dinardo S. (2010). Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12, 806-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R., Tautz D. (1994). RNA in situ hybridization. Methods Cell Biol. 44, 567-598 [DOI] [PubMed] [Google Scholar]
- McKearin D. M., Spradling A. C. (1990). bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 4, 2242-2251 [DOI] [PubMed] [Google Scholar]
- Nystul T., Spradling A. (2007). An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277-285 [DOI] [PubMed] [Google Scholar]
- Patel N. (1994). Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 44, 445-487 [DOI] [PubMed] [Google Scholar]
- Pietersen A. M., van Lohuizen M. (2008). Stem cell regulation by polycomb repressors: postponing commitment. Curr. Opin. Cell Biol. 20, 201-207 [DOI] [PubMed] [Google Scholar]
- Schulz C., Kiger A. A., Tazuke S. I., Yamashita Y. M., Pantalena-Filho L. C., Jones D. L., Wood C. G., Fuller M. T. (2004). A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics 167, 707-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima A., Konickova R., Czyzewski B. K., Kawasaki Y., Jeffrey P. D., Groisman R., Nakatani Y., Iwai S., Pavletich N. P., Thoma N. H. (2008). Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 135, 1213-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X. R., Posenau T., Gumulak-Smith J. J., Matunis E., Van Doren M., Wawersik M. (2009). Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev. Biol. 334, 335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani A. A., Ingham P. W. (2003). Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr. Biol. 13, 2065-2072 [DOI] [PubMed] [Google Scholar]
- Song X., Xie T. (2002). DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813-14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. (1975). Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell 4, 395-404 [DOI] [PubMed] [Google Scholar]
- Spradling A. C. (1993). Developmental genetics of oogenesis. In The Development of Drosophila melanogaster, Vol. 1 (ed. Bate M., Martinez Arias A.), pp. 1-70 Cold Spring Harbor: Cold Spring Harbor Press; [Google Scholar]
- Suka N., Nakashima E., Shinmyozu K., Hidaka M., Jingami H. (2006). The WD40-repeat protein Pwp1p associates in vivo with 25S ribosomal chromatin in a histone H4 tail-dependent manner. Nucleic Acids Res. 34, 3555-3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N., Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546-2549 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A., Lehmann R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243-246 [DOI] [PubMed] [Google Scholar]
- Wang K. C., Yang Y. W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B. R., Protacio A., Flynn R. A., Gupta R. A., et al. (2011). A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik M., Milutinovich A., Casper A. L., Matunis E., Williams B., Van Doren M. (2005). Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436, 563-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., et al. (2006). A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442, 86-90 [DOI] [PubMed] [Google Scholar]
- Xi R., Xie T. (2005). Stem cell self-renewal controlled by chromatin remodeling factors. Science 310, 1487-1489 [DOI] [PubMed] [Google Scholar]
- Xie T., Spradling A. C. (1998). decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251-260 [DOI] [PubMed] [Google Scholar]
- Yamashita Y. M., Jones D. L., Fuller M. T. (2003). Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547-1550 [DOI] [PubMed] [Google Scholar]
- Zhu C. H., Xie T. (2003). Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development 130, 2579-2588 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.