Abstract
Coordination of cell proliferation and differentiation is crucial for tissue formation, repair and regeneration. Some tissues, such as skin and blood, depend on differentiation of a pluripotent stem cell population, whereas others depend on the division of differentiated cells. In development and in the hair follicle, pigmented melanocytes are derived from undifferentiated precursor cells or stem cells. However, differentiated melanocytes may also have proliferative capacity in animals, and the potential for differentiated melanocyte cell division in development and regeneration remains largely unexplored. Here, we use time-lapse imaging of the developing zebrafish to show that while most melanocytes arise from undifferentiated precursor cells, an unexpected subpopulation of differentiated melanocytes arises by cell division. Depletion of the overall melanocyte population triggers a regeneration phase in which differentiated melanocyte division is significantly enhanced, particularly in young differentiated melanocytes. Additionally, we find reduced levels of Mitf activity using an mitfa temperature-sensitive line results in a dramatic increase in differentiated melanocyte cell division. This supports models that in addition to promoting differentiation, Mitf also promotes withdrawal from the cell cycle. We suggest differentiated cell division is relevant to melanoma progression because the human melanoma mutation MITF4TΔ2B promotes increased and serial differentiated melanocyte division in zebrafish. These results reveal a novel pathway of differentiated melanocyte division in vivo, and that Mitf activity is essential for maintaining cell cycle arrest in differentiated melanocytes.
Keywords: Melanocyte, Division, Imaging, Cell division, Regeneration, Zebrafish
INTRODUCTION
The coordination of cell proliferation with differentiation is important for both developmental and cancer biology. In some well-studied systems, proliferation and differentiation are mutually exclusive. For example, haematopoietic stem cells give rise to committed progenitor cells that produce mature, highly specialized and terminally differentiated blood cells. This is exploited in differentiation therapy, a successful treatment strategy for acute promyelocytic leukaemia, by promoting cancer cell maturation with the concomitant loss of proliferative capacity (de The and Chen, 2010). However, the coupling of differentiation to cell cycle arrest can be cell type specific, and some dividing cells can maintain differentiated characteristics, such as pancreatic β-cells, horizontal neurons in the retina and specific neural cell types in the spinal cord (Ajioka et al., 2007; Barnabe-Heider et al., 2010; Brennand et al., 2007; Davis and Dyer, 2010; Dor et al., 2004).
For melanocytes, differentiation involves cell shape changes, the expression of pigmentation enzymes and the generation of melanins to colour the skin, hair and eyes (Kelsh et al., 2009; Levy et al., 2006). In mouse development, unpigmented melanoblasts first undergo extensive proliferation in the dermis prior to migration into the epidemis, followed by rapid expansion in the epidermis before localizing to the hair follicle (Jordan and Jackson, 2000; Mackenzie et al., 1997; Nishikawa et al., 1991). At the hair follicle, unpigmented melanocyte stem cells (MSCs) in the bulge region undergo transient amplification to generate pigmenting melanocytes with each hair cycle and so colour the hair shaft (Nishimura et al., 2005; Nishimura et al., 2002). Differentiation seems to preclude proliferation in these MSCs because DNA damage appears to trigger differentiation and MSC pigmentation resulting in loss of melanocyte renewal (Inomata et al., 2009). Skin melanocyte numbers also expand following ultraviolet radiation exposure, and in the mouse these cells appear to arise from proliferative undifferentiated melanoblasts (Kawaguchi et al., 2001; van Schanke et al., 2005; Walker et al., 2009). In zebrafish, most embryonic melanocytes are directly derived from the neural crest, and adult melanocytes are added to the embryonic melanocyte pattern by an expansive wave of development from undifferentiated melanocyte progenitors or MSCs (Hultman et al., 2009; Parichy, 2003; Yang and Johnson, 2006). Thus, in developing animals, melanocyte population numbers are achieved primarily by proliferation of unpigmented melanocyte precursor cells and pigmentation is associated with differentiation without evidence of cell division.
In contrast to the observations in development, pigmented primary melanocytes and melanoma cells retain the potential for division in culture (Bennett, 1983; Bennett, 1989; Bennett et al., 1985). Time-lapse imaging of melanoma cell culture have captured cell division of pigmented melanoma cells in mitosis, and normal diploid pigmented human melanocytes can divide as quickly as unpigmented cells. Numerous pigmented mouse and human melanocyte and melanoma cell lines have been described in culture, supporting the notion that melanocyte differentiation features do not necessarily preclude the potential for cell division. In humans, a small percentage of melanocytes appear to divide in skin homeostasis (Jimbow et al., 1975) and differentiated melanocytes may contribute to re-pigmentation in wound-healing and vitiligo, although the primary melanocyte reservoir is thought to be from undifferentiated melanocytes (Cui et al., 1991; Falabella, 2009; Falabella and Barona, 2009; Hirobe, 1988; Tanimura et al., 2011). Many aggressive melanomas, including BRAFV600E melanomas, maintain differentiation characteristics in situ (Broekaert et al., 2010; Ohsie et al., 2008).
The apparent differences in the described potential for pigmented melanocytes to divide prompted us to revisit the fate of differentiated melanocytes in situ. The zebrafish system permits visualization of differentiating melanocytes in the natural context of the developing whole animal because the embryos are fertilized outside the mother, sustained by a yolk sac and are transparent. Developing zebrafish embryos can be imaged in real-time, allowing for the visualization of melanin and transgenic fluorescent reporters in melanocytes. Continuous imaging of differentiated melanocytes in living animals has not been previously reported. We used live-cell imaging of developing zebrafish embryos to address three questions: (1) does differentiated cell division play a role in zebrafish pigmentation?; (2) what controls cell cycle arrest in differentiated cells?; and (3) is differentiated cell division relevant for melanocyte regeneration or melanoma?
MATERIALS AND METHODS
Time-lapse Imaging of zebrafish
Zebrafish were cared for by standard procedures, as described (Westerfield, 2000). De-chorionated embryos were immobilized in a solution of 1.2% agarose, onto a six-well black polystyrene borosilicate glass-bottomed plate (IWAKI). Agarose around the head and tail of the embryo was removed to allow for growth of the embryo, and E3 embryo medium was added to each well. Time-lapse imaging took place on the Live Cell Imaging System, comprising a Zeiss Axiovert 200 fluorescence microscope equipped with 20×/1.5 EC plan neofluar, 10×/0.45 and 5×/0.16 plan apochromat objectives (Carl Zeiss, Welwyn, UK), a Lambda LS 300 W Xenon source with liquid light guide and 10-position excitation, and neutral density and emission filterwheels (Sutter Instrument, Novato, CA) containing #86000 Sedat Quad filter set (Chroma Technology, Rockingham, VT). For zebrafish work, the system was equipped with a Solent Scientific incubation chamber with CO2 enrichment (Solent Scientific, Segensworth, UK). Temperature was monitored by using an EL-USB-1 temperature sensor (Lascar Electronics, Hong Kong). Image capture was performed using MetaMorph software (Molecular Devices, Sunnyvale, CA). Time-lapse was set to capture an image every 20 minutes. Images were captured in bright field at 20 ms exposure and, when required, under FITC fluorescence at 500 ms exposure for Tyrp1-GFP respectively. Z-stacks of images were taken over a 120 μm z range with a slice interval of 20 μm or 30 μm as appropriate, to enable selection of the best plane of focus. For accurate lineage analysis in the tyrp1-GFP embryos, the seven z-stacks with seven 20 μm slices were compressed into a z-projection. Cell division events were defined as cells that clearly separated into two cells and migrated away from each other, remaining separated for the time-lapse analysis.
Drug treatment
The nitrofuran BTB05727 (Maybridge; referred to as NFN1 by H.I., K.L.T., Z.Z., S. Johnson, M. Tyers and E.C.P., unpublished) was dissolved in dimethyl sulphoxide (DMSO; Sigma) to make a stock solution of 10 mM and stored at –20°C. Embryos were incubated at 28.5°C in six-well plates with 20 μm BTB05727 dissolved in E3 embryo media (Westerfield, 2000). Upon washout into embryo media, embryos were allowed a 1-hour recovery before being immobilized in agarose for time-lapse microscopy.
Transgenic expression of MITF
The human MITF (wild type and mutant 4TΔ2B) cDNA (Cronin et al., 2009) and zebrafish mitfa cDNA were placed under the minimal 836 bp zebrafish mitfa promoter in the Gateway compatible Tol2 vector pT2Kmin-NP or the Gateway Tol2 vector pDestTol2CG2, respectively (J.A.L., unpublished) (Kwan et al., 2007). DNA constructs were injected into mitfavc7 (temperature sensitive) (Johnson et al., 2011) or mitfaw2 (nacre) (Lister et al., 1999) mutant embryos at approximately the one- to two-cell stage with Tol2 transposase RNA (Kwan et al., 2007). Each embryo was injected with ∼2 nl of mixed plasmid and RNA. The final concentrations of plasmid and RNA were 25 and 35 ng/μl, respectively. After injection, the nacre mutant embryos were incubated at 28.5°C, while the mitfa ts mutants were kept at 30-32°C to inhibit activity of endogenous mitfa.
Melanocyte counting
Embryos were dechorionated at 3 dpf, anesthetized in 160 μg/ml of tricaine and imaged under the stereomicroscope. After imaging, the embryos were fixed and stored in 4% paraformaldehyde (PFA, sigma) for melanocyte counting.
RESULTS
Time-lapse imaging of direct-developing melanocytes
We sought to establish whether cell division of pigmented cells contributes to the pigmentation pattern of the developing fish. Zebrafish melanocytes pigment the fish in two waves of development. First, during embryogenesis, most melanocytes are derived directly from the neural crest cells (NCCs; direct-developing melanocytes). During this period, quiescent adult melanocyte stem cells (MSCs) are also established and these cells primarily populate the adult stripe pattern in a second wave at metamorphosis (beginning at 15 dpf; stem-cell derived melanocytes) (Hultman et al., 2009; Parichy, 2003).
Zebrafish melanocytes first become visible at about 28 hpf with the formation of melanin. To visualize the differentiation of direct-developing melanocytes by time-lapse imaging, we embedded embryos in an imaging chamber at 28.5°C, and followed the development of melanocytes using pigment as a marker. We imaged the head of developing embryos because we could consistently image the same region in each fish, and because early and late developing melanocytes in the head appear to originate from direct-developing melanocytes (Fig. 1A) (Hultman and Johnson, 2010). Images were taken every 20 minutes, and individual melanocytes were followed from their first appearance to ∼92 hpf in nine embryos, and from 72 hpf to ∼116-144 hpf in another 10 embryos. We could clearly detect individual melanocytes emerging de novo in the dorsal head as very lightly pigmented cells that then rapidly darkened (Fig. 1A; see Movie 1 in the supplementary material). Cells were initially highly motile and then became mostly stationary by 72 hpf. Melanocytes were continually added to the pattern on the head from ∼25 hpf until 96 hpf, although the majority of melanocytes appeared established by 72 hpf, consistent with previous reports (Yang and Johnson, 2006). Crucially, we also noticed a previously undescribed population of melanocytes arising through division of pigmented cells (Fig. 1A,B). Cell division events were rare (n=8/174; 4.6%), but seen in five of the nine embryos that were followed to ∼90 hpf on the head region. In zebrafish, the distribution of melanin within a melanocyte can be altered (Logan et al., 2006), and thus the ability to analyze each frame in detail to follow each cell through time and position (z-stacks) was an important feature of our approach as it minimized the possibility of mis-scoring the division events. Melanocyte division also occurred outside the head region, as rare melanized cells in the yolk and embryonic body stripes were positive for the late-G2- and M-phase cell cycle marker phospho-histone H3 (see Fig. S2 in the supplementary material). These results identify a subpopulation of melanocytes that is added to the zebrafish pattern via division of differentiated melanocytes.
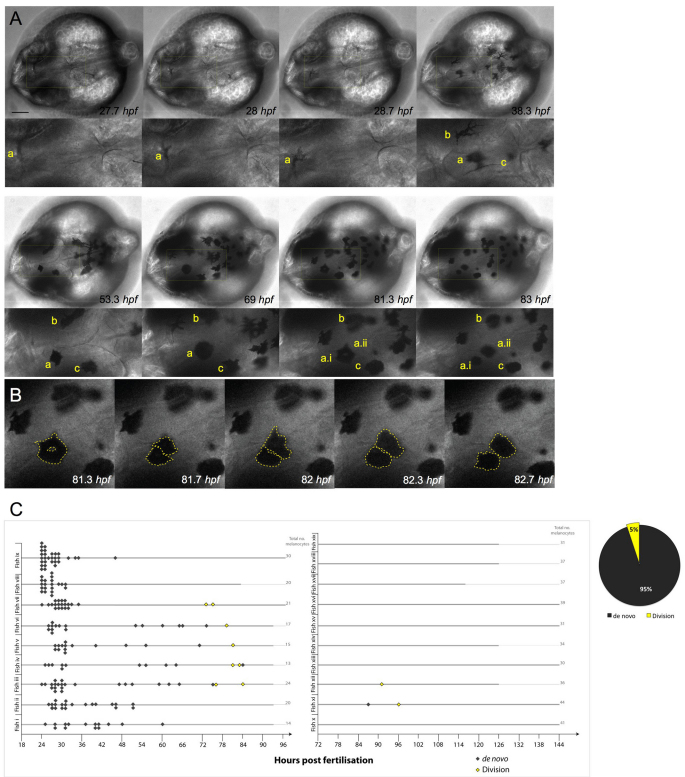
Fig. 1.
. Melanocytes develop from undifferentiated precursor cells and from pigmented melanocytes. (A) Still images of time-lapse microscopy at the onset of melanocyte differentiation. Melanocytes emerge in the dorsal head as highly dendritic and pigmenting cells. Most melanocytes arise de novo from the neural crest and do not divide (melanocytes b,c). A few pigmented melanocytes divide (melanocyte a), becoming rounded before division (to become two cells: a.i, a.ii), which then move away from each other. Scale bar: 100 μm. (B) High-magnification image of pigmented melanocyte a division during a 1.3 hour interval, with cells outlined (broken yellow lines depict the approximate outline of the cells as analyzed through all z-stacks using enhanced contrast techniques). (C) Quantitative analysis of melanocyte development in individual fish (labelled i-ix) over 1-4 dpf show the majority of melanocytes are derived de novo from unpigmented precursor cells (n=166; grey diamonds) and a subpopulation of melanocytes derived from cell division events (n=8; yellow diamonds). Division events were charted for ten other fish examples (fish x-xix) from 3 to 5/6 dpf. Only three new melanocytes were identified during this time, two of which were from division events. Thick grey lines indicate length of each individual time-lapse movie for each embryo.
Dividing embryonic melanocytes give rise to pigmented dendritic cells
As part of the differentiation programme, melanocytes express pigmentation enzymes, including tyrosinase related protein (Tyrp) 1 (Braasch et al., 2009; Smyth et al., 2006; Steel et al., 1992). We analyzed developing melanocytes in the zebrafish j900 line that expresses GFP from the fugu tyrp1 promoter in the neural crest-derived melanocytes (Hultman and Johnson, 2010). Expression of GFP could be seen prior to the first faint appearance of melanin (Fig. 2A; see Fig. S3 in the supplementary material). GFP expression was present throughout the cell, but in fully pigmented cells melanin obscured much of the GFP (Fig. 2A) (Zou et al., 2006). As in the wild-type zebrafish, rare cell division events were observed, with the GFP expression clearly showing the single cell becoming two cells in a rapid cell division of less than 20 minutes (Fig. 2A). The characteristically dendritic cells rounded up just prior to division, then resumed a dendritic shape immediately afterwards. Both mother and daughter melanocytes were always both pigmented, and we did not detect pigmented cells giving rise to GFP+ unpigmented cells. We did not see evidence of de-differentiation in dividing cell; however, this could be due to the stability of melanin or GFP preventing our ability to visualize dedifferentiation. No obvious differences were detected between the differentiation features, motility or distance to neighbouring melanocytes (data not shown) of the cells that divided and those that did not.
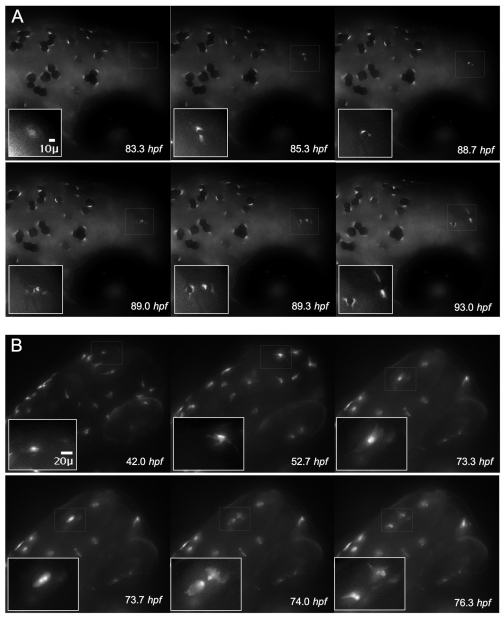
Fig. 2.
. Visualization of the differentiation marker tyrp1-GFP during cell division. (A) Expression of tyrp1-GFP in the developing melanocyte allows for visualization of the melanocyte cytoplasm and precedes pigment formation (83.3 hpf, thin white box and inset). Continued monitoring of this cell reveals pigmentation (85.3 hpf), followed by rounding (88.7 hpf) and division (89.0 hpf). Two cells with distinct cytoplasm are visible (89.0 hpf) that migrate apart and become dendritic (93.0 hpf). (B) A single albino tyrp1-GFP cell (73.3 hpf) clearly revealed the presence of two nuclei in the same cell (73.7 hpf) prior to division (74.0 hpf).
Because melanin obscured many of the intracellular features of the melanocyte during division, we also followed development of melanocytes in the j900 line crossed into an albino fish, which develop melanocytes but do not generate melanin (Hultman and Johnson, 2010). As with the pigmented fish, we did not detect division events in cells expressing tyrp1-GFP prior to 72 hpf in the head region. GFP expression was present throughout the cell and strongest in the nucleus (Fig. 2B). During cell division, two clear nuclear signals could be detected followed by rapid separation of the cells (Fig. 2B, 73.7 hpf and 74.0 hpf). Taken together with the observations described above, we conclude that a subpopulation of differentiated melanocytes have cell division potential in normal development.
Enhanced differentiated cell division during melanocyte regeneration
Having shown that differentiated melanocytes have the potential to divide, we next wanted to establish whether regenerating melanocytes derived from the MSCs also repopulate the embryonic pigment pattern by cell division. Recruitment of the MSC into differentiated melanocytes can be induced in embryogeneis during melanocyte repopulation after chemical or genetic ablation (Hultman et al., 2009; Parichy, 2003; Yang and Johnson, 2006). Yang and Johnson (Yang and Johnson, 2006) have previously shown that treatment of zebrafish embryos with MoTP, a phenol derivative, will specifically kill differentiated melanocytes and committed melanoblasts expressing tyrosinase (Yang and Johnson, 2006). After MoTP treatment, zebrafish regenerate melanocytes primarily from undifferentiated precursor cells. Following on from this, we have recently identified a series of nitrofuran compounds that also kill undifferentiated and differentiated melanocytes, depending on treatment dose (H.I., K.L.T., Z.Z., S. Johnson, M. Tyers and E.E.P., unpublished). We challenged the zebrafish embryo with a short nitrofuran treatment to kill melanocytes (∼30-50 hpf) and followed the repopulation of the melanocytes using time-lapse imaging (Fig. 3A,B; see Fig. S4A,B and Movie 3 in the supplementary material). Melanocyte cell division events were observed in seven of the nine fish imaged (Fig. 3C). Notably, these differentiated cell division events were more common in repopulating melanocytes (n=21/132; 15.9%; Fig. 3C,D) compared with embryonic development of the melanocytes [P=0.001; 95% CI (0.043, 0.18); binomial test of comparison of proportions].
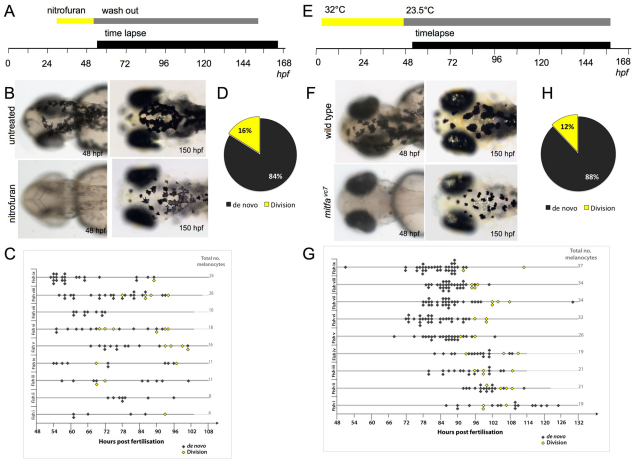
Fig. 3.
Melanocyte division events are enhanced during melanocyte regeneration. (A) Workflow of nitrofuran treatment from ∼30 hpf to 50 hpf to promote loss of differentiated melanocytes. After washout (grey bar), the embryos were immobilized in agarose and imaged by time-lapse microscopy (black bar). (B) Images of control and nitrofuran-treated embryos at the time of embedding in agarose and at the end of the time-lapse imaging. (C,D) Quantitative analysis of melanocytes derived de novo from unpigmented cells (grey diamonds; n=111) and from division of pigmented cells (yellow diamonds; n=21) in nine different zebrafish (i-ix). (E) Workflow of mitfavc7 temperature-sensitive experimental conditions. Zebrafish embryos with the mitfavc7 mutation grown at 32°C from 4 hpf until ∼48 hpf (yellow bar) do not develop ontogenic melanocytes. Upon shifting to the permissive conditions (grey bar), the embryos are immobilized in agarose and imaged by time-lapse microscopy (black bar). (F) Images of wild type and mitfavc7 embryos at the time of embedding in agarose and at the end of the time-lapse imaging. No neural crest-derived melanocytes are visible at 48 hpf (although non-neural crest pigmented cells in the eye remain pigmented) and melanocytes have repopulated the animal by 150 hpf. (G,H) Quantitative analysis of melanocytes derived de novo from unpigmented cells (grey diamonds; n=214) and from division of pigmented cells (yellow diamonds; n=29) in nine individual zebrafish (i-ix).
We next examined the role of division of differentiated cells in another melanocyte regeneration model. Microphthalmia-associated transcription factor (Mitf) is highly conserved and essential for melanocyte development in humans, mice and zebrafish; animals with mutations in mitf lack melanocytes or show hypopigmentation. Zebrafish have two Mitf genes (mitfa and mitfb) (Johnson et al., 2011; Lister et al., 2001), and all neural crest derived melanocytes require the activity of mitfa (Lister et al., 1999). A mitfa temperature-sensitive mutant line (mitfavc7, generating an intron 6 splice site mutation) has recently been described and re-establishment of Mitfa activity by shifting to the permissive temperature at 48 hpf allows for melanocyte regeneration from the presumed MSCs (Johnson et al., 2011). We grew mitfavc7 embryos at 32°C from ∼4-48 hpf so that melanocytes did not develop in the embryo (Fig. 3E,F), although the unpigmented MSCs are established (Johnson et al., 2011). Embryos were then shifted to 23-24°C and imaged (Fig. 3E,F; see Fig. S4C in the supplementary material). As with the nitrofuran-treated embryos, there was an increased proportion of cell divisions in the regenerating melanocytes compared with direct-developing melanocytes in normal development [n=29/243; 11.9%; Fig. 3G,H; P=0.009; 95% CI (0.022, 0.12); binomial test]. Thus, both chemical and genetic approaches to stimulate melanocyte repopulation from unpigmented progenitors or MSCs result in an increase in the proportion of pigmented cell divisions from differentiated melanocytes, suggesting cell division may be a regulated process and used where necessary to achieve completion of the melanocyte pattern.
Regenerating melanocytes are younger when they divide
Next, we asked whether the increased number of pigmented cell divisions was associated with a change in the time period from cell differentiation to division. Using time-lapse movies, we measured the time interval from onset of earliest visible pigmentation (or differentiation; TEVD) (Bennett, 1983) to cell separation. During normal development, there was a long latency between the TEVD and cell division, and dividing cells were pigmented for an average of 43 hours before division (Fig. 4). Analysis of the regenerating melanocytes in the nitrofuran-treated embryos and in the mitfavc7 mutant embryos revealed that the average time of initial pigmentation to cell division was significantly reduced, especially in the mitfavc7 regenerating melanocytes. In contrast to the direct-developing melanocytes, younger stem-cell derived melanocytes that had only been pigmented for an average of 12 hours were capable of division in the mitfavc7 mutant embryo (Fig. 4). In addition, regenerating melanocytes in the nitrofuran-treated embryos were pigmented for an average of 15 hours prior to division, also a significant reduction in the time from pigmentation to division compared with normal development. We suggest that, after melanocyte loss, the rapid division of differentiated melanocytes, in combination with the described expansion and differentiation of unpigmented precursors cells (Yang and Johnson, 2006), contributes to melanocyte repopulation in zebrafish.
Fig. 4.
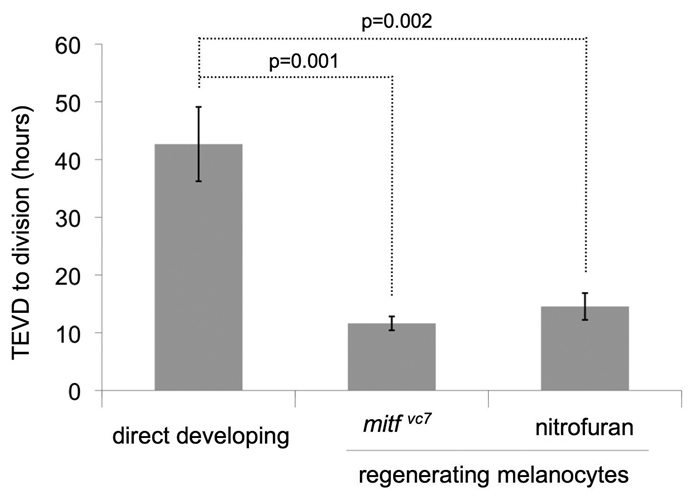
Time of earliest visible differentiation (pigmentation) to cell division is reduced in regeneration. Time of earliest visible differentiation (TEVD) until cell division was calculated for dividing melanocytes in development and regeneration conditions. During development, the average time of pigmentation to division is 43 hours (n=10 cell divisions; the origin and age of the dividing melanocyte in fish xii is unknown and has been excluded from the analysis). In regeneration, either after mitfavc7 or nitrofuran treatment, cell division occurred in younger melanocytes (12 hours or 15 hours post pigmentation, respectively). Data are mean±s.e.m. Significance is observed within the dataset (P<0.001); analysis of variance (ANOVA). Post-hoc analysis shows significance between TEVD of wild-type and nitrofuran treatment [mean and 95% confidence interval of differences; 26.81 (16.11; 37.52)] and between wild type and mitfavc7 [95% confidence interval; 31.05 (20.73; 41.37)].
Mitfa hypomorphic melanocytes undergo serial differentiated cell divisions
In melanocytes, Mitf controls both differentiation and cell cycle progression. MITF stimulates expression of differentiation genes (such as TYR encoding tyrosinase) and cell cycle regulators that both stimulate the cell cycle (e.g. CDK2) and inhibit cell cycle progression (e.g. p21) (Levy and Fisher, 2011). In melanoma, MITF has been proposed to act as a `rheostat': low levels of Mitf promote stem-like invasiveness, moderate levels stimulate proliferation, and high levels cause differentiation with cell cycle arrest (Hoek and Goding, 2010; Khaled et al., 2010). Much of this work has been done in cell lines and in cancer cells. The effects of reduced Mitf in differentiated cells has been difficult to study in animals because Mitf first affects melanocyte specification and survival (Hornyak et al., 2001; Lister et al., 1999; Opdecamp et al., 1997).
We hypothesized that reduced Mitf activity would cause division in differentiated melanocytes. We followed the development of the temperature-sensitive mitfavc7 embryos by time-lapse imaging from 30 hpf-151 hpf at a low (<24°C), intermediate (25-26.5°C) and high temperature (28.5°C). No melanocytes developed at the high temperature, consistent with the temperature-sensitive nature of the allele (Johnson et al., 2011). At low temperatures (<24°C), mitfavc7 mutants develop melanocytes but have somewhat compromised activity (Johnson et al., 2011). We found melanocytes developed and many of the differentiated melanocytes divided at low permissive temperatures (Fig. 5). In a few cases, the daughter melanocyte was also capable of additional divisions (Fig. 5; serial cell division). By contrast, at intermediate temperatures, most differentiated cells divided, and, surprisingly, many differentiated cells underwent serial cell division. Serial cell divisions significantly contributed to the pattern of the zebrafish. In one example, 12 differentiated melanocytes were derived from a single differentiated melanocyte (Fig. 5A). In the same embryo, of the final 39 melanocytes in the head region at 114 hpf, seven melanocytes were directly derived from undifferentiated precursor cells and 32 were from differentiated cells (Fig. 5A). The differences in differentiated melanocyte divisions were due to alterations in the temperature-sensitive nature of the allele (and not temperature itself) because wild-type fish grown at similar temperatures showed neither the high numbers of differentiated cell divisions, nor successive differentiated cell divisions (data not shown). Similarly, we attribute the increased differentiated melanocyte divisions to altered Mitf activity (and not to an unknown additional mutation) because we see a statistically significant difference between the low and intermediate temperatures (Fig. 5B,C). Thus, sustained reduction of Mitf activity in the developing embryo can permit appropriate melanocyte specification, differentiation and survival, but is not sufficient to inhibit cell cycle division in differentiated cells.
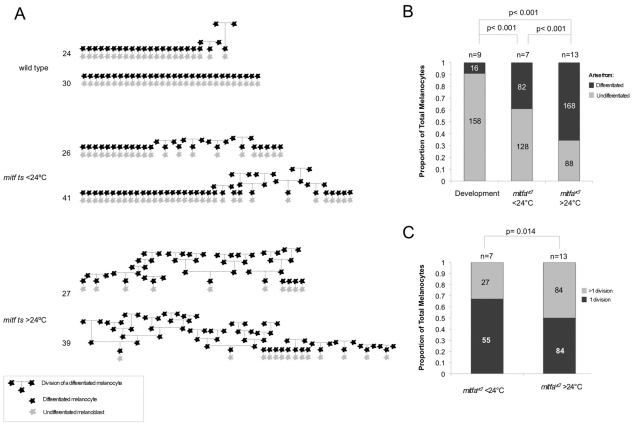
Fig. 5.
Hypomorphic Mitf activity enhances differentiated cell division. (A) Schematic representation of melanocyte development in embryos imaged by time-lapse microscopy. Two embryos are represented for each treatment condition. Wild-type or mitfavc7 mutant embryos were grown at 28.5°C for ∼20 hours, embedded in agarose, shifted to temperatures below 24°C (23-24°C) or over 24°C (25°C, 25.5°C or 26.0°C), and imaged by time-lapse microscopy until ∼108-151 hpf. All melanocytes begin as undifferentiated (grey) melanoblasts that become differentiated (black). Lineage is represented by broken lines: vertical broken lines indicate relative time between division events. The approximate order of melanocyte development is represented along the horizontal axis. Final total number of melanocytes in imaged region is indicated. (B) Stacked bar graph indicating the proportion of melanocytes that arise from differentiated or undifferentiated cells in wild type (n=9; 16/174 melanocytes) and mitf vc7 mutants grown at below 24°C (n=7; 82/210 total melanocytes) or over 24°C (n=13; 168/256 total melanocytes). The proportion of melanocytes arising from a differentiated cell is significantly greater in mitfvc7 mutants at below 24°C and over 24°C compared with wild-type fish; P<0.001; 95% CI (0.22, 0.38) and P<0.001; 95% CI (0.49, 0.64) respectively; binomial test of comparison of proportions. Additionally, the proportion of melanocytes arising from a differentiated cell between mitfvc7 mutants at below 24°C and over 24°C is significant; P<0.001; 95% CI (0.18, 0.36); binomial test of comparison of proportions. Datasets include only mitfavc7 time-lapse analysis that is longer than 108 hpf. (C) Bar graph indicating the proportion of mitfavc7 melanocytes that undergo serial division at below 24°C (n=7; 27/82) compared with those grown at over 24°C (n=13; 84/168). P=0.014, binomial test of comparison of proportions; 95% CI (0.04, 0.30). Datasets include only mitfavc7 time-lapse analyses that were longer than 108 hpf.
One possibility for the differentiated cell divisions in the mitfavc7 mutants is that the cue for cell division arose from a change in the embryonic environment, rather than from within the melanocyte itself. The mitfavc7 mutant embryos have fewer melanocytes when grown at the intermediate temperature compared with the lower temperature (Johnson et al., 2011). Therefore, differentiated cell division in the mitfavc7 mutants could be an indirect response to a reduction in the melanocyte population rather than a direct response of reduced Mitf activity. To test this idea, we rescued melanocytes in melanocyte-deficient embryos and assessed their cell division potential. Single-cell embryos (mitfavc7 at 30-32°C) were injected with DNA encoding zebrafish mitfa expressed from the mitfa promoter, and the embryos were grown for 3 dpf (Fig. 6A). In 31 injected zebrafish, one developed a single melanocyte and seven developed between two and 17 melanocytes (Fig. 6B). Uninjected control embryos (n=50) or embryos injected with a construct with GFP expressed from the mitfa promoter did not develop melanocytes (n=43). We imaged seven individual transgenic fish with one to a few melanocytes on the head by time-lapse microscopy (30-126 hpf; total 11 melanocytes). All melanocytes were derived from undifferentiated precursor cells, with no evidence of cell division. Taken together, we suggest that the increased melanocyte division in mitfvc7 (>24°C) is due to changes in Mitf activity, rather than to a reduced total number of melanocytes in the embryo.
Fig. 6.
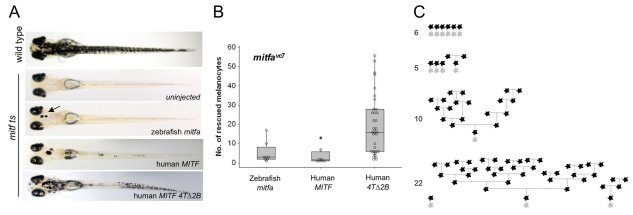
Human MITF 4TΔ2B promotes differentiated cell division. (A) Images of zebrafish embryos (day 5 postfertilization). Single-cell mitfavc7 embryos were injected with zebrafish mitfa, human MITF or human MITF4TΔ2B expressed from the mitfa promoter and grown at 30°C for 5 days. Uninjected mitfavc7 embryos were used as a control to monitor endogenous melanocyte development; no melanocytes developed in mitfavc embryos at 30°C (n=50). (B) Box plot of a representative experiment showing the range of melanocytes on individual zebrafish expressing zebrafish mitfa (n=8), human MITF (n=8) or human MITF4TΔ2B (n=35) from the mitfa promoter in mitfavc7 mutants grown at 30°C. Each data set has been repeated at least three times. Significance is observed within the dataset (P=0.001), analysis of variance (ANOVA). Post-hoc analysis shows significance between zebrafish mitfa and human MITF4TΔ2B; 95% confidence interval; 14.42 (2.03, 26.81), and between human MITF and MITF4TΔ2B; 95% CI; 16.04 (3.65, 28.43). (C) Schematic representation of melanocyte cell lineage analysis (as described in Fig. 5) for four embryos expressing MITF4TΔ2B. Total final number of melanocytes in imaged region is indicated.
Human melanoma MITF4TΔ2B stimulates differentiated cell division
MITF somatic mutations are found in human melanoma (Cronin et al., 2009). Because Mitf mutations in zebrafish could promote differentiated cell division, we asked whether human melanoma MITF mutations have the potential to promote differentiated cell division. First, we established whether human MITF could replace zebrafish mitfa function. As above, nacre (mitfa loss of function) or mitfavc7 (temperature sensitive grown at the restrictive temperature 30-32°C) were injected at the single-cell stage with a transgene expressing human MITF from the zebrafish mitfa promoter. Differentiated melanocytes were detected in nacre (n=22/55; see Fig. S6 in the supplementary material) and mitfa ts injected embryos (n=8/89; Fig. 6), whereas no melanocytes were seen in the uninjected controls. Thus, human MITF is functional in zebrafish, and can replace loss of mitfa function in neural crest melanocytes. We imaged eight individual transgenic fish with one to a few melanocytes on the head by time-lapse microscopy (30-126 hpf; total 27 melanocytes), and we did not observe the division of differentiated cells.
Second, we addressed the potential for a human melanoma mutation to rescue the melanocytes in the nacre and mitfavc7 mutant zebrafish. The human melanoma MITF4TΔ2B mutation is a splice variant that results in the loss of exon 2B (Cronin et al., 2009). Notably, compared with wild-type human MITF mutations, 4TΔ2B has increased transcriptional activity of the differentiation gene tyrosinase but reduced transcriptional activity of the cell cycle inhibitor p21 (Cronin et al., 2009). We expressed MITF4TΔ2B in zebrafish melanocytes from the mitfa promoter, and found that MITF4TΔ2B rescued zebrafish melanocyte development with a striking enhancement in total numbers of melanocytes (n=36/41 in nacre; n=35/61 in mitfavc7; Fig. 6; see Fig. S6 in the supplementary material).
Finally, we imaged ten embryos expressing melanocytes MITF4TΔ2B by time lapse microscopy (30-126 hpf) in mitfavc7 mutant zebrafish at 30°C. MITF4TΔ2B embryos had between one and 22 melanocytes in the head region, and a total of 75 melanocytes were imaged. No melanocytes were seen in mitfavc7 uninjected controls. In five embryos, melanocytes developed from undifferentiated precursor cells and did not divide. By contrast, in the other five embryos, two showed differentiated cell division and three showed serial differentiated cell division. Remarkably, in one example, a differentiated melanocyte gave rise to 18 differentiated cells. These data indicate that the melanoma mutation MITF4TΔ2B has the potential to both promote melanocyte cell division and differentiation.
DISCUSSION
Much of the extensive expansion required to populate the skin and hair in development is from undifferentiated melanocyte precursor cells. We were prompted by previous observations, that differentiated melanocytes in culture and in melanoma maintain proliferative capability, to examine closely the potential of differentiated zebrafish melanocytes to divide in situ. We find a subpopulation of differentiated melanocytes has cell division potential during melanocyte development and regeneration in zebrafish, and that Mitf activity is required to maintain cell cycle arrest in differentiated cells. The finding that a human melanoma MITF mutation permits cell division in differentiated cells underscores the importance of MITF anti-proliferative activity in vivo.
High or low levels of MITF activity in cell culture leads to G1 arrest with differentiation or invasion, respectively (Carreira et al., 2005; Carreira et al., 2006; Goodall et al., 2008). MITF also promotes the cell cycle in melanoma (Carreira et al., 2005; Du et al., 2004; Garraway et al., 2005; Widlund et al., 2002), and graded changes in MITF activity are thought to switch melanoma cells phenotypically from a proliferative to an invasive stem-cell like state or vice versa (the MITF rheostat model) (Hoek and Goding, 2010). Consistent with this idea, transient silencing of MITF generates invasive cells with stem-like properties that have increased efficiency of tumour initiation (Cheli et al., 2011; Carreira et al., 2006; Goodall et al., 2008; Pinner et al., 2009).
It is less clear how the rheostat model applies to melanocytes. TGFβ signalling maintains melanocyte stem cells in the hair follicle in mice, which is associated with low Mitf levels (Nishimura et al., 2010), and in zebrafish and frogs, loss of Mitf causes altered differentiation characteristics in shape and melanin distribution (Johnson et al., 2011; Kawasaki et al., 2008). The impact of altered MITF on the cell cycle in differentiated cells in animals has been difficult to study because the first role of MITF is in melanocyte specification and survival (Hornyak et al., 2001; Lister et al., 1999; Opdecamp et al., 1997). The development of mitfa temperature-sensitive lines in zebrafish (Johnson et al., 2011) is essential in order to address this issue because we can now assess the effects of graded Mitf activity during melanocyte development in vivo, and zebrafish are amenable to sustained live time-lapse imaging.
Our observations in live animals show that, during normal development, Mitf maintains cell cycle arrest in differentiated melanocytes (Figs 5, 6), and that differentiation is not necessarily terminal (Figs 1, 2, 3). Although our observations are consistent with the Mitf rheostat model that altered levels of Mitf promote different phenotypic outcomes in melanoma, they differ by suggesting that in some contexts melanocytes can be both differentiated and proliferative. In particular, differentiation and proliferation are frequency uncoupled in regenerating wild-type melanocytes (Fig. 3). How melanocytes in regeneration regulate differentiated cell division is unknown, but given our results that Mitf activity is required to maintain cell cycle arrest of differentiated cells in development, Mitf activity may be regulated during regeneration to permit cell division in differentiated cells. Additional factors may be involved, however, because from our initial experiments we do not see an enhancement of differentiated cell division in mitfavc7-regenerating melanocytes compared with melanocytes regenerating after nitrofuran treatment (Fig. 3E-H). For example, Kit signalling may be important in regulation of differentiated cell division (Rawls and Johnson, 2003). Our initial experiments also suggest a reduced number of melanocytes in the embryo is not sufficient to stimulate cell division in a differentiated cell (Fig. 6A), although transgenic melanocytes dependent on a fragment of the mitfa promoter may have less potential to respond to environmental stimuli than wild-type melanocytes. In vitro, the potential for both proliferation and differentiation in melanocytes is illustrated by the MITF4TΔ2B allele that has increased transcriptional activity of differentiation genes coupled with reduced expression of the cell cycle inhibitor p21 in vitro (Cronin et al., 2009). In zebrafish, we find that human MITF4TΔ2B has increased differentiated cell cycle division potential compared with wild-type human MITF in zebrafish (Fig. 6). We speculate that MITF4TΔ2B uncoupling of differentiation and cell cycle arrest in development may be relevant to the oncogenesis of MITF4TΔ2B mutant melanoma.
How melanocytes fine-tune Mitf activity in a temporal- and spatial-specific manner is a complex challenge in melanocyte biology (Levy and Fisher, 2011), and includes coordination of extrinsic signals, transcriptional regulation (Levy et al., 2006), interactions with related transcription factors (Hemesath et al., 1994), chromatin modifiers (de la Serna et al., 2006; Keenen et al., 2010; Price et al., 1998; Sato et al., 1997) and direct protein interactions (Bismuth et al., 2005). Our work illustrates the importance of fine tuning Mitf activity in melanocytes because Mitf activity that is sufficient for specification and differentiation is not sufficient for cell cycle arrest. Unlike in mammals, zebrafish maintain the potential for extensive regeneration in many tissues (O'Reilly-Pol and Johnson, 2009; Poss, 2007), and it is possible that differentiated melanocyte division is specific to zebrafish. Nonetheless, the molecular machinery that controls melanocyte development is highly conserved (Kelsh et al., 2009) and suggests that our observations in zebrafish may be relevant to stem cell and differentiation-based therapy in melanoma, and in melanocyte regeneration in vitiligo.
Supplementary Material
Acknowledgments
We thank Professors Mike Tyers, Dorothy Bennett and Nick Hastie for critical reading of the manuscript; Professor Colin Goding and Dr James Amatruda for helpful discussions; Professor Stephen Johnson for zebrafish lines; Catriona Graham for statistical advice and analysis; and Dr Yardena Samuels for the human MITF alleles. We thank Dr Karthika Paranthaman for zebrafish husbandry, and Dr Paul Perry and Matthew Pearson for the set-up and support of the imaging system. This work was supported by the Medical Research Council, the Wellcome Trust, the Concern Foundation, the Association for International Cancer Research, Medical Research Scotland and the European Union ZF-CANCER project. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.064014/-/DC1
References
- Ajioka I., Martins R. A., Bayazitov I. T., Donovan S., Johnson D. A., Frase S., Cicero S. A., Boyd K., Zakharenko S. S., Dyer M. A. (2007). Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 131, 378-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe-Heider F., Goritz C., Sabelstrom H., Takebayashi H., Pfrieger F. W., Meletis K., Frisen J. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470-482 [DOI] [PubMed] [Google Scholar]
- Bennett D. C. (1983). Differentiation in mouse melanoma cells: initial reversibility and an on-off stochastic model. Cell 34, 445-453 [DOI] [PubMed] [Google Scholar]
- Bennett D. C. (1989). Mechanisms of differentiation in melanoma cells and melanocytes. Environ. Health Perspect. 80, 49-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. C., Bridges K., McKay I. A. (1985). Clonal separation of mature melanocytes from premelanocytes in a diploid human cell strain: spontaneous and induced pigmentation of premelanocytes. J. Cell Sci. 77, 167-183 [DOI] [PubMed] [Google Scholar]
- Bismuth K., Maric D., Arnheiter H. (2005). MITF and cell proliferation: the role of alternative splice forms. Pigment Cell Res. 18, 349-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I., Liedtke D., Volff J. N., Schartl M. (2009). Pigmentary function and evolution of tyrp1 gene duplicates in fish. Pigment Cell Melanoma Res. 22, 839-850 [DOI] [PubMed] [Google Scholar]
- Brennand K., Huangfu D., Melton D. (2007). All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 5, e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert S. M., Roy R., Okamoto I., van den Oord J., Bauer J., Garbe C., Barnhill R. L., Busam K. J., Cochran A. J., Cook M. G., et al. (2010). Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 23, 763-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira S., Goodall J., Aksan I., La Rocca S. A., Galibert M. D., Denat L., Larue L., Goding C. R. (2005). Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433, 764-769 [DOI] [PubMed] [Google Scholar]
- Carreira S., Goodall J., Denat L., Rodriguez M., Nuciforo P., Hoek K. S., Testori A., Larue L., Goding C. R. (2006). Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 20, 3426-3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli Y., Guiliano S., Botton T., Rocchi S., Hofman V., Hofman P., Bahadoran P., Bertolotto C., Ballotti R. (2011). Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 30, 2307-2318 [DOI] [PubMed] [Google Scholar]
- Cronin J. C., Wunderlich J., Loftus S. K., Prickett T. D., Wei X., Ridd K., Vemula S., Burrell A. S., Agrawal N. S., Lin J. C., et al. (2009). Frequent mutations in the MITF pathway in melanoma. Pigment Cell Melanoma Res. 22, 435-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Shen L. Y., Wang G. C. (1991). Role of hair follicles in the repigmentation of vitiligo. J. Invest. Dermatol. 97, 410-416 [DOI] [PubMed] [Google Scholar]
- Davis D. M., Dyer M. A. (2010). Retinal progenitor cells, differentiation, and barriers to cell cycle reentry. Curr. Top. Dev. Biol. 93, 175-188 [DOI] [PubMed] [Google Scholar]
- de la Serna I. L., Ohkawa Y., Higashi C., Dutta C., Osias J., Kommajosyula N., Tachibana T., Imbalzano A. N. (2006). The microphthalmia-associated transcription factor requires SWI/SNF enzymes to activate melanocyte-specific genes. J. Biol. Chem. 281, 20233-20241 [DOI] [PubMed] [Google Scholar]
- de The H., Chen Z. (2010). Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat. Rev. Cancer 10, 775-783 [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I., Melton D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41-46 [DOI] [PubMed] [Google Scholar]
- Du J., Widlund H. R., Horstmann M. A., Ramaswamy S., Ross K., Huber W. E., Nishimura E. K., Golub T. R., Fisher D. E. (2004). Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565-576 [DOI] [PubMed] [Google Scholar]
- Falabella R. (2009). Vitiligo and the melanocyte reservoir. Indian J. Dermatol. 54, 313-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falabella R., Barona M. I. (2009). Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 22, 42-65 [DOI] [PubMed] [Google Scholar]
- Garraway L. A., Widlund H. R., Rubin M. A., Getz G., Berger A. J., Ramaswamy S., Beroukhim R., Milner D. A., Granter S. R., Du J., et al. (2005). Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117-122 [DOI] [PubMed] [Google Scholar]
- Goodall J., Carreira S., Denat L., Kobi D., Davidson I., Nuciforo P., Sturm R. A., Larue L., Goding C. R. (2008). Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res. 68, 7788-7794 [DOI] [PubMed] [Google Scholar]
- Hemesath T. J., Steingrimsson E., McGill G., Hansen M. J., Vaught J., Hodgkinson C. A., Arnheiter H., Copeland N. G., Jenkins N. A., Fisher D. E. (1994). microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 8, 2770-2780 [DOI] [PubMed] [Google Scholar]
- Hirobe T. (1988). Developmental changes of the proliferative response of mouse epidermal melanocytes to skin wounding. Development 102, 567-574 [DOI] [PubMed] [Google Scholar]
- Hoek K. S., Goding C. R. (2010). Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 23, 746-759 [DOI] [PubMed] [Google Scholar]
- Hornyak T. J., Hayes D. J., Chiu L. Y., Ziff E. B. (2001). Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech. Dev. 101, 47-59 [DOI] [PubMed] [Google Scholar]
- Hultman K. A., Johnson S. L. (2010). Differential contribution of direct-developing and stem cell-derived melanocytes to the zebrafish larval pigment pattern. Dev. Biol. 337, 425-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman K. A., Budi E. H., Teasley D. C., Gottlieb A. Y., Parichy D. M., Johnson S. L. (2009). Defects in ErbB-dependent establishment of adult melanocyte stem cells reveal independent origins for embryonic and regeneration melanocytes. PLoS Genet. 5, e1000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata K., Aoto T., Binh N. T., Okamoto N., Tanimura S., Wakayama T., Iseki S., Hara E., Masunaga T., Shimizu H., et al. (2009). Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137, 1088-1099 [DOI] [PubMed] [Google Scholar]
- Jimbow K., Roth S. I., Fitzpatrick T. B., Szabo G. (1975). Mitotic activity in non-neoplastic melanocytes in vivo as determined by histochemical, autoradiographic, and electron microscope studies. J. Cell Biol. 66, 663-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. L., Nguyen A. N., Lister J. A. (2011). mitfa is required at multiple stages of melanocyte differentiation but not to establish the melanocyte stem cell. Dev. Biol. 350, 405-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S. A., Jackson I. J. (2000). A late wave of melanoblast differentiation and rostrocaudal migration revealed in patch and rump-white embryos. Mech. Dev. 92, 135-143 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Mori N., Nakayama A. (2001). Kit(+) melanocytes seem to contribute to melanocyte proliferation after UV exposure as precursor cells. J. Invest. Dermatol. 116, 920-925 [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Kumasaka M., Satoh A., Suzuki M., Tamura K., Goto T., Asashima M., Yamamoto H. (2008). Mitf contributes to melanosome distribution and melanophore dendricity. Pigment Cell Melanoma Res. 21, 56-62 [DOI] [PubMed] [Google Scholar]
- Keenen B., Qi H., Saladi S. V., Yeung M., de la Serna I. L. (2010). Heterogeneous SWI/SNF chromatin remodeling complexes promote expression of microphthalmia-associated transcription factor target genes in melanoma. Oncogene 29, 81-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh R. N., Harris M. L., Colanesi S., Erickson C. A. (2009). Stripes and belly-spots – a review of pigment cell morphogenesis in vertebrates. Semin. Cell Dev. Biol. 20, 90-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled M., Levy C., Fisher D. E. (2010). Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 24, 2276-2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099 [DOI] [PubMed] [Google Scholar]
- Levy C., Fisher D. E. (2011). Dual roles of lineage restricted transcription factors: the case of MITF in melanocytes. Transcription 2, 19-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C., Khaled M., Fisher D. E. (2006). MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 12, 406-414 [DOI] [PubMed] [Google Scholar]
- Lister J. A., Robertson C. P., Lepage T., Johnson S. L., Raible D. W. (1999). nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757-3767 [DOI] [PubMed] [Google Scholar]
- Lister J. A., Close J., Raible D. W. (2001). Duplicate mitf genes in zebrafish: complementary expression and conservation of melanogenic potential. Dev. Biol. 237, 333-344 [DOI] [PubMed] [Google Scholar]
- Logan D. W., Burn S. F., Jackson I. J. (2006). Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res. 19, 206-213 [DOI] [PubMed] [Google Scholar]
- Mackenzie M. A., Jordan S. A., Budd P. S., Jackson I. J. (1997). Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol. 192, 99-107 [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Kusakabe M., Yoshinaga K., Ogawa M., Hayashi S., Kunisada T., Era T., Sakakura T. (1991). In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 10, 2111-2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura E. K., Jordan S. A., Oshima H., Yoshida H., Osawa M., Moriyama M., Jackson I. J., Barrandon Y., Miyachi Y., Nishikawa S. (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854-860 [DOI] [PubMed] [Google Scholar]
- Nishimura E. K., Granter S. R., Fisher D. E. (2005). Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720-724 [DOI] [PubMed] [Google Scholar]
- Nishimura E. K., Suzuki M., Igras V., Du J., Lonning S., Miyachi Y., Roes J., Beermann F., Fisher D. E. (2010). Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 6, 130-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly-Pol T., Johnson S. L. (2009). Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev. Biol. 20, 117-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsie S. J., Sarantopoulos G. P., Cochran A. J., Binder S. W. (2008). Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 35, 433-444 [DOI] [PubMed] [Google Scholar]
- Opdecamp K., Nakayama A., Nguyen M. T., Hodgkinson C. A., Pavan W. J., Arnheiter H. (1997). Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development 124, 2377-2386 [DOI] [PubMed] [Google Scholar]
- Parichy D. M. (2003). Pigment patterns: fish in stripes and spots. Curr. Biol. 13, R947-R950 [DOI] [PubMed] [Google Scholar]
- Pinner S., Jordan P., Sharrock K., Bazley L., Collinson L., Marais R., Bonvin E., Goding C., Sahai E. (2009). Intravital imaging reveals transient changes in pigment production and Brn2 expression during metastatic melanoma dissemination. Cancer Res. 69, 7969-7977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K. D. (2007). Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36-45 [DOI] [PubMed] [Google Scholar]
- Price E. R., Ding H. F., Badalian T., Bhattacharya S., Takemoto C., Yao T. P., Hemesath T. J., Fisher D. E. (1998). Lineage-specific signaling in melanocytes. C-kit stimulation recruits p300/CBP to microphthalmia. J. Biol. Chem. 273, 17983-17986 [DOI] [PubMed] [Google Scholar]
- Rawls J. F., Johnson S. L. (2003). Temporal and molecular separation of the kit receptor tyrosine kinase's roles in zebrafish melanocyte migration and survival. Dev. Biol. 262, 152-161 [DOI] [PubMed] [Google Scholar]
- Sato S., Roberts K., Gambino G., Cook A., Kouzarides T., Goding C. R. (1997). CBP/p300 as a co-factor for the Microphthalmia transcription factor. Oncogene 14, 3083-3092 [DOI] [PubMed] [Google Scholar]
- Smyth I. M., Wilming L., Lee A. W., Taylor M. S., Gautier P., Barlow K., Wallis J., Martin S., Glithero R., Phillimore B., et al. (2006). Genomic anatomy of the Tyrp1 (brown) deletion complex. Proc. Natl. Acad. Sci. USA 103, 3704-3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel K. P., Davidson D. R., Jackson I. J. (1992). TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development 115, 1111-1119 [DOI] [PubMed] [Google Scholar]
- Tanimura S., Tadokoro Y., Inomata K., Binh N. T., Nishie W., Yamazaki S., Nakauchi H., Tanaka Y., McMillan J. R., Sawamura D., et al. (2011). Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8, 177-187 [DOI] [PubMed] [Google Scholar]
- van Schanke A., Jongsma M. J., Bisschop R., van Venrooij G. M., Rebel H., de Gruijl F. R. (2005). Single UVB overexposure stimulates melanocyte proliferation in murine skin, in contrast to fractionated or UVA-1 exposure. J. Invest. Dermatol. 124, 241-247 [DOI] [PubMed] [Google Scholar]
- Walker G. J., Kimlin M. G., Hacker E., Ravishankar S., Muller H. K., Beermann F., Hayward N. K. (2009). Murine neonatal melanocytes exhibit a heightened proliferative response to ultraviolet radiation and migrate to the epidermal basal layer. J. Invest. Dermatol. 129, 184-193 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) (4th edn). Eugene, OR: University of Oregon Press; [Google Scholar]
- Widlund H. R., Horstmann M. A., Price E. R., Cui J., Lessnick S. L., Wu M., He X., Fisher D. E. (2002). Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J. Cell Biol. 158, 1079-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. T., Johnson S. L. (2006). Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development 133, 3563-3573 [DOI] [PubMed] [Google Scholar]
- Zou J., Beermann F., Wang J., Kawakami K., Wei X. (2006). The Fugu tyrp1 promoter directs specific GFP expression in zebrafish: tools to study the RPE and the neural crest derived melanophores. Pigment Cell Res. 19, 615-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.