Abstract
Protease-activated receptor-2 (PAR2) is expressed in endothelial cells and mediates endothelium-dependent vasodilation. We hypothesized that PAR2 regulates tumor necrosis factor-alpha (TNF-α)-induced coronary arteriolar dysfunction in type 2 diabetic (db/db) mice. To test this, coronary arterioles from WT control, db/db, db/db mice treated with PAR2 antagonist FSLLRY–NH2 (db/db+FSLLRY–NH2) and db/db mice null for TNF (dbTNF–/dbTNF–) were isolated and pressurized (60 cmH2O) without flow. Although vasodilation to the endothelium-independent vasodilator sodium nitroprusside (SNP) was not different among WT, db/db, db/db+FSLLRY–NH2 and dbTNF–/dbTNF–, endothelium-dependent acetylcholine (ACh)- and flow-mediated vasodilation were impaired in db/db mice but were enhanced in dbTNF–/dbTNF– mice and db/db mice treated with PAR2 antagonist. NOS inhibitor NG-nitro-l-arginine-methyl ester (l-NAME) significantly reduced ACh-induced dilation in WT, dbTNF–/dbTNF– and db/db+FSLLRY–NH2, but did not alter the vasodilation in db/db mice. In contrast, cyclooxygenase (COX) inhibitor indomethacin (Indo) did not alter ACh-induced vasodilation in these four groups of mice. PAR2-activating peptide (PAR2-AP, 2-Furoyl-LIGRLO-am)-induced dilation was higher in db/db mice than that in WT, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist. These effects were abolished by denudation, or in the presence of l-NAME or Indo. Protein expressions of TNF-α, PAR2, gp91phox and p47phox in the heart and isolated coronary arterioles were higher in db/db mice compared to WT mice. Administration of PAR2 antagonist to db/db mice reduced protein expression of TNF-α, gp91phox and PAR2. Protein expression of gp91phox and p47phox was lower in dbTNF–/dbTNF– compared to db/db mice. These results indicate that PAR2 plays a pivotal role in endothelial dysfunction in type 2 diabetes by up-regulating the expression/production of TNF-α and activating NAD(P)H oxidase subunit p47phox.
Keywords: Coronary microcirculation, Endothelium, Inflammation
Introduction
Protease (proteinase)-activated receptors (PARs) are the novel classes of seven transmembrane domain G-protein coupled receptors, and four PARs have been identified with distinct N-terminal cleavage sites and tethered ligand pharmacology, PAR1, PAR2, PAR3 and PAR4 [5, 15, 26, 27, 30]. Activation of PAR2 is initiated by cleavage of the N terminus of the receptor by (1) endogenous serine pro-teases such as trypsin, tryptase, membrane type protease-1, neutrophil protease 3, and factor Xa, and (2) exogenous stimulation of tethered ligand, where they interact with PAR2 activating peptides (PAR2-APs) including SLIGRL, SLIGKV, tc-LIGRLO and 2-furoyl-LIGRLO [5, 6, 15, 26, 27, 30, 36]. PAR2 is profoundly localized in the vasculature, especially in endothelial cells [6, 8, 16, 26], and is implicated in the control of vascular tone and homeostasis [6, 14, 34, 36]. Activation of endothelial PAR2 by endogenous serine protease or by PAR2-activating peptide (PAR2-APs, PAR2 agonist) causes vasodilation in vivo or isolated vessels mainly through nitric oxide (NO) and prostacyclin (PGI2) mechanisms [6, 14, 34, 36]. The role of PAR2 in vascular control may be more prevalent under inflammatory-induced pathological conditions such as in type 1 diabetes [24, 26, 36]. However, the role of PAR2 in coronary arteriolar dysfunction in type 2 diabetes has not been elucidated.
Type 2 diabetes generally co-occurs with metabolic cardiovascular risk factors associated with various complications of cardiovascular disease, including inflammation [17, 32]. Endothelial PAR2 plays a significant role in inflammation and tissue injury in the vascular system [14, 20, 33]. Vascular PAR2 expression is up-regulated by inflammatory cytokines such as TNF-α and, interestingly, TNF-α production is enhanced by activation of PAR2 indicating there may be a close link between TNF-α and PAR2 in pathophysiological situations [6, 14]. Increased TNF-α expression in type 2 diabetic coronary arterioles induces activation of reactive oxygen species (ROS), leading to endothelial dysfunction [3, 4, 11, 43]. However, the upstream mediator that is responsible for the elevations in TNF-α expression in type 2 diabetic coronary arterioles has not been elucidated. Furthermore, there is no direct evidence reported regarding the role of PAR2 in endothelial dysfunction in coronary microcirculation in type 2 diabetes. We propose that PAR2, at least in part, regulates inflammatory cytokines, especially TNF-α, leading to endothelial dysfunction in murine coronary microcirculation in type 2 diabetes. To test this, we evaluated (1) whether antagonizing PAR2 restores endothelial function in type 2 diabetic db/db mice; (2) whether PAR2-activating peptide PAR2-APs-induced vasodilation is altered in db/db mice; and (3) whether PAR2 regulates protein expression of TNF-α or NAD(P)H oxidase subunits in db/db mice.
Methods
Animal models
The procedures followed were in accordance with approved guidelines set by the Laboratory Animal Care Committee at University of Missouri. Wild type (WT, C57BL/6J) controls, type 2 diabetic (db/db, BKS.Cg-m +/+ Leprdb/J) mice and the breeding pairs of db/db mice null for TNF-α (dbTNF–/dbTNF–) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained on a normal rodent chow diet. The genotyping for dbTNF–/dbTNF– mice was performed to confirm that TNF-α was knocked down adequately. Our studies utilized 12–16-week-old male 25–30 g of WT, 45–50 g of db/db and dbTNF–/dbTNF– mice of either sex.
PAR2 antagonist treatment
Selective PAR2 antagonist (FSLLRY-Amide, H–Phe–Ser–Leu–Leu–Arg–Tyr–NH2) was purchased from PEPTIDES International Inc (Louisville, KY, USA). The PAR2 antagonist (4 mg/kg/day, i.p. for 5 days) was administered to both WT and db/db mice for 5 days to determine whether PAR2 affects the vasodilatory function of isolated coronary arterioles and the protein expression of TNF-α in the heart and coronary arterioles.
Functional assessment of isolated coronary arterioles
The techniques for identification and isolation of coronary microvessels were previously described in detail [42, 44]. Briefly, the heart from five groups of animals, WT, WT treated with PAR2 antagonist, db/db, db/db treated with PAR2 antagonist and dbTNF–/dbTNF– mice was excised and immediately placed in cold (4°C) saline solution. Each coronary arteriole (50–100 μm in internal diameter) was carefully isolated and cannulated with glass micropipettes. To determine the function of coronary arterioles, vessels were intraluminally pressurized at 60 cmH2O without flow. After developing a basal tone, the experimental interventions were performed.
The concentration–diameter relationships for an activator of endothelium-dependent vasodilation, acetylcholine (ACh, 1 nmol/L to 10 μmol/L), flow-induced dilation (endothelium-dependent but agonist-independent; pressure difference (ΔP) was 4–60 H2O), endothelium-independent NO donor sodium nitroprusside (SNP, 1 nmol/L to 10 μmol/L) and potent and selective PAR2-AP (0.1–10 μmol/L, 2-Furoyl-LIGRLO-amide, PEPTIDES International Inc., Louisville, KY, USA)-mediated dilation was then established. PAR2 agonist-induced vasodilation was also performed (1) after endothelial removal by passing approximately 5 mL of air through the lumen of the vessel. To ensure complete removal of the endothelium, the endothelium-dependent vasodilator ACh (3 × 10–5 M) was incubated in the denuded arterioles. Any vessel that was dilated more than 5% was discarded. Additionally, vasodilation in these denuded vessels to sodium nitroprusside (1 nmol/L to 10 μmol/L) was not altered indicating vascular smooth muscle function remained intact (data not shown); (2) in the presence of NOS inhibitor NPPGPP-nitro-l-arginine-methyl ester (l-NAME, 10 μmol/L, 20 min), or in the presence of cyclooxygenase (COX) inhibitor indomethacin (Indo, 10 μmol/L, 30 min); or (3) in the presence of TNF-α (1 μg/mL, 1 h) prior to beginning the protocols. At the end of each experiment, the vessel was exposed to 100-μmol/L SNP to obtain its maximal diameter at 60 cmH2O intraluminal pressure [44].
Protein expression of PAR2 and TNF-α by western blot analyses
Hearts or coronary arterioles were separately homogenized and sonicated in lysis buffer (Cellytic™ MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). Protein concentrations were assessed [BCA™ Protein Assay Kit (Pierce)], and equal amounts of protein (10 μg for PAR2 and 20 μg for TNF-α) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond, Amersham). Primary antibodies for TNF-α and PAR2 were purchased from Sigma (St. Louis, MO, USA). Horseradish peroxidase-conjugated goat anti-mouse was used as the secondary antibody (1:2,000 dilution) (Abcam). Signals were visualized by enhanced chemiluminescence (Santa Cruz), scanned with a Fuji LAS3000 densitometer, and quantified by Multigauge software (Fuji film). The relative amounts of protein expression were quantified and normalized to those of the corresponding internal reference and α-actin, and then normalized to corresponding WT control, which were set to a value of 1.0.
Mouse coronary artery endothelial cell (MCAEC) culture
Primary mouse coronary arterial endothelial cells (Celprogen, San Pedro, CA, USA) were maintained in culture in the presence or absence of recombinant TNF-α (R&D: control, 1, 3 and 10 ng/mL for 24 h). After the treatment with recombinant TNF-α, the MCAEC were collected, and the PAR-2 protein expression was determined by Western blotting.
Immunofluorescence staining
Immunohistochemistry was used to identify and localize proteins in sections of heart. Freshly isolated mouse heart was embedded in OCT and sectioned at 5 μm. Slides were incubated with blocking solution (10% donkey serum in PBS). Primary antibodies for PAR2 (goat polyclonal, Santa Cruz), and endothelial cell marker, von Willebrand factor (vWF, rabbit polyclonal, Abcam), or smooth muscle α-actin (rabbit polyclonal, Abcam), or macrophage marker, CD68 (rat monoclonal, Abcam) were used for sequential double-immunofluorescence staining. Negative control was performed with the use of goat polyclonal IgG (Abcam), rabbit polyclonal IgG (GeneTex) and rat monoclonal IgG (Abcam) isotype controls. Secondary fluorescent antibodies are either FITC or Texas Red conjugated. Sections were finally mounted in an anti-fading agent (Slowfade gold with DAPI, Invitrogen). Slides were observed and analyzed using a fluorescence microscope with a 40× objective (IX81, Olympus) [12].
Data analysis
At the end of each experiment, the vessel was exposed to 100-μmol/L SNP to obtain its maximal diameter at 60 cmH2O intraluminal pressure [11, 31, 44]. All diameter changes to pharmacological agonists were normalized to the control diameter. All data are presented as mean ± SEM, except as specifically stated (e.g. as mean ± SD for molecular study). Statistical comparisons of vasomotor responses under various treatments were performed with two-way ANOVA for repeated measure and intergroup differences were tested with Bonferonni inequality. The significance of intergroup differences observed in molecular studies was evaluated by one-way analyses of variance (one-way-ANOVA) using software SPSS11.5. Significance was accepted at P < 0.05.
Results
Animal characteristics
Table 1 shows that body weight, abdominal girth and glucose (non-fasting) concentration were higher in db/db, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 compared with WT and WT mice treated with FSLLRY–NH2.
Table 1.
Basic parameters
| WT | db/db | dbTNF–/dbTNF– | WT + FSLLRY–NH2 | db/db + FSLLRY–NH2 | |
|---|---|---|---|---|---|
| Body Weight, g | 27.0 ± 1.2 | 48.9 ± 1.4* | 47.3 ± 1.4* | 26.5 ± 0.7 | 48.5 ± 0.7* |
| Abdominal girth, cm | 7.8 ± 0.1 | 10.7 ± 0.2* | 10.9 ± 0.3* | 7.9.0 ± 0.2 | 11.0 ± 0.2* |
| Glucose, mg/dl | 155.7 ± 4 | 470 ± 10* | 423 ± 20* | 159.3 ± 3 | 438 ± 25* |
Body weight, abdominal girth and glucose concentration are higher in db/db, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 (PAR2 antagonist) compared to WT mice or WT treated with PAR2 antagonist (P < 0.05; n = 5~10)
Body weight, abdominal girth and glucose concentration of dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 are not different from that of db/db mice.
P < 0.05 versus WT
Role of PAR2 and TNF-α in coronary arteriolar function in type 2 diabetes
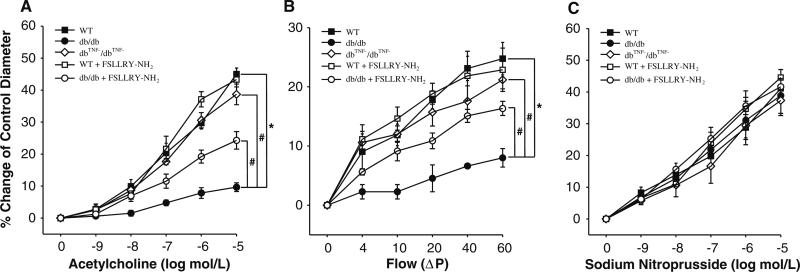
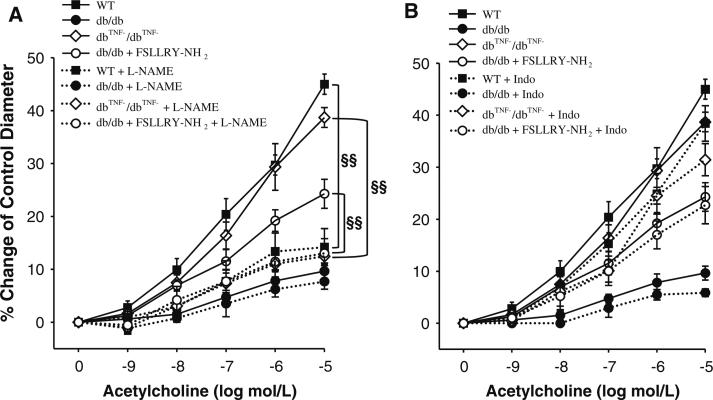
ACh- or flow-induced endothelial dependent vasodilation (Fig. 1a, b) was attenuated in coronary arterioles in db/db mice compared to WT. Administration of PAR2 antagonist (FSLLRY–NH2) partially restored impaired vasodilation in db/db mice (Fig. 1a, b), although it was not effective in WT mice. In dbTNF–/dbTNF– mice, coronary arteriolar dilation to ACh or to flow was greater than that in db/db mice, and comparable to that in WT control (Fig. 1a, b). Dilation to the NO donor SNP in WT, WT treated with FSLLRY–NH2, db/db, db/db treated with FSLLRY–NH2 and dbTNF–/dbTNF– mice are equal (Fig. 1c). l-NAME significantly attenuated vasodilation to ACh in WT mice (Fig. 2a). The dilations of the vessels incubated with l-NAME were not altered (P > 0/05) in db/db mice; however, incubation of l-NAME significantly inhibited vasodilation in dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist (Fig. 2a). Cyclooxygenase (COX) inhibitor indomethacin (Indo) did not alter vasodilation in WT, db/db, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist (Fig. 2b).
Fig. 1.
a Isolated coronary arterioles from WT (n = 14) and db/db (n = 10) mice were dilated in response to ACh in a concentration-dependent manner. ACh-induced vasodilation was significantly attenuated in db/db mice compared to WT mice. However, PAR2 antagonist (n = 6) partially restored ACh-induced vasodilation of diabetic coronary arterioles, but did not change vasodilation in WT mice (n = 5). In dbTNF–/dbTNF– mice (n = 5), ACh-induced vasodilation was greater than that in db/db mice and comparable to that in WT control (n = 5). b Flow-induced vasodilation was higher in WT mice compared to db/db mice. Administration of PAR2 antagonist FSLLRY–NH2 (i.p. injection for 5 days) to db/db mice partially improved flow-induced vasodilation, but flow-induced dilation was not altered in WT after administration of PAR2 antagonist. It was also higher in the dbTNF–/dbTNF– mice coronary arteriole than in db/db mice. ΔP pressure difference. c The endothelium-independent vasodilator (NO donor), SNP-induced vasodilation was not significantly different among the five groups (n = 5~8). Data were expressed as mean ± SEM. * P < 0.05 versus WT, # P < 0.05 versus db/db, or FSLLRY–NH2
Fig. 2.
a Incubation of l-NAME significantly reduced ACh-induced dilation in WT, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2, but not in db/db mice. l-NAME removed group differences in ACh-induced vasodilation in four groups, WT, db/db, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 (n = 5~8). b Incubation of Indo did not change ACh-induced dilation in four groups, WT, db/db, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 (n = 5~8). Data were expressed as mean ± SEM. §§ P < 0.05, effect of l-NAME. l-NAME incubated in the vessel for 20 min, Indo incubated in the vessel for 30 min
PAR2 agonist-induced vasodilation
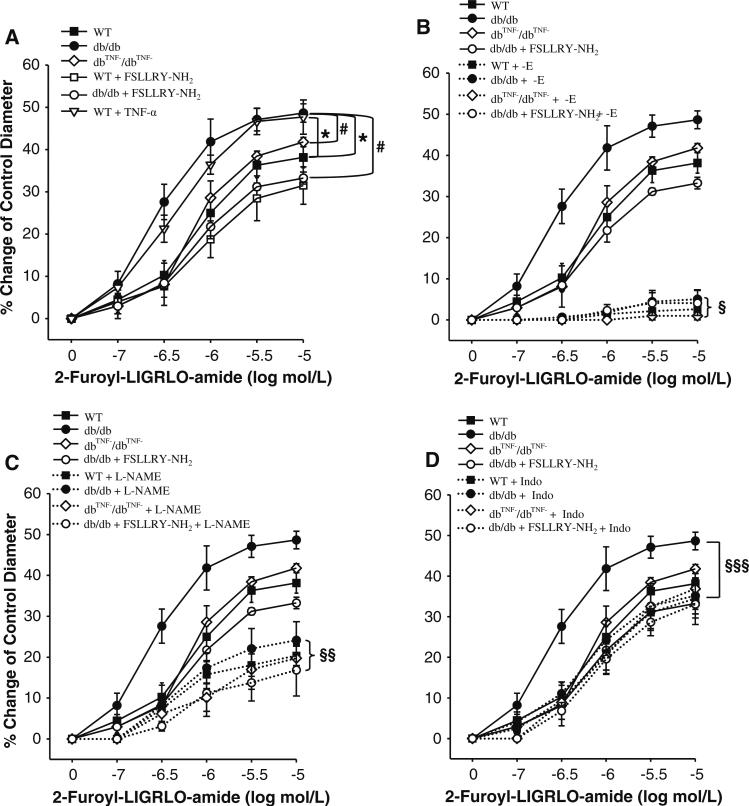
A potent and selective PAR2 agonist, 2-Furoyl-LIGRLO-amide, induced dilation of coronary arterioles in a concentration-dependent manner. 2-Furoyl-LIGRLO-amide-induced dilation was higher in coronary arterioles in db/db, and was equivalent in dbTNF–/dbTNF– mice compared to WT mice (Fig. 3a). 2-Furoyl-LIGRLO-amide-induced dilation was lower in db/db mice treated with PAR2 antagonist compared to db/db mice, but administration of PAR2 antagonist to WT mice did not change PAR2 agonist-induced dilation (Fig. 3a). Incubation of TNF-α with coronary arterioles isolated from WT mice increased PAR2 agonist-induced vasodilation suggesting PAR2 response is enhanced by TNF-α in WT coronary arterioles (Fig. 3a). The removal of endothelium (denudation) abolished PAR2 agonist-induced vasodilation (Fig. 3b) in all the five murine groups: WT, WT treated with FSLLRY–NH2 (data not shown), db/db, db/db treated with FSLLRY–NH2 and dbTNF–/dbTNF– mice. These results suggest that PAR2 agonist-induced vasodilation is endothelium-dependent. Vascular smooth muscle function remained intact in these denuded vessels, since vasodilation to sodium nitroprusside (1 nmol/L to 10 μmol/L) was not altered (data not shown). NOS inhibitor l-NAME significantly reduced PAR2 agonist-induced vasodilation in WT, WT treated with FSLLRY–NH2, db/db, dbTNF–/dbTNF– and db/db mice treated with FSLLRY–NH2 and eliminated the difference of PAR2-mediated dilation among these five murine groups (Fig. 3c), suggesting that PAR2 agonist-induced vasodilation is partially mediated by NO and the remaining vasodilation may be mediated by endothelium-dependent hyperpolarizing factor (EDHF). We then incubated COX inhibitor Indo with isolated coronary arterioles: attenuated vasodilation occurred only in db/db mice and dilation in WT, dbTNF–/dbTNF–, and WT and db/db mice treated with PAR2 antagonist was not affected. Indo incubation eliminated differences in PAR2-induced dilation in db/db mice (Fig. 3d).
Fig. 3.
PAR2-activating peptide caused vasodilation of mice coronary arterioles in a concentration-dependent manner. a PAR2-mediated vasodilation was significantly higher in db/db mice (n = 6) compared to WT mice (n = 5), but it was lower in dbTNF–/dbTNF– mice (n = 5) than that in db/db. PAR2 antagonist attenuated PAR2-induced vasodilation in db/db (n = 6) mice, but not in WT mice (n = 5). Incubation of TNF-α in arterioles (n = 5) significantly enhanced PAR2 agonist-induced vasodilation in WT mice compared to dbTNF–/dbTNF– mice. b PAR2 agonist-induced relaxation was largely blocked by endothelial denudation in WT, db/db, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist and differences of PAR2-mediated dilation among the groups were eliminated. c l-NAME caused a significant reduction in PAR2-mediated vasodilation in WT, db/db, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist and differences of PAR2-mediated dilation between groups were removed. d Indo did not significantly change PAR2-mediated vasodilation in WT, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist, but Indo significantly decreased PAR2-induced vasodilation only in db/db mice. Data were expressed as mean ± SEM. * P < 0.05 versus WT; # P < 0.05 versus db/db; § P < 0.05, effect of endothelium removal; §§ P < 0.05, effect of l-NAME; §§§ P < 0.05, effect of Indo. –E endothelium removal, FSLLRY-NH2 i.p. injection for 5 days, l-NAME incubated in the vessel for 20 min, Indo incubated in the vessel for 30 min, TNF-α incubated in the vessel for 1 h
Cellular source of PAR2 expression in type 2 diabetes
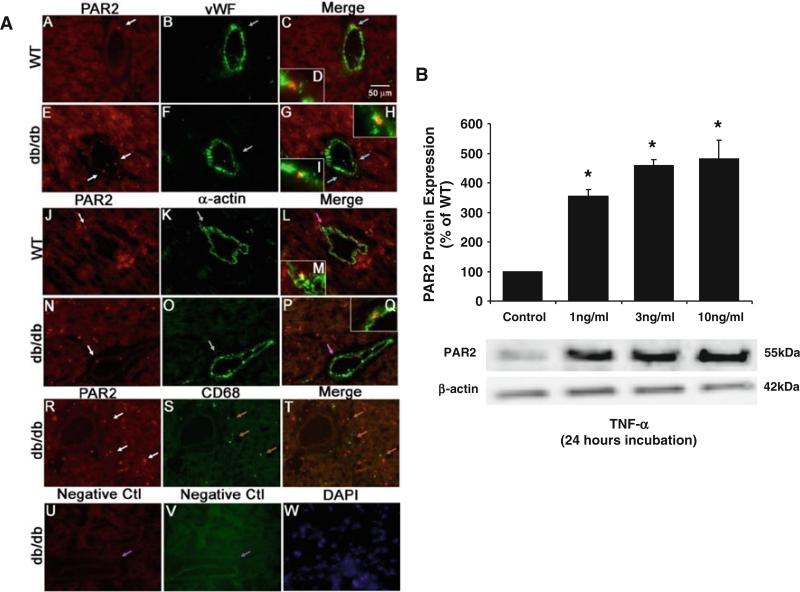
Dual immunostaining using a vascular smooth muscle cell marker α-actin or endothelial cell marker vWF determined whether PAR2 is localized in the vascular wall. PAR2 was expressed in endothelial cells in coronary microvessels of WT mice (Fig. 4a, A–D, red staining with blue arrows) and endothelial cells of coronary microvessels in db/db mice (Fig. 4a, E–I, red staining with blue arrows). PAR2 was co-localized with vascular smooth muscle cells in WT mice (Fig. 4a, J–M, red staining with pink arrows) and in db/db mice (Fig. 4a, N–Q, red staining with pink arrows).
Fig. 4.
a Dual fluorescence combining PAR2 with markers for endothelial cells [von Willebrand factor (vWF)], vascular smooth muscle cells (α-actin) and macrophages (CD68). A, B and C, dual labeling of PAR2 (red) and vWF (green) in control mouse heart tissue. E, F and G, dual labeling of PAR2 (red) and vWF (green) in db/db mouse heart tissue. The blue arrows in C and G show the colocalization of PAR2 and endothelial cells (yellow) in control and db/db mice. The inserts in C (D) and the inserts in G (H, I) show the higher magnification of the colocalization pointed by blue arrows in C and G. J, K and L, dual labeling of PAR2 (red) and α-actin (green) in control mice heart tissue. N, O and P, dual labeling of PAR2 (red) and α-actin (green) in db/db mice heart tissue. The pink arrows in L and P show the colocalization of PAR2 and vascular smooth muscle cells (yellow) in control and db/db mice. The inserts in L (M) and the inserts in P (Q) show the higher magnification of the colocalization pointed by pink arrows in L and P. R, S and T, dual labeling of PAR2 (red) and marker of macrophage in db/db mouse heart tissue. The brown arrows in T show the specific CD68 staining with absence of PAR2 staining. U and V, negative control: the purple arrows show the absence of staining in vessels without primary antibodies. W shows nuclear staining with DAPI (blue) in db/db mouse heart tissue. The immunostaining results suggest that in the heart of type 2 diabetic mice, PAR2 is expressed in endothelial cells and vascular smooth muscle cells, but not in macrophages. Magnification, ×40. Data shown are representative of four separate experiments. b PAR2 is expressed in mouse coronary arterial endothelial cells and it was significantly increased with the presence of TNF-α. However, there was no TNF-α concentration-dependent (1, 3, or 10 ng/mL) increase in PAR2 expression, n = 3. Data were expressed as mean ± SEM. * P < 0.05 versus WT
Immunohistochemistry for PAR2 and CD68 was performed to determine whether PAR2 was co-localized with cardiac macrophages. Figure 4a, R–T shows that PAR2 was not expressed in infiltrated macrophages in diabetic murine heart tissue.
PAR2 was expressed in murine coronary arterial endothelial cells (MCAEC) and incubation of TNF-α (at 1, 3, and 10 ng/mL) with MCAEC significantly increased PAR2 protein expression regardless of the level of TNF-α concentration (Fig. 4b).
Protein expression of TNF-α and PAR2 in type 2 diabetes
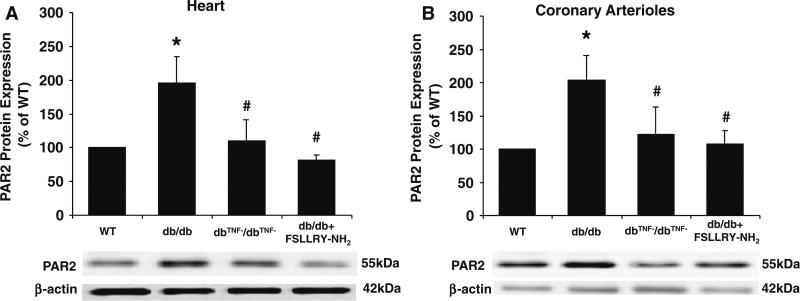
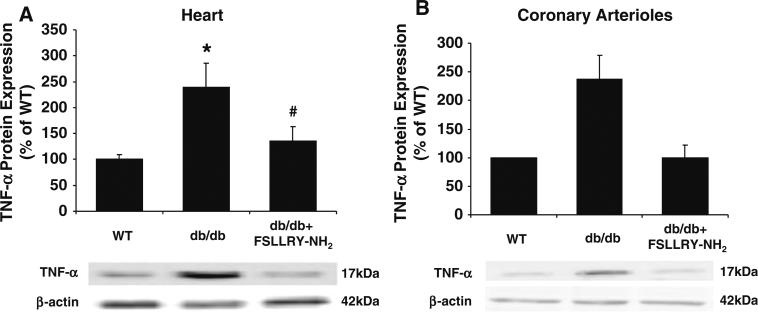
PAR2 protein expression in the heart tissue (Fig. 5a) and coronary arterioles (Fig. 5b) were approximately twofold higher in db/db mice than that in WT mice, but significantly attenuated in db/db mice null for TNF-α (dbTNF–/dbTNF–) suggesting that PAR2 expression may be regulated by TNF-α. Protein expression of TNF-α in the heart tissue (Fig. 6a) and coronary arterioles (Fig. 6b) of db/db mice was significantly elevated compared to WT mice, but TNF-α expression was markedly attenuated in db/db mice treated with PAR2 antagonist indicating that PAR2 regulates the expression of TNF-α
Fig. 5.
The protein expression of PAR2 in heart (a) and coronary arterioles (b) was significantly higher in db/db mice (n = 9) versus WT mice (n = 10), but the treatment of PAR2 antagonist (n = 9) decreased the protein expression of PAR2 from db/db mice heart and coronary arterioles comparable to WT mice. Also, PAR2 protein expression in heart and coronary arterioles from dbTNF–/dbTNF– mice (n = 7) was significantly lower than from db/db mice. Data were expressed as mean ± SEM. * P < 0.05 versus WT, # P < 0.05 versus db/db
Fig. 6.
TNF-α protein expression was more than twofold higher in db/db mice (n = 9) compared to that in WT mice (n = 10), but the treatment of PAR2 antagonist (n = 9) significantly reduced the protein contents of TNF-α expressed in heart (a) and coronary arterioles (b) from db/db mice. Data were expressed as mean ± SEM. * P < 0.05 versus WT, # P < 0.05 versus db/db
Protein expression of NAD(P)H oxidase subunits in type 2 diabetes
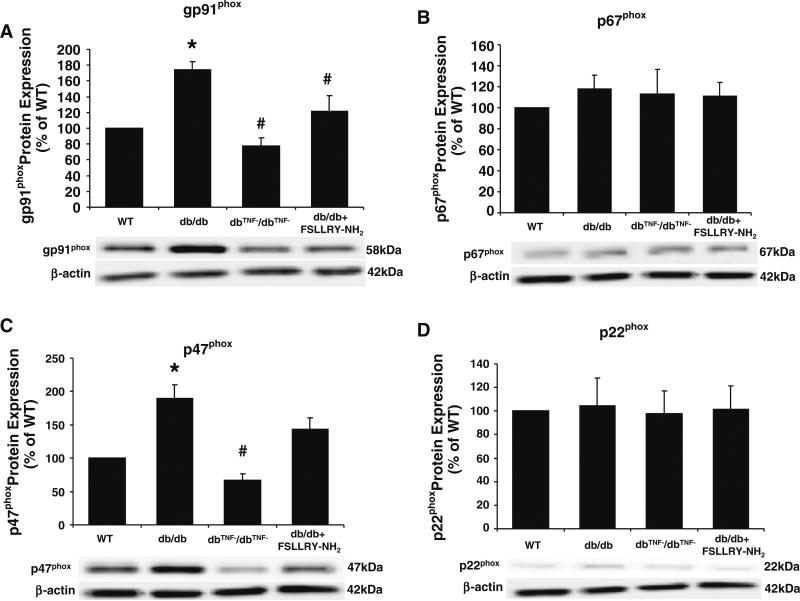
To test whether PAR2 regulates NAD(P)H oxidase in type 2 diabetes, protein expression of NAD(P)H oxidase subunits, gp91phox, p67phox, p47phox, and p22phox (Fig. 7a–d) were determined. Protein expression of gp91phox and p47phox were significantly greater in db/db mice compared to WT mice, although they were attenuated in dbTNF–/dbTNF– mice. PAR2 antagonist reduced gp91phox expression in db/db mice, but did not alter the expression of p47phox. Protein expression of p67phox and p22phox were not different among WT, db/db, dbTNF–/dbTNF and db/db treated with PAR2 antagonist.
Fig. 7.
Protein expression of NAD(P)H oxidase subunits, gp91phox (a) and p47phox (c) were significantly higher in db/db compared to WT mice, but they were lower in dbTNF–/dbTNF– mice comparable to WT mice. Treatment of PAR2 antagonist reduced protein expression of gp91phox without affecting protein expression of p47phox (b). However, protein expression of p67phox (b) and p22phox (d) were at the same level in WT, db/db, dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist. Data were expressed as mean ± SEM. n = 6~7. * P < 0.05 versus WT, # P < 0.05 versus db/db
Discussion
Our results indicate that PAR2 regulates TNF-α expression/production and activates NAD(P)H oxidase subunit p47phox resulting in endothelial dysfunction in coronary arterioles in type 2 diabetic db/db mice. Our major findings are: (1) endothelium-dependent vasodilation was impaired and SNP-induced endothelium-independent vasodilation was normal in type 2 diabetic mice; (2) Impaired endothelial function in db/db mice was fully restored in db/db mice null for TNF-α indicating that TNF-α plays a pivotal role in endothelial dysfunction in type 2 diabetes; (3) PAR2 antagonist improved endothelial function in db/db mice suggesting that PAR2 may also play a pivotal role in coronary arteriolar dysfunction in type 2 diabetic mice; (4) Protein expressions of TNF-α, PAR2, gp91phox and p47phox in the heart and isolated coronary arterioles were higher in db/db mice compared to that of WT mice. Administration of PAR2 antagonist to db/db mice reduced protein expression of TNF-α and p47phox and PAR2, and protein expression of gp91phox and p47phox was lower in dbTNF–/dbTNF– compared to db/db mice. This suggests that PAR2 may regulate TNF-α expression and then activates NAD(P)H oxidase subunit p47phox; (5) PAR2 was localized in vascular endothelial cells and vascular smooth muscle cells, but was not expressed in infiltrated macrophages in diabetic murine heart tissue; and (6) vasodilation induced by PAR2-activating peptide PAR2-AP was enhanced in db/db mice, but PAR2-mediated vasodilation was attenuated in dbTNF–/dbTNF– mice, suggesting TNF-α and PAR2 are closely linked to endothelial dysfunction in type 2 diabetes. These results suggest endothelium-dependent NO and/or PGI2-mediated mechanisms are involved in endothelial dysfunction in type 2 diabetes. Our molecular studies also support the concept that the interaction of PAR2 and TNF-α plays a key role in pathologies leading to endothelial dysfunction in the coronary microcirculation in type 2 diabetes.
Role of PAR2 and TNF-α in endothelial dysfunction in type 2 diabetes
Type 2 diabetes is closely associated with increased levels of inflammatory cytokines such as TNF-α [11, 32, 41], IL-6 [9, 31] and INF-γ [13]. TNF-α in the vasculature plays a critical role in endothelial dysfunction in type 2 diabetes [11, 32, 41]. TNF-α increases superoxide (O2α) production through enhanced activities of NAD(P)H oxidase [11, 21, 32, 41]. Enhanced oxidative stress interacts with TNF-α, NF-κB, AGE/RAGE and ox-LDL/LOX-1 inducing endothelial dysfunction in type 2 diabetes [12, 40, 41]. Our results indicate that endothelial function [agonist-activated (ACh) and agonist-independent flow-mediated] was prevented in db/db mice null for TNF-α (Fig. 1a, b). The oxidative stress occurring in type 2 diabetes leading to the endothelial dysfunction in the coronary microcirculation may be due to the prompt reaction between NO and that reduces NO bioavailability.
PAR2 is known to be up-regulated in various disease conditions such as radiation injury [39], asthma [22], viral lung infection [23], arthritis [28], and in vasculature after balloon angioplasty in rats and in advanced atherosclerotic lesions in human [15]. We determined the direct role of PAR2 in vascular function in coronary arterioles in type 2 diabetes, and our functional data in Figs. 1, 2, 3 show that PAR2 contributes to endothelial dysfunction by regulating TNF-α in type 2 diabetic mice. Our study is consistent with previous studies showing that PAR2 activation regulates the synthesis of inflammatory cytokines and stimulates the signaling pathway of NF-κB and MAPK leading to vascular dysfunction [1, 25, 26, 28, 35]. PAR2 triggers the generation of oxidative stress [2] and the activation of NAD(P)H oxidase, which then augments vascular oxidative stress leading to endothelial dysfunction in diabetes [10, 32]. However, how PAR2 interacts with NAD(P)H oxidase-induced vascular dysfunction has not yet been elucidated. Our present study shows that protein expression of NAD(P)H oxidase subunit gp91phox, but not p67phox, p47phox, or p22phox, is regulated by PAR2 in coronary arterioles in type 2 diabetic mice (Fig. 7). This may be one of the mechanisms whereby PAR2 regulates endothelial dysfunction in coronary arterioles in type 2 diabetes. Our results support the concept that gp91phox is a key protein within the NAD(P)H oxidase subunits that induces endothelial dysfunction in diabetes [45].
Unlike db/db mice null for TNF-α, administration of PAR2 antagonist (4 mg/kg/day) partially restored ACh- and flow-induced vasodilation (approximately 50% of WT normal mice). Higher dosage of PAR2 antagonist, at the concentration of 6 or 8 mg/kg/day, failed to show further improvement of endothelial dilation in db/db mice (data not shown), which implies that PAR2 may partially regulate endothelial dysfunction in coronary arterioles in type 2 diabetes. Other factors may also be involved independent of PAR2-mediated regulation. Protein expression of gp91phox was lower in dbTNF–/dbTNF– and db/db mice treated with PAR2 antagonist, compared to db/db mice. However, protein expression of p47phox was attenuated only in dbTNF–/dbTNF–, but not in db/db mice treated with PAR2 antagonist (Fig. 7). We posit that this may occur because PAR2 antagonist attenuated NO bioavailability to a greater extent in db/db mice versus that in dbTNF–/dbTNF– mice. Thus, limited inhibition of NAD(P)H-mediated oxidative stress by PAR2 antagonism would explain why vascular function was partially restored in db/db mice treated with the PAR2 antagonist.
PAR2-induced vasodilation in type 2 diabetes
PAR2 is involved in vascular function and the activation of this receptor by PAR2-APs such as SLIGRL, SLIGKV, tc-LIGRLO and 2-furoyl-LIGRLO results in endothelium-dependent dilation in the vasculature, both in vivo and in vitro [5, 6, 14, 15, 26, 27, 30, 34, 36]. Although various PAR2-APs have been used to investigate PAR2-mediated vasodilation, 2-furoyl-LIGRLO is the most selective and potent PAR2 activator specifically in calcium signaling and vasodilation in the murine model [27]. Thus, we selected 2-furoyl-LIGRLO as the PAR2 agonist for our study. We found that the vascular dilation in response to PAR2 agonist 2-furoyl-LIGRLO was enhanced in coronary arterioles of type 2 diabetic db/db mice compared to normal control WT mice. We also found that PAR2-induced dilation was reduced in dbTNF–/dbTNF– mice suggesting TNF-α plays a key role in PAR2-induced vasodilation in the diabetic vasculature. Our molecular data supports this concept and shows that PAR2 protein expression in coronary arterioles was attenuated in dbTNF–/dbTNF– mice (Fig. 5). Our results also indicate that PAR2 expression in TNF knockout (TNF KO) mice was similar versus WT and dbTNF–/dbTNF–, and there is no further decrease of PAR2 expression in TNF KO mice compared to WT or dbTNF–/dbTNF– (data not shown) suggesting that TNF-α plays an important role in regulating PAR2 expression in type 2 diabetic db/db mice, but not in the normal control mice. Various studies have reported that TNF-α stimulates PAR2 up-regulation in human isolated coronary arteries and human umbilical vein endothelial cells [7, 14, 29].
PAR2-induced vasodilation in type 2 diabetic mice was abolished by denudation or by NOS inhibitor l-NAME. The vasodilation induced by endothelial dependent vasodilator ACh in dbTNF–/dbTNF– mice is identical to denudation or by the incubation of coronary arterioles with l-NAME (Fig. 3b, c) compared with db/db mice treated with PAR2 antagonist. This suggests that the endothelial NO component was evident in the present study because l-NMMA and endothelial denudation attenuated the PAR2-induced vasodilation. The endothelium-dependent NOS signaling pathway is central to understand endothelial dysfunction in type 2 diabetes, as also shown by the findings in human in vivo and isolated coronary arteries [14] and in mice and rat aortas [36].
These results are paradoxical because, although both mechanisms are endothelium-dependent NO-mediated signaling pathways, ACh-induced vasodilation is impaired in type 2 diabetic coronary arterioles whereas vasodilation by PAR2-AP is enhanced in these vessels. The same paradoxical results have been reported by Roviezzo et al. [36] showing in type 1 diabetic mice aorta that PAR2-induced vasorelaxation was enhanced whereas ACh-induced dilation was impaired. How PAR2 mediates vasodilation in isolated coronary arterioles has not yet been clearly elucidated. Activation of PAR2 appears to have more than one consequence, particularly with regard to the diabetic organism. Our study shows how the PAR2 agonist induces vasodilation and increases NAD(P)H oxidase-associated oxidative stress; however, the same treatment shows that vascular dilatory function remains impaired in the type 2 diabetic organism, which indicates that the functionality of PAR2 may be different in the diseased organism. PAR2 may be affecting other, as yet unknown, processes among the many that occur in the living organism. One possible explanation is that enhanced PAR2 activation in type 2 diabetes may also decrease NO bioavailability. Alternatively, although activation of PAR2 increases NO production, it can also simultaneously stimulate NAD(P)H oxidase activity, which then increases production of . A prompt interaction between NO and would form peroxynitrite that causes a decrease in NO bioavailability and tissue damage. As a consequence, vasodilation would be impaired in type 2 diabetic coronary arterioles despite an enhanced PAR2 agonist-induced dilation. This may also involve a shift in the vasodilation mechanism towards the PAR2-mediated pathway in the pathological condition resulting from a compensatory reaction to the reduced ACh-induced NO-dependent dilation in type 2 diabetes. In living systems, intrinsic defensive mechanisms such as PGI2 and EDHF could be activated to compensate for diminished NO-dependent dilation in abnormal conditions such as in type 2 diabetes. We have previously reported that EDHF may compensate for diminished NO-dependent dilation in type 2 diabetic coronary arterioles [27]. This study found that dependence of EDHF-mediated dilation is increased in type 2 diabetic vasculature, in which NO-mediated dilation is substantially decreased. Like EDHF, enhanced PAR2-mediated dilation could compensate for impaired vasodilation in type 2 diabetes. Moreover, Roviezzo et al. [31] reported a gradual switch in the vascular relaxant mechanisms towards the PAR2 signaling pathway occurred with an increased contribution from the COX pathway in type 1 diabetic mice vasculature. We found that PGI2-mediated dilation was increased in diabetes (Fig. 3d), but lacked the means to measure COX2. However, Roviezzo et al. [31] did measure the COX2 level (mRNA and protein) and COX2 dependent vasodilation, and both were increased in diabetic vessels. These complementary findings imply that PAR2-induced endothelium-dependent relaxation may shift towards the COX2-dependent PGI2 signaling pathway in diabetes. Roviezzo et al. [31] suggest that enhanced PAR2 activation in diabetes potentiates the production of PGI2, which is one of the endothelium-dependent factors (EDRF), in endothelial cells via up-regulated COX2 expression. Contribution of PGI2-mediated vasodilation in endothelium-dependent PAR2-mediated dilation was increased in diabetes, which implies that PAR2-induced endothelium-dependent relaxation may shift towards the COX2-dependent PGI2 signaling pathway in diabetes. Our data show that the portion of PGI2-dependent vasodilation induced by the PAR2 agonist was significantly enhanced in coronary arterioles of db/db mice compared to WT mice. The difference in PAR2-induced dilation between db/db and WT mice was abolished after incubation with COX inhibitor Indo. This indicates the PGI2-mediated signaling pathway may play a dominant role in PAR2-induced dilation in type 2 diabetes (Fig. 3d). These results are supported by other works, which show that PGI2 in PAR2-mediated dilation exceeds that of ACh-mediated dilation for human forearm blood flow in vivo [34], and in isolated vascular beds of animals [6, 38]. Our present study shows that PAR2 can up-regulate TNF-α, which suggest that enhanced PAR2 activation may differentially stimulate COX2 mechanisms in the type 2 diabetic vasculature.
Whether PAR2-induced dilation is endothelium-dependent has not been elucidated. The physiological agonists of PAR2 tryptase and trypsin appear to play a minor role in vasodilatory function in normal conditions. Our study shows that higher PAR2 is expressed in diabetic coronary arterioles and that it causes higher vasodilatory response to PAR2 agonist (APR2-AP) than that in normal control mice. Moreover, administration of PAR2 antagonist restores vascular function (ACh-mediated) in db/db mice possibly through a systemic inhibition of the detrimental effect of pro-inflammatory cytokine TNF-α, and NAD(P)H oxidase on the vascular function and this may mask any diminished PAR2-mediated vasodilation.
Our results show that ACh- and flow-mediated vasodilation was impaired and PAR2-AP-induced vasodilation was enhanced in coronary arterioles in type 2 diabetes. This is consistent with previous reports [19, 27, 37], and explicable if the functionality of PAR2 is altered in the diseased organism compared to the healthy state. Our study suggests that additional NO release through enhanced PAR2 activation in diabetic vasculature may cause tissue damage due to peroxynitrite production via a prompt interaction between NO and [27]. In contrast, higher concentration/production of PAR2-mediated NO production in diabetic vessels may be beneficial to the living system through a preserved perfusion to the organs [19, 37], and this may play a compensatory role in diabetic vessels in which endothelial function is compromised.
Interaction between PAR2 and TNF-α in type 2 diabetes
PAR2 is present in vascular smooth muscle, nonvascular origin and stromal cells from a variety of tissues, as well as in endothelial and epithelial cells independent of tissue type [8], but PAR2 was not detected in cardiomyocytes in healthy human subjects [8]. More recent studies, however, report PAR2 localization in rat cardiomyocytes, endothelial cells and vascular smooth muscle cells [18]. In mice heart, PAR2 co-expressed with transient receptor potential vanilloid type 1 (TRPV1) channels on the cardiomyocytes, perivascular nerves and blood vessels [46]. Our study examined if PAR2 is co-localized in endothelial cells or vascular smooth muscle cells on coronary microvessels in type 2 diabetic mice by using specific cell markers. Double-immunofluorescence staining results clearly demonstrate that PAR2 was co-localized with both endothelial cells and vascular smooth muscle cells in coronary vessels of control and diabetic mice heart (Fig. 4), whereas PAR2 was not co-expressed with macrophages infiltrated into cardiacmyocytes.
Our immunohistochemical analyses confirm the previous work [8] that PAR2 is localized in endothelial cells (Fig. 4), and our western blotting data also show the enhanced expression of PAR2 in db/db mice heart and coronary arterioles (Fig. 5). Our data suggest that enhanced activity and expression of PAR2 is the key to potentiate the role of TNF-α in endothelial dysfunction in type 2 diabetes. We previously reported that TNF-α is an important mediator in type 2 diabetes-induced endothelial dysfunction [11, 12, 32, 40], and our present study shows that PAR2 directly regulates TNF-α expression in coronary microcirculation in type 2 diabetes (Fig. 6). Kim et al. reported that PAR2 agonist induces TNF-α secretion though the activation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinases (MAP kinase) in atrocytoma cells [20, 21], which support our study. Figure 4b shows that increasing TNF-α increases PAR2 protein, which is consistent with previous studies showing that TNF-α also controls PAR2 expression in various tissues [7, 14, 29]. Our present study shows that the protein expression of PAR2 was attenuated in both coronary arterioles and heart tissues in dbTNF–/dbTNF– mice versus db/db mice (Fig. 5). Taken together, our findings suggest that PAR2 and TNF-α appear to be closely linked and regulate each other to contribute to endothelial dysfunction in type 2 diabetes.
In conclusion, PAR2 plays a pivotal role in endothelial dysfunction by up-regulating TNF-α expression/production in coronary arterioles in type 2 diabetic mice. PAR2 agonist-induced vasodilation is endothelium-dependent, NO- and PGI2-mediated, and higher expression of PAR2 in vascular endothelial cells may result in greater PAR2 agonist-induced vasodilation in type 2 diabetes. PAR2 induces TNF-α expression and TNF-α can subsequently induce PAR2 expression. Thus, the interaction of PAR2 and TNF-α eventually induces endothelial dysfunction in coronary arterioles in type 2 diabetes.
Acknowledgments
This study was supported by grants from American Heart Association Scientist Development Grant (110350047A), Pfizer Atorvastatin Research Award (2004-37) and NIH grants (RO1-HL077566 and RO1-HL085119) to Dr. Cuihua Zhang.
Footnotes
Conflicts of Interest None.
References
- 1.Atsufumi K, Ryotaro K, Takeshi M, Kazuo K, Mamoru T. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfi C, Brioschi M, Barbieri SS, Eligini S, Barcella S, Tremoli E, Colli S, Mussoni L. Mitochondrial reactive oxygen species: a common pathway for PAR1- and PAR2-mediated tissue factor induction in human endothelial cells. J Thromb Haemost. 2009;7:206–216. doi: 10.1111/j.1538-7836.2008.03204.x. [DOI] [PubMed] [Google Scholar]
- 3.Bramos D, Ikonomidis I, Tsirikos N, Kottis G, Kostopoulou V, Pamboucas C, Papadopoulou E, Venetsanou K, Giatrakos N, Yang GZ, Nihoyannopoulos P, Toumanidis S. The association of coronary flow changes and inflammatory indices to ischaemia–reperfusion microvascular damage and left ventricular remodeling. Basic Res Cardiol. 2008;103:345–355. doi: 10.1007/s00395-008-0720-5. [DOI] [PubMed] [Google Scholar]
- 4.Chappell D, Hofmann-Kiefer K, Jacob K, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-α induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 5.Cicala C. Protease activated receptor 2 and the cardiovascular system. Br J Pharmacol. 2002;135:14–20. doi: 10.1038/sj.bjp.0704438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, Cruchley A, Kapas S, Howells GL, Cirino G. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- 7.Cocks TM, Moffatt JD. Protease-activated receptors: sentries for inflammation? Trends Pharmacol Sci. 2000;21:103–108. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 9.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre J-S. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in Type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2008;295:H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32:24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton JR, Frauman AG, Cocks TM. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res. 2001;89:92–98. doi: 10.1161/hh1301.092661. [DOI] [PubMed] [Google Scholar]
- 15.Hirano K. The roles of proteinase-activated receptors in the vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:27–36. doi: 10.1161/01.ATV.0000251995.73307.2d. [DOI] [PubMed] [Google Scholar]
- 16.Hwa JJ, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- 17.Ihm SH, Chang K, Kim HY, Baek SH, Youn HJ. Peroxi-some proliferator-activated receptor-γ activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol. 2010;105:399–407. doi: 10.1007/s00395-009-0071-x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang R, Zatta A, Kin H, Wang N, Reeves JG, Mykytenko J, Deneve J, Zhao ZQ, Guyton RA, Vinten-Johansen J. PAR-2 activation at the time of reperfusion salvages myocardium via an ERK1/2 pathway in in vivo rat hearts. Am J Physiol Heart Circ Physiol. 2007;293:H2845–H2852. doi: 10.1152/ajpheart.00209.2007. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata A, Nakaya Y, Kuroda R, Wakisaka M, Masuko T, Nishikawa H, Kawai K. Involvement of EDHF in the hypotension and increased gastric mucosal blood flow caused by PAR-2 activation in rats. Br J Pharmacol. 2003;140:247–254. doi: 10.1038/sj.bjp.0705433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M-S, Jo H, Um J-Y, Yi J-M, Kim D-K, Choi S-C, Kim T-H, Nah Y-H, Kim H-M, Lee Y-M. Agonists of proteinase-activated receptor 2 induce TNF-alpha secretion from astrocytoma cells. Cell Biochem Funct. 2002;20:339–345. doi: 10.1002/cbf.982. [DOI] [PubMed] [Google Scholar]
- 21.Kleinbongard P, Heusch G, Schulz R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 23.Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza a virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L388–L398. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- 24.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Zhong S, Zeng K, Luo Y, Fea Zhang. Blockade of NF-κB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous micro-thrombi in rats. Basic Res Cardiol. 2010;105:139–150. doi: 10.1007/s00395-009-0067-6. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 27.McGuire JJ. Proteinase-activated receptor 2 (PAR2): a challenging new target for treatment of vascular diseases. Curr Pharm Des. 2004;10:2769–2778. doi: 10.2174/1381612043383656. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh KA, Plevin R, Ferrell WR, Lockhart JC. The therapeutic potential of proteinase-activated receptors in arthritis. Curr Opin Pharmacol. 2007;7:334–338. doi: 10.1016/j.coph.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 30.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 31.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295:H1982–H1988. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie E, Saka M, MacKenzie C, Drummond R, Wheeler-Jones C, Kanke T, Plevin R. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase β in human endothelial cells. Br J Pharmacol. 2007;150:1044–1054. doi: 10.1038/sj.bjp.0707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robin J, Kharbanda R, McLean P, Campbell R, Vallance P. Protease-activated receptor 2-mediated vasodilatation in humans in vivo: role of nitric oxide and prostanoids. Circulation. 2003;107:954–959. doi: 10.1161/01.cir.0000050620.37260.75. [DOI] [PubMed] [Google Scholar]
- 35.Roman-Campos D, Duarte HLL, Sales PA, Natali AJ, Cea Ropert. Changes in cellular contractility and cytokines profile during Trypanosoma cruzi infection in mice. Basic Res Cardiol. 2009;104:238–246. doi: 10.1007/s00395-009-0776-x. [DOI] [PubMed] [Google Scholar]
- 36.Roviezzo F, Bucci M, Brancaleone V, Di Lorenzo A, Geppetti P, Farneti S, Parente L, Lungarella G, Fiorucci S, Cirino G. Proteinase-activated receptor-2 mediates arterial vasodilation in diabetes. Arterioscler Thromb Vasc Biol. 2005;25:2349–2354. doi: 10.1161/01.ATV.0000184770.01494.2e. [DOI] [PubMed] [Google Scholar]
- 37.Sobey CG, Moffatt JD, Cocks TM, Kontos HA. Evidence for selective effects of chronic hypertension on cerebral artery vasodilatation to protease-activated receptor-2 activation. Stroke. 1999;30:1933–1941. doi: 10.1161/01.str.30.9.1933. [DOI] [PubMed] [Google Scholar]
- 38.Trottier G, Hollenberg M, Wang X, Gui Y, Loutzenhiser K, Loutzenhiser R. PAR-2 elicits afferent arteriolar vasodilation by NO-dependent and NO-independent actions. Am J Physiol Renal Physiol. 2002;282:F891–F897. doi: 10.1152/ajprenal.00233.2001. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Zheng H, Hollenberg MD, Wijesuriya SJ, Ou X, Hauer-Jensen M. Up-regulation and activation of proteinase-activated receptor 2 in early and delayed radiation injury in the rat intestine: influence of biological activators of proteinase-activated receptor 2. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1850–H1858. doi: 10.1152/ajpheart.01199.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Wu C, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNF-α expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong B, Wang DH. Protease-activated receptor 2-mediated protection of myocardial ischemia-reperfusion injury: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1681–R1690. doi: 10.1152/ajpregu.90746.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]