Abstract
Functional MRI (fMRI) is a non-invasive brain imaging methodology that started in 1991 and allows human brain activation to be imaged at high resolution within only a few minutes. Because it has extremely high sensitivity, is relatively easy to implement, and can be performed on most standard clinical MRI scanners. It continues to grow at an explosive rate throughout the world. Over the years, at any given time, fMRI has been defined by only a handful of major topics that have been the focus of researchers using and developing the methodology. In this review, I attempt to take a snapshot of the field of fMRI as it is in mid-2009 by discussing the seven topics that I feel are most on the minds of fMRI researchers. The topics are, in no particular order or grouping: (1) Clinical impact, (2) Utilization of individual functional maps, (3) fMRI signal interpretation, (4) Pattern effect mapping and decoding, (5) Endogenous oscillations, (6) MRI technology, and (7) Alternative functional contrast mechanisms. Most of these topics are highly interdependent, each advancing as the others advance. While most fMRI involves applications towards clinical or neuroscience questions, all applications are fundamentally dependent on advances in basic methodology as well as advances in our understanding of the relationship between neuronal activity and fMRI signal changes. This review neglects almost completely an in-depth discussion of applications. Rather the discussions are on the methods and interpretation.
Keywords: fMRI, functional MRI, BOLD contrast, brain imaging, hemoglobin, cerebral blood flow, cognition, brain mapping, Magnetic Resonance Imaging, human brain, clinical applications
1. Introduction
In 1991, when functional MRI first emerged, the most pressing topics of discussion among researchers were about whether or not the observed signal changes were robustly and repeatedly localized to the “true” regions of activation, and whether or not “draining vein effects” occurring downstream from the true site of activation were a major confound in the interpretation of fMRI-derived maps [46, 140]. Other topics were related to what underlying neuronal activity these signal changes really represented [86]. Additional topics revolved around various dynamics of the signal change such as the “pre-undershoot” and the “post-undershoot” [16, 64, 65]. Topics focused on the contrast mechanisms themselves — about which MRI parameters (TR, TE, flip angle, field strength, and resolution) were most influential on the signal and why — were addressed by the physicists [12, 40, 48, 49, 98, 112]. These topics remain, to this day — seventeen years and about two months (as of this writing) after the first fMRI publications in June of 1992 [7, 73, 112], and after about 20,000 papers — incompletely answered. In fact, discussions and work on these topics have raised more questions than ever — more sophisticated, more insightful, more broad-ranging, and perhaps more practical.
The advancement of fMRI has been by all measures, explosive yet varied. Clinical applications — those in acute situations in the clinic — have advanced at a painstakingly slow rate, but advancements in human brain mapping and neuroscience have advanced rapidly and dramatically. The debate about the precise physiology behind of the signal may be slowing, since to most, this does not matter as the signal has been demonstrated to be representative enough and robust enough for healthy normal volunteers. Processing methods continue to pick up on highly sophisticated techniques developed years ago in other contexts and therefore continue to amaze, challenge, and advance the field. Currently, fMRI has made inroads into popular media and scientific journals with reports of “mind reading” [23, 54] and “lie detection” [27]. Companies performing “neuromarketing” have emerged. These companies ask what the brain itself thinks of new products rather than what individuals report in questionnaires.
Taken as a whole, the field of fMRI is very healthy, with well over 2000 papers being produced a year and a strong representation of researchers at scientific meetings throughout the world, including The Organization for Human Brain Mapping (OHBM), The Cognitive Neuroscience Society (CNS), The Society for Neuroscience (SFN), and the International Society for Magnetic Resonance in Medicine (ISMRM). In other manners, the field is lacking. The largest medical imaging meeting in the world, with over 60,000 attendees, the Radiological Society of North America (RSNA), has only a handful of fMRI presentations. That number is growing but at an anemic rate at this clinical meeting. Why is that so? Perhaps fMRI has not passed a critical threshold of sensitivity, repeatability, ease of use, robustness, and utility that is required in a demanding and unforgiving clinical setting. This is a topic of discussion below.
The task of any fMRI researcher is to propose research agendas that balance “achievability” with impact or importance. Towards achieving a high impact, the researcher, analogous to a miner, typically works along a promising vein in the rock. Currently, there are several promising “veins” of research in fMRI. The seven topics I discuss I believe are among these. Topics include areas of technology, interpretation, practical applications, fashionable trends, and the ultimate utility of fMRI. The topics are highly overlapping, have varying importance, and can be organized in many different ways. The way that I felt they most naturally grouped are: (1) Clinical impact, (2) Utilization of individual functional maps, (3) fMRI signal interpretation, (4) Pattern effect mapping and decoding, (5) Endogenous oscillations, (6) MRI technology, and (7) Alternative functional contrast mechanisms. In general, these discussions are not the last word on the topic but rather an overview of some of the more interesting recent and, sometimes less recent, work.
2. Clinical Impact
Since the inception of fMRI, the hope for a substantial clinical impact has remained mostly unfulfilled. “Clinical impact” is a broad term that ranges from use with patients who would immediately benefit from an fMRI procedure either for preor post-symptomatic diagnosis, presurgical mapping, or therapy monitoring, to the assessment of subtle differences in large clinical populations to try to identify a neuronal correlate of a disorder, or, secondarily, to begin to understand a mechanism behind the disorder [95]. In the USA, Current Procedural Terminology (CPT) codes for fMRI have been in place since January 2007. Hospitals are now able to be reimbursed for the use of fMRI in the context of presurgical mapping. While this development is promising, it only touches on the ultimate clinical impact of fMRI.
The bulk of the current clinical impact of fMRI involves the study of clinical populations: characterizing activation patterns associated with a specific task or characterizing resting state data and functionally connected regions. While the clinical impact of these studies is much more long term — not likely being used on patients needing acute care for five to 25 years — the importance of this research is clear. Revelations about how the brain is activated and internally connected in clinical populations provide useful information about regions that may be critical in specific disorders as well as altered mechanisms behind disorders. These studies promise to guide the development of specific therapies and targeted drug or surgical interventions in the future. Without this research, future work would be nearly impossible.
Regarding immediate clinical impact of fMRI, inroads have been made. As mentioned above, fMRI-based presurgical mapping is growing in popularity due to its non-invasiveness, ability to assess a wider area of the brain than electrode grids, and, importantly, a growing sophistication of techniques that allow fMRI maps to be used in the surgical suite [104, 125, 135, 145]. Methods for the surgeon to see the areas of activation registered to specific landmarks on the brain are non-trivial and highly critical to utility.
Uses of fMRI on individual patients have been growing in number. Functional MRI has been applied to “locked-in” patients as well as those in presumably vegetative states to allow communication and assurance that the patient is, in fact, conscious [114]. Along these lines, methods for allowing patients to see their own fMRI signal changes in real time have begun to allow for primitive fMRI-based brain-computer interfaces [132]. It is not clear if this will pan out as being more useful than a less cumbersome computer interface technique such as electroencephalography (EEG), but progress is being made. Functional MRI has also been used to assess which regions of the brain are becoming more — or less — activated after injury as the subject begins to recover from brain injury [24, 25]. This is important not only for assessing therapy benefits but perhaps for guiding therapeutic strategies, and more generally, understanding mechanisms of recovery and predicting final outcome. Lastly, what I think is one of the most creative uses of fMRI in clinical use is that of real time fMRI signal (i.e., the signal is obtained as the patient is being scanned) that is fed back to the subject in a form of “biofeedback” [78, 74, 147]. Specifically, this approach has been used therapeutically on patients with chronic pain [32, 33]. The patient, while lying in the scanner, is asked to “find a way” to reduce the signal in the anterior cingulate (known to be associated with pain perception). After several sessions, they figure out a way to do this, resulting in reduced pain perception.
Why is fMRI not yet a common clinical technique? Clinical utility is highly influenced by ease of use, expense, accuracy, importance and uniqueness of information provided. For example, anatomical MRI is on the other extreme of clinical utility, used almost always with regard to tumor assessment. Why is this so? The images are extremely accurate and critically useful and the technique can be performed with almost no error and minimal post-processing by the physician or technologist — all in about 30 minutes. Functional MRI has not passed a threshold on any of these variables. It will become easier to perform, and the information will prove to be unique and useful, but it is not clear when this critical threshold will be passed. The field is still waiting for that critical clinical application to thrust it into regular clinical use.
3. Utilization of Individual Functional Maps
Since the start of fMRI usage it has been clear that relatively high quality brain activation maps from individuals during single time series runs or single one hour sessions could be created. To increase sensitivity, group-averaged maps were used since the averaging of multiple subjects increases signal to noise ratio in approximate proportion to the square root of the number of samples. This multisubject averaging trend caught on quickly as methods for spatially normalizing and averaging group data had already been in place with previous studies using Positron Emission Tomography (PET).
While this method allows inferences about populations to be made, much of richness of individual subject data is lost. Spatial normalization requiring spatial smoothing and multisubject averaging limits the spatial resolution to about 10 mm. Nevertheless, as fMRI imaging resolution and sensitivity improves and the need for more clinical utility increases, the demand for the use of fMRI to assess individuals on a regular basis grows.
As mentioned, the approach involving averaging across subjects is a result of processing methods developed for use with PET. Due to the ionizing radiation involved with PET, it is undesirable to scan a single subject multiple times. PET also has intrinsically lower resolution and lower sensitivity than fMRI. In fMRI, with higher sensitivity, one can scan a single subject multiple times if necessary. Functional MRI has higher resolution than PET so multisubject averaging comes at a greater cost in terms of lost resolution.
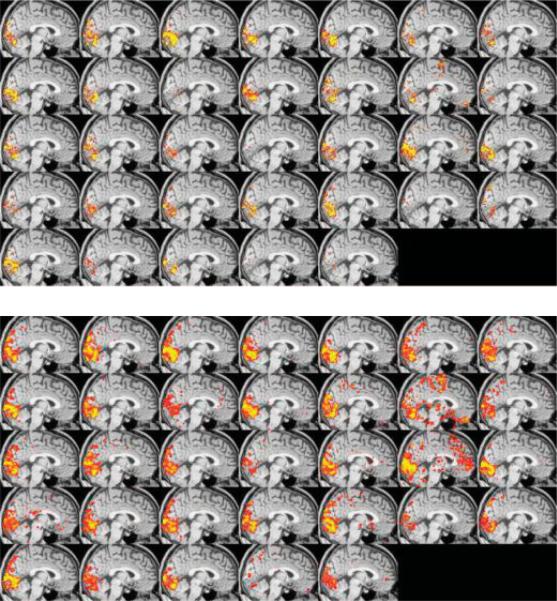
Problems and opportunities are associated with the assessment of individual fMRI data. A primary problem is variability in the data. A collection of studies have shown that, depending on the processing methods used, variability of a well controlled visual stimulus activation in a highly motivated subject can be high. Figure 1, shows a study by Smith et al. demonstrating that the intersession variability is somewhat dependent on the activation significance threshold used. On first glance this appears to be an illustration of a problem, while in fact, it is clear that the general area of activation is constant and the variability does not appear to be random. The interesting aspect of this figure is that there is not yet a fully understood reason for this variability.
Fig. 1.
Used with permission from Smith et al. showing that the apparent variability in the fMRI data is a function of the threshold level. At a high threshold, the variability appears substantial as the center of mass of the visual activation moves considerably from session to session. With a lower threshold, it is clear that the general area of activation remains relatively constant.
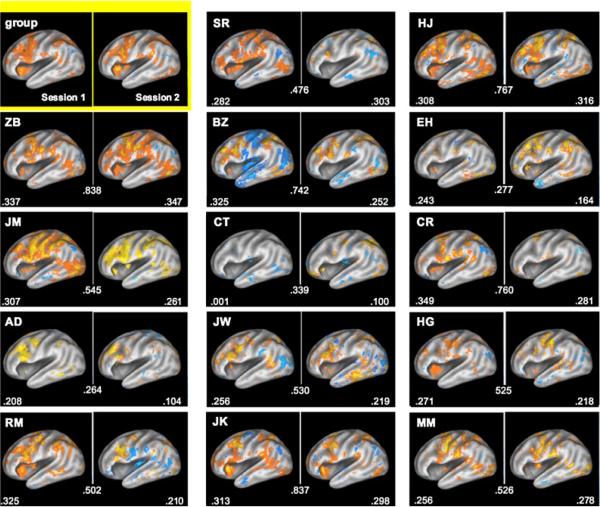
Different from intersession variability, interindividual variability is even more extreme — and more interesting. A study by Miller et al. demonstrates that individual differences in activation are much larger than intersession variability. Figure 2 [99], shows that with a working memory task, the group average map shows activation which is only approximately similar to any individual's map, and that each individual's map, while showing considerable difference from many of the other individual maps, does not show nearly as much difference in activation pattern across the two sessions. Again, while this variability some might say is a source of noise in creating the group average map, and best averaged out, I would emphasize that this variability is not random and perhaps represents individual differences in brain organization or cognitive strategy — all certainly worth further study.
Fig. 2.
Used with permission from Miller et al. [99]. This shows left hemisphere activations and deactivations for a group of 14 individuals during a working memory task (contrast is retrieval vs. baseline). On the upper left is the group-averaged map across both sessions. Displayed for each individual is the spatial correlation between the two sessions (number in the middle), and the average spatial correlation between each subject's activation map and every other subject's activation map. This clearly shows that the temporal variability within each subject is much lower than across subject.
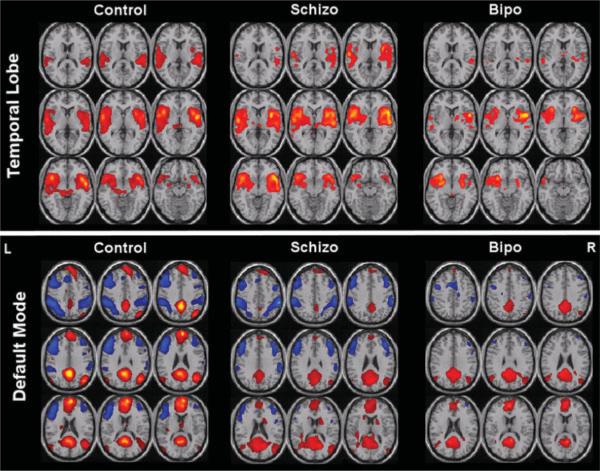
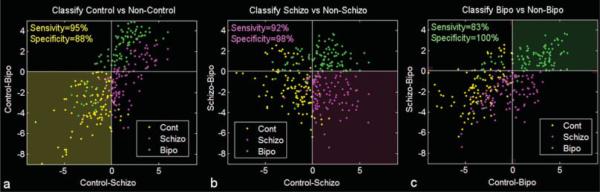
On the issue of comparing individuals, it is clear that many factors — pharmacologic, physiologic, cognitive, genetic, and those related to the general health of the subject — influence these activation maps in ways that are not easily predicted yet. In the presence of this clear variability an open question remains as to whether or not an individual subject's brain activation or resting state correlation data can be used to demonstrate their inclusion in or exclusion from populations showing a specific activation pattern. In other words, while differences in individual activation maps can be demonstrated, can they be used to classify subjects as being excluded from or belonging to a specific group? Studies have shown that this is certainly possible. For instance, Duncan et al. [39] have shown that individual differences in cortical magnification factor are correlated with acuity thresholds. In a work that focuses on resting state networks, Calhoun et al. [18] demonstrate clear differences between temporal lobe and default mode resting state networks of control subjects, individuals with Schizophrenia, and those with bipolar disorder (shown in Fig. 3). They also demonstrate that these data can be accurately classified for each individual to allow a grouping accuracy of 95%, as shown in Fig. 4. These are extremely encouraging results showing that fMRI data and resting state fluctuation data can be potentially used to complement diagnoses.
Fig. 3.
Used with permission from Calhoun et al. [18]. Two useful resting state networks: temporal lobe and default mode. These are group averages extracted from fMRI data from controls, schizophrenia patients, and bipolar patients, showing clear differences.
Fig. 4.
Used with permission from Calhoun et al. [18]. Classification results: Decision regions for (a) Control (dark yellow) vs. non-control (black), (b) schizophrenia (dark pink) vs. non-schizophrenia (black), and (c) bipolar (dark green) vs. non-bipolar (black). The diagnosis of the individual is indicated by the color of the dot where controls are yellow, schizophrenia patients are pink, and bipolar patients are green. Sensitivity and specificity values were 90% and 95% respectively.
Overall, these types of studies and positive results are increasing as sources of variability and instability are being either characterized or suppressed. Importantly, work that can characterize and classify activation maps of individuals relies on the continual development of sophisticated classification methods that use as much information about the true activation and artifact as possible.
4. Signal Interpretation
What is the relationship between neuronal activity and BOLD contrast? Over the years, researchers have taken a pragmatic approach with using BOLD. It is not exactly clear what aspect of neuronal activity it is representing, but from early work looking at the spatial relationship between known functional brain regions and BOLD contrast changes with activation, as well as the parametric relationship between BOLD contrast and the degree of neuronal activity in visual, motor, and auditory paradigms, the relationship is sufficiently clear to make inferences about brain organization with a very wide range of paradigms and spatial resolutions. Even though BOLD contrast is extremely robust and consistent, many assumptions have to be made if more in-depth interpretation of the signal is desired. The field has benefitted from efforts to better understand not only the neuronal correlates of fMRI but the sources of fMRI signal variability. These efforts have continued as indicated by a large number of new fMRI contrast mechanism papers as well as some excellent review articles.
Recently published review articles by Logothetis [84, 85] do an excellent job in laying out some limits and misuses of fMRI while also discussing its potential. At the heart of a long-recognized problem with some fMRI studies is the issue of data interpretation. Data interpretation can be confounded on several levels. First, the fMRI signal is based on the complex interaction of neuronal activity, neuronal metabolism, blood flow and blood volume on a spatial scale that lumps together hundreds of thousands of neurons in each MRI voxel.
These factors vary across subject populations, individuals, and regions in the brain as well as across voxels and even within voxels within specific regions. This hemodynamic variability poses a severe limit on how fMRI can be used. On an individual level, this limit prevents an investigator from suggesting that one part of the brain shows “more” activity simply because the fMRI signal is greater. Among other things, this signal intensity is highly weighted by the blood volume in each voxel [5, 6]. Logothetis goes further in his review to suggest that the limits in our knowledge of precisely what neuronal activity (excitation, inhibition, sub-threshold activity, top-down or bottom-up modulation) is manifested by the hemodynamic response that limits the depth to which we can draw inferences even from parametrically modulated signal within a region. While most researchers have known these caveats all along, this particular one is a potentially significant confound in the interpretation of the many parametric studies that have been performed over the years.
Due to the variability of the timing of hemodynamics, we have also understood well the limits (on the order of seconds) on the inferences of the relative timing of brain activity between regions. Without a clear understanding of the non-neuronal sources of this spread in latency, inferences about causality based on relative timing (below 2 second-differences) are inherently weak and likely to be so weighted by the underlying vasculature-influenced timing that they are incorrect. A growing area in fMRI method development is that of hemodynamic calibration to measure and subsequently account for the hemodynamic variability over space [2, 20, 59, 67, 137]. Nevertheless, the problem of parsing excitatory and inhibitory brain activity, for example, remains unable to be “calibrated” with fMRI.
Another perhaps more common problematic level of interpretation in fMRI is not that of inferring the level, degree, or timing of BOLD signal change but that of drawing unsubstantiated inferences about mental states or conditions from assessment of brain activation maps. Functional MRI is not immune to the problem of researchers creating the “just so” story that can explain any complicated pattern of activation shown in the data. The best fMRI studies are those with testable hypotheses, tightly controlled paradigms, appropriate calibrations, and careful inferences. It is typically correct to say “this area is associated with processing visual stimuli”. It is much more risky (but not necessarily impossible) to say “Because there is more activation in this area, this subject prefers this political candidate more”.
Regarding fMRI interpretation, an encouraging amount of fascinating progress has been made recently. An interesting study by Schummers et al. [124] describes in detail the spatial and temporal behavior of astrocyte activation relative to neuronal activation, and convincingly demonstrates that it is astrocytes that signal blood flow to increase with activation. Interestingly, this work shows that the spatial tuning of visual cortex astrocytes to orientation column stimulation is sharper than that of neurons. In addition, the activation timing of astrocytes is delayed about 4 seconds from that of neurons. To show astrocyte influence on hemodynamics, astrocyte activation was selectively modulated using isoflurane, which has less of an effect on neurons. When astrocyte activity was suppressed, the measured hemodynamic response to stimulation was also shown to be suppressed. These findings certainly shed light on the mechanism of fMRI contrast mechanisms and will cause us to refine our models of neurovascular coupling.
As is well known, considerable controversy continues as to the physiologic origin of several specific aspects of BOLD contrast, including BOLD nonlinearity, preundershoot, post-undershoot, and fluctuations. Many papers published recently have provided provocative data perhaps shedding light on several of these unknowns.
One example is that of the post-stimulus undershoot. As is well understood, BOLD signal increases with an increase in blood oxygenation or decrease in blood volume. Three popular hypotheses for the post-undershoot exist: The first is that there is a perseveration of increased blood volume, after blood flow and oxygenation have returned to baseline. The second is that there is a perseveration of oxidative metabolic rate after blood flow and volume have returned to baseline, thus reducing blood oxygenation. The third is that there is a post-stimulus decrease in flow, causing blood oxygenation to decrease since the time for oxygen to be delivered to active tissue increases, thus enhancing delivery. The work by Devor et al. [35] may suggest the latter explanation. Other groups have experimental evidence for a blood volume perseveration [131], while yet another set of researchers have experimental evidence for oxidative metabolic rate perseveration [89] or just against blood volume perseveration [45]. There exist different assumptions, experimental conditions, and spatial scales with all of these studies. As with most fMRI contrast mechanism work, the explanation for an effect, when discovered, is always more complicated than we expect it to be. To most people outside of the subspecialty of fMRI contrast mechanism studies, this seems like an esoteric discussion since the post-stimulus undershoot is not typically used as a source of contrast. Understanding it fully is nevertheless extremely important as this understanding may lend insight into other aspects of BOLD contrast and potentially lead to an enhanced capability of using BOLD contrast to understand neuronal activity.
Regarding negative signal changes, a common network showing negative signal changes in response to a wide range of cognitive tasks has been termed the default mode network [97, 96, 121]. An ongoing debate continues with regard to precisely what these negative signal changes imply, and what the functional role of this network is. As shown above in the “individual subject assessment” section, Calhoun et al. [18] have clearly shown that the default mode network, among others, has a clinical significance.
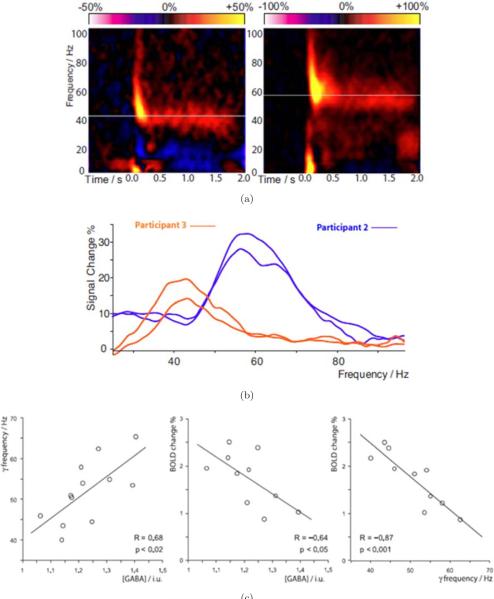
Some recent work involving the relationship between GABA (an inhibitory neurotransmitter) concentrations, MEG contrast, and BOLD contrast has shed some light on mechanisms underlying BOLD. In work by Muthukumaraswamy et al. [107] GABA concentrations, Magnetoencephalography (MEG) signal, and BOLD signal were measured in visual cortex. The results of this study show that across individuals, gamma oscillation frequency is positively correlated with resting GABA concentration in visual cortex, BOLD magnitude is inversely correlated with resting GABA, and gamma oscillation frequency is strongly inversely correlated with the magnitude of the BOLD response. These results, shown in Fig. 5, clearly demonstrate that neuroimaging measures are highly dependent on the excitation/inhibition balance in an individual's cortex and have implications for the interpretation of functional imaging results, particularly when making between-group comparisons in clinical research.
Fig. 5.
Used with permission from Muthukumaraswamy et al. [107]. (a) Time-frequency plots for MEG activity at the maximal response position. Orange/yellow represents power increases and blue/purple indicates power decreases from baseline. In each plot, the white line indicates the peak sustained gamma frequency that showed the greatest change in response to the stimulus. (b) The difference power spectrum, averaged over the period from 0.5 to 2.0 s after stimulus onset. Intersubject differences are visible in both the frequency and amplitude of the gamma response. Note the stability of peak frequency across sessions, which were separated by at least 2 weeks for both participants. (c) Graphs with best-fit linear regression lines, showing for each participant (Left) peak gamma oscillation frequency versus GABA concentration (Center) BOLD response magnitude versus GABA concentration and (Right) BOLD response magnitude versus peak gamma oscillation frequency.
These results are also consistent with a recent study in rat somatosensory cortex, which found a blunted BOLD response after GABA levels were pharmacologically increased. In humans, a study combining GABA MRS and fMRI [109] demonstrated that the negative BOLD response in the anterior cingulate region of the default mode network increased with increasing GABA across participants. All these recent data suggest that individuals with relatively high GABA concentrations in an area will exhibit relatively small positive BOLD responses and relatively large negative BOLD responses. Other studies provide results which are less explainable but are interesting in that they suggest that BOLD contrast is not necessarily measuring the same aspects of neuronal activity as MEG contrast [108]. Muthukumaraswamy and Singh compared fMRI and MEG Gamma frequency (40 to 60 Hz) responses to a visual stimulus which was varied in spatial frequency and temporal frequency. While the spatial overlap between brain activation measured with the two modalities was substantial, the parametric dependence of the signals dependence curves were generated for both spatial frequencies. With MEG, high spatial frequency showed much higher power across temporal frequency than low spatial frequency.
5. Pattern Effect Mapping and Decoding
The powerful approach of analyzing fMRI that involves extraction of subtle voxel-wise activation patterns rather than mapping blobs of activation has, in the past few years, seen a tremendous surge of interest due the dramatic results that it has produced [19, 53, 66, 23, 74, 27, 42, 47, 55–57, 103, 71, 68, 69, 72, 100, 117, 118, 110, 111, 126, 127, 134]. Essentially, these approaches are able to pull information from fMRI data that does not conform to a clear mapping of blobs of activity. This new class of techniques is generally known as multivariate pattern recognition, classification, or decoding (i.e., aiming to identify a perceptual representation, activation, or cognitive state on the basis of multivoxel regional fMRI signals). When a perceptual representation can be decoded from the activity pattern, the brain region studied contains information about the stimulus. In general, such “information-based” analysis requires multivariate techniques, but not necessarily decoding. In many instances “decoding” has been performed using univariate techniques.
Multivariate techniques in neuroimaging were introduced over a decade ago [151], but the current interest in the information-based approach is derived from the idea that brain activation patterns reveal information carried by a neuronal population code at the scale of imaging voxels. This idea motivates multivariate analysis in single subjects without smoothing of the data. An information-based analysis determines whether there is a statistical dependency (i.e., mutual information) between the experimental conditions and the regional spatiotemporal activity patterns. Information undetected by activation-based mapping is often present in neuroimaging data. If the information resides in the fine-scale pattern of the activity, the spatial average may be similar between conditions, so no effect may be found by conventional methods with the data spatially averaged for ROI analysis or smoothed for statistical mapping. Information-based analysis can be applied to predefined ROIs. Alternatively, a continuous information-based mapping can be performed with a multivariate searchlight in order to discover regions carrying a particular type of information [72, 105].
It should be emphasized that this approach to neuroimaging, while, on the surface, appears to approach the brain as a black box from which to extract information about what the subject is doing, can be used as a highly sensitive probe to understand which aspects of a response are most informative, and therefore most relevant to behavior. In addition, as researchers begin to explore and understand precisely why certain classification algorithms work better in the context of extracting information from messy biological systems, perhaps this information will also be useful in lending insight into brain function that could not be probed by any other way. Ultimately, the technique may, at the moment, appear to be a flashy way of doing tricks with fMRI (i.e., brain reading, etc.), but in fact, this approach has tremendous potential to pose and answer extremely subtle questions about how the brain is organized. The basics of the techniques are provided in some recent papers [101, 105]. In the past year, two outstanding studies have further pushed the limits of decoding mental content from brain activity. Previous decoding studies, the experiments have involved two components: a training set in which the spatial pattern of brain activity was associated with an object (or object category), orientation, or position; and an experiment set in which the same stimuli used in the training set were used. The training set and test set was the same or highly similar stimuli. The two studies described below extend fMRI decoding to allow the accurate identification of brain activation associated with completely novel stimuli or tasks.
An exciting paper by Kay et al. [66] has demonstrated the ability for fMRI patterns to be used to identify the perception of completely novel images that the subject was viewing. This study consisted of a training set in which subjects viewed 1750 natural images. From this training set a quantitative receptive field model for each voxel was created using Gabor wavelets, characterizing tuning dimensions in space, orientation, and spatial frequency. Subjects then viewed 120 completely novel images. The corresponding brain activity patterns were compared with the receptive field models obtained from the training set. Accuracy ranged from 72 to 92%. In addition, the decrease of accuracy was extremely small as the size of the test set increased, providing hope that with the appropriate training set size and tuning dimensions, activation for just about any object that exists may be characterized with a high level of accuracy.
In the second study, Mitchell et al. [102] describe a method by which whole-brain patterns associated with statistical relationships between words representing abstract semantic concepts are determined and used to predict mental states not present in the training set. These results establish a direct predictive relationship between the statistics of word occurrences in text and the activation patterns associated with processing word meanings. A new insight into how the brain processes concrete nouns is also put forth in this study. Essentially, the meaningful fMRI patterns for these nouns are distributed across the brain (i.e., also in prefrontal regions) rather than only existing in typical sensory-motor regions.
Both of these studies represent an important paradigm shift in fMRI decoding—that of using a large training set focused on more elemental aspects of a brain state (i.e., from visual stimulus or word set) to predict the brain activation pattern associated with novel stimuli or brain states associated with processing novel semantic content. Not only do these studies pave the way for extensive applications of fMRI for “brain reading” but also lend a unique insight into how the brain is processing information by being able to identify the “most informative” voxels and regions. Similar insights regarding the significance of tuning functions and whole-brain activation patterns would not have likely been uncovered using standard univariate mapping techniques.
A related study by Williams et al. [149] addresses a previously overlooked aspect of pattern information — regarding which aspects of a specific pattern in the brain are actually used in the processing of a visual stimulus or word and which are non-essential epiphenomenon. In their study, subjects were scanned while observing novel objects that belonged in one of three categories. They were asked to decide which category each viewed object fell into. The corresponding fMRI activation patterns in retinotopic and lateral occipital cortex (LOC) were shown to contain category information associated with objects, but only in the LOC were the patterns stronger for correct than for incorrect trials. Put another way, in trials in which the subjects did not correctly categorize the stimulus, the correct stimulus information was present in retinotopic cortex but not in LOC. This work represents an alternative direction in fMRI pattern effect imaging — that of determining pattern representations and then comparing with corresponding behavior to further classify cortical areas as they influence behavior.
6. Endogenous Oscillations
One area of rapid development in fMRI is that of using endogenous or “resting state” fluctuations or oscillations to explore resting state networks as well as their correlation with neuronal pathology. The basic observation is that when subjects are not performing any task in the scanner, the time series signal consists of fluctuations that are not simply thermal noise or random, but rather, of interest. Components of these fluctuations include cardiac pulsations of blood and CSF (inflow effects), breathing oscillations (changes in Bo field from chest expansion), bulk motion, scanner instability, vasomotion, BOLD and flow fluctuations that occur with slow changes in end tidal CO2 variations causing a chance in blood CO2 level, and BOLD and flow fluctuations that occur with spontaneous neuronal activity. The last component is of course what is most interesting to neuroscientists, but it is first important to ascertain that they are related to neuronal activity. The predominant frequency range of these “neuronally related” fluctuations is at or below 0.1 Hz.
Interestingly, since the first observation of this effect — in which the endogenous oscillatory signal from one motor cortex was found to most correlate with the other motor cortex [11], it actually took several years for others to start to take interest. It was only after year 2000, that the field started expanding rapidly. Now, the expansion is explosive, with over a hundred papers a year being produced addressing endogenous oscillations.
Research related to this phenomenon has been along three avenues: (a) How to most efficiently and robustly extract and map resting state fluctuations — or, rather, how to process the data, (b) How to interpret these fluctuations, as well as to best separate neuronal from physiologic fluctuations, and (c) How to best apply the phenomenon of resting state fluctuations towards neuroscience questions and towards understanding of patient populations. Hundreds of papers have covered these topics in only the past 5 years. In the past year, the trend of an explosive interest in endogenous oscillations continues. A recent special issue of the journal Human Brain Mapping (Volume 29, Issue 7, July 2008) was completely devoted to the publication of studies about endogenous oscillations. Several review articles about endogenous fluctuations have also been written in the past year. Regarding each of the three areas of endogenous oscillations research, the following are some notable recent advancements.
a. Endogenous oscillation processing
A current problem in resting state processing is as follows: Given that a single plane of data consists of 64 × 64 voxels, and that 30 planes are typically collected per volume, and that 300 time points are collected per run, about 37 million time points are collected in a single 5-minute volumetric fMRI scan, the task of determining the correlation of every voxel time series with every other voxel in the brain is, to date, computationally prohibitive. Two alternative solutions have been popular. The first is to chose “seed voxel” regions [11, 52] and the second is to use relatively unsupervised model-free approaches such as independent component analysis (ICA) [26]. Each of the methods has limitations. With the seed voxel approach, the results are highly dependent on the choice of ROI or voxel from which the “seed” reference time series is chosen from. Within this chosen time series, much of the energy in the signal driving the correlation may also be artifactual. In addition, many correlated areas are of course, missed, depending on how many “seeds” are chosen. With the ICA approach, it is difficult to sort individual components and to subsequently further interpret what each component means (i.e., What frequency components are correlated? What about phase offsets over space?). As ICA methodology improves, it appears that the problem of robustly extracting a stable set of networks for comparison and clinical use is being solved. Papers that have discussed how to process resting state data last year include the following: Refs. [17, 18, 22, 38, 43, 122, 142, 150, 159].
b. Endogenous oscillation interpretation and separation
A central issue is whether or not the extracted networks actually mean anything neuronal, and if they do contain neuronally-relevant fluctuations, how are these separated robustly from data that contain non-neuronal fluctuations? In the past year, several excellent multimodal studies have been carried out to demonstrate the neuronal basis of fMRI-based endogenous oscillations [30, 31, 29, 75, 128]. In addition, several studies have recently addressed the issue of how physiologic processes such as breathing and cardiac function can influence fluctuations [8–10, 130]. Other studies have shown functionally correlated endogenous oscillations in the generally “cleaner signal” perfusion data [21]. Others have demonstrated that, in non-living samples, artifactual correlations in the resting state data can be seen as they relate to how MR images are formed (adjacent voxels share a considerable amount of image “energy”) coupled with an imperfect stability of the scanner [70]. While identification of potentially confounding artifacts is relatively straightforward, a clear assessment in each subject of what regions show artifactual correlation and which do not is much more difficult. It is problematic to cleanly “regress out” respiration or other variations since there is a considerable amount of overlap. Work is ongoing, and will be ongoing for quite a few years, to minimize any false positives in the identification of these networks.
One other point that is not discussed as much as other aspects of endogenous oscillations is in regard to what the physiologic mechanisms of these neuronally mediated oscillations in flow and BOLD are. What “purpose” do they serve? Are they only an epiphenomenon of regions being physically connected or functionally related, or do they serve a specific critical function to how we interact with the world? Several studies have came out recently which speak of a functional role, or at least an effect on behavior — of endogenous oscillations.
One study, carried out by Boly et al. [13], starts with the observation that baseline signal magnitude in the brain spontaneously varies. They then demonstrate that a temporal variance in perception of identical stimuli is positively correlated with pre-trial resting state oscillation signal phase in medial thalamus and lateral frontoparietal network (thought to be associated with vigilance and external monitoring) and negatively correlated with posterior cingulate/precuneus and temporoparietal cortices, which are hypothesized to be related to introspection and incidentally, the primary areas associated with what has been identified as the “default network”. A second study, by Fox et al. [44] demonstrates not only that endogenous oscillations persist during tasks and that they contribute to a significant fraction of the trial to trial variance of the BOLD response in supplementary motor cortex with a simple finger tapping task, but, interestingly, they are also directly related to trial to trial variation in motor performance. The performance of the task itself (force of the button press) is affected by when the task is performed relative to the phase of the endogenous oscillation. It appears that when the oscillations are at the peak of their cycle at the beginning of activation-induced BOLD changes, the force of the finger tap force is lightest, and vice versa. When the trough is at the beginning of the BOLD-signal change, the finger tap force is greatest. About 74% of the behavioral (finger tapping force) variance is attributed to ongoing endogenous oscillations in supplementary motor cortex. A third study, a comparison study of the resting state networks (default, oculomotor, somatomotor, and visual) in anesthetized monkeys, was carried out by Vincent et al. [144]. Not only did they show that the same “default” network was activated in humans and monkey but it also provided anatomical connectivity and evoked response pattern support for the oculomotor network. This work strongly argues that the “default” network is not strongly dependent on the level of consciousness nor is it a uniquely human system. Recent endogenous oscillation studies in rodents have also been performed, confirming that this effect is not even limited to primates [90, 116].
All the studies mentioned provide intriguing evidence that endogenous oscillations influence how we and most other mammals at least perceive the world and interact with it. What to make of this information with regard to understanding the functional significance of endogenous oscillations remains to be fully worked out.
c. Applications of neuronal fluctuation assessment
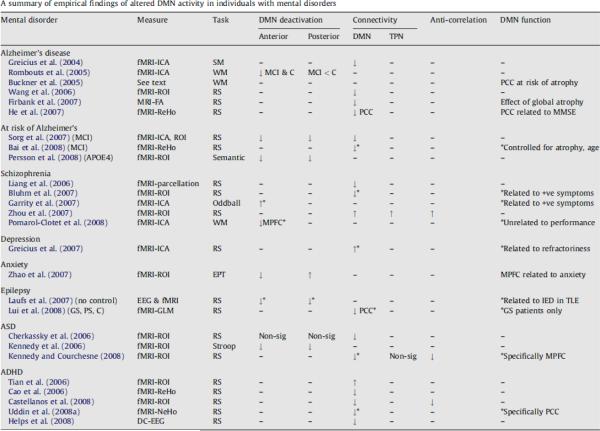
A very large number of applications of studies involving endogenous oscillations have already been reported in a very short time. A recent outstanding review article by Broyd et al. has summarized the myriad of applications of spontaneous brain oscillation fMRI data to brain dysfunction in mental disorders [15]. A comprehensive table, obtained with Elsevier's permission from that review article is reproduced in Fig. 6. Recent studies have included those of children and infants [81, 136], populations with multiple sclerosis [87], intelligence [133], acupuncture [36], sleep [62, 63], sedation [51], seizure activity [93], Alzheimer's disease [83], schizophrenia [82, 158], chronic pain [3], ADHD [141], depression [50], and consciousness [14, 123]. For future clinical studies, it is endogenous oscillations that clinicians are looking to with hope as these are fMRI signal changes that do not require a self-limiting probe task and are sensitive to patient population and conscious state.
Fig. 6.
Used with permission from Broyd et al. [15]. Abbreviations and symbols used in this table: DMN, default-mode network; TPN, task-positive network; MRI, magnetic resonance imaging; fMRI, functional magnetic resonance imaging; EEG, electroencephalogram; ICA, independent component analysis; ROI, region-of-interest (or seed voxel); ReHo, regional homogeneity; NeHo, network homogeneity; FA, fractional anisotropy; RS, resting-state; SM, sensory motor task; EPT, emotional processing task; WM, working memory task; ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; MCI, mild cognitive impairment; GS, generalized seizure patients; PS, partial seizure patients; C, controls; MMSE, mini mental state exam; (−) indicates that this was not tested; (↑) reflects an increase, for DMN deactivation it refers to increased deactivation; (↓) reflects a decrease, for DMN deactivation it refers to reduced deactivation; “non-sig” indicates the groups did not differ significantly; (*) indicates association between DMN function and particular result. All results are considered in comparison to a normal control group unless otherwise specified.
7. MRI Technology
Just about every significant step in the advancement in fMRI applications has been made is a direct result of advancements in MRI technology. MRI technology includes magnetic field, radio frequency (RF) coil configuration, and acquisition and/or image reconstruction strategies. Advancements in MRI technology allows an increase in sensitivity, resolution, robustness, or immunity to artifact, imaging speed, and information obtained. In the past year, several dramatic improvements in MRI technology have been published. These are discussed below.
First, magnetic field strength has exhibited a surprisingly linear increase over the years, starting in 1984 when the first clinical 1.5T scanner was available and projecting to 2011, when an 11.7T human scanner is planned to be operational. At least three centers have plans for an 11.7T for humans. They include the National Institutes of Health, Massachusetts General Hospital, and NeuroSpin — located just outside of Paris. At the time of writing this article, there are about 30 human 7T scanners in operation in the world. The push for higher field strength is driven not only by a direct proportionality of sensitivity to field strength: With this increased sensitivity comes the ability to collect high SNR functional and anatomical images faster and at higher resolution, allowing investigation of more subtle signal changes or anatomic features or allowing scanning of volunteers or patients at a faster rate. A recent article summarizing the gains in functional contrast at 7T [143] shows a linear increase in percent signal change, and a small gain in small vessel specificity. Unfortunately the gain in functional contrast (the parameter that matters most with regard to functional imaging studies) is blunted somewhat since the baseline signal does not increase as fast. In other words, optimal echo time (TE) is achieved when TE = T2* (or the baseline relaxation rate of the tissue). With an increase in field strength, the activation-induced change in relaxation rate increases linearly, but the baseline relaxation rate increases at a slightly greater rate than linear rate. This pushes down the contrast somewhat. Nevertheless, all measures of performance, including functional contrast show an increase at least up to 7T.
Anatomic images at 7T not only have higher signal to noise ratio or higher resolution, but also have qualitatively different anatomical contrast. New anatomic contrast is apparent in 7T anatomical images. An example of the qualitative difference in contrast at 7T is a study by Duyn et al. [41] demonstrating that MRI phase is a highly sensitive contrast at 7T, revealing a 10 × improvement in gray matter–white matter delineation over magnitude contrast at lower field strengths, as well as cortical layers, and perhaps even white matter tracts. The authors speculate that the phase shifts are mostly due to differences in susceptibility effects, but other hypotheses have been put forward [157], suggesting that macromolecules play a role.
Performance of fMRI at high field is technically extremely challenging. Challenges include making sure of a high magnetic field homogeneity throughout the brain, minimizing RF power deposition in some sequences, maintaining an acceptable RF excitation homogeneity, minimizing acoustic noise, managing an increased amount of physiologic noise [139], and minimizing patient discomfort, among others. Nevertheless, the benefits are beginning to manifest themselves in fMRI. A recent publication by Yacoub et al. [153] has convincingly demonstrated that activation of human orientation columns (about a third of the size of ocular dominance columns) can be imaged. To achieve this, scanning was performed at 7T on a Siemens/Varian console using a four shot spin-echo sequence, resulting in an in-plane resolution of 0.5 × 0.5mm2. A novel finding from this study is that there is a bias toward the brain processing orientations around the vertical direction. An important point also to be taken from this study is that at 7T, the intravascular contribution is reduced such that spin-echo sequences do not suffer from large vessel intravascular effects — as occurs at lower field strengths. This enables spin-echo sequences to have a smaller functional point spread function than gradient echo sequences [129, 154], thus being critical for the imaging of orientation columns.
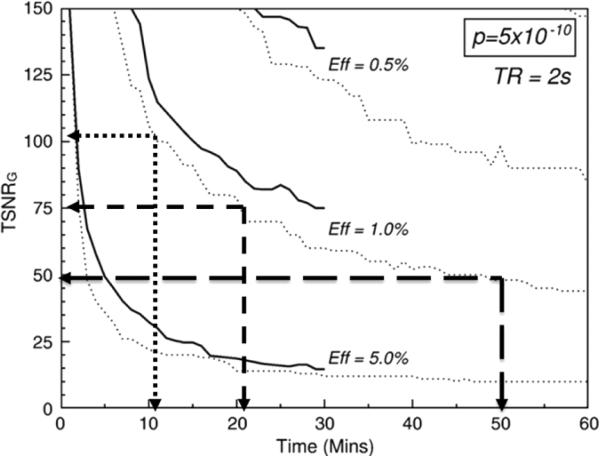
The motivation for increasing sensitivity is strong for many reasons. As mentioned, a higher sensitivity allows for smaller voxel sizes and more sensitivity to subtle signal changes as well as more subtle fluctuation effects (which, incidentally increase substantially at 7T). A higher sensitivity will also allow for faster assessment of individual subjects. Figure 7, used with permission, is adopted from Murphy et al. [106] showing that, in the realm of a relatively low temporal signal to noise — common when imaging at high resolution — the reduction of necessary scan time to create an adequate quality functional image is a nonlinear function of temporal signal to noise. Simply doubling the temporal signal to noise in this regime would decrease the necessary time to create an adequate image by a factor of 5. This type of time reduction opens up the opportunity for an extremely different set of experiments that were previously limited by low sensitivity.
Fig. 7.
Used with permission from Murphy et al. [106]. This shows the relationship between temporal signal to noise (y-axis) and necessary time to scan to achieve a functional image with a sufficient p value, shown top right corner. The three curves are for different effect sizes. If one is working at a temporal signal to noise of 50 (typical for high resolution fMRI at 7T) it would take about 50 minutes to perform enough temporal averaging to create a sufficient image. If the temporal signal to noise is increased by 50%, the time is reduced to just over 20 minutes. If temporal signal to noise is doubled, the time is reduced by a factor of 5 to a very manageable 10 minutes.
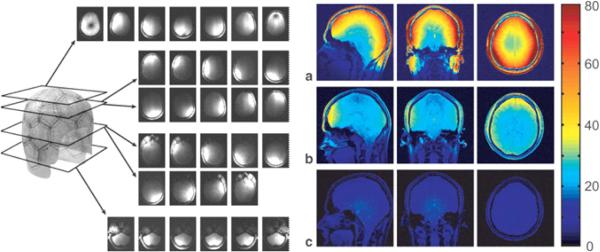
A much less expensive alternative to increasing field strength is decreasing the size but increasing the number of RF receivers around the head. Moving up from a single quadrature receive coil to a 32-channel coil (about the state-of-the-art technology at 3T), will increase image signal to noise ratio (SNR) up to a factor of 4 [148]. Figure 8, obtained with permission from Wiggins et al. [148], shows the clear and dramatic advantages in terms of signal to noise ratio increasing as a function of the number of coil elements — at least to 32. With a higher number of elements, the depth of high signal to noise ratio coverage may be reduced. At higher field strengths, this would be reduced less.
Fig. 8.
Used with permission from Wiggins et al. [148], showing, on the left, uncombined images for the 32-channel coil from a gradient-echo scan obtained with all elements active, demonstrating isolation between all of the coil elements at 3T. As the coils are distributed in 3D, their sensitivity profiles must be visualized by viewing the images in different planes that pass through different rows of coils. On the right are signal to noise maps derived from gradient-echo scans for (a) a 32-channel coil, (b) a commercial eight-channel coil, and (c) a commercial volume coil. All maps are generated with the same color scale.
Other technological innovations have been in the direction of using these multielement coils for faster image acquisition. Currently, almost all functional MRI data involves the collection of a stack of 2D images which takes about 2 seconds. With higher bandwidth receivers, parallel RF coil arrays, stronger gradients, and novel image reconstruction strategies, the goal of obtaining a relatively high resolution volume of data in a single excitation or with a single acquisition is drawing closer. In the past year, two papers have described novel echo volume imaging (EVI) techniques [80, 120]. In the near future, EVI will emerge as a new standard in image acquisition for those researchers performing multisubject averaging (who do not need high resolution) or interesting in studying subtle or rapid temporal dynamics of the response across the brain.
Lin et al. [79] and Hennig et al. [58] have taken parallel acquisition to a new level in minimizing the need for time-consuming spatial encoding gradients. Lin et al., using a 32-channel parallel array with the coil configuration resembling EEG or MEG sensor geometries, apply similar source localization algorithms to reconstruct these images. They have named this approach magnetic resonance Inverse Imaging (InI). Hennig et al. have reduced the need for spatial encoding gradients even further with their one-voxel-one-coil (OVOC) imaging method. While the obvious advantage of these methods is the extreme rapidity with which useful volumes are obtained, another advantage is the presumably silent acquisition. This avenue of technology development appears to be just beginning. It is easy to imagine improvement in coil configurations optimized for specific brain areas or more efficient sampling schemes. Higher bandwidth acquisition rates will also lead to further improvements in resolution. Perhaps in the next several years, relatively silent, whole brain fMRI will be the standard.
8. Alternative Functional Contrast Mechanisms
As BOLD contrast is based on blood oxygenation changes that are dependent on the precise interplay of flow increase and metabolic rate increase, the temporal and spatial accuracy as well as the interpretability of the signal is fundamentally limited. Many researchers are busy trying to develop an MRI-based functional contrast that may somehow be a more direct, sensitive, quantitative measure of neuronal activity. Indeed, many other functional contrasts have been put forward as possible. These include the non-invasive measures of activation induced changes in perfusion itself [34], blood volume [88], diffusion [77], CMRO2 [28, 59] temperature [152], and presumably magnetic field changes in the vicinity of active neurons [4]. These results have always been met with extreme interest by the imaging community but none have proven superior to BOLD, because the techniques are more technically challenging or, more often, the effect size produced using the specific contrast sensitivity is considerably less than BOLD contrast. Perfusion imaging has come the closest to BOLD in terms of utility and functional contrast. While it generally has less brain coverage, lower temporal resolution, and lower functional contrast to noise than BOLD, it does have the advantages of higher specificity, baseline information, and less baseline drift. The latter advantage is significant when probing very slow brain activation timing [1].
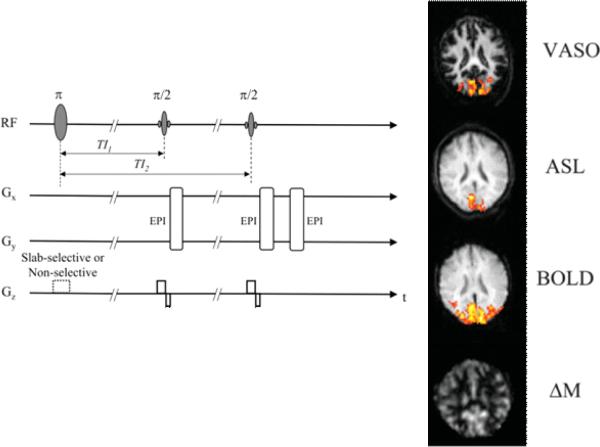
Each of these functional contrasts has the potential to providing unique information not only about brain activity location, timing, and magnitude but also about cerebral physiology. This information is not only useful for brain mapping but may be useful for understanding more deeply, neurovascular coupling and variations of this with disease. What is exciting about fMRI is that the potential impact of the field is not limited to mapping brain function, but rather to understanding many more aspects of brain physiology at a depth not previously achieved with more invasive methods. An exciting direction that I feel has significant potential in fMRI is the simultaneous collection of multiple contrasts [92, 37, 60, 146, 61, 91] and even the simultaneous collection of data across multiple modalities [119, 113, 138, 155, 76, 115, 94]. The simultaneous collection of this information allows detailed temporal behavior of the signal changes and the noise to be carefully studied in a temporally and spatially registered manner. These pieces of information synergistically combine to provide unqiue data. Just one example of this approach is that of Yang et al. [156]. Figure 9 shows an example in which baseline and activation induced perfusion was obtained simultaneously with BOLD and blood volume changes. This particular approach holds the potential for mapping changes in CMRO2 from these various measures without the need for a global flow change calibration scan. This is one example of what is possible. Many more pulse sequence approaches are also on the horizon.
Fig. 9.
Modified with permission from Yang et al. [156]. This is a figure showing a pulse sequence strategy, on the left, for the virtually simultaneous and relatively rapid collection of multiple hemodynamic contrasts: blood volume (VASO — vascular space occupancy), blood flow (ASL — arterial spin labeling), blood oxygenation (BOLD — blood oxygen level dependent contrast), and baseline perfusion (ΔM).
9. Conclusions
This overview of fMRI is an attempt to convey not only some of the most interesting current work, but also a solid sense that fMRI is becoming a rich field from neuroscience, physiology, engineering, signal processing, and physics perspectives. Clinical applications, while not yet here, will steadily move from the realm of “potential” to “practice”. Neuroscience applications, while high in number, are in reality just beginning as we continue to learn more about fMRI contrast and develop better processing methods and better technology for extracting even more about brain anatomy, physiology, and function.
References
- [1].Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. NeuroImage. 2002;15:488. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- [2].Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: Implications for BOLD-fMRI. NeuroImage. 2008;39:1510. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Appl Magn Reson. 2005;29:65. [Google Scholar]
- [5].Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [6].Bandettini PA, Wong EC. Magnetic resonance imaging of human brain function — Principles, practicalities, and possibilities. Neurosurg Clin North Am. 1997;8:345. [PubMed] [Google Scholar]
- [7].Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course epi of human brain-function during task activation. Magn Reson Med. 1992;25:390. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- [8].Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: A 7 T study. Magn Reson Imag. 2009 doi: 10.1016/j.mri.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Human Brain Map. 2008;29:740. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- [12].Blamire AM, Ogawa S, Ugurbil K, Rothman D, McCarthy G, Ellermann JM, Hyder F, Rattner Z, Shulman RG. Dynamic mapping of the human visual-cortex by high-speed magnetic-resonance-imaging. Proc Natl Acad Sci USA. 1992;89:11069. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boly M, Phillips C, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Peigneux P, Faymonville ME, Maquet P, Laureys S. Consciousness and cerebral baseline activity fluctuations. Human Brain Map. 2008;29:868. doi: 10.1002/hbm.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33:279. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [16].Buxton RB. The elusive initial dip. NeuroImage. 2001;13:953. doi: 10.1006/nimg.2001.0814. [DOI] [PubMed] [Google Scholar]
- [17].Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Map. 2008;29:828. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Human Brain Map. 2008;29:1265. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carlson TA, Schrater P, He S. Patterns of activity in the categorical representations of objects. JCogn Neurosci. 2003;15:704. doi: 10.1162/089892903322307429. [DOI] [PubMed] [Google Scholar]
- [20].Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. NeuroImage. 2007;37:808. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- [21].Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, Duyn JH, Talagala SL. Mapping resting-state functional connectivity using perfusion MRI. NeuroImage. 2008;40:1595. doi: 10.1016/j.neuroimage.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. NeuroImage. 2008;41:45. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: Detecting and classifying distributed patterns of fMRI activity in human visual cortex. NeuroImage. 2003;19:261. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- [24].Cramer SC, Benson RR, Burra VC, Himes D, Crafton KR, Janowsky JS, Brown JA, Lutsep HL. Mapping individual brains to guide restorative therapy after stroke: Rationale and pilot studies. Neurol Res. 2003;25:811. doi: 10.1179/016164103771953899. [DOI] [PubMed] [Google Scholar]
- [25].Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, Trouard TP, Squire SW, Weinand ME, Savage CR, Wilkinson SB, Juranek J, Leu SY, Himes DM. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- [26].Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, Gur RC, Langleben DD. Classifying spatial patterns of brain activity with machine learning methods: Application to lie detection. NeuroImage. 2005;28:663. doi: 10.1016/j.neuroimage.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [28].Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Munck JC, Gonçalves SI, Mammoliti R, Heethaar RM, Lopes da Silva FH. Interactions between different EEG frequency bands and their effect on alpha-fMRI correlations. NeuroImage. 2009;47:69. doi: 10.1016/j.neuroimage.2009.04.029. [DOI] [PubMed] [Google Scholar]
- [30].de Munck JC, Gonçalves SI, Huijboom L, Kuijer JPA, Pouwels PJW, Heethaar RM, Lopes da Silva FH. The hemodynamic response of the alpha rhythm: An EEG/fMRI study. NeuroImage. 2007;35:1142. doi: 10.1016/j.neuroimage.2007.01.022. [DOI] [PubMed] [Google Scholar]
- [31].de Munck JC, Gonçalves SI, Faes TJC, Kuijer JPA, Pouwels PJW, Heethaar RM, Lopes da Silva FH. A study of the brain's resting state based on alpha band power, heart rate and fMRI. NeuroImage. 2008;42:112. doi: 10.1016/j.neuroimage.2008.04.244. [DOI] [PubMed] [Google Scholar]
- [32].deCharms RC. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cogn Sci. 2007;11:473. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [33].DeCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JDE, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA. 2005;102:18626. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion Imaging. Magn Reson Med. 1992;23:37. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- [35].Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Donahue KM, Van Kylen J, Guven S, El-Bershawi A, Luh WM, Bandettini PA, Cox RW, Hyde JS, Kissebah AH. Simultaneous gradient-echo/spin-echo EPI of graded ischemia in human skeletal muscle. J Magn Reson Imag. 1998;8:1106. doi: 10.1002/jmri.1880080516. [DOI] [PubMed] [Google Scholar]
- [38].Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Human Brain Map. 2008;29:778. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Duncan RO, Boynton GM. Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron. 2003;38:659. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- [40].Duyn JH, Moonen CTW, Vanyperen GH, Deboer RW, Luyten PR. Inflow versus deoxyhemoglobin effects in bold functional MRI using gradient echoes at 1.5 T. NMR Biomed. 1994;7:83. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- [41].Duyn JH, Van Gelderen P, Li TQ, De Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci USA. 2007;104:11796. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Eger E, Ashburner J, Haynes JD, Dolan RJ, Rees G. fMRI activity patterns in human LOC carry information about object exemplars within category. J Cogn Neurosci. 2008;20:356. doi: 10.1162/jocn.2008.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R, Di Salle F. Independent component model of the default-mode brain function: Combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imag. 2008;26:905. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- [44].Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- [45].Frahm J, Baudewig J, Kallenberg K, Kastrup A, Merboldt KD, Dechent P. The post-stimulation undershoot in BOLD fMRI of human brain is not caused by elevated cerebral blood volume. NeuroImage. 2008;40:473. doi: 10.1016/j.neuroimage.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [46].Frahm J, Merboldt KD, Hanicke W, Kleinschmidt A, Boecker H. Brain or vein-oxygenation or flow on signal physiology in functional MRI of human brain activation. NMR Biomed. 1994;7:45. doi: 10.1002/nbm.1940070108. [DOI] [PubMed] [Google Scholar]
- [47].Fu CHY, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SCR, Brammer MJ. Pattern classification of sad facial processing: Toward the development of neurobiological markers in depression. Biol Psych. 2008;63:656. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- [48].Gati JS, Menon RS, Ugurbil K, Rutt BK. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med. 1997;38:296. doi: 10.1002/mrm.1910380220. [DOI] [PubMed] [Google Scholar]
- [49].Glover GH, Lemieux SK, Drangova M, Pauly JM. Decomposition of inflow and blood oxygen level-dependent (BOLD) effects with dual-echo spiral gradient-recalled echo (GRE) fMRI. Magn Reson Med. 1996;35:299. doi: 10.1002/mrm.1910350306. [DOI] [PubMed] [Google Scholar]
- [50].Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psych. 2007;62:429. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Greicius MD, Kiviniemi V, Tervonen O, Vainionpää V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Human Brain Map. 2008;29:839. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S. PyMVPA: A python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics. 2009;7:37. doi: 10.1007/s12021-008-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hanson SJ, Halchenko YO. Brain reading using full brain support vector machines for object recognition: There is no “face” identification area. Neural Comput. 2008;20:486. doi: 10.1162/neco.2007.09-06-340. [DOI] [PubMed] [Google Scholar]
- [55].Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: Is there a “face” area? NeuroImage. 2004;23:156. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- [56].Haynes JD, Rees G. Predicting the stream of consciousness from activity in human visual cortex. Curr Biol. 2005;15:1301. doi: 10.1016/j.cub.2005.06.026. [DOI] [PubMed] [Google Scholar]
- [57].Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading hidden intentions in the human brain. Curr Biol. 2007;17:323. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- [58].Hennig J, Zhong K, Speck O. MR-Encephalography: Fast multi-channel monitoring of brain physiology with magnetic resonance. NeuroImage. 2007;34:212. doi: 10.1016/j.neuroimage.2006.08.036. [DOI] [PubMed] [Google Scholar]
- [59].Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Stimulus-dependent BOLD and perfusion dynamics in human V1. NeuroImage. 1999;9:573. doi: 10.1006/nimg.1999.0443. [DOI] [PubMed] [Google Scholar]
- [61].Hoge RD, Franceschini MA, Covolan RJM, Huppert T, Mandeville JB, Boas DA. Simultaneous recording of task-induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin-labeling MRI. NeuroImage. 2005;25:701. doi: 10.1016/j.neuroimage.2004.12.032. [DOI] [PubMed] [Google Scholar]
- [62].Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci USA. 2009;106:11376. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Horovitz SG, Fukunaga M, De Zwart JA, Van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Human Brain Map. 2008;29:671. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu XP, Le TH, Ugurbil K. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn Reson Med. 1997;37:877. doi: 10.1002/mrm.1910370612. [DOI] [PubMed] [Google Scholar]
- [65].Jones RA, Schirmer T, Lipinski B, Elbel GK, Auer DP. Signal undershoots following visual stimulation: A comparison of gradient and spin-echo BOLD sequences. Magn Reson Med. 1998;40:112. doi: 10.1002/mrm.1910400116. [DOI] [PubMed] [Google Scholar]
- [66].Kay KN, Naselaris T, Prenger RJ, Gallant JL. Identifying natural images from human brain activity. Nature. 2008;452:352. doi: 10.1038/nature06713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: Implications for dynamic functional magnetic resonance imaging calibration. J Cerebral Blood Flow Metab. 2007;27:690. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. NeuroImage. 2007;38:649. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kriegeskorte N, Bandettini P. Combining the tools: Activation- and information-based fMRI analysis. NeuroImage. 2007;38:666. doi: 10.1016/j.neuroimage.2007.06.030. [DOI] [PubMed] [Google Scholar]
- [70].Kriegeskorte N, Bodurka J, Bandettini P. Artifactual time-course correlations in echo-planar fMRI with implications for studies of brain function. Int J Imaging Syst Technol. 2008;18:345. [Google Scholar]
- [71].Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA. 2006;103:3863. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic-resonance-imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].LaConte SM, Peltier SJ, Hu XP. Real-time fMRI using brain-state classification. Human Brain Map. 2007;28:1033. doi: 10.1002/hbm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Laufs H. Endogenous brain oscillations and related networks detected by surface EEG-combined fMRI. Human Brain Map. 2008;29:762. doi: 10.1002/hbm.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Laufs H, Daunizeau J, Carmichael DW, Kleinschmidt A. Recent advances in recording electrophysiological data simultaneously with magnetic resonance imaging. NeuroImage. 2008;40:515. doi: 10.1016/j.neuroimage.2007.11.039. [DOI] [PubMed] [Google Scholar]
- [77].Le Bihan D, Urayama SI, Aso T, Hanakawa T, Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci USA. 2006;103:8263. doi: 10.1073/pnas.0600644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lee JH, Ryu J, Jolesz FA, Cho ZH, Yoo SS. Brain-machine interface via real-time fMRI: Preliminary study on thought-controlled robotic arm. Neurosci Lett. 2009;450:1. doi: 10.1016/j.neulet.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lin FH, Witzel T, Mandeville JB, Polimeni JR, Zeffro TA, Greve DN, Wiggins G, Wald LL, Belliveau JW. Event-related single-shot volumetric functional magnetic resonance inverse imaging of visual processing. NeuroImage. 2008;42:230. doi: 10.1016/j.neuroimage.2008.04.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lindquist MA, Zhang CH, Glover G, Shepp L. Rapid three-dimensional functional magnetic resonance imaging of the initial negative BOLD response. J Magn Res. 2008;191:100. doi: 10.1016/j.jmr.2007.12.016. [DOI] [PubMed] [Google Scholar]
- [81].Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Res. 2008;1223:42. doi: 10.1016/j.brainres.2008.05.054. [DOI] [PubMed] [Google Scholar]
- [82].Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- [83].Liu Y, Wang K, Yu C, He Y, Zhou Y, Liang M, Wang L, Jiang T. Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: A review of resting-state fMRI studies. Neuropsychologia. 2008;46:1648. doi: 10.1016/j.neuropsychologia.2008.01.027. [DOI] [PubMed] [Google Scholar]
- [84].Logothetis NK. The ins and outs of fMRI signals. Nature Neurosci. 2007;10:1230. doi: 10.1038/nn1007-1230. [DOI] [PubMed] [Google Scholar]
- [85].Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- [86].Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- [87].Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, Phillips MD. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Human Brain Map. 2008;29:818. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lu H, Golay X, Pekar JJ, Van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- [89].Lu H, Golay X, Pekar JJ, Van Zijl PCM. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. J Cerebral Blood Flow Metab. 2004;24:764. doi: 10.1097/01.WCB.0000124322.60992.5C. [DOI] [PubMed] [Google Scholar]
- [90].Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104:18265. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Luh WM, Wong EC, Bandettini PA, Ward BD, Hyde JS. Comparison of simultaneously measured perfusion and bold signal increases during brain activation with T1-based tissue identification. Magn Reson Med. 2000;44:137. doi: 10.1002/1522-2594(200007)44:1<137::aid-mrm20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [92].Luh WM, Wong EC, Bandettini PA, Ward BD, Hyde JS. Comparison of simultaneously measured perfusion and BOLD signal increases during brain activation with T-1-based tissue identification. Magn Reson Med. 2000;44:137. doi: 10.1002/1522-2594(200007)44:1<137::aid-mrm20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [93].Lui S, Ouyang L, Chen Q, Huang X, Tang H, Chen H, Zhou D, Kemp GJ, Gong Q. Differential interictal activity of the precuneus/posterior cingulate cortex revealed by resting state functional MRI at 3T in generalized vs. partial seizure. J Magn Reson Imag. 2008;27:1214. doi: 10.1002/jmri.21370. [DOI] [PubMed] [Google Scholar]
- [94].MacIntosh BJ, Baker SN, Mraz R, Ives JR, Martel AL, McIlroy WE, Graham SJ. Improving functional magnetic resonance imaging motor studies through simultaneous electromyography recordings. Human Brain Map. 2007;28:835. doi: 10.1002/hbm.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nature Rev Neurosci. 2006;7:732. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- [96].McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. NeuroImage. 2006;29:1185. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- [98].Menon RS, Ogawa S, Tank DW, Ugurbil K. Tesla gradient recalled echo characteristics of photic stimulation-induced signal changes in the human primary visual-cortex. Magn Reson Med. 1993;30:380. doi: 10.1002/mrm.1910300317. [DOI] [PubMed] [Google Scholar]
- [99].Miller MB, Donovan CL, Van Horn JD, German E, Sokol-Hessner P, Wolford GL. Unique and persistent individual patterns of brain activity across different memory retrieval tasks. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. JNeurosci. 2004;24:4912. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mitchell TM, Hutchinson R, Niculescu RS, Pereira F, Wang XR, Just M, Newman S. Learning to decode cognitive states from brain images. Machine Learning. 2004;57:145. [Google Scholar]
- [102].Mitchell TM, Shinkareva SV, Carlson A, Chang KM, Malave VL, Mason RA, Just MA. Predicting human brain activity associated with the meanings of nouns. Science. 2008;320:1191. doi: 10.1126/science.1152876. [DOI] [PubMed] [Google Scholar]
- [103].Mourao-Miranda J, Ecker C, Sato JR, Brammer M. Dynamic changes in the mental rotation network revealed by pattern recognition analysis of fMRI data. J Cogn Neurosci. 2009;21:890. doi: 10.1162/jocn.2009.21078. [DOI] [PubMed] [Google Scholar]
- [104].Munk S, Forchhammer HB, Brennum J, Hansen AE, Larsson HBW. Possibilities and limitations in the use of presurgical functional MR imaging in the mapping of language function. Magnetisk resonans-skanning til prækirurgisk kortlægning af sprogfunktion. 2007;169:3571. [PubMed] [Google Scholar]
- [105].Mur M, Bandettini PA, Kriegeskorte N. Revealing representational content with pattern-information fMRI — An introductory guide. Soc Cogn Affective Neurosci. 2009;4:101. doi: 10.1093/scan/nsn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage. 2007;34:565. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106:8356. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Muthukumaraswamy SD, Singh KD. Spatiotemporal frequency tuning of BOLD and gamma band MEG responses compared in primary visual cortex. NeuroImage. 2008;40:1552. doi: 10.1016/j.neuroimage.2008.01.052. [DOI] [PubMed] [Google Scholar]
- [109].Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neurosci. 2007;10:1515. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- [110].O'Toole AJ, Jiang F, Abdi H, Haxby JV. Partially distributed representations of objects and faces in ventral temporal cortex. J Cogn Neurosci. 2005;17:580. doi: 10.1162/0898929053467550. [DOI] [PubMed] [Google Scholar]
- [111].O'Toole AJ, Jiang F, Abdi H, Pénard N, Dunlop JP, Parent MA. Theoretical, statistical, and practical perspectives on pattern-based classification approaches to the analysis of functional neuroimaging data. J Cogn Neurosci. 2007;19:1735. doi: 10.1162/jocn.2007.19.11.1735. [DOI] [PubMed] [Google Scholar]
- [112].Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation — Functional brain mapping with magnetic-resonance-imaging. Proc Natl Acad Sci USA. 1992;89:5951. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Olbrich S, Mulert C, Karch S, Trenner M, Leicht G, Pogarell O, Hegerl U. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. NeuroImage. 2009;45:319. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- [114].Owen AM, Coleman MR. Functional neuroimaging of the vegetative state. Nature Rev Neurosci. 2008;9:235. doi: 10.1038/nrn2330. [DOI] [PubMed] [Google Scholar]
- [115].Patterson J, Bandettini P, Ungerleider L. Simultaneous skin conductance measurement and fMRI during cognitive tasks: Correlations of skin conductance activity with ventromedial prefrontal cortex (PFC) and orbitofrontal cortex (OFC) activity. NeuroImage. 11:2000. [Google Scholar]
- [116].Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59:1021. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pessoa L, Padmala S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proc Natl Acad Sci USA. 2005;102:5612. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pessoa L, Padmala S. Decoding near-threshold perception of fear from distributed single-trial brain activation. Cerebral Cortex. 2007;17:691. doi: 10.1093/cercor/bhk020. [DOI] [PubMed] [Google Scholar]
- [119].Purdon PL, Pierce ET, Bonmassar G, Walsh J, Harrell PG, Kwo J, Deschler D, Barlow M, Merhar RC, Lamus C, Mullaly CM, Sullivan M, Maginnis S, Skoniecki D, Higgins HA, Brown EN. Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Annals of the New York Academy of Sciences. 2009:61. doi: 10.1111/j.1749-6632.2008.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rabrait C, Ciuciu P, Ribés A, Poupon C, Le Roux P, Dehaine-Lambertz G, Le Bihan D, Lethimonnier F. High temporal resolution functional MRI using parallel echo volumar imaging. J Magn Reson Imag. 2008;27:744. doi: 10.1002/jmri.21329. [DOI] [PubMed] [Google Scholar]
- [121].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Salvador R, Martínez A, Pomarol-Clotet E, Gomar J, Vila F, Sarró S, Capdevila A, Bullmore E. A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage. 2008;39:279. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- [123].Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [124].Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- [125].Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. Resting-state spontaneous fluctuations in brain activity. A new paradigm for presurgical planning using fMRI. Acad Radiol. 2009;16:578. doi: 10.1016/j.acra.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Shinkareva S, Ombao H, Sutton B, Gutchess A, Park D. Model free classification of fMRI brain images. JCogn Neurosci. 35:2005. [Google Scholar]
- [127].Shinkareva SV, Mason RA, Malave VL, Wang W, Mitchell TM, Just MA. Using fMRI brain activation to identify cognitive states associated with perception of tools and dwellings. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Human Brain Map. 2008;29:751. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. NeuroImage. 2007;35:539. doi: 10.1016/j.neuroimage.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38:306. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Silva AC, Koretsky AP, Duyn JH. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magn Reson Med. 2007;57:1110. doi: 10.1002/mrm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sitaram R, Caria A, Birbaumer N. Hemodynamic brain-computer interfaces for communication and rehabilitation. Neural Networks. 2009 doi: 10.1016/j.neunet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [133].Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T. Brain spontaneous functional connectivity and intelligence. NeuroImage. 2008;41:1168. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- [134].Strother SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, Frutiger S, Muley S, LaConte S, Rottenberg D. The quantitative evaluation of functional neuroimaging experiments: The NPAIRS data analysis framework. NeuroImage. 2002;15:747. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- [135].Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12:74. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Thomason ME, Burrows BE, Gabrieli JDE, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005;25:824. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- [137].Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Human Brain Map. 2007;28:59. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Toyoda H, Kashikura K, Okada T, Nakashita S, Honda M, Yonekura Y, Kawaguchi H, Maki A, Sadato N. Source of nonlinearity of the BOLD response revealed by simultaneous fMRI and NIRS. NeuroImage. 2008;39:997. doi: 10.1016/j.neuroimage.2007.09.053. [DOI] [PubMed] [Google Scholar]
- [139].Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage. 2005;26:243. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [140].Turner R. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. NeuroImage. 2002;16:1062. doi: 10.1006/nimg.2002.1082. [DOI] [PubMed] [Google Scholar]
- [141].Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Met. 2008;169:249. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- [142].van den Heuvel M, Mandl R, Pol HH. Normalized cut group clustering of resting-state fMRI data. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].van der Zwaag W, Francis S, Head K, Peters A, Gowland P, Morris P, Bowtell R. fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. NeuroImage. 2009;47:1425. doi: 10.1016/j.neuroimage.2009.05.015. [DOI] [PubMed] [Google Scholar]
- [144].Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447:83. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- [145].Voyvodic JT, Petrella JR, Friedman AH. FMRI activation mapping as a percentage of local excitation: Consistent presurgical motor maps without threshold adjustment. J Magn Reson Imag. 2009;29:751. doi: 10.1002/jmri.21716. [DOI] [PubMed] [Google Scholar]
- [146].Weiskopf N, Klose U, Birbaumer N, Mathiak K. Single-shot compensation of image optimization using multi-echo EPI distortions and BOLD contrast for real-time fMRI. NeuroImage. 2005;24:1068. doi: 10.1016/j.neuroimage.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [147].Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K. Real-time functional magnetic resonance imaging: Methods and applications. Magn Reson Imag. 2007;25:989. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- [148].Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-Channel 3 tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- [149].Williams MA, Dang S, Kanwisher NG. Only some spatial patterns of fMRI response are read out in task performance. Nature Neurosci. 2007;10:685. doi: 10.1038/nn1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wink AM, Bullmore E, Barnes A, Bernard F, Suckling J. Monofractal and multifractal dynamics of low frequency endogenous brain oscillations in functional MRI. Human Brain Map. 2008;29:791. doi: 10.1002/hbm.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. NeuroImage. 1997;6:305. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- [152].Yablonskiy DA, Ackerman JJH, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA. 2000;97:7603. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci USA. 2008;105:10607. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn spin echo BOLD functional MRI at 7 Tesla. NeuroImage. 2007;37:1161. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yang YH, Gu H, Silbersweig DA, Stern E. Simultaneous perfusion and blood-oxygenation-level-dependent measurements using single-shot interleaved z-shim echo-planar imaging. Magn Reson Med. 2005;53:1207. doi: 10.1002/mrm.20431. [DOI] [PubMed] [Google Scholar]
- [156].Yang YH, Gu H, Stein EA. Simultaneous MRI acquisition of blood volume, blood flow, and blood oxygenation information during brain activation. Magn Reson Med. 2004;52:1407. doi: 10.1002/mrm.20302. [DOI] [PubMed] [Google Scholar]
- [157].Zhong K, Leupold J, Hennig J, Speck O. Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn Reson Med. 2007;57:67. doi: 10.1002/mrm.21103. [DOI] [PubMed] [Google Scholar]
- [158].Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Res. 2007;97:194. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [159].Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, Sui MQ, Wang YF. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. NeuroImage. 2008;40:110. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]