Neurofibromatosis is one of the most common genetic disorders, with type-I neurofibromatosis having a global prevalence of one in 3000 individuals1-4. Inherited in an autosomal dominant manner, type-1 neurofibromatosis may be known best for its cutaneous manifestations. Café au lait spots and peripheral neurofibromas arise as a result of unchecked proliferation of neural crest-derived melanocytes and Schwann cells, respectively5,6. These superficial lesions are generally benign and are often considered to be purely a cosmetic issue7. In contrast, the osteopathological manifestations of type-1 neurofibromatosis are of far greater clinical concern. Spinal deformity, particularly kyphoscoliosis of the thoracic spine, is the most common abnormality (present in 10% to 60% of cases)4,8-10.
Although the precise etiology of these spinal abnormalities is not well understood and most are probably multifactorial, a variety of pathologic processes have been implicated4,11-13. Dural ectasia may result from cerebral spinal fluid pulsations, which lead to dilatation of the weakened dural sac, with erosion of the surrounding vertebral elements as the dural sac enlarges14. Peripheral neurofibromas can expand into adjacent ribs, facet joints, pedicles, and paravertebral musculature4,12. Intrinsic pathologic conditions, such as osteomalacia and general mesodermal dysplasia, can also contribute to spinal instability in a more occult fashion13. Evidence of these processes on imaging studies includes rib penciling, meningoceles, expanded neural foramina, vertebral scalloping and wedging, and soft-tissue masses15-17. Clinical sequelae, ranging from simple back pain to decreased pulmonary function and quadriparesis, have been associated with the spinal manifestations of type-1 neurofibromatosis18,19. Despite the potential for major neurologic complications, a substantial proportion of patients with type-1 neurofibromatosis exhibit normal neurologic function4,13,20. In some cases, spinal cord compression is avoided because of an ectatic thecal sac and widened spinal canal4,13.
In the absence of dysplastic lesions, the spinal deformity is not likely to decompensate rapidly, and early treatment can be conservative (observation and bracing)4,21,22. When there are dysplastic lesions in the spine, swift progression of the spinal deformity can be expected, and more aggressive surgical intervention is recommended21,23-25. While surgical arthrodesis and instrumentation is often indicated to prevent or reverse a neurologic deficit, pedicle erosion often precludes the use of pedicle screws for segmental fixation at the involved levels. Furthermore, the osteopenic nature of type-1 neurofibromatosis predisposes patients to higher pseudarthrosis rates after spinal fusion21,25-27.
While substantially less common than scoliosis and kyphosis, vertebral dislocation has been reported in patients with type-1 neurofibromatosis10,18,20,28-30. Three published cases of thoracic dislocation of the dystrophic subtype were corrected with a combined anterior-posterior surgical approach to attain circumferential spinal fusion18,28,30. In one of these patients, Kim et al.30 utilized a pedicle screw/rod spinal instrumentation construct to treat the deformity.
Despite the success of staged anterior-posterior spinal procedures, circumferential spinal fusion may be associated with greater surgical morbidity than is a single surgical exposure. Two separate surgical exposures increase operative time and blood loss, especially when one considers the extensive vascularity of neurofibromatous tissue adjacent to the anterior aspect of the spine17. Thus, Stone et al.10 described the use of posterior-only instrumentation and fusion to correct upper thoracic spontaneous vertebral dislocation associated with dural ectasia in a patient with type-1 neurofibromatosis. Eichhorn et al.14 subsequently presented the case of a patient with type-1 neurofibromatosis with severe lumbar dural ectasia without dislocation that was treated with posterior-only fusion with pedicle screw/rod spinal instrumentation.
In this report, we describe two patients with type-1 neurofibromatosis in whom dystrophic spinal deformities were successfully treated with posterior-only pedicle screw-based instrumented spinal fusion and use of recombinant human bone morphogenetic protein-2 (rhBMP-2) as a biologic agent to achieve solid fusion.
Case Reports
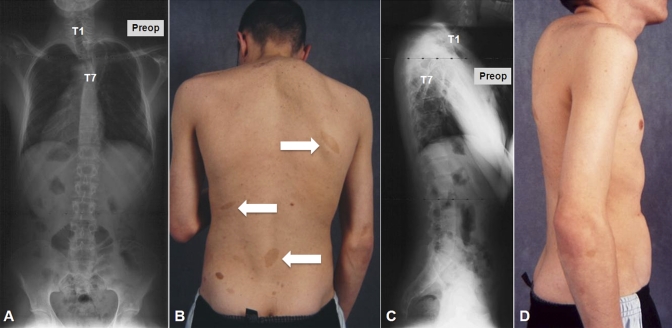
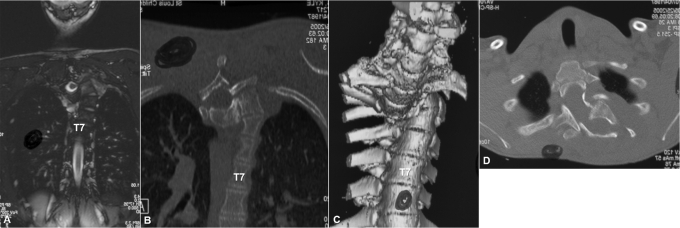
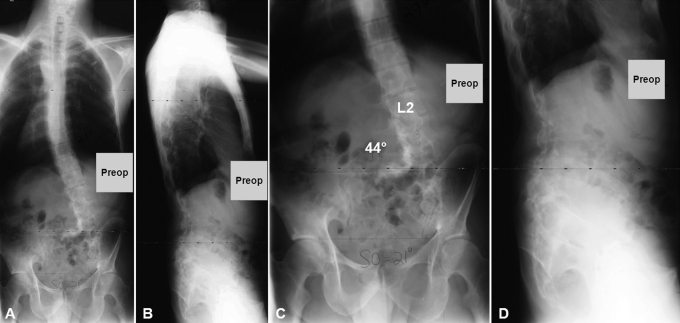
Case 1. A seventeen-year-old boy with type-1 neurofibromatosis presented with increasing upper back pain, which he had had for several months. He had no neurologic symptoms, and bowel function and bladder function were normal. There was no history of trauma. On physical examination, he was found to be a well-developed, lean boy with multiple café-au-lait spots throughout his trunk. He had increased cervical lordosis and severe upper thoracic kyphosis with a right-sided posterior prominence at the cervicothoracic junction (Fig. 1). He was neurologically intact in all extremities with no long-tract signs. Initial radiographs, magnetic resonance imaging (MRI), and computed tomography (CT) scans showed complete spontaneous dislocation of T3 on T4 with marked angular kyphosis and dural ectasia with a widened spinal canal (Figs. 1 and 2). The extent of deformity was such that two different planes of the spine, axial and coronal, could be visualized on the same MRI and CT cut (Fig. 2, A and B). Also, the classic double-vertebrae sign of rotational dislocation of the spine was observed on axial CT images (Fig. 2, D).
Fig. 1.
Case 1. Preoperative anteroposterior (A) and lateral (C) radiographs show complete spontaneous dislocation of the upper thoracic spine. Preoperative clinical photographs show café-au-lait spots (B, arrows) and severe cervicothoracic kyphosis with a right-sided prominence (D).
Fig. 2.
Case 1. Coronal MRI (A) and CT (B) images show two different planes, axial and coronal, of the dislocated spine in the same cut (the double-plane sign). Three-dimensional reconstruction of the CT scan shows complete dislocation of the spine (C). Axial cut of the CT scan shows the classic double-vertebrae sign of rotational dislocation of the spine.
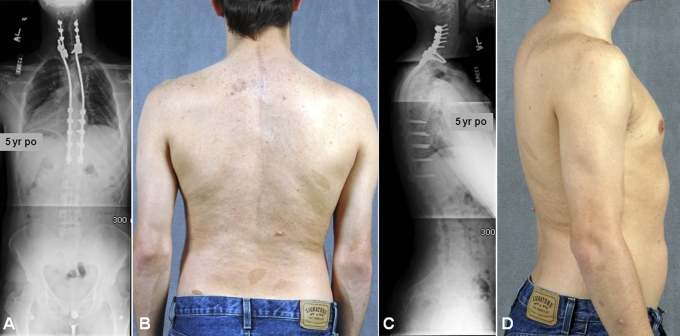
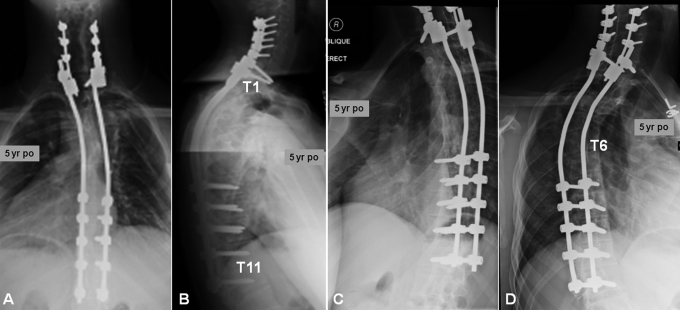
The patient underwent halo-gravity traction (with up to 30 lb [14 kg]) to reduce the dislocation. Definitive posterior spinal fusion was then performed with segmental instrumentation with use of lateral mass screws from C4 to C6 and pedicle screws at T1 and from T8 to T12 bilaterally. Dural ectasia and subsequent erosion of pedicles precluded the safe use of pedicle screws from T2 to T7. Therefore, rhBMP-2 (48 mg) was utilized in addition to allograft (50 mL) and autologous iliac bone graft (30 mL) to aid fusion. The patient wore a cervicothoracolumbosacral orthosis for four months postoperatively. At his five-year follow-up visit, radiographs demonstrated intact spinal instrumentation with robust bone formation. He continued to report high satisfaction without pain, deformity, or a neurologic deficit (Figs. 3, 4, and 5).
Fig. 3.
Case 1. Five-year postoperative anteroposterior (A) and lateral (C) radiographs show corrected alignment and an intact, instrumented fusion construct. Five-year postoperative clinical photographs (B and D) show a markedly improved clinical appearance.
Fig. 4.
Case 1. Five-year postoperative anteroposterior (A), lateral (B), right oblique (C), and left oblique (D) radiographs focused on the cervicothoracic fusion construct.
Fig. 5.
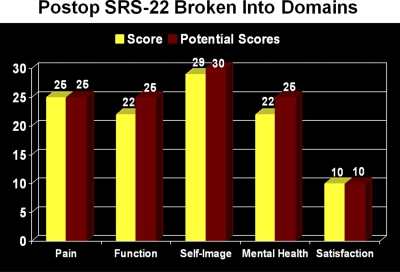
Case 1. Five-year postoperative Scoliosis Research Society-2258 (SRS-22) Questionnaire scores.
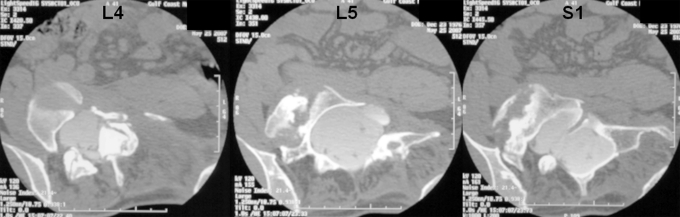
Case 2. A thirty-year-old man with type-1 neurofibromatosis presented with severe back and right lower-extremity pain. The patient recalled no antecedent trauma. On physical examination, multiple café-au-lait spots were observed. He was neurologically intact in all extremities. Radiographs and a CT myelogram confirmed the presence of severe dural ectasia from L3 to L5 and more extensively throughout the sacrum (Figs. 6 and 7).
Fig. 6.
Case 2. Preoperative long (A) and focused (C) anteroposterior and long (B) and focused (D) lateral radiographs showing multiple subluxations of the lumbar vertebrae.
Fig. 7.
Case 2. Axial cuts of the preoperative CT myelogram showing extensive osseous erosion with dural ectasia and rotational subluxation of the lumbosacral spine.
Over the next several months, the pain in the right lower extremity continued to worsen, and the patient's ability to walk gradually declined. Radiographs showed progressive rotatory kyphoscoliosis and multiple vertebral subluxations from L2 to the sacrum. The patient initially underwent halo-gravity traction for a short period of time (two weeks) with a decrease in the lower-extremity pain but negligible correction of the deformity. A posterior spinal fusion with instrumentation was then performed from T12 to the ilium. Extensive dural ectasia and osseous dysplasia precluded the use of pedicle screws from L3 to the sacrum. Furthermore, the need for distal fixation at the ilium prevented the harvesting of an autologous bone graft. Thus, eleven total fixation points were established with six bilateral pedicle screws (from T12 to L2) and five iliac screws, two on the left side and three on the right side. Because of the poor local bone stock, rhBMP-2 (280 mg) was utilized in a compression-resistant matrix carrier (140 mL) at a concentration of 2 mg/mL, and 40 mg/level of rhBMP-2 was applied as previously described by Dimar et al.31. Substantially more rhBMP-2 was used in this case, as compared with the amount used in Case 1 (48 mg), as no autogenous local bone was available to supplement the biologic agent.
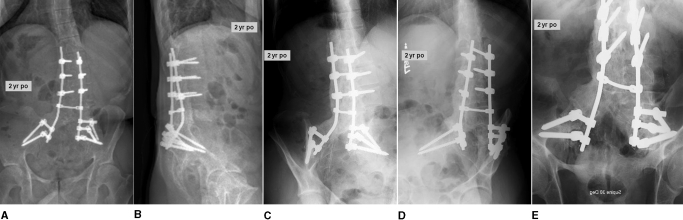
The patient was maintained in a thoracolumbosacral orthosis with a thigh cuff for four months postoperatively. We believed that the thigh cuff was an important addition to protect the pelvic portion of the reconstruction. At the two-year follow-up visit, the pain in the back and right lower extremity had fully resolved, and a solid fusion was noted throughout (Figs. 8 and 9). There was no radiographic evidence of deformity progression.
Fig. 8.
Case 2. Two-year postoperative short anteroposterior (A), short lateral (B), right oblique (C), left oblique (D), and Ferguson (E) radiographs of the robust fusion mass.
Fig. 9.
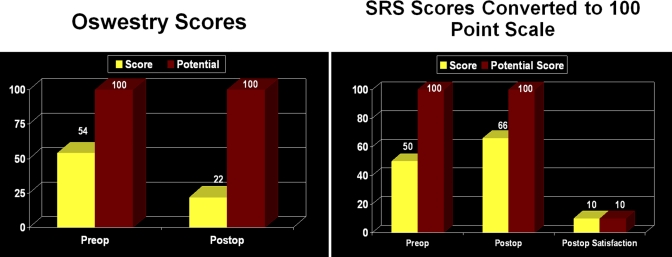
Case 2. Preoperative and two-year postoperative Oswestry Disability Index59 and Scoliosis Research Society-2258 (SRS) Questionnaire scores.
Discussion
Spinal deformity is a common manifestation of type-1 neurofibromatosis4,13. Dural ectasia and other dystrophic lesions have been shown to cause rapid erosion of osseous structures that surround the spinal cord and generate spinal instability, which results in complete dislocation of the spine in severe cases4,14,23. Most patients with type-1 neurofibromatosis, however, exhibit normal neurologic function even in the setting of complete spinal dislocation, partially as a result of a pathologically ectatic thecal sac and widened spinal canal4,13,20.
Traditionally, combined anterior and posterior surgical approaches have been employed to achieve circumferential spinal fusion. More recently, treatment of spinal dislocations in patients with type-1 neurofibromatosis with use of posterior-only instrumented fusion has been described10,32,33. Erosion and weakening of the bone, however, render posterior instrumentation challenging. Dysplastic bone in type-1 neurofibromatosis leads to a high incidence of hook dislodgement22. Decreased bone mineral density predisposes the instrumentation construct to screw pullout34.
Despite the superior biomechanical properties of pedicle screws compared with hooks and wires and the routine use of pedicle screw instrumentation in many spinal deformity procedures, we are not aware of any reported cases in which pedicle screw-based instrumentation, without supplemental anterior surgery, has been used to treat dislocations at the spinal cord level in patients with type-1 neurofibromatosis35. To our knowledge, Case 1 is the first reported case of successful posterior-only spinal fusion with pedicle screw-based instrumentation for treatment of spontaneous dislocation of the upper thoracic spine in type-1 neurofibromatosis. Sublaminar wires alone10,28 or in combination with pedicle screws36 could have been an acceptable form of spinal instrumentation in these patients. However, sublaminar wires are known to be poor anchors, especially proximally, in the presence of kyphosis. Furthermore, the posterior elements were very dysplastic, which could have predisposed sublaminar wires to pull-out through those posterior elements due to erosion. In our two cases, it did not appear that use of sublaminar wires would provide the ideal spinal instrumentation construct because of the dysplastic posterior elements and dural ectasia.
As described in Cases 1 and 2, dural ectasia and pedicular erosion prevented the safe use of pedicle screws at the deformity apices. Ironically, vertebral levels adjacent to a dislocation are where segmental fixation is most needed to achieve optimal biomechanical stability by minimizing the bending moment37,38. In Case 1, pedicle screws could not be safely inserted from T2 to T7. In Case 2, no screws were placed from L3 to the sacrum. This made the instrumentation more tenuous while fusion took place. Furthermore, patients with type-1 neurofibromatosis are known to be osteopenic and reportedly have high pseudarthrosis rates of up to 60%13,21,26,27.
We circumvented this problem with the off-label use of a biologic agent, rhBMP-2. Use of rhBMP-2 in spinal arthrodesis has been studied extensively and has demonstrated equivalent or better fusion rates than autologous iliac bone graft39-41. Investigations of animals and humans have demonstrated faster fusion with the biologic agent rhBMP-242-45. We believe that rhBMP-2 allowed faster, more robust bone formation and eventual fusion, either synergistically with the autologous graft (Case 1) or alone (Case 2), and was essential in these challenging cases. It is conceivable that solid fusion could have been achieved without rhBMP-210,14. However, bilateral harvest of iliac crest bone graft may have been necessary in addition to multilevel anterior spinal fusion. Had there been pseudarthrosis, an anterior fusion to supplement the posterior procedure would have been considered. Although not seen in our patients, there are reports of curve progression even after achievement of solid fusion in patients with type-1 neurofibromatosis16,46, and for this reason, further follow-up is necessary.
Despite the demonstrated efficacy of rhBMP-2, the BMP family of endogenous growth factors has been associated with the promotion of tumor formation in animals47. Many BMP receptors are upregulated and expressed on cell membranes of certain neoplasms48-50. We are not aware of any reports of BMP-induced cancer in humans, and no definitive association between BMP and the promotion of tumorigenesis or metastasis has been documented47. Moreover, the rapid pharmacokinetics of rhBMP-2, with a half-life of only two days, makes tumorigenesis unlikely51,52. Nevertheless, interaction of the rhBMP-2 with hyperproliferative neurofibromata was a potential concern, especially in a young patient (Case 1). For this reason, care was taken to avoid direct contact with neurofibromatous tissue when the rhBMP-2 was applied.
Heterotopic ossification is another rare but known complication associated with rhBMP-253. Neurologic compromise associated with rhBMP-2-induced ectopic bone formation seems to occur primarily during posterior or transforaminal lumbar interbody fusion, procedures in which the dura is exposed to rhBMP-2. In our cases, care was taken to preserve the lamina and avoid exposing the adjacent dura to prevent direct application of rhBMP-2 on neural elements.
Ong et al.54 recently reported that BMP use during spinal procedures is on the rise and 85% of its application is off-label. In addition to ethical and medical concerns, the question of whether BMP use is justified financially remains unresolved. Some have argued that rhBMP-2 is cost-effective in the long-term55-57. The authors of one study concluded that the up-front initial increased cost of rhBMP-2 compared with that of iliac crest autograft in anterior lumbar interbody fusion would be offset by other medical costs incurred over a two-year period after use of iliac crest autograft55. In our two cases, the short-term success with regard to achieving solid fusion without the need for additional anterior procedures resulted from the off-label use of rhBMP-2.
In conclusion, substantial vertebral subluxation or even complete dislocation of the spine can occur in patients with dystrophic type-1 neurofibromatosis whose neurologic function is spared. Following gradual halo-gravity traction, surgical stabilization should be considered for these challenging cases. Posterior-only procedures with pedicle screw-based instrumentation and rhBMP-2 as a biologic adjuvant can be used to achieve fusion and avoid anterior spinal procedures. However, both the risks and the benefits of off-label use of rhBMP-2 should be carefully considered on a case-by-case basis.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Crawford AH, Jr, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6:72-88 [DOI] [PubMed] [Google Scholar]
- 2.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124-33 [DOI] [PubMed] [Google Scholar]
- 3.Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). J Med Genet. 1996;33:2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsirikos AI, Saifuddin A, Noordeen MH. Spinal deformity in neurofibromatosis type-1: diagnosis and treatment. Eur Spine J. 2005;14:427-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maertens O, De Schepper S, Vandesompele J, Brams H, Heyns I, Janssens S, Speleman F, Legius E, Messiaen L. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir D, Neubauer D, Lim IT, Yachnis AT, Wallace MR. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am J Pathol. 2001;158:501-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, Towers R, Gleeson M, Steiger C, Kirby A. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbarnia BA, Gabriel KR, Beckman E, Chalk D. Prevalence of scoliosis in neurofibromatosis. Spine (Phila Pa 1976). 1992;17(8 Suppl):S244-8 [DOI] [PubMed] [Google Scholar]
- 9.Rezaian SM. The incidence of scoliosis due to neurofibromatosis. Acta Orthop Scand. 1976;47:534-9 [DOI] [PubMed] [Google Scholar]
- 10.Stone JW, Bridwell KH, Shackelford GD, Abramson CL. Dural ectasia associated with spontaneous dislocation of the upper part of the thoracic spine in neurofibromatosis. A case report and review of the literature. J Bone Joint Surg Am. 1987;69:1079-83 [PubMed] [Google Scholar]
- 11.Funasaki H, Winter RB, Lonstein JB, Denis F. Pathophysiology of spinal deformities in neurofibromatosis. An analysis of seventy-one patients who had curves associated with dystrophic changes. J Bone Joint Surg Am. 1994;76:692-700 [DOI] [PubMed] [Google Scholar]
- 12.Kim HW, Weinstein SL. Spine update. The management of scoliosis in neurofibromatosis. Spine (Phila Pa 1976). 1997;22:2770-6 [DOI] [PubMed] [Google Scholar]
- 13.Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am. 2007;38:553-62, vii [DOI] [PubMed] [Google Scholar]
- 14.Eichhorn C, Wendt G, Staudte HW, Gilsbach JM. Dural ectasia in von Recklinghausen's disease of the lumbar spine: a case report. J Bone Joint Surg Br. 1995;77:834-5 [PubMed] [Google Scholar]
- 15.Durrani AA, Crawford AH, Chouhdry SN, Saifuddin A, Morley TR. Modulation of spinal deformities in patients with neurofibromatosis type 1. Spine (Phila Pa 1976). 2000;25:69-75 [DOI] [PubMed] [Google Scholar]
- 16.Wilde PH, Upadhyay SS, Leong JC. Deterioration of operative correction in dystrophic spinal neurofibromatosis. Spine (Phila Pa 1976). 1994;19:1264-70 [DOI] [PubMed] [Google Scholar]
- 17.Hsu LC, Lee PC, Leong JC. Dystrophic spinal deformities in neurofibromatosis. Treatment by anterior and posterior fusion. J Bone Joint Surg Br. 1984;66:495-9 [DOI] [PubMed] [Google Scholar]
- 18.Rockower S, McKay D, Nason S. Dislocation of the spine in neurofibromatosis. A report of two cases. J Bone Joint Surg Am. 1982;64:1240-2 [PubMed] [Google Scholar]
- 19.Winter RB, Lovell WW, Moe JH. Excessive thoracic lordosis and loss of pulmonary function in patients with idiopathic scoliosis. J Bone Joint Surg Am. 1975;57:972-7 [PubMed] [Google Scholar]
- 20.Curtis BH, Fisher RL, Butterfield WL, Saunders FP. Neurofibromatosis with paraplegia. Report of eight cases. J Bone Joint Surg Am. 1969;51:843-61 [PubMed] [Google Scholar]
- 21.Betz RR, Iorio R, Lombardi AV, Clancy M, Steel HH. Scoliosis surgery in neurofibromatosis. Clin Orthop Relat Res. 1989;245:53-6 [PubMed] [Google Scholar]
- 22.Savini R, Parisini P, Cervellati S, Gualdrini G. Surgical treatment of vertebral deformities in neurofibromatosis. Ital J Orthop Traumatol. 1983;9:13-24 [PubMed] [Google Scholar]
- 23.Calvert PT, Edgar MA, Webb PJ. Scoliosis in neurofibromatosis. The natural history with and without operation. J Bone Joint Surg Br. 1989;71:246-51 [DOI] [PubMed] [Google Scholar]
- 24.Winter RB, Lonstein JE, Anderson M. Neurofibromatosis hyperkyphosis: a review of 33 patients with kyphosis of 80 degrees or greater. J Spinal Disord. 1988;1:39-49 [PubMed] [Google Scholar]
- 25.Winter RB, Moe JH, Bradford DS, Lonstein JE, Pedras CV, Weber AH. Spine deformity in neurofibromatosis. A review of one hundred and two patients. J Bone Joint Surg Am. 1979;61:677-94 [PubMed] [Google Scholar]
- 26.Crawford AH. Pitfalls of spinal deformities associated with neurofibromatosis in children. Clin Orthop Relat Res. 1989;245:29-42 [PubMed] [Google Scholar]
- 27.Sirois JL, 3rd, Drennan JC. Dystrophic spinal deformity in neurofibromatosis. J Pediatr Orthop. 1990;10:522-6 [PubMed] [Google Scholar]
- 28.Winter RB. Spontaneous dislocation of a vertebra in a patient who had neurofibromatosis. Report of a case with dural ectasia. J Bone Joint Surg Am. 1991;73:1402-4 [PubMed] [Google Scholar]
- 29.Isu T, Miyasaka K, Abe H, Ito T, Iwasaki Y, Tsuru M, Kitaoka K, Tsunoda M. Atlantoaxial dislocation associated with neurofibromatosis. Report of three cases. J Neurosurg. 1983;58:451-3 [DOI] [PubMed] [Google Scholar]
- 30.Kim K-T, Lee S-H, Suk K-S, Lee J-H, Seo E-M, Jeong B- O. Spontaneous vertebral column dislocation in neurofibromatosis. A Case report. J Korean Orthop Assoc. 2007;42:822-7 [Google Scholar]
- 31.Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976). 2006;31:2534-40 [DOI] [PubMed] [Google Scholar]
- 32.Cho SK, Lenke LG, Hanson D. Traumatic noncontiguous double fracture-dislocation of the lumbosacral spine. Spine J. 2006;6:534-8 [DOI] [PubMed] [Google Scholar]
- 33.Vialle R, Court C. Traumatic lateral lumbosacral dislocation: one case and review of literature. J Spinal Disord Tech. 2005;18:286-9 [PubMed] [Google Scholar]
- 34.Illés T, Halmai V, de Jonge T, Dubousset J. Decreased bone mineral density in neurofibromatosis-1 patients with spinal deformities. Osteoporos Int. 2001;12:823-7 [DOI] [PubMed] [Google Scholar]
- 35.Bess RS, Lenke LG, Bridwell KH, Cheh G, Mandel S, Sides B. Comparison of thoracic pedicle screw to hook instrumentation for the treatment of adult spinal deformity. Spine (Phila Pa 1976). 2007;32:555-61 [DOI] [PubMed] [Google Scholar]
- 36.Sun E, Alkalay R, Vader D, Snyder BD. Preventing distal pullout of posterior spine instrumentation in thoracic hyperkyphosis: a biomechanical analysis. J Spinal Disord Tech. 2009;22:270-7 [DOI] [PubMed] [Google Scholar]
- 37.McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine (Phila Pa 1976). 2006;31(11 Suppl):S70-9, S104 [DOI] [PubMed] [Google Scholar]
- 38.Krag MH. Biomechanics of thoracolumbar spinal fixation. A review. Spine (Phila Pa 1976). 1991;16:S84-99 [DOI] [PubMed] [Google Scholar]
- 39.Maeda T, Buchowski JM, Kim YJ, Mishiro T, Bridwell KH. Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine (Phila Pa 1976). 2009;34:2205-12 [DOI] [PubMed] [Google Scholar]
- 40.Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976). 2008;33:2153-9 [DOI] [PubMed] [Google Scholar]
- 41.Papakostidis C, Kontakis G, Bhandari M, Giannoudis PV. Efficacy of autologous iliac crest bone graft and bone morphogenetic proteins for posterolateral fusion of lumbar spine: a meta-analysis of the results. Spine (Phila Pa 1976). 2008;33:E680-92 [DOI] [PubMed] [Google Scholar]
- 42.Rogozinski A, Rogozinski C, Cloud G. Accelerating autograft maturation in instrumented posterolateral lumbar spinal fusions without donor site morbidity. Orthopedics. 2009;32:809. [DOI] [PubMed] [Google Scholar]
- 43.Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301-7 [DOI] [PubMed] [Google Scholar]
- 44.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976). 2002;27:2662-73 [DOI] [PubMed] [Google Scholar]
- 45.Lovell TP, Dawson EG, Nilsson OS, Urist MR. Augmentation of spinal fusion with bone morphogenetic protein in dogs. Clin Orthop Relat Res. 1989:266-74 [PubMed] [Google Scholar]
- 46.Holt RT, Johnson JR. Cotrel-Dubousset instrumentation in neurofibromatosis spine curves. A preliminary report. Clin Orthop Relat Res. 1989;245:19-23 [PubMed] [Google Scholar]
- 47.Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66:233-46 [DOI] [PubMed] [Google Scholar]
- 48.Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Büchler MW, Korc M. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202-16 [DOI] [PubMed] [Google Scholar]
- 49.Laitinen M, Jortikka L, Halttunen T, Nevalainen J, Aho AJ, Marttinen A, Lindholm TS. Measurement of total and local bone morphogenetic protein concentration in bone tumours. Int Orthop. 1997;21:188-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshikawa H, Rettig WJ, Lane JM, Takaoka K, Alderman E, Rup B, Rosen V, Healey JH, Huvos AG, Garin-Chesa P. Immunohistochemical detection of bone morphogenetic proteins in bone and soft-tissue sarcomas. Cancer. 1994;74:842-7 [DOI] [PubMed] [Google Scholar]
- 51.Uludag H, Gao T, Porter TJ, Friess W, Wozney JM. Delivery systems for BMPs: factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83 Suppl 1 (Pt 2):S128-35 [PubMed] [Google Scholar]
- 52.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613-29 [DOI] [PubMed] [Google Scholar]
- 53.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62(5 Suppl 2):ONS423-31, ONS431 [DOI] [PubMed] [Google Scholar]
- 54.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976). 2010;35:1794-800 [DOI] [PubMed] [Google Scholar]
- 55.Ackerman SJ, Mafilios MS, Polly DW., Jr Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion: an evidence-based modeling approach. Spine (Phila Pa 1976). 2002;27(16 Suppl 1):S94-9 [DOI] [PubMed] [Google Scholar]
- 56.Glassman SD, Carreon LY, Campbell MJ, Johnson JR, Puno RM, Djurasovic M, Dimar JR. The perioperative cost of Infuse bone graft in posterolateral lumbar spine fusion. Spine J. 2008;8:443-8 [DOI] [PubMed] [Google Scholar]
- 57.Polly DW, Jr, Ackerman SJ, Shaffrey CI, Ogilvie JW, Wang JC, Stralka SW, Mafilios MS, Heim SE, Sandhu HS. A cost analysis of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. Orthopedics. 2003;26:1027-37 [DOI] [PubMed] [Google Scholar]
- 58.Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society-22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine (Phila Pa 1976). 2003;28:70-3 [DOI] [PubMed] [Google Scholar]
- 59.Smith JS, Shaffrey CI, Berven S, Glassman S, Hamill C, Horton W, Ondra S, Schwab F, Shainline M, Fu KM, Bridwell K; Spinal Deformity Study Group Improvement of back pain with operative and nonoperative treatment in adults with scoliosis. Neurosurgery. 2009;65:86-93; discussion 93–4 [DOI] [PubMed] [Google Scholar]