Abstract
Objective
To describe the patterns and clinical features of toxicity related to recreational use of mephedrone and other cathinones in the UK using data collected by the National Poisons Information Service (NPIS).
Methods
The number of accesses to TOXBASE, the NPIS online poisons information database, details of consecutive cases uploaded onto TOXBASE and the number and details of telephone enquiries made to the NPIS by health professionals in the UK were collected for the period March 2009 to February 2010.
Results
Over the year of study there were 2901 TOXBASE accesses and 188 telephone enquiries relating to cathinones, the majority relating to mephedrone (TOXBASE 1664, telephone 157), with a month-on-month increase in numbers. In 131 telephone enquiries concerning mephedrone, alone or in combination with alcohol, common clinical features reported included agitation or aggression (n=32, 24%, 95% CI 18% to 33%), tachycardia (n=29, 22%, 95% CI 16% to 30%), confusion or psychosis (n=18, 14%, 95% CI 9% to 21%), chest pain (n=17, 13%, 95% CI 8% to 20%), nausea (n=15, 11%, 95% CI 7% to 18%), palpitations (n=14, 11%, 95% CI 6% to 18%), peripheral vasoconstriction (n=10, 8%, 95% CI 4% to 14%) and headache (n=7, 5%, 95% CI 2% to 11%). Convulsions were reported in four cases (3%, 95% CI 1% to 8%). One exposed person died following cardiac arrest (1%, 95% CI 0% to 4%), although subsequent investigation suggested that mephedrone was not responsible.
Conclusions
Toxicity associated with recreational mephedrone use is increasingly common in the UK. Sympathomimetic adverse effects are common and severe effects are also reported. Structured data collected by the NPIS may be of use in identifying trends in poisoning and in establishing toxidromes for new drugs of abuse.
Keywords: Toxicology
Introduction
Mephedrone (4-methylmethcathinone, 4-MMC) is one of several synthetic cathinones structurally related to the naturally occurring phenylpropylamine alkaloid cathinone found in the khat plant (Catha edulis).1 This is commonly chewed for its stimulant properties in Somalia and Yemen. Cathinones are β-ketoamfetamine derivatives which possess a ketone group at the β carbon position of the amfetamine backbone (figure 1).1 While cathinone and some of its derivatives are controlled under misuse of drugs regulations in the UK, mephedrone has not been controlled2 until April 2010 when it became classified as a class B drug. It also remains legal in many other countries worldwide. Synthetic cathinones including mephedrone have been freely available and inexpensive for purchase as research chemicals or plant foods in ‘head shops’ and especially via the internet.
Figure 1.
Chemical structures.
Other than some preliminary data on routes of metabolism in rodents and humans,3 4 information currently available about the pharmacology of mephedrone is extremely limited although, as would be expected from their chemical similarity, the properties of other cathinones resemble closely those of amfetamines.5–8 However, although it has been suggested that significant structure–activity similarities exist between specific cathinones and their amfetamine analogues, caution should be used in attempting to draw conclusions or make predictions about the activity and potency of individual analogues.9
Severe clinical effects and deaths apparently associated with mephedrone have been reported widely in the media2 although, to date, analytical confirmation of mephedrone exposure has only been established in a few cases.1 10
The National Poisons Information Service (NPIS) provides information and advice to health professionals in the UK about the management of poisoning via information held on its website TOXBASE11 and by answering enquiries made by telephone. Over the last year the NPIS has received increasing numbers of enquiries relating to synthetic cathinones, predominantly mephedrone. This paper describes the epidemiology and clinical effects of poisoning with these agents as reported to the NPIS by the healthcare professionals involved in their care.
Methods
TOXBASE accesses and telephone enquiry data relating to mephedrone and other synthetic cathinones were sought for the year 1 March 2009 to 28th February 2010. Data for methylenedioxymethamfetamine (MDMA, ‘ecstasy’) and cocaine were also extracted for comparison.
TOXBASE accesses were quantified for clinical users within the UK, excluding users from Ireland, other overseas countries, the Channel Islands and the Isle of Man. Users within the ‘Educational’ (eg, medical schools) and ‘Government Office’ categories were also excluded, as well as those from NPIS units, to avoid double counting. Accesses were classified into ‘sessions’ to consolidate multiple instances of the same entry being accessed during the same session.
TOXBASE provides the opportunity for health professionals to upload structured clinical information about exposures,12 including those involving new or uncommon agents. Information uploaded for cathinones since these entries were added to TOXBASE (table 1) was also collated.
Table 1.
TOXBASE accesses and telephone enquiries relating to selected stimulants March 2009–February 2010
| Substance | Synonyms | TOXBASE accesses | Telephone enquiries | ||
| Date of entry on TOXBASE | n | n | |||
| Mephedrone | 4-methylmethcathinone, 4-MMC, MCAT, ‘bubbles’, ‘meow’, ‘sub coca’, ‘methylamino’, ‘drone’, ‘meph’ | July 2009 | 1664 | 157 | |
| Methedrone | Methoxymethcathinone, bk-PMMA, PMMA, ‘methoxymethcath’ | Nov 2009 | 618 | 6 | |
| Methylone | bk-MDMA, ‘methylene dioxy’ | Nov 2009 | 53 | 6 | |
| Methcathinone | ephedrone, m-cat, methylcathinone | Oct 2009 | 566 | 0 | |
| Ethylone | bk-MDEA, MDEC | No entry | – | 0 | |
| Butylone | bk-MBDB | No entry | – | 2 | |
| Methylenedioxy-pyrovalerone | MDPV, hyperfocusine | No entry | – | 11 | |
| Methelenedioxy-methamfetamine | MDMA, ‘Ecstasy’ | Throughout study | 3524 | 151 | |
| Cocaine | Throughout study | 3707 | 196 | ||
Telephone enquiries to the NPIS are handled by information scientists from the four NPIS units in Birmingham, Cardiff, Edinburgh and Newcastle. Clinical details provided by the enquirer are recorded on the stored on the UK Poisons Information Database which is held on a central server, allowing rapid access to information collected nationally. Data on demographic characteristics (age, sex), severity and clinical features of poisoning relating to mephedrone and other cathinones as reported by enquirers were extracted from the UK Poisons Information Database. Ethical approval was not required for this study since involved analysis of routinely collected clinical information.
Results
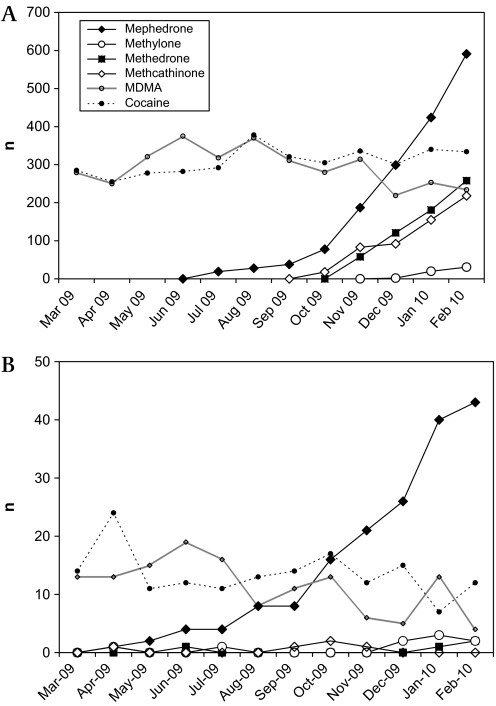
The numbers of telephone and TOXBASE enquiries relating to synthetic cathinones increased steeply over the year of the study, especially those involving mephedrone which has recently become more commonly involved in enquiries than MDMA or cocaine (figure 2). Telephone enquiry numbers for cocaine and MDMA declined over the course of the year.
Figure 2.
(A) TOXBASE accesses and (B) telephone enquiries relating to selected stimulants during study period.
Over the study period the NPIS handled telephone enquiries from about 188 people reported to be exposed to cathinones (table 1). Of these, 157 involved mephedrone and in 131 (77 males, 49 females, 5 sex not known; median age 20 years) the drug was reported to have been used alone or in combination with alcohol only. In the remaining 26 cases other agents were also reported to be involved, including cocaine (n=13), cannabis (n=6), amfetamine, ketamine, growth hormone, buprenorphine, risperidone, quetiapine and methedrone (n=1 for each).
Details of 27 episodes involving cathinones (23 mephedrone, 4 methedrone) have also been uploaded by health professionals onto TOXBASE. In 18 of these cases (13 males, 7 females, median age 20 years) mephedrone was used alone or with alcohol and in five cases it was used in combination with other agents (cocaine, diazepam, heroin, amfetamine, cannabis, trifluoperazine).
Clinical features reported in the cases involving mephedrone taken alone or in combination with alcohol are shown in table 2. Because the methods of data collection are different, telephone enquiry data and TOXBASE upload data are shown separately. For telephone enquiries, ingestion was more common than insufflation (‘snorting’), while in the cases uploaded to TOXBASE, insufflation was more common although details of route of exposure were often not provided. The median mephedrone dose reported was 1 g for both telephone enquiries (n=30) and TOXBASE reports (n=8). In telephone enquiries the median dose was 1 g for both ingestion (n=19) and insufflation (n=11). In most cases, however, this information was not available or not provided.
Table 2.
Clinical features reported with exposure to mephedrone alone or in combination with alcohol as reported in telephone enquiries (n=131) or uploaded to TOXBASE (n=18)
| Telephone enquiries | TOXBASE reports | |||
| n | % (95% CI) | n | % (95% CI) | |
| Route of exposure | ||||
| Ingestion | 69 | 53 (44 to 61) | 2 | 11 (2 to 36) |
| Insufflation | 42 | 32 (24 to 41) | 5 | 28 (11 to 54) |
| Parenteral | 2 | 2 (0 to 6) | 0 | 0 (0 to 22) |
| Other/multiple | 2 | 2 (0 to 6) | 2 | 11 (2 to 36) |
| Not known | 16 | 12 (5 to 16) | 9 | 50 (26 to 73) |
| Clinical features | ||||
| Agitation, aggression | 32 | 24 (18 to 33) | 9 | 50 (26 to 73) |
| Tachycardia | 29 | 22 (16 to 30) | 7 | 39 (18 to 64) |
| Anxiety | 19 | 15 (9 to 22) | 3 | 17 (4 to 42) |
| Confusion, psychosis | 18 | 14 (9 to 21) | 3 | 17 (4 to 42) |
| Chest pain | 17 | 13 (8 to 20) | 5 | 28 (11 to 54) |
| No features | 17 | 13 (8 to 20) | 3 | 17 (4 to 42) |
| Nausea | 15 | 11 (7 to 18) | 4 | 22 (7 to 48) |
| Palpitations | 14 | 11 (6 to 18) | 5 | 28 (11 to 54) |
| Fever, sweating | 12 | 9 (5 to 16) | 2 | 11 (2 to 36) |
| Breathlessness | 11 | 8 (4 to 15) | 2 | 11 (2 to 36) |
| Dizziness | 10 | 8 (4 to 14) | 2 | 11 (2 to 36) |
| Peripheral vasoconstriction | 10 | 8 (4 to 14) | 1 | 6 (3 to 29) |
| Mydriasis | 9 | 7 (3 to 13) | 2 | 11 (2 to 36) |
| Skin changes, rash | 9 | 7 (3 to 13) | 1 | 6 (3 to 29) |
| Headache | 7 | 5 (2 to 11) | 3 | 17 (4 to 42) |
| Reduced level of consciousness | 7 | 5 (2 to 11) | 4 | 22 (7 to 48) |
| Abdominal pain | 6 | 5 (2 to 10) | 2 | 11 (2 to 36) |
| Hypertension | 5 | 4 (1 to 9) | 0 | 0 (0 to 22) |
| Parasthesiae | 5 | 4 (1 to 9) | 0 | 0 (0 to 22) |
| Insomnia | 5 | 4 (1 to 9) | 0 | 0 (0 to 22) |
| Convulsions | 4 | 3 (1 to 8) | 2 | 11 (2 to 36) |
| Loin pain | 4 | 3 (1 to 8) | 1 | 6 (3 to 29) |
| Tongue disorder | 4 | 3 (1 to 8) | 0 | 0 (0 to 22) |
| Myoclonus/abnormal movements | 3 | 2 (1 to 7) | 2 | 11 (2 to 36) |
| Tremor | 3 | 2 (1 to 7) | 2 | 11 (2 to 36) |
| ECG abnormal | 3 | 2 (1 to 7) | 0 | 0 (0 to 22) |
| Local effects (mouth/pharynx) | 3 | 2 (1 to 7) | 0 | 0 (0 to 22) |
| Dystonic reaction | 2 | 2 (1 to 7) | 0 | 0 (0 to 22) |
| Abnormal vision | 2 | 2 (1 to 7) | 1 | 6 (3 to 29) |
| Liver function tests abnormal | 2 | 2 (1 to 7) | 0 | 0 (0 to 22) |
| Raised creatine kinase | 1 | 1 (0 to 4) | 3 | 17 (4 to 42) |
| Acidosis | 1 | 1 (0 to 4) | 1 | 6 (3 to 29) |
| Epistaxis | 1 | 1 (0 to 4) | 1 | 6 (3 to 29) |
| Renal function abnormal | 1 | 1 (0 to 4) | 0 | 0 (0 to 22) |
| Death | 1 | 1 (0 to 4) | 0 | 0 (0 to 22) |
| Persistence of symptoms after exposure | ||||
| >24 h | 59 | 45 (36 to 54) | 4 | 22 (7 to 48) |
| >48 h | 39 | 30 (22 to 38) | 1 | 6 (3 to 29) |
The most common clinical features reported by health professionals with mephedrone exposure were those typical for a sympathomimetic agent including tachycardia, palpitations, agitation, anxiety, mydriasis, tremor, fever or sweating and hypertension (table 2). Some patients reported features suggesting peripheral vasoconstriction such as white or blue extremities which were sometimes painful. Other common features included nausea, breathlessness, dizziness and headache. Skin rashes and local effects in the mouth, pharynx or nose were also occasionally reported. Symptoms were often reported to be prolonged after mephedrone exposure (table 2).
Chest pain was a common symptom; in one case this was associated with ECG changes suggesting acute myocardial infarction. Confusion and/or psychosis were also frequent. Three patients were reported to have generalised convulsions; in one episode this was following an apparent cardiac arrest and in two episodes no prior history of epilepsy was recorded. A further patient was described as having focal convulsions and another patient had undiagnosed blackouts. In other episodes convulsions were not reported but a reduced level of consciousness or raised creatine kinase was documented. There were also occasional reports of acidosis and abnormal liver function tests (raised transaminases) and a single report of spontaneous pneumomediastinum associated with insufflation.
One NPIS enquiry was made during unsuccessful resuscitation attempts following an apparent cardiac arrest in a patient exposed to mephedrone.
Discussion
NPIS data have a number of limitations that need to be considered in interpretation. Enquiry numbers are not a direct measurement of patient presentations to hospital since advice is more likely to be sought for sicker patients or when unfamiliar agents are involved. The data analysed relies on users and healthcare professionals knowing and providing accurate information on the agents involved since toxicological confirmation is not generally available. A particular problem in this respect is the similarity of drug names, which may be confused. Clinical features may be underestimated because they are not reported or recorded at the time of enquiry or because they occur after an enquiry has been made. Incomplete follow-up of enquiries results in limited information being available about later complications or final outcome. Information uploaded to TOXBASE by health professionals is more likely to be complete than telephone data, but patients with more severe or unusual outcomes may be more likely to be reported. For both methods the reported clinical features may reflect the effects of other agents used concurrently and details of these agents may not be reported.
Allowing for these limitations, however, it is apparent that structured data collected by the NPIS may be of use in identifying trends in poisoning and in establishing toxidromes for new drugs of abuse. Enquiries relating to mephedrone in particular have become commonplace in the UK, reflecting a substantial workload for healthcare professionals, especially those working in emergency departments. This may be partly offset by reductions in presentations associated with other stimulants such as MDMA and cocaine, although it is too early to draw reliable conclusions.
Most mephedrone exposures are not associated with severe toxicity although, as predicted from its amfetamine-like chemical structure, sympathomimetic effects are common and have been described previously in one case of confirmed mephedrone exposure13 and in a series of exposed people attending an emergency department in London.1 A similar pattern of common effects was also reported from a survey of users, with sweating, headaches, nausea, palpitations and cold or blue fingers being common.14
The occurrence of severe features including hallucinations, chest pains and convulsions is of particular concern. The reported episode of apparent myocardial infarction is of interest; it is not possible to demonstrate causality from this single report, but an increased risk of myocardial infarction has been reported in users of khat.15 Other effects such as confusion, fever or myoclonus may reflect serotoninergic actions of the drug.
One patient in this series died having experienced cardiac arrest, apparently in the context of mephedrone use, but subsequent investigation suggested that mephedrone was not responsible. Deaths have previously been reported in mephedrone users and in some cases toxicological confirmation of the presence of mephedrone is available,1 10 although this does not prove that death was caused by mephedrone exposure.
It is of interest that clinical features, including severe effects, sometimes appear to persist for (or occur) more than 24 h after the most recent reported exposure. The explanation for this is unclear; elimination of other cathinones appears rapid, with elimination half-lives in humans reported as 1.5–2.3 h for cathinone and 5.2 h for cathine.7 16 Pharmacokinetic information is not currently available for mephedrone. For amfetamines, repetitive use has been reported to increase the apparent half-life and duration of effect.17
NPIS data are not helpful for predicting the longer term toxic effects of mephedrone, but available data for other cathinones are not reassuring. Use of Khat has been associated with an increased risk of psychosis18 while methcathinone (ephedrone), a dopamine and serotonin transporter substrate, reduces frontal concentrations of dopamine and serotonin19 and has toxic actions against dopaminergic and serotoninergic neurons.20 A Parkinsonian syndrome has been reported in the intravenous use of methcathinone synthesised from pseudoephedrone using potassium permanganate, although this appears to result from chronic manganese toxicity rather than as a direct neurotoxic effect of the cathinone.21
It remains to be seen what effects recent changes in legal status may have on the pattern of presentations associated with the toxicity of mephedrone and other cathinones. In the meantime, health professionals, especially those working in emergency departments and drug rehabilitation services, should be aware of mephedrone and its acute toxic effects.
Footnotes
Funding: The National Poisons Information Service is commissioned by the Health Protection Agency. No specific research funding was allocated for this project.
Competing interests: None.
Contributors: All authors were involved in the design of the study and approved the final manuscript. DJ, RA, RS, GC and DJL obtained and analysed the data. SHLT and JPT drafted the manuscript. SHLT acts as guarantor.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Advisory Council on the Misuse of Drugs Consideration of the cathinones. 31 March 2010. http://drugs.homeoffice.gov.uk/publication-search/acmd/ACMD-cathinones-report.html (accessed 1 Apr 2010).
- 2.Winstock AR, Marsden J, Mitcheson L. What should be done about mephedrone? BMJ 2010;340:c1605. [DOI] [PubMed] [Google Scholar]
- 3.Kamata HT, Shima N, Zaitsu K, et al. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica 2006;36:709–23 [DOI] [PubMed] [Google Scholar]
- 4.Markus R, Meyer MR, Wilhelm J, et al. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry. Anal Bioanal Chem 2010;397:1225–33 [DOI] [PubMed] [Google Scholar]
- 5.Glennon RA, Yousif MY, Naiman N, et al. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav 1987;26:547–51 [DOI] [PubMed] [Google Scholar]
- 6.Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol 1992;70:77–86 [DOI] [PubMed] [Google Scholar]
- 7.Widler P, Mathys K, Brenneisen R, et al. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clin Pharmacol Ther 1994;55:556–62 [DOI] [PubMed] [Google Scholar]
- 8.Cozzi NV, Sievert MK, Shulgin AT, et al. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol 1999;381:63–9 [DOI] [PubMed] [Google Scholar]
- 9.Dal Casona TA, Young R, Glenon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogues. Pharmacol Biochem Behav 1997;58:1109–16 [DOI] [PubMed] [Google Scholar]
- 10.Gustaffsson D, Escher C. Mefedron – Internetdrog som tycks ha kommit för att stanna. (Mephedrone – Internet drug that seems to have come to stay). Läkartidningen 2009;106:2769–71 [PubMed] [Google Scholar]
- 11.Bateman DN, Good AM, Laing WJ, et al. TOXBASE: Poisons information on the internet. Emerg Med J 2002;19:31–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams RD, Lupton D, Good AM, et al. UK childhood exposures to pesticides 2004–2007: a TOXBASE toxicovigilance study. Arch Dis Child 2009;94:417–20 [DOI] [PubMed] [Google Scholar]
- 13.Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of 4-methylmethcathinone (4-MMC) presenting with sympathomimetic toxicity and confirmed by toxicological screening. Clin Toxicol 2009;47:733 [Google Scholar]
- 14.Mixmag Mephedrone: meet the UK's favourite new drug. 2010. http://www.mixmag.net/mephedrone (accessed 23 Mar 2010).
- 15.Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat checwing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol 2005;59:574–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toennes SW, Harder S, Schramm M, et al. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br J Clin Pharmacol 2003;56:125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jönsson LE, Anggård E, Gunne LM. Blockade of intravenous amphetamine euphoria in man. Clin Pharmacol Ther 1971;12:889–96 [DOI] [PubMed] [Google Scholar]
- 18.Odenwald M, Neuner F, Schauer M, et al. Khat use as risk factor for psychotic disorders: a cross-sectional and case-control study in Somalia. BMC Med 2005;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cozzi NV, Foley KF. Methcathinone is a substrate for the serotonin uptake transporter. Pharmacol Toxicol 2003;93:219–25 [DOI] [PubMed] [Google Scholar]
- 20.Sparago M, Wlos J, Yuan J, et al. Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J Pharmacol Exp Ther 1996;279:1043–52 [PubMed] [Google Scholar]
- 21.Stepens A, Logina I, Liguts V, et al. A Parkinsonian syndrome in methcathinone users and the role of manganese. N Engl J Med 2008;358:1009–17 [DOI] [PubMed] [Google Scholar]