Abstract
ATP released from airway epithelial cells promotes purinergic receptor-regulated mucociliary clearance activities necessary for innate lung defense. Cell swelling-induced membrane stretch/strain is a common stimulus that promotes airway epithelial ATP release, but the mechanisms transducing cell swelling into ATP release are incompletely understood. Using knockdown and knockout approaches, we tested the hypothesis that pannexin 1 mediates ATP release from hypotonically swollen airway epithelia and investigated mechanisms regulating this activity. Well differentiated primary cultures of human bronchial epithelial cells subjected to hypotonic challenge exhibited enhanced ATP release, which was paralleled by the uptake of the pannexin probe propidium iodide. Both responses were reduced by pannexin 1 inhibitors and by knocking down pannexin 1. Importantly, hypotonicity-evoked ATP release from freshly excised tracheas and dye uptake in primary tracheal epithelial cells were impaired in pannexin 1 knockout mice. Hypotonicity-promoted ATP release and dye uptake in primary well differentiated human bronchial epithelial cells was accompanied by RhoA activation and myosin light chain phosphorylation and was reduced by the RhoA dominant negative mutant RhoA(T19N) and Rho and myosin light chain kinase inhibitors. ATP release and Rho activation were reduced by highly selective inhibitors of transient receptor potential vanilloid 4 (TRPV4). Lastly, knocking down TRPV4 impaired hypotonicity-evoked airway epithelial ATP release. Our data suggest that TRPV4 and Rho transduce cell membrane stretch/strain into pannexin 1-mediated ATP release in airway epithelia.
Keywords: ATP, Epithelial Cell, Purinergic Receptor, Rho, TRP Channels, ATP Release, Airway Epithelia, Pannexin 1
Introduction
The mucociliary clearance process that removes foreign particles and pathogens from the airways is the primary innate defense mechanism in the lung (1). Nucleotides and nucleosides within the airway surface liquid (ASL)3 regulate key components of mucociliary clearance via activation of epithelial cell surface purinergic receptors (2, 3). ATP activates the Gq-coupled P2Y2 receptor that promotes mucin secretion and ciliary beat frequency and regulates electrolyte transport and ASL volume production by inhibiting sodium absorption (4–10) and promoting cystic fibrosis transmembrane conductance regulator and Ca2+-activated Cl− channel activity (11–16). Adenosine, generated from the hydrolysis of ATP, activates the Gs-coupled A2b receptor that promotes cyclic AMP-regulated cystic fibrosis transmembrane conductance regulator Cl− channel activity (17) and increases cilia beat frequency (5). Although ATP and adenosine are naturally occurring signaling molecules in ASL (18–22), the mechanisms of airway epithelial ATP release into the ASL are poorly understood.
The lung epithelia exhibit a complex cellular composition, and thus, several mechanisms and pathways are likely involved in the release of nucleotides onto airway surfaces. Our recent studies with goblet-like cell models indicate that ATP and other nucleotides are released concomitantly with MUC5AC, a secretory mucin, during Ca2+-regulated exocytosis of mucin granules (23, 24). Thus, mucin-secreting granules constitute an important source of ASL ATP, providing a pathway for paracrine signaling to ciliated cells to hydrate secreted mucins (24). A vesicular mechanism for nucleotide release may also operate in non-mucous cells. For example, by selectively manipulating the levels of expression of Golgi-resident nucleotide-sugar transporters in 16HBE14o− cells, a cell line that mimics aspects of ciliated epithelia, we demonstrated that the Golgi lumen is an important source of extracellular UDP-sugar constitutively released from cells (25). Although not formally demonstrated, a similar mechanism may apply for the constitutive release of ATP (26).
Mechanical forces during tidal breathing and coughing and cell swelling during hypotonic gland secretions are ubiquitous stimuli imparting robust ATP release in the airways, but the mechanism involved in mechanically promoted airway epithelial ATP release are not well defined (21, 22, 27–29). Recently, Ransford et al. (30) reported that ATP release from hypotonically swollen primary cultures of human bronchial epithelial (HBE) cells was nearly 60% inhibited by pannexin channel blockers or by knocking down pannexin 1 via shRNA. Thus, pannexin 1 is a candidate ATP release pathway in hypotonically swollen HBE cells. However, regulatory signaling elements transducing hypotonic/mechanical stress into ATP release have not been identified. Moreover, the contribution of pannexin 1 to the physiological release of ATP from native airways is not known. We recently discovered that activation of lung epithelial cell G protein-coupled protease-activated receptors (PAR) resulted in enhanced release of ATP and uptake of propidium iodide in a Rho-dependent manner (31), suggesting a link between Rho activation and the opening of a propidium iodide-permeable plasma membrane channel. In the present study, we tested the hypothesis that ATP release from hypotonically stimulated airway epithelial cells involves Rho-regulated opening of pannexin 1 channels and used a pannexin 1 knockout mouse model to assess the contribution of pannexin 1 to the release of ATP from a physiologically relevant airway tissue, i.e. excised tracheas.
EXPERIMENTAL PROCEDURES
Reagents
2-Phenyl-1,2-benzisoselenazol-3(2H)-one (ebselen), β,γ-methylene ATP, carbenoxolone, flufenamic acid, propidium iodide, luciferase from Photinus pyralis, and anti-α tubulin monoclonal antibody were obtained from Sigma. Luciferin and monoclonal antibodies against Golgi proteins G-p28 and G-p84 were obtained from BD Biosciences. The Rho activation assay biochem kit was purchased from Cytoskeleton (Denver, CO). ML-7 and H1152 were purchased from Calbiochem. The TRPV4 inhibitor HC67047 (32) was a kind gift from Drs. David Clapham and Magdalene Moran (Hydra Biosciences). The pannexin 1 blocking peptide WRQAAFVDSY (10Panx1) (33) and its scrambled version, (SCRPanx1) SADYRVAFWQ, were synthesized at the University of North Carolina Microprotein Sequencing and Peptide Synthesis Facility. Antibodies directed against the carboxyl-terminal tails of human and mouse pannexin 1, respectively, have been previously characterized (34). Other chemicals were from sources reported previously (19, 35).
Generation of Pannexin 1 Knockout Mice
Pannexin 1-targeted heterozygous C57B1/6N mice were obtained from the Knockout Mouse Project (KOMP) (36), which generates disruption of pannexin 1 by insertion of a strong splice acceptor site between exons 1 and 2 (pannexin1tm1a(KOMP)Wtsi). Heterozygous mice were bred to produce homozygous pannexin 1-deficient animals. All animal studies were approved by the Institutional Animal Care and Use Committee and were performed according to principles outlined by the Animal Welfare and the National Institutes of Health guidelines for the care and use of animals in biomedical research. Genotyping was conducted using primers for WT and targeted alleles (supplemental Table 1). Pannexin 1 expression was assessed by RT-PCR and Western blot analysis, as indicated further below (supplemental Figs. 4 and 5). Heterozygous pannexin 1-targeted mice are fertile and produce homozygous-targeted mice at the expected frequency. Homozygous deletion of pannexin 1 has not caused grossly observed abnormalities.
All experiments involving animals were performed in accordance with the protocols approved by the UNC Institutional Animal Care and Use Committee.
Cell Culture and Incubations
Primary cultures of polarized, well differentiated HBE (WD-HBE) cells were provided by the UNC Cystic Fibrosis Center Tissue Culture Core. WD-HBE cells were grown on 12-mm Transwell supports (Costar) and maintained at an air-liquid interface, as described previously (37). A549 lung epithelial cells were grown to confluence on plastic dishes as described previously (31). Calu-3 cells were grown as polarized monolayers, as described (19). Primary cultures of mouse trachea epithelial (MTE) cells were grown on 6-mm coated supports, as described previously (38).
Cells were rinsed twice with Hanks' balanced salt solution (HBSS) supplemented with 1.6 mm CaCl2, 1.8 mm MgCl2, and 25 mm HEPES (pH 7.4) (HBSS+) and preincubated as indicated in the corresponding assay sections. The hypotonic challenge was applied by gently replacing one third of the volume of the extracellular solution with a HEPES-buffered (pH 7.4) solution containing 1.6 mm CaCl2 and1.8 mm MgCl2, thus reducing the solution tonicity to 200 mOsm, as described previously (21). A saline-based (isotonic) solution containing the above additions was used for volume replacement in control cultures.
Measurement of ATP Release
ATP release was quantified using the luciferin/luciferase assay, as described previously (31). Calibration curves using known concentrations of ATP were generated at the end of each experiment. None of the reagents used during ATP release measurements interfered with the luciferase reaction.
Uptake of Propidium Iodide
We have described this assay in detail (31). Briefly, cultures were challenged for 5 min in the presence of 20 μm propidium iodide (added mucosally on polarized primary cultures). At the end of the incubation, the bathing solution was replaced with HBSS+ containing 4% paraformaldehyde. Acquisition of confocal images and quantification of nuclei staining were performed on a Leica SP5 confocal microscope as described (31). Alternatively, to measure propidium iodide uptake in real time, WD-HBE cell cultures were mounted on the microscope platform, and propidium iodide was added. After a 5-min recording, cells were challenged, and serial images were taken during an additional 5 min. Confocal microscopy scanning in the xz axis of WD-HBE and MTE cell cultures verified that nuclei labeled with propidium iodide were localized in the most lumenal cell layer of the cultures. In primary (multilayered) cultures, total nuclei were quantified from the differential interference contrast images. In A549 cells (monolayers), total nuclei were assessed either from the differential interference contrast images or by quantifying propidium iodide staining after permeabilizing the cells with 0.05% Triton X-100. Both methods yield similar results.
RT-PCR Analysis
Total RNA was prepared using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) and reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen). Standard RT-PCR was performed as described (31), except that 45 thermocycles (rather than 35) were used to amplify Panx2 and Panx3 in WD-HBE cells. Amplified products were sequenced at the UNC Genome Analysis. Semi-quantitative PCR was performed in a LightCycler PCR machineR thermal cycler, as described previously (31). Pannexin primers are described in supplemental Table 1. Connexin primers were as follows: forward, 5′-GGGTTAAGGGAAAGAGCGACC-3′ and reverse, 5′-CCCCATTCGATTTTGTTCTGC-3′.
siRNA
Oligonucleotides targeting human pannexin 1 (siRNA-70) and its scrambled control (supplemental Table 2) were purchased from Dharmacon, Inc. A549 cells were transfected with 1 μg of oligonucleotide using the Amaxa Nucleofector Devicetm and Cell Line Nucleofector® Kit T (Amaxa Biosystems, Gaithersburg, MD), following the manufacturer's instructions. Transfected cells were cultured in serum-supplemented DMEM for 48 h prior to assays.
shRNA
Lentiviral vector expression clones (pLKO1/puromycin) containing shRNAs (supplemental Table 2) were obtained from the Lenti-shRNA core facility of the UNC. Cells were infected with the desired lentivirus (106cfu/35-mm dish) and subsequently selected with 0.5 μg/ml puromycin. Cells were used within five passages post-infection.
Overexpression of RhoA(T19N)
A549 cells were transfected with empty pcDNA3.1 vector or vector containing RhoA(T19N) insert using the Amaxa Nucleofector Devicetm and used 48 h post-transfection, as described (31).
RhoA Pull-down Assay and MLC Phosphorylation
Measurements of GTP-bound RhoA were performed using the Rho activation assay biochem kit (Rhoketing pull-down assay), as described previously (31). Duplicated membranes were separately blotted with anti-phospho-MLC(Ser-19) antibody or anti-MLC antibodies (Cell Signaling Technology, Inc., Danvers, MA), and immunoblots were revealed and quantified as described (31). To minimize autocrine feedback via ATP release, hypotonic stress-promoted Rho activation and MLC phosphorylation were assessed in the presence of 5 units/ml apyrase (31).
Cell Volume Regulation
Changes in cell height were measured to estimate cell volume changes, as described previously (21). In brief, WD-HBE cells were loaded with 5 μm calcein-acetoxymethyl ester (AM) (Molecular Probes, Eugene, OR) for 30 min at 37 °C. The apical surface of cultures was equilibrated for 10 min with HBSS+, and the osmolarity of the solution was reduced to 200 mOsm, as indicated above. Images of confocal microscopy scanning in the xz axis were obtained every second for the initial 15 s and then every 5 s for the next 75 s.
Perfusion of Mouse Tracheas
Tracheas were excised from euthanized mice (5–6 weeks old), cannulated/connected to a peristaltic pump, and perfused under controlled flow rates (50 μl/min) for 90 min with HBSS+ to remove cell debris/inflammatory cells, as described (39). After rinsing, effluents were collected at 5-min intervals under isotonic conditions followed by hypotonic (50% tonicity) perfusion conditions, as indicated.
Data Analysis
Data were analyzed by Student's t test or, where indicated, analysis of variance with GraphPad InStat software (21). Statistical significance was defined as p < 0.05 or p < 0.01, as indicated.
RESULTS
Hypotonic Stress Promotes Pannexin 1-mediated Dye Uptake and ATP Release
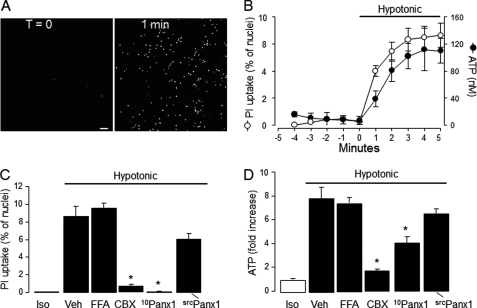
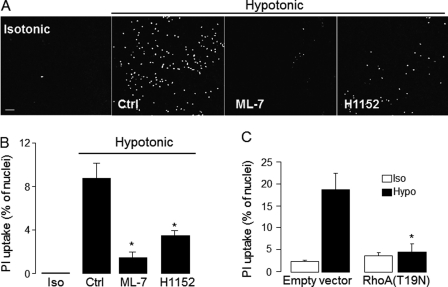
Pannexins and connexins form non-junctional plasma membrane channels that, upon activation, allow the passage of <1 kDa molecules, including ATP and small molecular weight (MW) dyes (40–42). As an initial test for the expression of functional pannexin channels on airway epithelial cells, the uptake of the pannexin/connexin channel-permeable reporter dye propidium iodide (31) was investigated. Propidium iodide displays low intrinsic fluorescence in solution, but its fluorescence increases 20- to 30-fold upon binding to nucleic acids. Resting cultures of WD-HBE cells displayed negligible nuclear labeling with propidium iodide, but exposure of cells to a hypotonic challenge resulted in enhanced uptake of the dye, as judged by the sharp increase of nuclear fluorescence (Fig. 1A). The time course of propidium iodide uptake in hypotonically challenged WD-HBE cells was nearly identical to that of hypotonicity-stimulated ATP release (Fig. 1B).
FIGURE 1.
Hypotonicity-induced dye uptake and ATP release in WD-HBE cells. A, the uptake of propidium iodide (PI) was assessed in real time in response to a 33% hypotonic stress as described under “Experimental Procedures.” The images represent PI-associated nuclear fluorescence at T = 0 and 60 s after the hypotonic challenge. Scale bar = 100 μm. B, time course of hypotonic stress-promoted PI uptake (assessed as in A) and ATP release. Dye uptake is expressed as the percent of nuclei displaying red fluorescence. Similar results were obtained in at least three separate experiments performed in quadruplicate. C and D, WD-HBE cells were preincubated for 15 min with vehicle or with 10 μm carbenoxolone (CBX), 100 μm flufenamic acid (FFA), 30 μm 10Panx1 or its scrambled control (srcPanx1), and exposed for 5 min to either isotonic (Iso) or hypotonic solution containing vehicle (Veh) or the indicated reagent. The results are mean ± S.E., n = 4. *, significant inhibition of hypotonic stress-evoked responses, p < 0.001 (analysis of variance).
Both hypotonicity-stimulated propidium iodide uptake (Fig. 1C) and ATP release (D) were markedly impaired in the presence of 10 μm carbenoxolone, a licorice root derivative that preferentially inhibits pannexin channels over connexin hemichannels and volume-regulated anion channels (43–45). In contrast, 100 μm flufenamic acid, a potent inhibitor of connexin hemichannels that displays low affinity for pannexin 1 (43, 46), had no significant effect on ATP release and propidium iodide uptake in WD-HBE cells (Fig. 1, C and D).
The potential involvement of pannexin 1 in ATP release and dye uptake was further tested by assessing the effect of the pannexin 1-selective blocking peptide 10Panx1. 10Panx1 (30 μm) completely blocked the uptake of propidium iodide in hypotonically challenged WD-HBE cells, whereas its scrambled control peptide (scrPanx1) had a negligible effect (Fig. 1C). In parallel experiments, 10Panx1 (but not scrPanx1) inhibited ATP release (Fig. 1D). These results strongly suggest that pannexin 1 is an important mediator of ATP release from WD-HBE cells. It is worth noting, however, that inhibition of ATP release by 10Panx1 (50–60%) was less robust than the nearly 100% inhibition observed on dye uptake (compare Fig. 1, C and D), suggesting that other mechanisms in addition to pannexin channel activation contributed to ATP release from these cells.
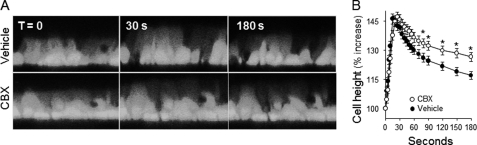
An important control for these studies was to rule out the potential effect of the pharmacological inhibitors on hypotonicity-triggered cell swelling. As shown in Fig. 2, the extent of hypotonic cell swelling was not affected by carbenoxolone, although carbenoxolone delayed the regulatory volume decrease (RVD) (Fig. 2, A and B). Similar to carbenoxolone, 10Panx1 had no effect on hypotonicity-induced cell swelling but reduced RVD (supplemental Fig. 1). The inhibitory effect of carbenoxolone and 10Panx1 on RVD likely reflected less ATP release. That is, ATP released from swollen cells promotes P2Y2 receptor-mediated activation of intermediate-conductance Ca2+-dependent K+ channels, which are essential for the RVD response (21, 47, 48). These results are consistent with the notion that carbenoxolone and 10Panx1 acted downstream of the hypotonic stress sensor but upstream of ATP release-evoked RVD (21).
FIGURE 2.
Hypotonicity induces cell swelling. Calcein-labeled WD-HBE cells were preincubated for 15 min with vehicle or 10 μm carbenoxolone (CBX). A, images of representative confocal microscopy scanning in the xz axis of cells undergoing hypotonic cell swelling. B, quantification of hypotonicity-elicited cell volume changes. The data are mean ± S.E. of three independent experiments performed in triplicate. *, significant difference from control RVD.
RT-PCR analysis indicated that pannexin 1 is expressed in WD-HBE cells (supplemental Fig. 2) as reported (30). Sequence analysis of the PCR reaction product demonstrated the insertion of ggt atg aac ata 66 bp upstream of the termination codon of pannexin 1, suggesting that the 426 amino acid-long pannexin 1b (GenBank accession number NP_056183.2 (49)) is the major pannexin 1 subvariant expressed in HBE cells. Pannexin 2 (49) and pannexin 3 RNAs were weakly or not amplified, respectively (supplemental Fig. 2). These results are consistent with a previous report indicating that pannexin 1 is the major pannexin subtype expressed in HBE cells (30).
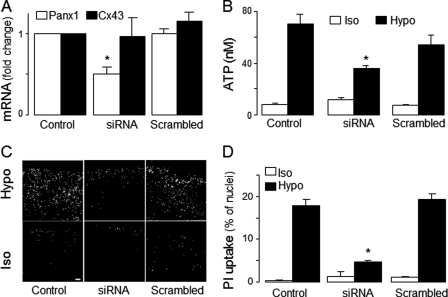
Previously, we demonstrated that regulatory features associated with G protein-coupled receptor-promoted ATP release from WD-HB cells, e.g. sensitivity to carbenoxolone, are also present in lung epithelial carcinoma A549 cells, and that agonist-evoked ATP release from A549 cells was accompanied by carbenoxolone-sensitive uptake of propidium iodide (31). Moreover, like WD-HBE cells, A549 cells displayed enhanced propidium iodide uptake (supplemental Fig. 3A) and ATP release (supplemental Fig. 3B) in response to a hypotonic challenge, which were inhibited by carbenoxolone and 10Panx1. Therefore, given the suitability of A549 cells for siRNA oligonucleotide transfection (31), we utilized siRNA approaches to investigate the involvement of pannexin 1 in ATP release from A549 cells. A549 cells transfected with siRNA-70 (supplemental Table 2), a pannexin 1 siRNA oligonucleotide that knocked down pannexin 1 in various cell lines (40, 50) exhibited a ∼50% reduction of pannexin 1 transcript levels (Fig. 3A). The siRNA approach did not affect the expression of connexin 43 transcripts (Fig. 3A). Pannexin 1 siRNA-transfected cells exhibited reduced hypotonic challenge-promoted ATP release (Fig. 3B) and propidium iodide uptake (C and D). A scrambled oligonucleotide had no effect (Fig. 3).
FIGURE 3.
Pannexin 1 mediates hypotonicity-induced ATP release in A549 cells. A549 cells were sham transfected (Control) or transfected with either pannexin 1 siRNA oligonucleotides (siRNA) or its scrambled control, as described under “Experimental Procedures.” A, Panx1 siRNA decreased pannexin 1 (but not connexin 43 (Cx43)). The data represent mean ± S.E., n = 4. B, ATP release was measured in A549 cells transfected as above and incubated for 5 min in isotonic (Iso) or hypotonic (Hypo) solutions. The data represent mean ± S.E. of three separate experiments performed in triplicate. C, representative images of PI uptake assessed under the conditions described in B. Scale bar = 100 μm. D, quantification of PI uptake (mean ± S.D., n = 4). Similar results were obtained in two independent experiments performed in quadruplicate. *, significantly different from control and scrambled, p < 0.05.
As a complementary approach to the siRNA-70 studies, shRNA lentiviral vectors (supplemental Table 2) were used in A549 cells as well as airway epithelial Calu-3 Cells. A549 and Calu-3 cells stably expressing shRNA-N1 (supplemental Table 2) exhibited a 66% reduction in pannexin 1 transcript levels, relative to uninfected (WT) cells or empty vector-infected (mock) cells. Importantly, shRNA-N1 reduced hypotonicity-evoked ATP release in A549 cells and Calu-3 cells grown as polarized cultures (supplemental Fig. 4B). Western blot analysis of WT Calu-3 cells indicated strong immunoreactivity consistent with pannexin 1 mobility (∼50 kDa (50)). Pannexin 1 immunoreactivity was markedly reduced in shRNA-N1-expressing but not in mock-infected Calu-3 cells (supplemental Fig. 4C).
In sum, our results in Figs. 1–3 are in close agreement with and expand recent observations by Ransford et al. (30), indicating that pharmacological inhibitors of pannexin 1 and pannexin 1 knockdown decreased ATP release and hypotonicity-stimulated airway epithelia cells.
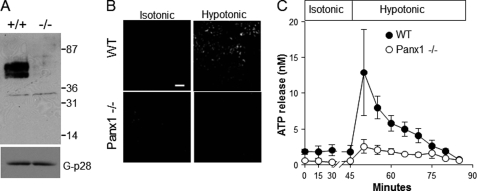
Next, we tested the hypothesis that pannexin 1 contributes to physiological nucleotide release from native airways using a pannexin 1-targeted mouse. RT-PCR analysis of mouse ear punches and Western blot analysis of murine brain (the tissue exhibiting the highest level of pannexin 1 immunoreactivity (34)) verified the absence of pannexin 1 expression in the pannexin 1 knockout mouse and reduced expression in pannexin 1 ± mice (supplemental Fig. 5, A and B). Western blot analysis of primary cultures of MTE cells indicated a strong anti-pannexin 1 immunoreactive double band of ∼50 kDa in WT MTE cells that was completely absent in cells from a pannexin 1-deficient mouse (Fig. 4A). Hypotonicity evoked propidium iodide uptake in WT MTE cells but not in MTE cells from pannexin 1−/− mice (Fig. 4B). Next, utilizing a perfusion approach (39, 51), we assessed ATP levels in luminal secretions from tracheas excised from WT versus pannexin 1 knockout mice under controlled flow conditions. As shown in Fig. 4C, a baseline ATP concentration of 2 ± 1 nm was observed in tracheas from WT mice under isotonic perfusion that imparts minor shear stress (50 μl/min = 0.08 dyn/cm2 (22, 39, 52)). ATP levels increased sharply, up to 12.6 ± 4 nm, after 5 min of hypotonicity and gradually decayed to initial values after 30 min. In contrast, tracheas from pannexin 1−/− animals exhibited slightly decreased baseline ATP levels (1 ± 0.4 nm) and strikingly impaired hypotonicity-stimulated ATP release (3.6 ± 1.8 nm at peak (Fig. 4C)).
FIGURE 4.
Reduced ATP release from pannexin 1−/− tracheas. A, Western blot analysis of cultured MTE cells (15 μg prot) from WT and pannexin 1−/− littermates. Molecular weight standards are indicated on the right (in kDa). G-p28, a Golgi marker, was used as loading control. B, propidium iodide uptake was performed in WT and pannexin 1-deficient MTE cells incubated for 5 min under isotonic or hypotonic conditions. C, tracheas excised from WT and pannexin 1−/− littermates were perfused (50 μl/min) with HBSS+ (isotonic) for 45 min. Tonicity was reduced to 50% (hypotonic), as indicated. The data (mean ± S.D., n = 3) is representative of two independent experiments performed with separate litters.
Having demonstrated that cell swelling-promoted ATP release is associated with pannexin 1-mediated dye uptake, we assessed the potential reversibility of this phenomenon. WD-HBE cells exposed to hypotonic solution exhibited enhanced propidium iodide uptake only if the dye was added concurrently with the hypotonic challenge (Fig. 5, a and b). That is, no changes in propidium iodide uptake were observed if the dye was added to cells after the isotonic conditions were restored (Fig. 5, c and d). Similar results were observed in A549 cells (supplemental Fig. 6). Thus, opening of the propidium iodide uptake permeable channel/pore during hypotonic shock reflected a transient (i.e. regulated) phenomenon rather than irreversible plasma membrane damage.
FIGURE 5.
Dye uptake in WD-HBE cells reflects a reversible phenomenon. Representative images (A) and quantification (B) of dye uptake in WD-HBE cells that were incubated for 5 min with isotonic (iso) or hypotonic (hypo) solutions in the presence of propidium iodide (a and b) or in its absence (c and d). Isotonicity was restored in d, and propidium iodide subsequently added to c and d for an additional 5 min. The images are representative of two independent experiments performed in quadruplicate. Scale bar = 100 μm. Nuclei staining was quantified (B) and expressed as mean ± S.E. *, significantly different from a, p < 0.01.
Collectively, the data described above indicate that pannexin 1 contributes to the regulated release of ATP from hypotonic stress-stimulated airway epithelial cells.
Rho GTPases Regulate ATP Release from Hypotonically Stimulated Airway Epithelial Cells
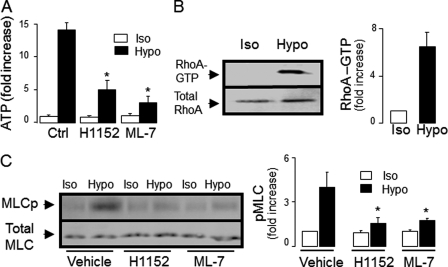
On the basis of recent studies suggesting that Rho GTPases are important regulators of ATP release in cells stimulated with the serine protease thrombin (31, 53), we hypothesized that Rho signaling is also involved in ATP release from hypotonic stress-stimulated WD-HBE cells. Therefore, the effects of inhibitors of Rho kinase and Rho kinase downstream effectors were investigated in WD-HBE cultures subjected to hypotonic stress. Hypotonic challenge-promoted ATP release was reduced in the presence of H1152 (Fig. 6A), a highly selective Rho kinase inhibitor (54, 55). The regulatory domain of MLC is a major downstream effector of Rho kinase. By phosphorylating and inactivating MLC phosphatase, Rho kinase facilitates MLC phosphorylation by MLC kinase (56). Consistent with the possibility that MLC phosphorylation was involved in ATP release from hypotonicity-stimulated HBE cells, the MLC kinase inhibitor ML-7 markedly reduced ATP release from these cells (Fig. 6A). ML-7 and H1152 had no effect on hypotonic stress-elicited cell swelling, although, like carbenoxolone and 10Panx1, they delayed RVD (supplemental Fig. 1). These results suggest that hypotonic stress-promoted Rho/Rho kinase activation and enhanced MLC phosphorylation are upstream of ATP release.
FIGURE 6.
Hypotonicity-induced ATP release in WD-HBE cells is associated with enhanced Rho activation and MLC phosphorylation. A, WD-HBE cells were preincubated for 45 min with 1 μm H1152 or 1 μm ML-7, and ATP release was measured after a 5-min incubation in hypotonic solution (Hypo) or isotonic control (Iso). n = 4. *, significantly different from Ctrl/Hypo, p < 0.05. B, representative Western blot for RhoA (left panel) and quantification of RhoA activation (right panel, mean ± S.E., n = 7) in response to a 5-min hypotonic challenge. C, effect of 1 μm H1152 or 1 μm ML-7 on hypotonicity-promoted MLC phosphorylation. A representative Western blot analysis and the quantification of phosphorylated MLC (MLCp) are shown in the left and right panels, respectively (mean ± S.D., n = 4). *, significantly different from Vehicle/Hypo, p < 0.05.
To directly test whether hypotonic stress induced Rho activation and MLC phosphorylation in WD-HBE cells, RhoA-GTP and MLC phosphorylation were measured by pull-down assays and phospho-MLC(Ser-19) immunoblots, respectively. As illustrated in Fig. 6, B and C, hypotonic stress promoted RhoA activation and enhanced MLC phosphorylation, respectively, relative to control cells. As predicted, H1152 and ML-7 reduced MLC phosphorylation in hypotonicity challenged HBE cells (Fig. 6C). Control experiments indicated that H1152 and ML-7 had no effect on hypotonicity-promoted RhoA-GTP formation, i.e. upstream of Rho kinase (supplemental Fig. 7).
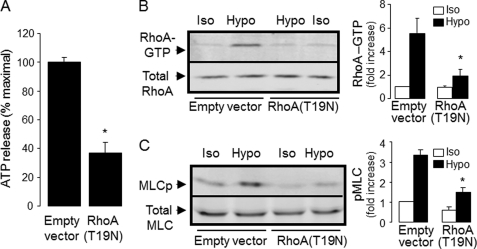
Although the experiments described above indicated that RhoA was activated in response to a hypotonic challenge, evidence that Rho activation is involved in ATP release has relied exclusively on pharmacological inhibitors. To more conclusively assess the involvement of Rho in ATP release, the effect of a dominant negative mutant of RhoA, RhoA(T19N) (31), was examined. A549 cells transiently transfected with RhoA(T19N) cDNA displayed reduced hypotonic shock-evoked ATP release relative to empty vector-transfected cells (Fig. 7A). As expected, RhoA activation and MLC phosphorylation were impaired in A549 cells transfected with RhoA(T19N) (Fig. 7, B and C).
FIGURE 7.
Inhibition of RhoA activation reduces hypotonic stress-induced ATP release. A549 cells were transfected with empty vector or RhoA(T19N), as indicated under “Experimental Procedures.” A, hypotonicity-elicited ATP release (mean ± S.E., from three independent experiments performed in quadruplicate; *, p < 0.05). B and C, RhoA activation and phosphorylated MLC (MLCp) in response to hypotonic stress. Representative Western blot analysis and quantifications (mean ± S.E., n = 4) are shown in the left and right panels, respectively. *, significantly different from empty vector/Hypo, p < 0.05.
Rho Signaling Regulates Dye Uptake in Airway Epithelial Cells
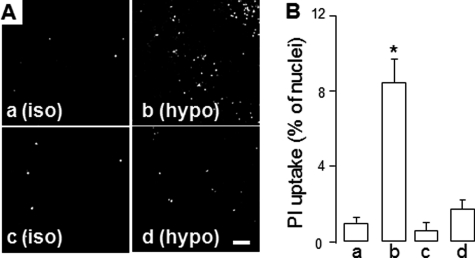
Having determined that ATP release from hypotonic stress-stimulated cells reflects a Rho-dependent process (Figs. 6 and 7) and is associated with pannexin 1 activation (Figs. 1–5), the potential link between Rho signaling and pannexin channel opening was examined. Both the Rho kinase inhibitor H1152 and the MLC kinase inhibitor ML-7 caused a nearly complete inhibition of hypotonic challenge-promoted propidium iodide uptake in WD-HBE cells (Fig. 8, A and B). Moreover, transfection of A549 cells with RhoA(T19N) markedly reduced the uptake of propidium iodide in these cells (Fig. 8C).
FIGURE 8.
Rho-dependent regulation of dye uptake. A, representative images illustrating PI uptake in WD-HBE cells preincubated with vehicle, H1152, or ML-7 (as in Fig. 5) and incubated for an additional 5 min with isotonic (Iso) or hypotonic (hypo) solutions in the presence of PI. B, quantification of PI uptake in WD-HBE cells treated as in A. Data are the mean ± S.E., n = 4. Scale bar = 200 μm. C, PI uptake was measured (as above) in A549 cells transfected with empty vector or RhoA(T19N). The results represent the mean ± S.E. of four separate experiments performed in triplicate. *, significantly different from control (p < 0.05, B) or empty vector/hypotonic (p < 0.01, C).
TRPV4 as a Potential Upstream Effector of ATP Release
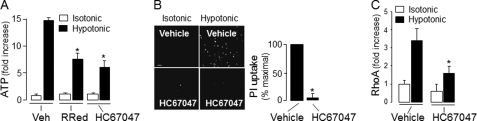
The TRPV4 channel is a broadly expressed cation channel that acts as a sensor of various physical stimuli such as heat, osmotic stress, shear stress, and stretch (57–59). Relevant to our study, it has been reported recently that TRPV4 mediated ATP release in response to osmotic stress in the thick ascending limb of the renal medulla (60) and in stretch-stimulated urothelia (61). Because TRPV4 is abundantly expressed in the airways (62, 63), we examined the possibility that TRPV4 transduces hypotonic stress into Rho/pannexin 1-mediated ATP release in airway epithelia. An initial assessment of this hypothesis indicated that ruthenium red (10 μm), a general inhibitor of TRPV channels, significantly reduced (by ∼50%) hypotonic stress-promoted ATP release in WD-HBE cells (Fig. 9A). Moreover, HC67047, a potent and highly selective inhibitor of TRPV4 (32), markedly impaired ATP release (Fig. 9A), dye uptake (B), and RhoA activation in hypotonicity-challenged WD-HBE cells (C). Control experiments showed that HC67047 did not affect hypotonic stress-induced cell swelling (supplemental Fig. 1). Altogether, the data indicated that the target of HC67047 was downstream of the cell volume change and triggered by the osmotic swelling.
FIGURE 9.
Hypotonic challenge-induced Rho activation and pannexin 1-mediated ATP release is sensitive to TRPV4 inhibitors. A, WD-HBE cells were preincubated for 30 min with vehicle, 10 μm ruthenium red (RuRed), or 10 μm HC67047 followed by a 5-min hypotonic challenge, and ATP release was measured as indicated under “Experimental Procedures.” *, p < 0.05 against vehicle/hypotonic. B, cells were preincubated with 10 μm HC67047 and propidium iodide uptake was assessed as described in previous figures. Scale bar = 200 μm. The data (mean ± S.E., n = 4, right panel) are expressed as the % maximal PI uptake in response to a hypotonic challenge. *, p < 0.01. C, RhoA activation was measured in cells preincubated as above and challenged for 5 min with isotonic or hypotonic solution. Data are mean ± S.D., n = 3. *, significantly different from vehicle/hypotonic, p < 0.05.
To more definitively assess the involvement of TRPV4 in hypotonicity-evoked ATP release, A549 were infected with lentiviruses bearing TRPV4 shRNAs (supplemental Table 2). shRNA-E12 reduced TRPV4 transcript levels to less than 20% of control cells, whereas other shRNAs caused only partial reduction (shRNA-F12, 43% reduction) or no reduction at all (shRNA-F11). Importantly, A549 cells stably expressing shRNA-E12 exhibited reduced ATP release in response to hypotonicity. shRNA-E12 virtually abolished the HC67047-sensitive responses observed in these cells (supplemental Fig. 8A). Similar results were obtained with Calu-3 cells (supplemental Fig. 8B).
DISCUSSION
Our study demonstrates that pannexin 1 mediates ATP release from hypotonic stress-activated airway epithelia, both in vitro and ex vivo. We illustrated that WD-HBE cells displayed hypotonic stress-promoted uptake of the hemichannel probe propidium iodide with kinetics overlapping that of ATP release (Fig. 1). The non-selective hemichannel inhibitor carbenoxolone, the pannexin 1-selective blocking peptide 10Panx1, and pannexin 1 RNAi markedly decreased dye uptake in addition to reducing ATP release (Figs. 1 and 3 and supplemental Figs. 3 and 4). Thus, pannexin 1 is functionally expressed at the human airway epithelial plasma membrane, i.e. as an ATP- (and dye)-permeable channel or channel regulator. Importantly, using a pannexin 1-targeted mouse model, we demonstrated for the first time that pannexin 1 mediates the release of ATP from a physiologically relevant tissue, i.e. freshly excised trachea (Fig. 4).
An additional important finding of the current study is the identification of regulatory mechanisms upstream of pannexin 1-mediated ATP release. Given the non-selective large conductance of pannexin 1 (64), strict control mechanisms should be in place to regulate pannexin 1 activity under physiological conditions to prevent collapse of the cellular ion gradients. Recent studies demonstrated that irreversible opening of pannexin 1 resulted in increased release of cellular ATP/UTP from apoptotic T lymphocytes (50, 65). Therefore, an important goal of our studies was to address whether responses attributed to pannexin 1 in airway epithelia reflect a regulated function as opposed to the irreversible opening of a plasma membrane pore and cell death.
We have addressed this question by demonstrating that WD-HBE and A549 cells exposed to a hypotonic solution exhibited enhanced dye uptake only if the dye was added concurrently with the hypotonic challenge; i.e. no changes in propidium iodide uptake were observed if the dye was added to cells after the isotonic conditions were restored (Fig. 5 and supplemental Fig. 6). Thus, opening of the propidium iodide permeable channel/pore during hypotonic cell swelling reflected a reversible phenomenon rather than irreversible plasma membrane damage.
Furthermore, regulatory components of the ATP release response triggered by hypotonic cell swelling were identified. For example, we demonstrated that hypotonic stress elicited RhoA activation and Rho kinase-dependent MLC phosphorylation and that inhibition of RhoA activation, Rho kinase, and MCL kinase decreased ATP release and impaired dye uptake (Figs. 6–8). These results strongly suggest that RhoA/Rho kinase activation (and subsequent MLC phosphorylation) is a step upstream of pannexin 1-mediated ATP release in hypotonic stress-stimulated epithelia. These results are consistent with the notion that hypotonic cell swelling/stretch promotes Rho activity, as reported previously with stretch-activated smooth muscle (66, 67), kidney mesangial cells (68), and endothelial cells subjected to hypotonic stress (69, 70).
Our study also provides a clue to the potential mechanism by which hypotonic challenge resulted in Rho activation. We demonstrated that TRPV4 channel inhibitors markedly impaired hypotonicity-evoked Rho activation and reduce ATP release and dye uptake in WD-HBE cells (Fig. 9), and we also showed that knocking down TRPV4 reduced hypotonicity-promoted ATP release (supplemental Fig. 8). The simplest interpretation of our results is that TRPV4 channels transduce hypotonic cell swelling into Rho activation. The mechanisms by which TRPV4 activate promotes Rho activation remain to be elucidated.
We have not addressed the mechanism by which Rho regulates pannexin 1-mediated ATP release. However, given the actions exerted by Rho/Rho kinase on cytoskeletal components (e.g. regulating MLC phosphorylation and actin polymerization (71)), one speculation is that Rho-promoted membrane-cytoskeletal rearrangements facilitate the insertion of pannexin 1 (or a pannexin 1 regulator) within the plasma membrane.
It is worth nothing, however, that pathways in addition to pannexin 1 likely contribute to ATP release in WD-HBE cells. Indeed, our results indicate that residual ATP release activity is evident under conditions in which dye uptake has been completely or nearly completely abrogated by 10Panx1, ML-7, or H1152. Observation of ATP release in the absence of dye uptake together with the lack of effect of flufenamic acid (Fig. 1) and the fact that incubations were in the presence of extracellular 1.6 mm CaCl2 (a condition that inhibits connexin hemichannel opening (42, 72)) argues against the involvement of connexins in the residual ATP release. Volume-regulated and maxi anion channels (73) and vesicle exocytosis from non-mucous cells (25, 74, 75) are potential mechanisms for pannexin1-independent nucleotide release in hypotonic stress-stimulated WD-HBE cells.
In sum, we have shown that pannexin 1 is an important contributor to ATP release in hypotonic stress-stimulated airway epithelia. Our data also indicate that hypotonic stress induces Rho activation upstream of pannexin channel opening and that TRPV4 channels likely transduce hypo-osmotic stress into RhoA-promoted pannexin 1-mediated ATP release.
Supplementary Material
Acknowledgments
We thank Dr. Robert Tarran for the use of the Leica SP5 confocal microscope system. We also thank Dr. Scott Randell for providing primary cultures of airway epithelial cells and for useful comments, Dr. William C. Davis for the use of the peristaltic pump and incubator for tracheal perfusions, Alessandra Livraghi for assistance with mouse protocols, and Dr. Tal Kafri (UNC Lenti-shRNA Core Facility) for providing shRNA lentiviruses and for useful suggestions. We also thank Nadya Mamoozadeh for tissue culture assistance and Lisa Brown for editorial assistance with the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant P01-HL034322. This work was also supported by Cystic Fibrosis Foundation Grant CFF-SEMINA08FO.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–8 and Tables 1 and 2.
- ASL
- airway surface liquid
- HBE
- human bronchial epithelial
- PAR
- protease-activated receptor(s)
- TRPV4
- transient receptor potential vanilloid 4
- Panx1
- pannexin 1
- WD-HBE
- primary well differentiated human bronchial epithelial
- UNC
- University of North Carolina
- MTE
- primary mouse tracheal epithelial
- HBSS
- Hanks' balanced salt solution
- MLC
- myosin light chain
- RVD
- regulatory volume decrease
- PI
- propidium iodide.
REFERENCES
- 1. Boucher R. C. (2003) Pflugers Arch. 445, 495–498 [DOI] [PubMed] [Google Scholar]
- 2. Davis C. W., Lazarowski E. (2008) Respir. Physiol. Neurobiol. 163, 208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazarowski E. R., Boucher R. C. (2009) Curr. Opin. Pharmacol. 9, 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis C. W., Dickey B. F. (2008) Annu. Rev. Physiol. 70, 487–512 [DOI] [PubMed] [Google Scholar]
- 5. Morse D. M., Smullen J. L., Davis C. W. (2001) Am. J. Physiol. Cell Physiol. 280, C1485–C1497 [DOI] [PubMed] [Google Scholar]
- 6. Jia Y., Mathews C. J., Hanrahan J. W. (1997) J. Biol. Chem. 272, 4978–4984 [DOI] [PubMed] [Google Scholar]
- 7. Devor D. C., Pilewski J. M. (1999) Am. J. Physiol. 276, C827–C837 [DOI] [PubMed] [Google Scholar]
- 8. Yue G., Malik B., Yue G., Eaton D. C. (2002) J. Biol. Chem. 277, 11965–11969 [DOI] [PubMed] [Google Scholar]
- 9. Ma H. P., Saxena S., Warnock D. G. (2002) J. Biol. Chem. 277, 7641–7644 [DOI] [PubMed] [Google Scholar]
- 10. Kunzelmann K., Bachhuber T., Regeer R., Markovich D., Sun J., Schreiber R. (2005) FASEB J. 19, 142–143 [DOI] [PubMed] [Google Scholar]
- 11. Mason S. J., Paradiso A. M., Boucher R. C. (1991) Br. J. Pharmacol 103, 1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cressman V. L., Lazarowski E., Homolya L., Boucher R. C., Koller B. H., Grubb B. R. (1999) J. Biol. Chem. 274, 26461–26468 [DOI] [PubMed] [Google Scholar]
- 13. Zsembery A., Fortenberry J. A., Liang L., Bebok Z., Tucker T. A., Boyce A. T., Braunstein G. M., Welty E., Bell P. D., Sorscher E. J., Clancy J. P., Schwiebert E. M. (2004) J. Biol. Chem. 279, 10720–10729 [DOI] [PubMed] [Google Scholar]
- 14. Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) Science 322, 590–594 [DOI] [PubMed] [Google Scholar]
- 15. Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) Nature 455, 1210–1215 [DOI] [PubMed] [Google Scholar]
- 16. Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Cell 134, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boucher R. C. (2002) Adv. Drug Delivery Rev. 54, 1359–1371 [DOI] [PubMed] [Google Scholar]
- 18. Donaldson S. H., Lazarowski E. R., Picher M., Knowles M. R., Stutts M. J., Boucher R. C. (2000) Mol. Med. 6, 969–982 [PMC free article] [PubMed] [Google Scholar]
- 19. Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang P., Lazarowski E. R., Tarran R., Milgram S. L., Boucher R. C., Stutts M. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14120–14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okada S. F., Nicholas R. A., Kreda S. M., Lazarowski E. R., Boucher R. C. (2006) J. Biol. Chem. 281, 22992–23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarran R., Button B., Picher M., Paradiso A. M., Ribeiro C. M., Lazarowski E. R., Zhang L., Collins P. L., Pickles R. J., Fredberg J. J., Boucher R. C. (2005) J. Biol. Chem. 280, 35751–35759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreda S. M., Okada S. F., van Heusden C. A., O'Neal W., Gabriel S., Abdullah L., Davis C. W., Boucher R. C., Lazarowski E. R. (2007) J. Physiol. 584, 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreda S. M., Seminario-Vidal L., van Heusden C. A., O'Neal W., Jones L., Boucher R. C., Lazarowski E. R. (2010) J. Physiol. 588, 2255–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sesma J. I., Esther C. R., Jr., Kreda S. M., Jones L., O'Neal W., Nishihara S., Nicholas R. A., Lazarowski E. R. (2009) J. Biol. Chem. 284, 12572–12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazarowski E. R., Boucher R. C., Harden T. K. (2003) Mol. Pharmacol. 64, 785–795 [DOI] [PubMed] [Google Scholar]
- 27. Button B., Picher M., Boucher R. C. (2007) J. Physiol. 580, 577–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grygorczyk R., Tabcharani J. A., Hanrahan J. W. (1996) J. Membr. Biol. 151, 139–148 [DOI] [PubMed] [Google Scholar]
- 29. Watt W. C., Lazarowski E. R., Boucher R. C. (1998) J. Biol. Chem. 273, 14053–14058 [DOI] [PubMed] [Google Scholar]
- 30. Ransford G. A., Fregien N., Qiu F., Dahl G., Conner G. E., Salathe M. (2009) Am. J. Respir. Cell Mol. Biol. 41, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seminario-Vidal L., Kreda S., Jones L., O'Neal W., Trejo J., Boucher R. C., Lazarowski E. R. (2009) J. Biol. Chem. 284, 20638–20648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Everaerts W., Zhen X., Ghosh D., Vriens J., Gevaert T., Gilbert J. P., Hayward N. J., McNamara C. R., Xue F., Moran M. M., Strassmaier T., Uykal E., Owsianik G., Vennekens R., De Ridder D., Nilius B., Fanger C. M., Voets T. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 19084–19089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J., Ma M., Locovei S., Keane R. W., Dahl G. (2007) Am. J. Physiol. Cell Physiol. 293, C1112–C1119 [DOI] [PubMed] [Google Scholar]
- 34. Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) J. Cell Sci. 120, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 35. Kreda S. M., Seminario-Vidal L., Heusden C., Lazarowski E. R. (2008) Br. J. Pharmacol. 153, 1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guan C., Ye C., Yang X., Gao J. (2010) Genesis 48, 73–85 [DOI] [PubMed] [Google Scholar]
- 37. Fulcher M. L., Gabriel S., Burns K. A., Yankaskas J. R., Randell S. H. (2005) Methods Mol. Med. 107, 183–206 [DOI] [PubMed] [Google Scholar]
- 38. Okada S. F., O'Neal W. K., Huang P., Nicholas R. A., Ostrowski L. E., Craigen W. J., Lazarowski E. R., Boucher R. C. (2004) J. Gen. Physiol. 124, 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu Y., Ehre C., Abdullah L. H., Sheehan J. K., Roy M., Evans C. M., Dickey B. F., Davis C. W. (2008) J. Physiol. 586, 1977–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelegrin P., Surprenant A. (2006) EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Locovei S., Bao L., Dahl G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scemes E., Spray D. C., Meda P. (2009) Pflugers Arch. 457, 1207–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma W., Hui H., Pelegrin P., Surprenant A. (2009) J. Pharmacol. Exp. Ther. 328, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benfenati V., Caprini M., Nicchia G. P., Rossi A., Dovizio M., Cervetto C., Nobile M., Ferroni S. (2009) Channels (Austin.) 3, 323–336 [DOI] [PubMed] [Google Scholar]
- 45. Blum A. E., Walsh B. C., Dubyak G. R. (2009) Am. J. Physiol. Cell Physiol. 298, C386–C396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eskandari S., Zampighi G. A., Leung D. W., Wright E. M., Loo D. D. (2002) J. Membr. Biol. 185, 93–102 [DOI] [PubMed] [Google Scholar]
- 47. Okada Y., Maeno E., Shimizu T., Dezaki K., Wang J., Morishima S. (2001) J. Physiol. 532, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harron S. A., Clarke C. M., Jones C. L., Babin-Muise D., Cowley E. A. (2009) Can. J. Physiol. Pharmacol. 87, 337–346 [DOI] [PubMed] [Google Scholar]
- 49. Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., Tiunova A., Born T. L., Usman N., Staroverov D., Lukyanov S., Panchin Y. (2004) Genomics 83, 706–716 [DOI] [PubMed] [Google Scholar]
- 50. Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., Ravichandran K. S. (2010) Nature 467, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ehre C., Zhu Y., Abdullah L. H., Olsen J., Nakayama K. I., Nakayama K., Messing R. O., Davis C. W. (2007) Am. J. Physiol. Cell Physiol. 293, C1445–C1454 [DOI] [PubMed] [Google Scholar]
- 52. Tarran R., Button B., Boucher R. C. (2006) Annu. Rev. Physiol. 68, 543–561 [DOI] [PubMed] [Google Scholar]
- 53. Blum A. E., Joseph S. M., Przybylski R. J., Dubyak G. R. (2008) Am. J. Physiol. Cell Physiol. 295, C231–C241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ikenoya M., Hidaka H., Hosoya T., Suzuki M., Yamamoto N., Sasaki Y. (2002) J. Neurochem. 81, 9–16 [DOI] [PubMed] [Google Scholar]
- 55. Sasaki Y., Suzuki M., Hidaka H. (2002) Pharmacol. Ther. 93, 225–232 [DOI] [PubMed] [Google Scholar]
- 56. Schwartz M. (2004) J. Cell Sci. 117, 5457–5458 [DOI] [PubMed] [Google Scholar]
- 57. Venkatachalam K., Montell C. (2007) Annu. Rev. Biochem. 76, 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. O'Neil R. G., Heller S. (2005) Pflugers Arch. 451, 193–203 [DOI] [PubMed] [Google Scholar]
- 59. Wu L., Gao X., Brown R. C., Heller S., O'Neil R. G. (2007) Am. J. Physiol. Renal Physiol. 293, F1699–F1713 [DOI] [PubMed] [Google Scholar]
- 60. Silva G. B., Garvin J. L. (2008) Am. J. Physiol. Renal Physiol. 295, F1090–F1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mochizuki T., Sokabe T., Araki I., Fujishita K., Shibasaki K., Uchida K., Naruse K., Koizumi S., Takeda M., Tominaga M. (2009) J. Biol. Chem. 284, 21257–21264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sidhaye V. K., Schweitzer K. S., Caterina M. J., Shimoda L., King L. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3345–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lorenzo I. M., Liedtke W., Sanderson M. J., Valverde M. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12611–12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bao L., Locovei S., Dahl G. (2004) FEBS Lett. 572, 65–68 [DOI] [PubMed] [Google Scholar]
- 65. Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., Ravichandran K. S. (2009) Nature 461, 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith P. G., Roy C., Zhang Y. N., Chauduri S. (2003) Am. J. Respir. Cell Mol. Biol. 28, 436–442 [DOI] [PubMed] [Google Scholar]
- 67. Kawamura S., Miyamoto S., Brown J. H. (2003) J. Biol. Chem. 278, 31111–31117 [DOI] [PubMed] [Google Scholar]
- 68. Krepinsky J. C., Ingram A. J., Tang D., Wu D., Liu L., Scholey J. W. (2003) J. Am. Soc. Nephrol. 14, 2790–2800 [DOI] [PubMed] [Google Scholar]
- 69. Koyama T., Oike M., Ito Y. (2001) J. Physiol. 532, 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hirakawa M., Oike M., Karashima Y., Ito Y. (2004) J. Physiol. 558, 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Riento K., Ridley A. J. (2003) Nat. Rev. Mol. Cell Biol. 4, 446–456 [DOI] [PubMed] [Google Scholar]
- 72. Dahl G., Locovei S. (2006) IUBMB Life 58, 409–419 [DOI] [PubMed] [Google Scholar]
- 73. Sabirov R. Z., Okada Y. (2005) Purinergic Signal. 1, 311–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tatur S., Groulx N., Orlov S. N., Grygorczyk R. (2007) J. Physiol. 584, 419–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feranchak A. P., Lewis M. A., Kresge C., Sathe M., Bugde A., Luby-Phelps K., Antich P. P., Fitz J. G. (2010) J. Biol. Chem. 285, 8138–8147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.