Abstract
Thyroglobulin (precursor for thyroid hormone synthesis) is a large secreted glycoprotein comprising contiguous region I (multiple type-1 repeating units engaging the first ∼1,191 residues, followed by a ∼245-residue hinge region), regions II-III (multiple type-2 and 3 repeating units, comprising ∼720 residues), and the C-terminal cholinesterase-like (ChEL) domain (∼570 residues). A signal peptide attached to ChEL makes an independent secretory protein that binds to I-II-III, stabilizing it and rescuing the secretion of I-II-III that would otherwise be trapped in the endoplasmic reticulum (ER). In this study, we found that a signal peptide attached to regions II-III also makes for an efficient secretory protein that neither demonstrably interacts nor has its secretion enhanced by the presence of secretory ChEL. By contrast, region I, either with or without the hinge region, cannot be secreted on its own and remains in the ER where it is bound to ER chaperones BiP and GRP94. Whereas ChEL can rescue secretion of I-II-III, it can rescue I-II only very weakly, and region I not at all. Yet, ChEL begins to rescue region I in cells that also co-express secretory II-III. The data suggest that conformational maturation of region I is a limiting step in the thyroglobulin maturation process, and this step is facilitated by the presence of both regions II-III and ChEL. Mutations causing hypothyroidism might induce solely local/regional misfolding or may interfere more globally by impeding interactions between regions that are required for thyroglobulin secretion.
Keywords: Endoplasmic Reticulum (ER), Intracellular Trafficking, Protein Assembly, Protein Folding, Secretion, Thyroid
Introduction
In vertebrates, thyroid hormones are first created in the apical luminal cavity of follicles of the thyroid gland, upon iodination of the thyroglobulin (Tg)3 protein (1, 2). It is the structure of Tg (3, 4) rather than the specificity of the peroxidase (5) that is thought to catalyze the coupling of di-iodotyrosyl residues 5 and 130 at the N terminus of Tg (6, 7), forming thyroxine within the Tg molecule. Even in the endostyle of protochordates (the earliest species in which thyroid hormone synthesis is known to occur), a Tg-like protein has been reported (8), whereas no other thyroidal proteins have yet been discovered to substitute effectively for Tg in this role.
The primary sequence of Tg (∼2,746 residues in mouse Tg after signal peptide cleavage) comprises disulfide-rich contiguous regions: region I (multiple type-1 repeating units engaging the first ∼1,191 residues followed by a ∼245-residue hinge region); II-III (multiple type-2 and type-3 repeating units, spanning ∼720 residues); and the C-terminal cholinesterase-like (ChEL) domain (∼570 residues). The genetic origins of these units are still being discovered, but the type-1 repeat is a structural motif that is appears in evolution as a protease inhibitor (9); the type-2 and type-3 repeats have limited homology to other proteins; whereas ChEL exhibits homology to acetylcholinesterase (10–12). Because no crystallographic data are yet available, the precise physical relationships between the Tg regions remain unidentified, but mutations in each of these regions can account for genetic hypothyroidism (13).
What is known is that region I-II-III itself is incompetent for hormonogenesis because the protein cannot be secreted (14), whereas when attached to a signal peptide, ChEL itself is an excellent secretory protein that facilitates oxidative maturation of co-expressed I-II-III and rescues I-II-III secretion (15). However, the regions required to enable ChEL to permit secretory rescue of Tg have remained unknown.
At our current state of knowledge, all Tg mutants causing genetic hypothyroidism are defective for intracellular transport (16). To understand the molecular/cellular basis for defective transport of a wide variety of Tg mutants (13), it is essential to determine the rate-limiting step(s) in normal Tg maturation and export. In this report we have further dissected Tg structure and pinpointed region I as the “problematic” portion of Tg for folding and secretion, requiring assistance both from regions II-III and ChEL.
EXPERIMENTAL PROCEDURES
Materials
Lipofectamine 2000, Zysorbin, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were from Invitrogen. Zysorbin was from Zymed Laboratories Inc. Complete protease inhibitor mixture was from Roche Applied Bioscience. Brefeldin A and protein A-agarose were from Sigma. Protein G-agarose was from EMD Chemicals (Gibbstown, NJ). Dithiobis(succinimidyl) propionate was from ThermoFisher Scientific. Peptide-N-glycosidase F was from New England Biolabs. Trans35S-Label was from MP Biomedicals (Irvine, CA). Rabbit polyclonal anti-myc was from Immunology Consultants, Inc. (Newberg, OR). Mouse monoclonal anti-HA epitope was from Covance (Princeton, NJ). Rabbit polyclonal anti-Tg (containing antibodies against epitopes at both N- and C-terminal regions of the protein) has been described previously (17).
Mutagenesis of Mouse Tg
cDNAs encoding secretory ChEL and truncated Tg regions I-II-III (L2169X) and secretory ChEL and ChEL-myc have been described previously (14, 15, 18). Other constructs described in this paper were made using the QuikChange site-directed mutagenesis kit (Stratagene) using following the mutagenic primers paired with their complements: Tg-Q2139X and Q2139X in secretory II-III (5′-GGTTGTGAGATGTGTGTTCTACCCTGATATATAGTAATGCATACACAGTCTACGGAGCC-3′); C1973S in secretory II-III-HA (5′-CCAATGGGTTCTTTGAGAGTGAGCGGCTCTGTGACAGGG-3′); Tg-E1192X (5′-GGTAGGCACCCAGCCTGCCTGTTAAAGCCCACAGTGTCCACTGCC-3′); Tg-A1435X (5′-CCCAACCACCAGACAGGATTGACTGGGCTGTGTGAAATGCCC-3′); Tg-Q1509X (5′-CCAGAATGGTCAGTACTAAGCCAGCCAGAAGAACAGGG-3′). To make secretory II-III-HA, a SalI restriction site was introduced into 5′ end of the first type-2 repeat by using a mutagenesis primer and its complement (5′-GGTTCTACAGAGTCCCAACCACCAGTCGACATGCTCTGGGCTGTGTGAAATGC-3′). The prolactin signal peptide was then fused in-frame at 5′ end of region II using SalI as described previously (15). An HA epitope tag was added using the following primer and its complement (5′-CCGGAAGTCTTACCCCTATGACGTCCCAGATTATGCATGATCCACACCTTCTGTACGC-3′). I-ChEL was made by introducing SalI sites first, at the 5′ end of the first type-2 repeat as described above and second, at the 5′ end of the ChEL domain using primers described previously (15). The intervening sequence between region I and ChEL was excised by SalI digestion and re-ligation to produce an in-frame I-ChEL construct. For I-II-ChEL, SalI sites were introduced first, at the 5′ end of region III using the following primer and its complement (5′-CCCCAGTGCTGACTCCCCGCGTCGACAGTGCTTGACAGATTGTGC-3′) and second, at the 5′ end of the ChEL domain as described above. The intervening sequence was excised as above to produce an in-frame I-II-ChEL. Each final product was confirmed by direct DNA sequencing.
Cell Culture and Transfection
HEK293 cells (simply called 293 cells) were cultured in DMEM with 10% FBS in plates at 37 °C in a humidified 5% CO2 incubator. Plasmids were transiently transfected using Lipofectamine 2000 transfection reagent, following the manufacturer's instructions.
Metabolic Labeling and Immunoprecipitation
Transfected 293 cells were starved for 30 min in Met/Cys-free DMEM and then pulse-labeled with 180 μCi/ml 35S-amino acids. The cells were then washed with an excess of cold Met/Cys and chased in complete DMEM plus serum. At each time point, cells were lysed in buffer containing 1% Nonidet P-40, 20 mm N-ethylmaleimide, 0.1% SDS, 0.1 m NaCl, 2 mm EDTA, 25 mm Tris, pH 7.4, and a protease inhibitor mixture. For immunoprecipitation, anti-Tg antibody was incubated with cell or media samples overnight at 4 °C, and the immunoprecipitate was recovered with protein A-agarose. For co-immunoprecipitation studies, cells were either cross-linked or prepared without cross-linking in the same lysis buffer lacking SDS and were incubated overnight at 4 °C with immunoprecipitating antibodies and protein A- or protein G-agarose. For cross-linking, cells before lysis were incubated in PBS containing 2 mm dithiobis(succinimidyl) propionate for 30 min at room temperature. Cells were then washed in PBS containing 20 mm N-ethylmaleimide and lysed in buffer containing both 20 mm N-ethylmaleimide and 50 mm glycine to quench residual cross-linker. Immunoprecipitates (or co-precipitates) were washed three times before boiling in SDS sample buffer with or without reducing agent, resolved by reducing SDS-PAGE, and analyzed by fluorography or phosphorimaging.
RESULTS
Loss of ChEL Rescue upon Loss of an Intact I-II-III
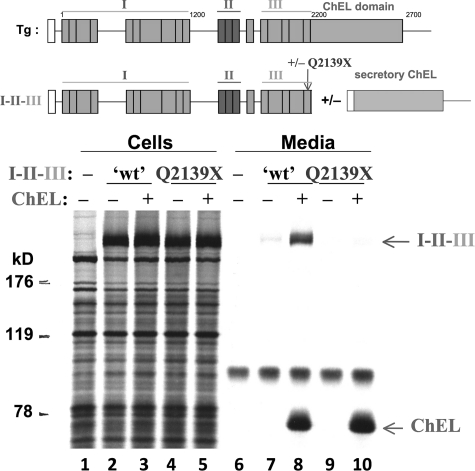
ChEL is known to function as an intramolecular chaperone and escort for I-II-III (15). A stop codon at residue 2,142 of human Tg (equivalent to residue 2,139 of mouse Tg) linked to hypothyroidism (19) truncates a portion of the very last type-3 repeat, creating a naturally occurring I-II-III truncation. To determine whether secretory ChEL is competent to rescue this naturally occurring truncation, we co-expressed these constructs and found comparable intracellular protein expression of recombinant Tg-Q2139X (Fig. 1 lanes 2–5). However, unlike unmutagenized I-II-III (lane 8), secretory ChEL could not rescue Tg-Q2139X (lane 10). Thus, loss of information from the end of region III prevents ChEL-mediated rescue.
FIGURE 1.
Secretory ChEL rescue of I-II-III requires a fully intact region III. A schematic of the full-length Tg protein is shown at the top, initiated by the signal peptide (open box) and then comprising region I bearing multiple type-1 repeat units (shown as boxes) connected via a hinge region to regions II-III, and finally the ChEL domain. As indicated in the schematic, a final, eleventh, type-1 repeat is contained in between the clustered type-2 and type-3 repeats that comprise regions II-III, but for the purposes of the experiments in this paper, region I is not considered further than the hinge region linking to regions II-III. Above the data, a second schematic shows the constructs used in this experiment. The intact region I-II-III terminating at L2169X has been described previously (15). Secretory ChEL was engineered by inclusion of an artificial signal peptide (open box). The mouse Tg-Q2139X mutation precisely matches the truncation found in Tg-Q2142X associated with human hypothyroidism (19). 293 cells transfected to co-express these constructs were pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 4 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography. The partial rescue observed for wild-type I-II-III is precisely as reported previously (15); however, secretion of Tg-Q2139X could not be rescued by ChEL. The positions of molecular mass marker proteins is indicated, at left.
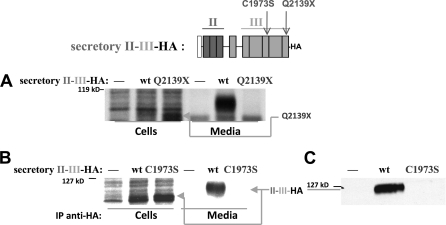
To understand this further, we engineered a construct in which region II-III (HA epitope-tagged) immediately follows a signal peptide sequence to direct the protein into the ER. First, we found that II-III-HA was efficiently secreted; and second, we found that introduction of the Q2139X mutation blocked this secretion (Fig. 2A), indicating that Q2139X introduces local misfolding into II-III. However, it is unclear, within the context of full-length Tg, whether II-III local misfolding overrides all other factors to bring about Tg retention in the ER, or whether II-III conformational integrity is needed to help optimize ChEL-mediated facilitation of Tg export (see below).
FIGURE 2.
An artificial signal peptide converts II-III into an independent secretory protein. Above the data, a schematic shows the construct(s) used in this experiment. Secretory II-III was engineered by inclusion of an artificial signal peptide (open box) and a C-terminal HA epitope. The mouse C1973S and Q2139X mutations precisely match the Tg-C1977S and Tg-Q2142X associated with human hypothyroidism (19, 22). A and B, 293 cells were either untransfected (—) or transfected with wild-type II-III-HA (wt), or those bearing the Q2139X mutation were pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg (A) or anti-HA (B) and analyzed by reducing SDS-PAGE and fluorography. C, media bathing cells either untransfected (—) or transfected with wild-type II-III-HA or those bearing the C1973S mutation were collected for 24 h, resolved by reducing SDS-PAGE, and analyzed by immunoblotting with anti-HA. The positions of molecular mass marker proteins are indicated at the left of each panel.
Japanese patients with mild hypothyroidism and goiter bearing a Tg-C1977S missense mutation (C1973S of mouse, located in domain 3-2b) have been described (20). We found that export of secretory II-III-HA was totally blocked by this mutation, as examined either by pulse-chase experiment (Fig. 2B) or by immunoblotting of collected secretion (Fig. 2C). Importantly, however, in similar experiments Hishinuma et al. clearly showed that a portion of full-length Tg-C1977S molecules does acquire endoglycosidase H resistance, indicating that in the context of the full-length Tg protein, including a wild-type ChEL domain, some rescue of Tg export occurs despite local misfolding caused by mutation in region III (20). These findings seem to argue against the idea that local misfolding within II-III overrides all other factors to bring about Tg retention in the ER. As an alternative, it seemed plausible that II-III may help to optimize ChEL-mediated facilitation of Tg export, and in that case it seemed reasonable to think that II-III and ChEL might directly associate.
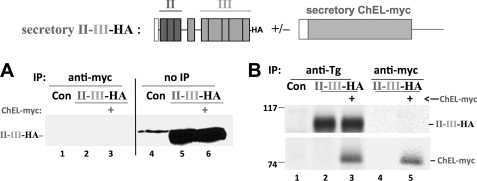
We therefore chose to examine whether secretory ChEL could assist in the export of wild-type secretory II-III-HA. However, the amount of II-III-HA secreted was unaffected by co-expression of secretory ChEL-myc, again measured either by immunoblotting of collected secretion (Fig. 3A, lanes 5 and 6) or by release of newly synthesized protein from metabolically labeled cells (Fig. 3B, lanes 2 and 3). Moreover, immunoprecipitation of secreted ChEL-myc could not co-precipitate secreted II-III-HA (Fig. 3A, lanes 2 and 3, and 3B, lanes 4 and 5) even though secretory ChEL-myc does indeed co-precipitate intact I-II-III (15, 21).
FIGURE 3.
II-III and ChEL are secreted independently without evidence of interaction. Above the data, a schematic shows the construct(s) used in this experiment. 293 cells were either untransfected controls (Con) or transfected with secretory II-III-HA (see Fig. 2) +/− secretory ChEL-myc (see Fig. 1). A, bathing media were collected for 24 h and either immunoprecipitated (IP) with anti-myc and then resolved by reducing SDS-PAGE, or resolved directly by SDS-PAGE without IP (no IP), and analyzed by immunoblotting with anti-HA. Although a large quantity of II-III-HA was secreted, there was no change in this amount upon co-expression of ChEL-myc, and none was co-precipitated with ChEL-myc. B, cells as in A were pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h. The collected chase media were either immunoprecipitated with anti-Tg (recovering all secreted Tg constructs) or anti-myc (recovering secreted ChEL) and analyzed by reducing SDS-PAGE and fluorography. Note that there was no increase in II-III-HA secretion in the presence of secretory ChEL-myc (lanes 2 and 3), and co-precipitation of II-III-HA with ChEL-myc was not detected when the two were co-expressed (lane 5). The positions of molecular mass marker proteins are indicated at left.
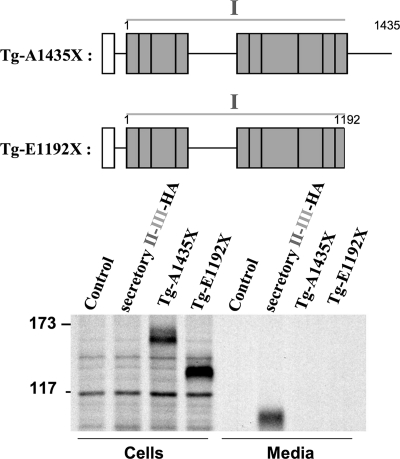
Region I Is Incompetent for Secretion
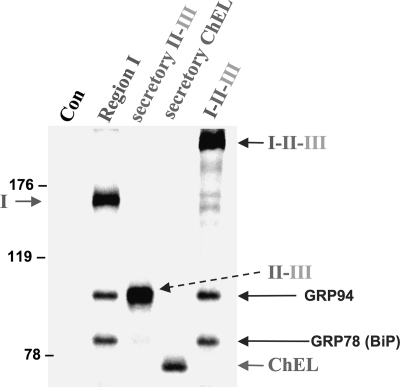
Because II-III and ChEL are both readily secreted, it was important to examine the export of region I alone. Two mouse Tg truncations were prepared for this purpose: E1192X, which contains the first 10 type-1 repeats but no hinge region, and A1435X, which contains these same repeats plus the entire hinge region (see schematic, Fig. 4). There is reason to think that the latter construct might not be secretion-competent because it is so similar to the human Tg-W1418X mutation that has been reported as a heterozygous allele in human hypothyroidism (22). In fact, with or without the ∼245-residue hinge, region I alone was incompetent for secretion (Fig. 4), very similar to the behavior of region I-II-III in the absence of ChEL (Fig. 1, lane 7). To explore further the molecular basis for this secretion-incompetence, we performed chemical cross-linking with dithiobis(succinimidyl) propionate to identify ER-interacting proteins associated either with region I alone or region I-II-III in the absence of ChEL. Secretory II-III-HA and secretory ChEL were used as positive controls, and all cells were treated with brefeldin A to prevent any of the proteins from being exported. Impressively, after immunoprecipitation with anti-Tg antibodies and analysis by reducing SDS-PAGE, both I-II-III and region I alone were found to interact selectively with proteins of 94 and 78 kDa, which correspond to ER chaperones GRP94 and BiP (Fig. 5). (Indeed, upon repeat immunoprecipitation with anti-GRP94- and anti-BiP-specific antibodies, identification of both of the cross-linked chaperones was confirmed (data not shown)). By contrast, neither secretory II-III-HA nor secretory ChEL-myc appeared to be appreciably cross-linked to these ER resident proteins (although the SDS-PAGE mobility of secretory II-III is sufficiently close to that of GRP94 that it obscured unequivocal distinction; Fig. 5). The data suggest that in the absence of ChEL facilitation, region I, either alone or embedded within I-II-III, remains conformationally immature, being retained in the ER in association with ER molecular chaperone proteins GRP94 and BiP.
FIGURE 4.
Unlike secretory II-III or ChEL, region I, with or without the hinge region, is secretion-incompetent. Above the data, a schematic shows the two region I construct(s) used in this experiment: mouse Tg-A1435X contains the full hinge region, Tg-E1192X contains no hinge. Secretory II-III-HA (Fig. 2) was included as a control. 293 cells were either untransfected (Control) or transfected to express the constructs indicated. The cells were pulse-labeled with 35S-amino acids for 20 min and chased in complete medium for 4 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography. The positions of molecular mass marker proteins are indicated at left.
FIGURE 5.
Tg region I-II-III in the absence of ChEL, or region I alone, is bound by ER molecular chaperones. 293 cells were either untransfected controls (Con) or transfected to express the constructs indicated, with region I bearing a partial hinge (Tg-L1363X). The cells were pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 6 h in the presence of 5 μg/ml brefeldin A (to prevent export of secretory II-III or secretory ChEL). The cells were then exposed to cross-linker for 30 min as described under “Experimental Procedures,” before lysis and immunoprecipitation with anti-Tg, followed by reducing SDS-PAGE and fluorography. Independent immunoprecipitation with specific antibodies (data not shown) confirmed the identification of GRP94 and BiP. The positions of molecular mass marker proteins are indicated at left.
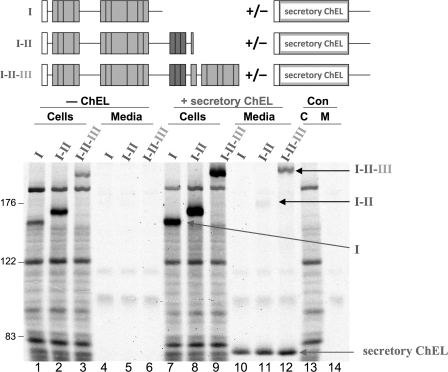
ChEL Interactions with Regions I-II-III, I-II, or Region I Alone
To determine the extent to which ChEL could directly enhance export of upstream regions of Tg, we co-expressed secretory ChEL with either region I alone, I-II, or I-II-III. Co-expression of secretory ChEL did seem to increase the intracellular recovery of each of these newly synthesized constructs (Fig. 6 compare lanes 7–9 with lanes 1–3); however, unlike for I-II-III, secretory ChEL rescue of I-II was minimal (Fig. 6), consistent with the observation that the I-II construct (terminating at mouse Tg-Q1509X) is homologous to the Tg-R1511X mutation reported to be associated with human hypothyroidism (23). In addition, there was no detectable ChEL rescue of region I alone (Fig. 6, compare lanes 10–12).
FIGURE 6.
ChEL rescue of region I-containing constructs requires the later repeating units contained within II-III. Above the data, a schematic shows the construct(s) used in this experiment. 293 cells were either untransfected controls (Con, lanes 13 and 14) or transfected with the cDNA constructs indicated with region I bearing a partial hinge (Tg-L1363X); I-II terminating at Tg-Q1509X which is homologous to the Tg-R1511X mutation associated with human hypothyroidism (23) (and unrelated to translation of an exon 35-deleted mRNA) (27, 28); and I-II-III terminating at L2169X. Each of these constructs was expressed alone (0.5 μg of plasmid DNA/transfection) or co-expressed from the same plasmid DNA co-transfected with 1.5 μg of plasmid DNA expressing secretory ChEL. The cells were then pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography. The positions of molecular mass marker proteins are indicated at left.
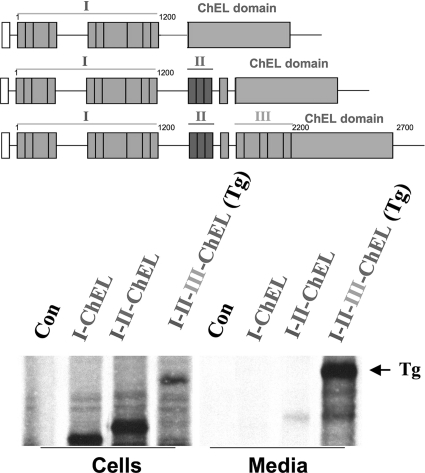
We considered the possibility that direct contiguity of the ChEL domain within the same polypeptide might increase favorable interactions between these regions to enhance secretion. Therefore, we engineered fusion proteins I-ChEL and I-II-ChEL (in contrast to I-II-III-ChEL, which is in fact full-length wild-type Tg). However, direct tethering of the ChEL domain to either region I or I-II did not improve secretory efficiency (Fig. 7) over that observed when secretory ChEL was expressed separately (Fig. 6).
FIGURE 7.
Even when tethered within a contiguous polypeptide, ChEL interaction rescue of region I-containing constructs requires information contained within II-III. Above the data, a schematic shows the construct(s) used in this experiment. 293 cells were either untransfected controls (Con) or transfected with the cDNA constructs indicated, with region I bearing its full hinge, and region I-II terminating after the final, 11th type-1 repeat, both contiguous with the full ChEL domain. I-II-III contiguous with the full ChEL domain is in fact full-length wild-type Tg. The cells were then pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography.
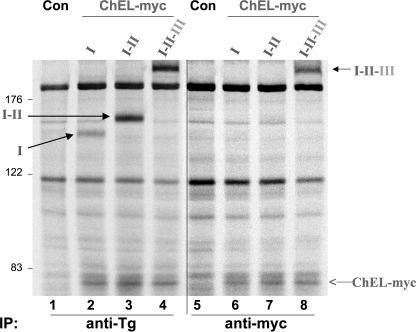
Presumably, the inability of ChEL to improve secretory efficiency of region I or I-II relates at least in part to its (in)ability to make a strong independent contact with these regions. To test this, we examined co-precipitation of metabolically labeled region I or I-II in cells co-expressing secretory ChEL-myc and treated with brefeldin A to block ChEL export. Upon immunoprecipitation, co-expressed I-II-III was efficiently co-precipitated with ChEL (Fig. 8, lane 8), consistent with previous reports (15, 21). Secretory ChEL-myc was also directly immunoprecipitated from other transfections in which it was included, but there was little or no co-precipitation of region I or I-II with ChEL (Fig. 8, lanes 6 and 7).
FIGURE 8.
Stable ChEL interaction with region I-containing constructs requires II-III. 293 cells were either untransfected controls (Con, lanes 1 and 5) or transfected/co-transfected with cDNA constructs identical to those described in Fig. 6. The cells were then pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h in the presence of 5 μg/ml brefeldin A (to prevent export of I-II-III or secretory ChEL). The cells were then exposed to cross-linker for 30 min as described in under “Experimental Procedures” before lysis and immunoprecipitation either with anti-Tg (recovering all Tg constructs) or anti-myc (recovering ChEL) and analyzed by reducing SDS-PAGE and fluorography. Note that although there was excellent co-precipitation of intracellular I-II-III with ChEL-myc (lane 8), co-precipitation of either region I or I-II was not detected when co-expressed with secretory ChEL-myc (lanes 6 and 7). The positions of molecular mass marker proteins are indicated at left.
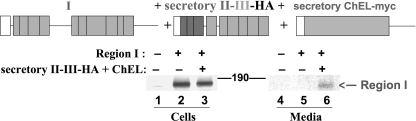
Because ChEL is unable to rescue region I (or I-II), the foregoing data seemed to suggest that II-III may be required for ChEL-mediated facilitation of Tg export, but this occurs without a strong binary interaction between II-III and ChEL. We therefore triply co-transfected plasmids co-expressing secretory II-III-HA, secretory ChEL, and region I. For the first time (but repeated and confirmed), evidence of rescue of region I secretion was detected (Fig. 9, lane 6). Thus, the data suggest that regions II-III and ChEL both participate in facilitating the maturation and export of region I within Tg.
FIGURE 9.
Partial rescue of region I secretion in the presence of secretory ChEL and secretory II-III. Above the data, a second schematic shows the constructs used in this experiment. As before, secretory II-III-HA and ChEL, when used, each included an artificial signal peptide (open box), and region I bears a partial hinge (Tg-L1363X). Transfected 293 cells were then pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 5 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography. The position of the 190-kDa molecular mass standard is indicated.
DISCUSSION
In the last 20 years, ER storage diseases have emerged as one of the central problems in modern secretory biology (24). Quality control machinery of the ER, designed to distinguish normal and abnormal versions of exportable proteins, is largely based on the exposure of surface features that are ultimately buried within the core of properly folded globular proteins (25). For Tg as for other secretory proteins, sites designed for interaction between secretory protein partners, subunits, or even distinct regions within the same protein, are typically among the sites recognized by ER quality control proteins and ER retention factors (26).
A growing number of Tg mutations have been identified as a genetic cause of hypothyroidism (13), and it seems increasingly likely that some of these mutations (e.g. Tg-Q2139X), which bring about transport blockade within the ER, primarily disrupt regional interactions (Fig. 1).
We previously showed that ChEL can be folded and exported as an independent secretory protein (15), yet ChEL is strongly implicated in Tg conformational stability and dimerization, allowing for Tg export from the ER (18). If we wish to understand the mechanism for Tg maturation and export, we must dissect the way in which local domain-specific folding (and folding defects) impact(s) on regional interactions within the larger Tg polypeptide.
In this report, we have discovered that region II-III behaves as a fully competent secretory protein (Fig. 2). These data indicate that II-III can fold independently of the rest of the Tg molecule. Indeed, II-III does not require assistance from secretory ChEL for its export; nor can we find evidence for direct physical interaction of II-III with ChEL (Fig. 3). Further, although a local C1973S mutation in II-III results in a total blockade of secretion (proof of local misfolding; Fig. 2) that same mutation is at least partially suppressed in the context of full-length Tg, which includes a wild-type ChEL domain (20). These findings highlight the importance of regional interactions as an overriding determinant of Tg quality control in the ER.
Interestingly, upon dissection of the Tg molecule, despite being initiated by its natural signal peptide, the data indicate that region I conformational maturation is likely to provide the greatest structural challenge for Tg to become competent for export and thyroid hormonogenesis, because region I alone is intrinsically defective for secretion. This observation is true in the absence of the ensuing hinge sequence (mouse Tg-E1192X) or in its presence (mouse Tg-A1435X), and it is also true for Tg bearing a partial hinge sequence (mouse Tg-L1363X). Indeed, we have learned of a homozygous hypothyroid patient bearing the equivalent human Tg-L1364X mutation,4 and these findings are also consistent with Tg-W1418X as a mutation-linked to human hypothyroidism (22). From our studies, it appears that virtually all of the association of I-II-III with molecular chaperones BiP and GRP94 (a feature linked to Tg retention within the ER) can be attributed to region I alone (Fig. 5). Importantly, this class of truncations cannot be secreted (Fig. 4) even in the presence of secretory ChEL (Fig. 6).
The inability of ChEL to rescue region I is not because of its deployment in trans; indeed, even when directly tethered, ChEL could not promote secretion of region I alone. Although ChEL does exhibit trace rescue of region I-II, it is not until the type-3 repeats are included that ChEL promotion of Tg secretion begins to become efficient (Fig. 7). This conclusion is supported by the inability to detect a stable intracellular interaction between ChEL and either region I or I-II, whereas an obviously stable intracellular association is detected between ChEL and I-II-III (15, 21).
We began these studies by postulating that region III might provide the primary ChEL binding site, but the fact that II-III does not interact with ChEL (Fig. 3) supports a more complex interaction. In our current view, we suggest that structural constraints brought about through binary interactions facilitate conformational stabilization (satisfying ER quality control and export requirements) by promoting ternary complexes of region I, II-III, and ChEL. It is likely that inappropriate or insufficient binary interactions will not support a stabilized and export-competent ternary structure (21). In support of such a view is the finding that only after triple transfection in which secretory II-III and secretory ChEL are co-expressed can secretion of region I begin to become evident (Fig. 9). More studies are needed to map the regional interactions sites, culminating in a complete three-dimensional Tg structure.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-DK40344 (to P. A.). This work was also supported by University of Michigan Diabetes Research and Training Center Grant NIH5P60-DK20572 for the Molecular Biology and Sequencing Cores and University of Michigan Cancer Center Core Laboratories Grant P30 CA46592.
S. Refetoff, unpublished observation.
- Tg
- thyroglobulin
- ChEL
- cholinesterase-like
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Taurog A. (1999) Biochimie 81, 557–562 [DOI] [PubMed] [Google Scholar]
- 2. Arvan P., Di Jeso B. (2004) in The Thyroid (Braverman L. E., Utiger R. eds) 9th Ed., pp. 77–95, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 3. Turakulov I., Saatov T., Babaev T. A., Rasuleva G., Makhmudov V. (1976) Biokhimiia 41, 1004–1007 [PubMed] [Google Scholar]
- 4. Xiao S., Dorris M. L., Rawitch A. B., Taurog A. (1996) Arch. Biochem. Biophys. 334, 284–294 [DOI] [PubMed] [Google Scholar]
- 5. Lamas L., Taurog A. (1977) Endocrinology 100, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 6. Marriq C., Lejeune P. J., Venot N., Vinet L. (1991) Mol. Cell. Endocrinol. 81, 155–164 [DOI] [PubMed] [Google Scholar]
- 7. Dunn A. D., Corsi C. M., Myers H. E., Dunn J. T. (1998) J. Biol. Chem. 273, 25223–25229 [DOI] [PubMed] [Google Scholar]
- 8. Monaco F., Dominici R., Andreoli M., Pirro R. D., Roche J. (1981) Comp. Biochem. Physiol. B 70, 341–343 [Google Scholar]
- 9. Mihelic M., Turk D. (2007) Biol. Chem. 388, 1123–1130 [DOI] [PubMed] [Google Scholar]
- 10. Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. (1986) Nature 319, 407–409 [DOI] [PubMed] [Google Scholar]
- 11. Swillens S., Ludgate M., Mercken L., Dumont J. E., Vassart G. (1986) Biochem. Biophys. Res. Comm. 137, 142–148 [DOI] [PubMed] [Google Scholar]
- 12. Mori N., Itoh N., Salvaterra P. M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 2813–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Targovnik H. M., Esperante S. A., Rivolta C. M. (2010) Mol. Cell. Endocrinol. 322, 44–55 [DOI] [PubMed] [Google Scholar]
- 14. Park Y. N., Arvan P. (2004) J. Biol. Chem. 279, 17085–17089 [DOI] [PubMed] [Google Scholar]
- 15. Lee J., Di Jeso B., Arvan P. (2008) J. Clin. Invest. 118, 2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vono-Toniolo J., Rivolta C. M., Targovnik H. M., Medeiros-Neto G., Kopp P. (2005) Thyroid 15, 1021–1033 [DOI] [PubMed] [Google Scholar]
- 17. Kim P. S., Kwon O. Y., Arvan P. (1996) J. Cell Biol. 133, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J., Wang X., Di Jeso B., Arvan P. (2009) J. Biol. Chem. 284, 12752–12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pardo V., Vono-Toniolo J., Rubio I. G., Knobel M., Possato R. F., Targovnik H. M., Kopp P., Medeiros-Neto G. (2009) J. Clin. Endocrinol. Metab. 94, 2938–2944 [DOI] [PubMed] [Google Scholar]
- 20. Hishinuma A., Takamatsum J., Ohyama Y., Yokozawa T., Kanno Y., Kuma K., Yoshida S., Matsuura N., Ieiri T. (1999) J. Clin. Endocrinol. Metab. 84, 1438–1444 [DOI] [PubMed] [Google Scholar]
- 21. Wang X., Lee J., Di Jeso B., Treglia A. S., Comoletti D., Dubi N., Taylor P., Arvan P. (2010) J. Biol. Chem. 285, 17564–17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hishinuma A., Fukata S., Nishiyama S., Nishi Y., Oh-Ishi M., Murata Y., Ohyama Y., Matsuura N., Kasai K., Harada S., Kitanaka S., Takamatsu J., Kiwaki K., Ohye H., Uruno T., Tomoda C., Tajima T., Kuma K., Miyauchi A., Ieiri T. (2006) J. Clin. Endocrinol. Metab. 91, 3100–3104 [DOI] [PubMed] [Google Scholar]
- 23. Gutnisky V. J., Moya C. M., Rivolta C. M., Domené S., Varela V., Toniolo J. V., Medeiros-Neto G., Targovnik H. M. (2004) J. Clin. Endocrinol. Metab. 89, 646–657 [DOI] [PubMed] [Google Scholar]
- 24. Kim P. S., Arvan P. (1998) Endocr. Rev. 19, 173–202 [DOI] [PubMed] [Google Scholar]
- 25. Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. (1991) Nature 353, 726–730 [DOI] [PubMed] [Google Scholar]
- 26. Hendershot L., Bole D., Köhler G., Kearney J. F. (1987) J. Cell Biol. 104, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Targovnik H. M., Medeiros-Neto G., Varela V., Cochaux P., Wajchenberg B. L., Vassart G. (1993) J. Clin. Endocrinol. Metab. 77, 210–215 [DOI] [PubMed] [Google Scholar]
- 28. Peteiro-Gonzalez D., Lee J., Rodriguez-Fontan J., Castro-Piedras I., Cameselle-Teijeiro J., Beiras A., Bravo S. B., Alvarez C. V., Hardy D. M., Targovnik H. M., Arvan P., Lado-Abeal J. (2010) J. Clin. Endocrinol. Metab. 95, 3522–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]