Abstract
Fibronectin (FN) is an extracellular matrix protein that is assembled into fibrils by cells during tissue morphogenesis and wound healing. FN matrix fibrils are highly elastic, but the mechanism of elasticity has been debated: it may be achieved by mechanical unfolding of FN-III domains or by a conformational change of the molecule without domain unfolding. Here, we investigate the folded state of FN-III domains in FN fibrils by measuring the accessibility of buried cysteines. Four of the 15 FN-III domains (III-2, -3, -9, and -11) appear to unfold in both stretched fibrils and in solution, suggesting that these domains spontaneously open and close even in the absence of tension. Two FN-III domains (III-6 and -12) appear to unfold only in fibrils and not in solution. These results suggest that domain unfolding can at best contribute partially to the 4-fold extensibility of fibronectin fibrils.
Keywords: Extracellular Matrix, Extracellular Matrix Proteins, Fibronectin, Protein Folding, Thiol, Domain Unfolding, Elasticity, Fibrillogenesis, Traction Force
Introduction
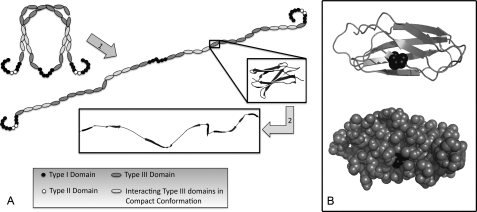
Fibronectin (FN)2 is a dimer of a 250-kDa protein that forms insoluble fibrils that are a critical component of the extracellular matrix. It is made up of a series of three domain types connected end-to-end (see Fig. 1A) (1). The N terminus of FN consists of nine FN-I and two FN-II domains, each of which contains an internal disulfide bond. The C terminus of FN consists of three FN-I domains and contains intermolecular disulfides that link the two FN monomers to form the dimer. The central portion of the FN molecule is made up of 15 tandem FN-III domains; these domains each consist of seven β-strands folded into a β-sandwich structure and contain no internal disulfides (see Fig. 1B). FN in solution adopts a compact conformation in which the second to fourth FN-III domains (III-2–4) of one subunit interact with the 12th to 14th FN-III domains (III-12–14) of the other, causing the FN dimer to fold onto itself (see Fig. 1A) (2).
FIGURE 1.
Models for FN elasticity. A, there are two possible mechanisms to explain the elasticity of FN fibrils. Soluble FN is known to fold into a compact conformation, and stretching of fibrils may extend it to an elongated conformation (block arrow 1). Alternatively, each FN-III domain is composed of seven β-strands and may be unfolded when fibrils are stretched (block arrow 2). B, the structure of the III-7 domain is shown as a ribbon structure (top) and a space-filling model (bottom). The free cysteine is shown in black, and the space-filling model shows that this residue has limited surface accessibility when the domain is folded.
FN interacts with both cells and other extracellular matrix proteins. The N-terminal domains contain binding sites for the extracellular matrix proteins collagen and fibrin, whereas the ninth and tenth FN-III domains bind to integrins at the cell surface (3–6). Upon attachment to cells, FN is stretched and assembled into fibrils by cell contraction: matrix assembly is inhibited if cell contractility is inhibited (7) or if tension within the matrix is released (8). Although the exact mechanism of FN fibril formation is still unknown, studies have shown that FN fibrils are stretched to up to 4 times their resting length by cells and are highly elastic structures (9–12). This elasticity requires cell contraction; disruption of cytoskeletal contraction relaxes stretched fibrils (10).
Two different mechanisms have been proposed to explain the elasticity of FN matrix fibrils (see Fig. 1A) (13). One theory contends that the elasticity is a function of FN-III domains unfolding under tension (see Fig. 1A, mechanism 2) (14, 15). These domains contain no internal disulfides and can be mechanically unfolded by atomic force microscopy (16, 17). It is unclear how relevant these atomic force microscopy pulling forces are to the in vivo assembly of fibrils; the unfolding by atomic force microscopy occurred at high forces on the order of 100 pN, due to high pulling rates. In cell-assembled fibrils, the pulling speed is nearly zero, so it is difficult to extrapolate the cell-applied force that would be needed to unfold these domains. In addition to mechanical unfolding of FN-III domains, transient opening of certain FN-III domains has been demonstrated in the absence of applied force during the assembly of in vitro assembled super-fibronectin (18, 19).
The second theory to explain fibril elasticity contends that FN molecules are in a compact conformation in relaxed fibrils and are stretched to the extended conformation in elongated fibrils (see Fig. 1A, mechanism 1) (20). These two mechanisms are not exclusive; elasticity of fibrils may be a combination of the two.
In the current study, we have investigated the folded state of FN-III domains by assaying exposure of buried free cysteines, as was done previously to examine the folded state of the cytoskeletal protein spectrin (21). In this assay, proteins containing free cysteines (i.e. not in a disulfide bond) buried within certain domains are probed with a thiol-reactive dye. If the domain has opened, then the cysteine will be exposed to the dye, but if the domain remains folded, the buried cysteine will be blocked from labeling. Here, we use thiol-reactive dyes to investigate the folded state of FN-III domains both in stretched fibrils, where FN is subjected to cellular forces, and in solution, where relaxed FN is subjected to no external stretch.
EXPERIMENTAL PROCEDURES
Expression of Recombinant Fibronectin
FN cDNA was inserted into the pHLSec2 vector, which has previously been shown to be effective in polyethylenimine-based transfections (22). Vector DNA was incubated with polyethylenimine at a 1:2 ratio for 10 min, and was subsequently transfected into Human Embryonic Kidney 293 cells. Cell cultures were incubated for 10 days, conditioned medium was collected from the cell cultures, and recombinant FN was purified from the conditioned medium by running it over a gelatin agarose resin column (Sigma). Protein was eluted using 1 m l-arginine (Sigma Aldrich). Expression was confirmed by polyacrylamide gel electrophoresis on a 5% acrylamide cross-linked gel.
Introduction of Cysteines into FN-III Domains
Cysteines were introduced into full-length FN using site-directed mutagenesis. Single amino acid cysteine substitutions were incorporated into a pBlueScript vector containing a fragment of FN DNA that contained the 15 FN-III domains using PfuTurbo polymerase (Stratagene). The cysteine-containing FN fragment was excised from the vector and cloned back into the FN-pHLSec2 vector. Introduction of cysteines was confirmed by DNA sequencing.
Expression of Individual Domains
cDNAs coding for single FN-III domains containing the introduced cysteine were amplified via PCR with pfu polymerase (Stratagene) and introduced into the pET15b vector (Novagen) using NdeI and BamHI cloning sites. FN-III-pET15b vectors were transformed in C41 competent Escherichia coli cells (23) and grown on ampicillin-resistant LB agar plates overnight. Individual colonies were selected and amplified in LB containing 0.1 mg/ml ampicillin. Cultures were grown to an A600 of 0.5, at which point protein expression was induced with 0.5 mm IPTG (Sigma-Aldrich). Cultures were incubated overnight at room temperature to facilitate protein expression. Poly-histidine-tagged protein was purified from bacterial lysates by running over a cobalt resin column (Clontech) using standard protein purification techniques. Protein was eluted from the column with 0.2 m imidazole.
Labeling of FN Fibrils in Cell Culture
Glass coverslips were cleaned with 0.8 m KOH, rinsed in PBS, and coated with 10 μg/ml recombinant FN. NIH3T3 cells were plated onto the coverslips in standard DMEM supplemented with 10% calf serum, 1% l-glutamine, and 10 μg/ml of recombinant FN and were left to assemble FN fibrils for 24 h. Subsequently, culture medium was replaced with 50 μm fluorescein-5-maleimide in cysteine-free, serum-free DMEM to label exposed free cysteines for 1 h. Cells were then rinsed with PBS and fixed with 3.7% formaldehyde for 20 min. Images of the labeled FN were collected at 40× magnification using an upright fluorescence microscope (Zeiss Axiophot) at room temperature and a GFP filter set. Images were captured with an internally cooled CCD camera (COOLSNAPHQ, Roper Scientific) and Axiovision software (Zeiss). Additionally, some matrices were labeled with a monoclonal anti-human FN antibody. To ensure FN-specific labeling, recombinant FNs that showed significant maleimide labeling were preincubated with 5 mm iodoacetamide (Sigma) overnight. Free iodoacetamide was removed by passing the recombinant FN over a G-25 Sepahadex buffer exchange column (GE Healthcare). Iodoacetamide-blocked FN was then used to assemble a matrix and subsequently probed with maleimide.
Labeling of Individual Domains with CPM
Exposed free thiols in individual FN-III domains were probed in solution by incubating 10 μm of protein with 10-fold excess 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin (CPM). Labeled proteins were scanned on a fluorospectrophotometer (Shimadzu) with an excitation wavelength of 384 nm and an emission range of 400–500 nm.
Measuring Stability by Tryptophan Fluorescence
Domain stability was assessed by measuring the tryptophan fluorescence in increasing urea concentrations. Samples were scanned on a fluorospectrophotometer (Shimadzu) with an excitation wavelength of 280 nm, and fluorescence intensity was recorded over an emission range of 300–400 nm.
RESULTS
Probing Folded State of FN-III Domains in Cell-stretched Fibrils
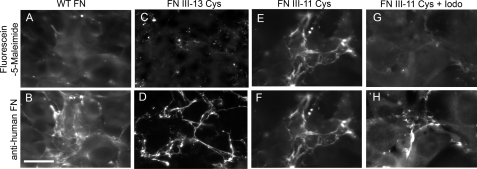
Native FN contains multiple disulfide bonds in the FN-I and FN-II domains, as well as intramolecular disulfide bonds that join the two monomers in the dimer; however, FN only has two free cysteines, one within domain III-7 and one within domain III-15. Previous studies indicated that these cysteines are buried within their respective domains because the cysteines could only be labeled with thiol-reactive dyes when denatured (15). Furthermore, the crystal structure of domain III-7 shows that the cysteine has little surface accessibility (Fig. 1B) (24). We first investigated whether these domains were unfolded in cell-stretched fibrils by examining the accessibility of the buried cysteines in III-7 and III-15 with thiol-reactive dyes. NIH3T3 fibroblasts were seeded onto FN-coated coverslips and cultured for 24 h to allow cells to assemble FN fibrils. Subsequently, cell medium was replaced with 50 μm of the thiol-reactive probe, fluorescein-5-maleimide, in cysteine-free, serum-free medium for 1 h. The cultures were thoroughly rinsed, fixed in formaldehyde, and imaged. Results showed no specific labeling of FN fibrils by the thiol-reactive probe (Fig. 2A), suggesting that these domains are not unfolded in cell-stretched fibrils. However, this approach only allowed us to explore the folded state of two of the 15 FN-III domains.
FIGURE 2.
Labeling of buried free thiols to assess domain unfolding. FN was assembled into fibrils by cells for 24 h and subsequently probed with fluorescein-5-maleimide to detect exposed free thiols. A and B, representative images of FN matrix labeled with fluorescein-5-maleimide (A) and anti-human FN antibody (B) show no substantial maleimide labeling of FN. C–H, to ensure that recombinant FN was integrated into growing fibrils and cysteine-labeling was specific to FN, cells were dual-labeled with fluorescein-5-maleimide and anti-human FN. Representative maleimide (C) and anti-FN (D) images from recombinant FN, which shows no thiol reactivity (III-13). Representative maleimide (E) and anti-FN (F) images from recombinant FN that reacts with the thiol-reactive dye (III-11) indicated colocalization of cysteine-labeling and FN fibrils. Representative maleimide (G) and anti-FN (H) images from recombinant FN that was prelabeled with iodoacetamide (Iodo) to block free cysteines. Scale bar is 20 μm.
To explore the folded state of other FN-III domains, we introduced a free cysteine within each domain at a location that was predicted to be buried (Fig. 1B and Table 1). All FN domains have a similar β-sandwich structure; in domain III-7, the cysteine is the second residue within the “E” strand. Crystal structures of domains III-7–10 (24) and III-12–14 (25) show that the equivalent residue in the E strand is also buried in each of these domains, as does the NMR structure of domains III-1–2 (26). We therefore replaced the second residue of the E strand of each domain with a cysteine. Thirteen recombinant FN mutants were engineered such that each mutant had a single buried free cysteine in one of its FN-III domains: for each mutant, the two native cysteines in III-7 and III-15 were substituted with non-cysteine residues (C1232A/C2227V) (Table 1). Additionally, positive and negative control FN constructs were created: the negative control contained no free cysteines (C1232A/C2227V), whereas the positive control contained a single exposed free cysteine on the surface of domain III-7 (N1214C). NIH3T3 fibroblasts were cultured for 24 h in the presence of each recombinant FN, allowed to assemble FN fibrils, and were subsequently probed with fluorescein-5-maleimide. The FN fibrils are comprised of a mixture of mouse FN synthesized by the NIH3T3 cells, bovine FN from the serum, and the exogenously added recombinant human FN.
TABLE 1.
Introduction of engineered cysteines into FN-III domains
Single amino acid substitutions were introduced into FN-III domains at the equivalent position to the free cysteine in III-7. Introduced amino acid is underlined in amino acid sequence.
| Domain | Mutation | aa Sequence |
|---|---|---|
| III-1 | Y666C | GHLNSCTIKGL |
| III-2 | V775C | STATSCNIDPL |
| III-3 | V865C | ETANSCTLSDL |
| III-4 | A962C | SRNTFCEVTGL |
| III-5 | Y1051C | PSVSKCPLRNL |
| III-6 | I1138C | SDSGSCVVSGL |
| III-7 | C1232A | ADQSSATFDNL |
| III-8 | V1414C | PSDNACVLTNL |
| III-9 | I1504C | HSRNSCTLTNL |
| III-10 | A1594C | GSKSTCTISGL |
| III-11 | M1688C | PDQTECTIEGL |
| III-12 | V1868C | PDSSSCVVSGL |
| III-13 | Y1959C | PDVRSCTITGL |
| III-14 | A2049C | PGVTECTITGL |
| III-15 | C2227V | EYIISVHPVGT |
We found that buried cysteines in several FN-III domains could be labeled with the thiol-reactive dye, whereas others could not (Fig. 2, C and E). To confirm that recombinant FN was integrating into fibrils, we simultaneously labeled fibrils with both fluorescein-5-maleimide and an anti-human FN antibody. This monoclonal antibody (a gift from Dr. Luciano Zardi) shows no cross-reactivity with mouse or bovine FN and thus specifically labeled recombinant FN and not FN derived from either the seeded fibroblasts or serum. Images indicated that recombinant FN was indeed incorporated into fibrils (Fig. 2, D and F). Some matrices that showed positive maleimide labeling were co-labeled with the anti-human FN antibody to confirm that the maleimide labeling corresponded with the FN matrix (Fig. 2, E and F). To ensure that maleimide labeling was specific to FN, we preincubated cysteine-containing recombinant FNs with iodoacetamide to block the free cysteine and then used these iodoacetamide-treated FNs in matrix assembly. Maleimide labeling was significantly reduced in these matrices (Fig. 2, G and H).
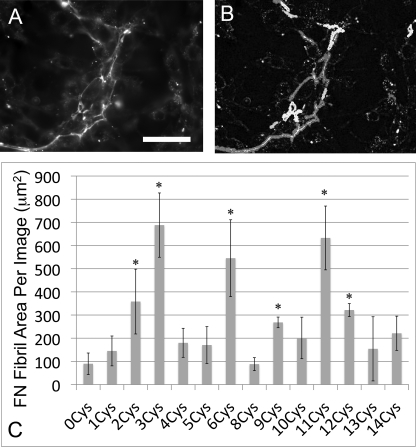
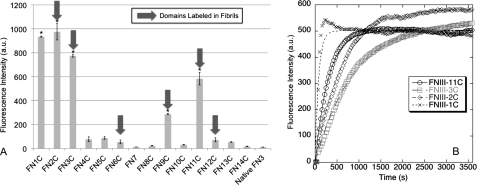
Representative images of matrices assembled from each cysteine-containing recombinant FN are shown in Fig. 3. Fibril labeling was quantified for each recombinant FN by processing fluorescein-5-maleimide images with an author-written Matlab edge detection and quantification algorithm (Fig. 4, A and B). Briefly, each image was converted to a binary image using a uniform threshold value for the entire image set. Areas within each image were calculated, summed, and converted to square microns. The total labeled fibril area was determined for at least three images from each recombinant FN. Results indicate that six of the 15 recombinant FNs showed significant labeling relative to the cysteine-free control (C1232A/C2227V FN) (Fig. 4C). Specifically, fibrils with buried cysteines in III-3, III-6, or III-11 were substantially labeled with fluorescein-5-maleimide, whereas cysteines in III-2, III-9, and III-12 showed a more moderate (but still significant) degree of labeling. Fibrils with free cysteines in all other domains showed no significant reactivity with the maleimide probe, suggesting that these domains are not unfolded.
FIGURE 3.
Labeling of introduced free thiols in FN-III domains. Engineered buried cysteines were introduced into each FN-III domain, and the cell-assembled matrix was labeled with fluorescein-5-maleimide. For each image, the FN-III domain containing the buried cysteine is listed in the lower right corner. 0Cys FN (top left, −) was used as a negative control, whereas N1214C FN (top left, +) was used as a positive control (see text for details of these mutants). Scale bar is 50 μm.
FIGURE 4.
Quantification of fibril labeling. An author-written Matlab edge-detection program was used to quantify labeled fibrils in fluorescein-maleimide images. A representative fluorescein maleimide image (A) and the resulting quantified areas from the Matlab program (B) are shown. C, quantification of fibril area shows six domains with statistically significant labeling relative to the 0Cys FN negative control. *, p < .05, n > 3 for each FN. Scale bar is 50 μm.
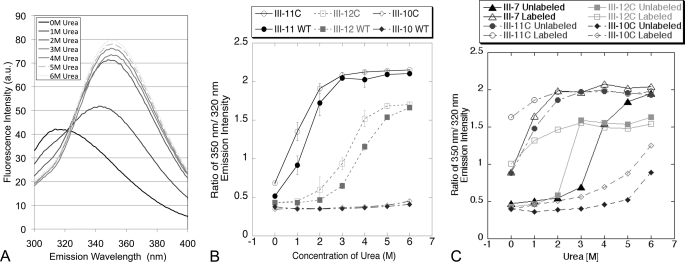
It was possible that the introduction of the free cysteine into FN-III domains weakened the domain relative to the native structure and that the exposure of the buried cysteine was a result of this weakening. We therefore investigated the stability of the cysteine-containing domains by assaying the unfolding of the domain under urea denaturation. This was done by measuring the tryptophan fluorescence of individual domains; each FN-III domain has a single buried tryptophan (with the exception of domain III-1, which has three). Unfolding of single FN-III domains can be determined by monitoring changes in fluorescence of tryptophan, excited at 280 nm, as a function of increasing urea concentration. As the domain unfolds, the buried tryptophan is exposed, which results in the emission peak shifting from ∼320 to 350 nm (Fig. 5A). The ratio of fluorescence intensity at these wavelengths gives an indication of the folded state of the domain.
FIGURE 5.
Stability of recombinant fibronectins. Domain stability was quantified by measuring tryptophan fluorescence in both native and cysteine-containing FN-III domains. Each FN-III domain contains a single buried tryptophan, whose emission peak shifts from ∼320 to ∼350 nm as the domain is denatured. A, urea denaturation of domain III-11 shows typical tryptophan fluorescence profiles as a function of urea concentration. a.u., arbitrary units. B, ratios of 350/320 nm fluorescence intensities are shown for three FN-III domains as a function of urea. C, labeling urea-unfolded FN-III domains with fluorescein-5-maleimide followed by renaturation caused a significant increase in the 350/320 tryptophan fluorescence ratio, suggesting that FN-III domains are not properly refolded following fluorescein-5-maleimide labeling.
Wild type FN-III domains were expressed individually in Escherichia coli cells, as were FN-III domains containing the introduced cysteines detailed in Table 1. Domains were denatured in increasing concentrations of urea, and tryptophan fluorescence was measured. Fig. 5A shows a representative tryptophan emission spectrum for wild type III-11 in increasing urea concentrations. The ratio of tryptophan emission at 350/320 nm was calculated for each FN-III domain as a function of urea. This ratio was ∼0.5 for folded domains and increased to ∼1.5 as the tryptophan was exposed. Tryptophan fluorescence ratios are shown in Fig. 5B for domains III-10, III-11, and III-12. III-11 is chemically the weakest FN-III domain; it is substantially unfolded at 1 m urea and almost completely unfolded at 2 m urea. III-12 requires 4 m urea for ∼50% denaturation, whereas III-10 is not denatured at the highest urea concentration. We found that for each of the labeled domains, there were only small changes in stability between the cysteine-containing and wild type domains. Furthermore, this reduction in stability appears to be uniform across all FN-III domains; weakly stable domains (such as III-11) are affected as much as moderately stable domains (such as III-12) and stable domains (III-10). This indicates that maleimide labeling cannot be attributed to a weakening of domains by the introduced cysteines but instead represents an exposure of the buried residues during normal opening and closing of FN-III domains.
Stability of FN-III domains was also investigated after labeling with fluorescein-5-maleimide. For this assay, FN-III domains containing the engineered cysteines were denatured in 6 m urea, labeled with fluorescein-5-maleimide, and refolded by exchanging the buffer to PBS using a buffer exchange column. We found that the label prevented the complete refolding of FN-III domains (Fig. 5C). This is expected because the cysteine is normally buried in the interior of the domain, and the attachment of fluorescein would force it to the exterior. This is not a concern for the present study, where the fluorescein labeling was only used to assay the opening of domains. However, previous studies (15) have used FN in which the cysteines in III-7 and III-15 are prelabeled and used to assemble a matrix to be assayed for domain unfolding. Data show that unlabeled III-7 required 4 m urea for substantial unfolding, but once labeled with fluorescein-5-maleimide, it is partially unfolded in the absence of urea and extensively unfolded at 1 m urea.
Probing Folded State of Single FN Domains in Absence of Force
The fibril-labeling experiments show that nine of the 15 FN-III domains are not labeled in stretched fibrils. This indicates that these nine domains do not contribute to the fibril stretching by unfolding. However, this does not mean that the six domains that were labeled are opened in fibrils by mechanical stretching. Previous studies have shown that certain FN-III domains spontaneously unfold in solution (18). They spontaneously refold more rapidly than they unfold, such that the folded state dominates. However, the open state occurs at a high enough frequency that III-3 is capable of reacting with anastellin (a fragment of III-1) to generate in vitro polymerized superfibronectin (18). Thus, it is possible that the labeling of domains III-2, -3, -6, -9, -11, and -12 might also occur during spontaneous opening in the absence of cell-applied force.
To probe the folded state of FN-III domains in the absence of force, we examined the accessibility of the engineered buried cysteines in solution. 10 μm of each FN-III domain was labeled with 100 μm CPM for 24 h (Fig. 6). CPM is a thiol-reactive dye that fluoresces only when coupled to free thiols; thus, it can be used to quantify exposed cysteines without the competing background signal of unbound dye. (Although CPM is ideal for solution experiments due to its lack of fluorescence in its unbound state, it is cell-permeable, so it could not be used for fibril-labeling experiments.) Wild type III-3 was used as a negative control, as it contains no free thiol, and previous studies have shown that it spontaneously opens in solution (18). Results from CPM labeling indicated that five domains were significantly labeled in solution: III-1, III-2, III-3, III-9, and III-11 (Fig. 6A). All other domains showed no significant labeling relative to the thiol-free control. Note that although these domains are unfolding enough to allow labeling of the buried cysteine, tryptophan fluorescence indicates that the ensemble-averaged protein is folded.
FIGURE 6.
Labeling of isolated FN-III domains with CPM in solution. 10 μm individual FN-III domains were labeled in solution with 100 μm thiol-reactive dye, CPM. CPM is not fluorescent until bound to free thiols. A, fluorescence intensity for each of the FN-III domains (n = 3). a.u., arbitrary units. Five domains showed significant CPM labeling: III-1, III-2, III-3, III-9, and III-11. Native III-3 (which lacks a free thiol) was used as a negative control. Domains that labeled in the fibril-labeling experiment (Fig. 3) are indicated with block arrows. B, CPM reactivity kinetics were measured for four of the domains that showed CPM reactivity. All reached steady state labeling within 1 h. *, p < .05 (n = 3).
Due to its lack of fluorescence when unbound to free thiols, CPM can also be used to measure the relative kinetics of the buried thiol exposure (Fig. 6B). CPM fluorescence intensity reached a plateau within 1 h of labeling, indicating that the 24-hour time point used in the labeling data (Fig. 6A) was more than sufficient as a steady-state measurement. The rate constant was calculated for each domain that showed significant labeling (Table 2). This rate constant is the net constant for two steps: the rate of opening/closing of the FN-III domain and the rate of the CPM-thiol reaction. BSA, which has one exposed free thiol, was used to determine the rate constant of the CPM-thiol reaction, and the rate of labeling of each FN-III domain was calculated relative to the BSA label. III-1 labeled at a similar rate to BSA, which suggests that the engineered cysteine in III-1 in solution may not actually be buried. (Note that the stability of III-1 could not be assessed by tryptophan fluorescence as other FN-III domains were, due to the presence of multiple tryptophans within III-1). In contrast, III-2, III-3, and III-11 labeled at a 2.5–9-fold slower rate relative to BSA. Although these experiments cannot give an exact measurement of the opening and closing frequency of these domains, it does suggest that the cysteine is indeed buried in these domains and is labeled as domains spontaneously open and close with time.
TABLE 2.
Relative rates of FN-III domain opening
FN-III domains were labeled with CPM, and CPM fluorescence intensity was recorded as a function of time (Fig 6B). Data were fit to an exponential association equation, and the rate constant k was calculated for each domain that showed significant CPM reactivity (n = 3).
| Domain | k (×103) | t50 | Fold-change relative to BSA |
|---|---|---|---|
| 1/s | min | ||
| BSA | 15 | 0.78 | 1.0 |
| III-1 | 12 ± 0.8 | 0.93 ± 0.06 | 1.2 ± 0.1 |
| III-11 | 6.4 ± 3.0 | 2.0 ± 0.8 | 2.6 ± 1.0 |
| III-2 | 2.0 ± 0.1 | 5.9 ± 0.4 | 7.6 ± 0.5 |
| III-3 | 1.7 ± 0.4 | 7.2 ± 1.7 | 9.3 ± 2.2 |
Comparing results from the solution data to the fibril-labeling data shows that four domains are labeled in both conditions: III-2, III-3, III-9, and III-11. This suggests that these domains are spontaneously opening in the absence of force, so their labeling in fibrils may not be due to mechanical tension. Domain III-1 showed significant labeling in solution but not in fibrils; this is addressed under “Discussion.” Only domains III-6 and III-12 were labeled in fibrils but not in solution. This suggests that these two domains may be mechanically stretched open in fibrils.
DISCUSSION
We investigated the unfolding of FN-III domains in FN by introducing buried free cysteines and measuring their exposure with thiol-reactive dyes in both FN matrix fibrils (assembled and stretched by cells) and in solution (in the absence of force). Successful labeling of buried cysteines indicated that domains were opened sufficiently to allow chemical attachment of the thiol reagent. In the original application of the buried thiol labeling approach, Johnson et al. (21) labeled red blood cell ghosts with IAEDANS; the cells were either static in solution or subjected to shear. The sheared cells showed 50% more labeling of spectrin, whereas other cytoskeletal proteins showed no change. Six shear-sensitive cysteines were identified; two of these cysteines were tested in folded domains in solution, and their reactivity to IAEDANS closely followed their denaturation with increasing temperature. This suggested that a buried cysteine became susceptible to labeling only upon denaturation of the domain and that shear caused an equivalent force-induced unfolding.
Our work reveals an additional nuance of the technique: in some FN-III domains, the apparently buried cysteine was exposed to labeling when the isolated domain was assayed in solution. One possibility is that the cysteine is not actually buried but instead remains surface exposed. This seems unlikely since the corresponding residue is buried in known FN-III domain structures. Additionally, with the exception of III-1, the labeling was 2–9-fold slower than for the exposed cysteine on BSA. We suggest that these domains are actually folded, as indicated by tryptophan fluorescence, but are transiently unfolding and refolding. We previously suggested that domains III-1, III-2, III-3, and III-11 transiently open and bind anastellin, a fragment of domain III-1, when forming the in vitro induced FN matrix known as superfibronectin (18, 19). We suggest that the labeling of the buried cysteine in these domains, and in III-9, occurs during this transient opening.
In addition to the four domains that were labeled in both solution and stretched fibrils, three domains showed situation-specific labeling. Domain III-1 was significantly labeled in solution in the absence of tension; however, there was no apparent labeling in stretched fibrils. One possible explanation is that domain III-1 is opened in fibrils, but the cysteine becomes buried in an FN–FN bond. Previous work has implicated III-1 in FN fibril assembly (27–29). Conversely, domains III-6 and III-12 showed no labeling in solution but showed significant labeling in stretched fibrils, suggesting that these domains are unfolded specifically in matrix fibrils. This unfolding could be induced by either the assembly of FN into fibrils or the mechanical stretching of fibrils.
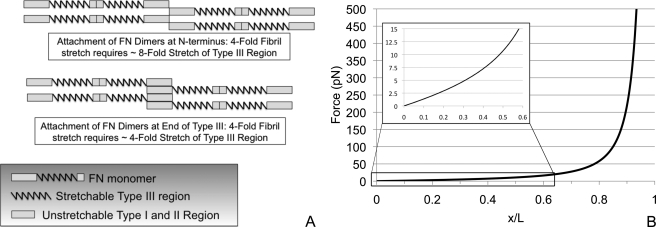
These results suggest that nine of the 15 FN-III domains do not unfold and thus do not contribute to the 4-fold extensibility of FN fibrils. The other six FN-III domains may contribute to this extensibility; however, this depends on two unanswered questions: 1) to what degree are these domains extended and 2) where do the FN-FN intermolecular bonds occur in fibrils. If one assumes that FN-FN bonds occur at the 70-kDa N-terminal region, which has been shown to be involved in FN fibrillogenesis (30, 31), and that FN molecules are connected end-to-end, then the region containing the FN-III domains would make up roughly 50% of the FN contour length (Fig. 7A). Thus, a 4-fold stretch of FN would require an 8-fold stretch of the FN-III domain region. Because each domain is a seven-strand β-sandwich, this would mean that all FN-III domains would need to be stretched to their full contour length; in this scenario, the six domains that open could account for maximally 40% of the observed stretch, and only if each domain was completely unfolded and fully extended. However, if one assumes that FN-FN bonds occur immediately adjacent to the FN-III domain region, then this region would only need to stretch 4-fold (Fig. 7A). If each of the six domains that open were stretched to their full contour length, then the resulting fibril would have an ∼3-fold extensibility, which is on the order of magnitude of the observed fibril stretch. However, this would again require that these domains be stretched to their full contour length.
FIGURE 7.
Estimating the potential contribution of FN-III domain unfolding to fibril elasticity. The contribution of FN-III domain unfolding to fibril elasticity depends on both the location of FN-FN bonds in fibrils as well as the degree of extension in the unfolded domains. A, effect of the location of FN-FN binding sites: if FN-FN interactions are at the extreme ends of the molecule, then all FN-III domains must completely unfold to their fully extended length. If binding is at the end of the FN-III region, then roughly half of the domains must unfold to their fully extended length. B, an unfolded domain can be modeled as a 90-amino acid unstructured polypeptide. The force required to extend this polypeptide can be predicted using the worm-like chain model.
The degree of unfolding in the opened domains is subject to two conditions. First, the tension needs to be sufficient to prevent domain refolding. Carrion-Vasquez et al. (32) have demonstrated that an Ig domain unfolded by force will spontaneously refold even while subjected to a continuous tension. For the particular I27 domain they studied, Li et al. (33) calculated that a force of 13.7 pN would cause 50% of the domains to be unfolded. This is a high force relative to the ∼5 pN generated by myosin motor molecules. The force for blocking domain refolding will likely depend on the parameters for each domain, which are not known for FN-III domains. It is worth keeping in mind, however, that domains will tend to refold even against a tensile force.
Even for a domain that has been unfolded, extending it to a stretched conformation will require force, because the unstructured polypeptide will act as an entropic spring. This is usually modeled as a worm-like chain, where the entropic force is given as a function of the end-to-end distance (34–36):
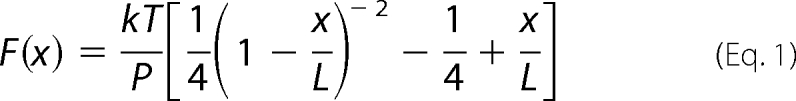 |
where P is the persistence length, L is the contour length, and x is the end-to-end distance of the chain. Using a persistence length of 0.6 nm, we can predict the force needed to extend a 90-amino acid unstructured polypeptide, which is the length of a typical FN-III domain (Fig. 7B). The force of 15 pN, which is needed to prevent refolding, would stretch the polypeptide to 60% of its contour length. For a domain subjected to a 5 pN force, the peptide would only have an approximate 30% extension. These extensions are significantly less than the complete, 7-fold extension that would be necessary to explain 4-fold stretch that is observed in FN fibrils. Thus, we conclude that the unfolding of domains III-2, III-3, III-6, III-9, III-11, and III-12 can only account for a fraction of the observed extensibility of FN fibrils. The majority of fibril elongation would have to be produced by global conformational changes of the entire FN molecule.
This work was supported, in whole or in part, by National Institutes of Health Grants CA47056 (to H. P. E.) and GM089331 (to C. A. L.).
- FN
- fibronectin
- CPM
- 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin
- FN-I
- type I domain of fibronectin
- N
- newtons.
REFERENCES
- 1. Potts J. R., Campbell I. D. (1996) Matrix Biol. 15, 313–320; discussion 321 [DOI] [PubMed] [Google Scholar]
- 2. Johnson K. J., Sage H., Briscoe G., Erickson H. P. (1999) J. Biol. Chem. 274, 15473–15479 [DOI] [PubMed] [Google Scholar]
- 3. Ruoslahti E., Pierschbacher M. D. (1987) Science 238, 491–497 [DOI] [PubMed] [Google Scholar]
- 4. Fogerty F. J., Akiyama S. K., Yamada K. M., Mosher D. F. (1990) J. Cell Biol. 111, 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowditch R. D., Hariharan M., Tominna E. F., Smith J. W., Yamada K. M., Getzoff E. D., Ginsberg M. H. (1994) J. Biol. Chem. 269, 10856–10863 [PubMed] [Google Scholar]
- 6. Nagai T., Yamakawa N., Aota S., Yamada S. S., Akiyama S. K., Olden K., Yamada K. M. (1991) J. Cell Biol. 114, 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A. M., Burridge K. (1998) J. Cell Biol. 141, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halliday N. L., Tomasek J. J. (1995) Exp. Cell Res. 217, 109–117 [DOI] [PubMed] [Google Scholar]
- 9. Ohashi T., Kiehart D. P., Erickson H. P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2153–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohashi T., Kiehart D. P., Erickson H. P. (2002) J. Cell Sci. 115, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 11. Sivakumar P., Czirok A., Rongish B. J., Divakara V. P., Wang Y. P., Dallas S. L. (2006) J. Cell Sci. 119, 1350–1360 [DOI] [PubMed] [Google Scholar]
- 12. Baneyx G., Baugh L., Vogel V. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5139–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson H. P. (2002) J. Muscle Res. Cell Motil. 23, 575–580 [DOI] [PubMed] [Google Scholar]
- 14. Erickson H. P. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10114–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith M. L., Gourdon D., Little W. C., Kubow K. E., Eguiluz R. A., Luna-Morris S., Vogel V. (2007) PLOS Biol. 5, e268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oberhauser A. F., Badilla-Fernandez C., Carrion-Vazquez M., Fernandez J. M. (2002) J. Mol. Biol. 319, 433–447 [DOI] [PubMed] [Google Scholar]
- 17. Abu-Lail N. I., Ohashi T., Clark R. L., Erickson H. P., Zauscher S. (2006) Matrix Biol. 25, 175–184 [DOI] [PubMed] [Google Scholar]
- 18. Ohashi T., Augustus A., Erickson H. (2009) Biochemistry 48, 4189–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohashi T., Erickson H. P. (2005) J. Biol. Chem. 280, 39143–39151 [DOI] [PubMed] [Google Scholar]
- 20. Erickson H. P., Carrell N. A. (1983) J. Biol. Chem. 258, 14539–14544 [PubMed] [Google Scholar]
- 21. Johnson C. P., Tang H. Y., Carag C., Speicher D. W., Discher D. E. (2007) Science 317, 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aricescu A. R., Lu W., Jones E. Y. (2006) Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 23. Miroux B., Walker J. E. (1996) J. Mol. Biol. 260, 289–298 [DOI] [PubMed] [Google Scholar]
- 24. Leahy D. J., Aukhil I., Erickson H. P. (1996) Cell 84, 155–164 [DOI] [PubMed] [Google Scholar]
- 25. Sharma A., Askari J. A., Humphries M. J., Jones E. Y., Stuart D. I. (1999) EMBO J. 18, 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vakonakis I., Staunton D., Rooney L. M., Campbell I. D. (2007) EMBO J. 26, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chernousov M. A., Faerman A. I., Frid M. G., Printseva OYu, Koteliansky V. E. (1987) FEBS Lett. 217, 124–128 [DOI] [PubMed] [Google Scholar]
- 28. Sechler J. L., Rao H., Cumiskey A. M., Vega-Colón I., Smith M. S., Murata T., Schwarzbauer J. E. (2001) J. Cell Biol. 154, 1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hocking D. C., Sottile J., McKeown-Longo P. J. (1994) J. Biol. Chem. 269, 19183–19187 [PubMed] [Google Scholar]
- 30. McKeown-Longo P. J., Mosher D. F. (1985) J. Cell Biol. 100, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sottile J., Schwarzbauer J., Selegue J., Mosher D. F. (1991) J. Biol. Chem. 266, 12840–12843 [PubMed] [Google Scholar]
- 32. Carrion-Vazquez M., Oberhauser A. F., Fowler S. B., Marszalek P. E., Broedel S. E., Clarke J., Fernandez J. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3694–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H., Linke W. A., Oberhauser A. F., Carrion-Vazquez M., Kerkvliet J. G., Lu H., Marszalek P. E., Fernandez J. M. (2002) Nature 418, 998–1002 [DOI] [PubMed] [Google Scholar]
- 34. Bustamante C., Marko J. F., Siggia E. D., Smith S. (1994) Science 265, 1599–1600 [DOI] [PubMed] [Google Scholar]
- 35. Bouchiat C., Wang M. D., Allemand J., Strick T., Block S. M., Croquette V. (1999) Biophys. J. 76, 409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher T. E., Oberhauser A. F., Carrion-Vazquez M., Marszalek P. E., Fernandez J. M. (1999) Trends Biochem. Sci. 24, 379–384 [DOI] [PubMed] [Google Scholar]