Abstract
The primary site of mercury-induced injury is the kidney due to uptake of the reactive Hg2+-conjugated organic anions in the proximal tubule. Here, we investigated the in vivo role of Oat1 (organic anion transporter 1; originally NKT (Lopez-Nieto, C. E., You, G., Bush, K. T., Barros, E. J., Beier, D. R., and Nigam, S. K. (1997) J. Biol. Chem. 272, 6471–6478)) in handling of known nephrotoxic doses of HgCl2. Oat1 (Slc22a6) is a multispecific organic anion drug transporter that is expressed on the basolateral aspects of renal proximal tubule cells and that mediates the initial steps of elimination of a broad range of endogenous metabolites and commonly prescribed pharmaceuticals. Mercury-induced nephrotoxicity was observed in a wild-type model. We then used the Oat1 knock-out to determine in vivo whether the renal injury effects of mercury are mediated by Oat1. Most of the renal injury (both histologically and biochemically as measured by blood urea nitrogen and creatinine) was abolished following HgCl2 treatment of Oat1 knock-outs. Thus, acute kidney injury by HgCl2 was found to be mediated mainly by Oat1. Our findings raise the possibility that pharmacological modulation of the expression and/or function of Oat1 might be an effective therapeutic strategy for reducing renal injury by mercury. This is one of the most striking phenotypes so far identified in the Oat1 knock-out. (Eraly, S. A., Vallon, V., Vaughn, D. A., Gangoiti, J. A., Richter, K., Nagle, M., Monte, J. C., Rieg, T., Truong, D. M., Long, J. M., Barshop, B. A., Kaler, G., and Nigam, S. K. (2006) J. Biol. Chem. 281, 5072–5083).
Keywords: ABC Transporter, Brain, Creatinine, Drug Transport, Exchangers, Metals, Multidrug Transporters, Transport Metals, Urine
Introduction
Environmental exposure to mercury represents a major threat in an increasingly industrialized world. Children exposed to high levels of the metal before and after birth suffer from chorea, ataxia, seizures, tremors, and mental retardation (2, 3). The ingestion of mercury-contaminated seafood represents the major source of exposure, although other sources such as many manufacturing processes, salvaging of electronics, and mining practices (particularly in the developing world) contribute to the accumulation of heavy metals in the environment. Metallic mercury, methyl mercury, and ethyl mercury are neurotoxic compounds that can be converted in the body to inorganic mercury, which is highly nephrotoxic. Mercuric (Hg2+) ions are found in the plasma primarily as conjugates of thiol-containing biomolecules such as glutathione, cysteine, homocysteine, N-acetylcysteine, and albumin. Mercuric S-conjugates of these thiols are considered primary substrates for transport by the proximal tubule cells of the kidney (4, 5).
Although the renal transport of mercury and the mechanism of renal toxicity are not well characterized, accumulating evidence suggests that the uptake is mediated, at least in part, via the organic anion transporter system (6). Oat1, which was originally identified as NKT by us (1), is expressed on the basolateral membrane of proximal tubular cells and is a multispecific organic solute transporter mediating the initial steps of elimination of various substrates, including nutrients, drugs, toxins and metabolites, from the circulation (7–9). In vitro assays demonstrated that Oat1 is able to mediate the uptake of mercuric compounds such as conjugates of homocysteine, cysteine, N-acetylcysteine, and glutathione (10–12). Moreover, p-aminohippurate (a high affinity competitive substrate transported by this organic anion/dicarboxylate exchanger) and probenecid (an Oat1 inhibitor) can block the uptake of mercuric conjugates (13, 14). Nevertheless, in vivo evidence directly linking mercury toxicity and an individual Oat transporter is lacking. In this study, we investigated the mercury-induced renal injury in both rats and Oat1−/− mice by intraperitoneal administration of 4 mg/kg of body weight of HgCl2. Our results indicate that, although the rodents are susceptible to mercury, mice deficient in Oat1 due to genetic deletion are largely protected from the mercury-induced kidney injury. This is consistent with the notion that Oat1 is the major transporter mediating cellular uptake of mercuric conjugates and provides direct genetic evidence implicating Oat1 in the renal injury from HgCl2 by mediating its transport into the proximal tubular cells.
EXPERIMENTAL PROCEDURES
In Vivo Studies (16)
Wistar Rats (110–150 days of age) were treated with a single injection (intraperitoneal) of HgCl2 at a nephrotoxic dose of 4 mg/kg of body weight (w/v in 1 ml of saline/kg; HgCl2 group, n = 4) (4). All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD) and were approved by the local animal ethics committee. Controls (control group, n = 4) received the vehicle alone (1 ml of saline/kg). Urine volumes were collected for 18 h after the injection in metabolic cages. Blood was collected, and kidneys were decapsulated, weighed, and processed for histopathological studies. The volume of urine was determined by gravimetry. The plasma was separated by centrifugation (3000 rpm, 3 min). Creatinine concentrations were determined in urine and plasma samples. Blood urea nitrogen (BUN)2 levels were evaluated in plasma samples. Creatinine and BUN concentrations were assayed employing commercial kits (Wiener Laboratory, Rosario, Argentina). Creatinine clearance was calculated by conventional formulas for each animal.
Mice between 12 and 20 weeks of age (matched within 2 weeks) with the Oat1 null allele were backcrossed with C57BL/6J mice for a total of 10 generations. Oat1 knock-out mice and wild-type C57BL/6J controls were used in the experiments described. After 18 h of HgCl2 treatment as described above, urine, blood, and tissues were collected. The plasma was separated by centrifugation (3000 rpm, 3 min). BUN concentrations were assayed in plasma samples employing commercial kits (QuantiChromTM, BioAssay Systems, Hayward, CA).
Histological Studies
Kidneys were rinsed with 0.9% saline, fixed in 4% paraformaldehyde for 24 h, transferred to 70% ethanol, processed, and finally embedded in paraffin. 5-μm sections were cut and stained with hematoxylin and eosin for histological examination using a light microscope.
Materials
Chemicals were purchased from Sigma and were analytical grade.
Statistical Analysis
Statistical analysis was performed using an unpaired t test. When variances were not homogeneous, Welch's correction was employed. p values <0.05 were considered significant. Values are expressed as means ± S.E.
RESULTS
HgCl2-induced Renal Insufficiency
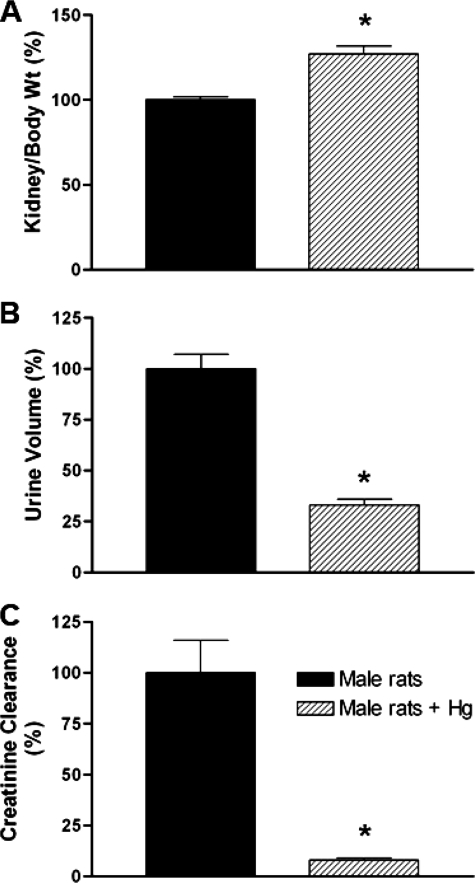
A substantial body of evidence indicates that mercuric conjugates are the major form in which mercury is transported by the kidney (Table 1). Nevertheless, a major environmental source is inorganic mercury, even though it is believed to be eliminated as mercury organic conjugates transported predominantly by Oat1 (see “Discussion”). HgCl2 treatment is a well established model for studying this process in vivo (11, 16). Thus, treatment of rats with HgCl2 significantly increased plasma BUN levels (Table 2), suggesting impairment of renal function. In addition, blood and urine chemistry analyses revealed significant renal disturbance under the HgCl2-treated conditions. When both HgCl2-treated and vehicle-treated rats were placed in metabolic cages for 18 h immediately following the injection, significantly reduced amounts of urine were excreted by the HgCl2-treated rats versus the controls treated with vehicle alone. This was accompanied by a reduction in the clearance of creatinine, further supporting the notion that HgCl2 treatment results in renal impairment. Fig. 1 shows that the ratio of kidney weight to body weight was increased (Fig. 1A) and that the urine volume (Fig. 1B) and clearance of creatinine (Fig. 1C) were decreased in the HgCl2-treated rats.
TABLE 1.
Known mercuric conjugates that are substrates for OAT1
MDCK, Madin-Darby canine kidney; h, human; r, rat; NAC, N-acetylcysteine.
| Substrate | Assay | Transporter | Km | Ref. |
|---|---|---|---|---|
| μm | ||||
| Cys-S-Hg-S-Cys | MDCK II | hOAT1 | 91 | 11 |
| Hcy-S-Hg-S-Hcy | MDCK II | hOAT1 | 128 | 11 |
| Hg2+ (NAC-S-Hg-S-NAC) | MDCK II | hOAT1 | 144 | 10 |
| MeHg-2,3-dimercapto-1-propanesulfonic acid | X. laevis | rOAT1 | 9 | 31 |
| MeHg-N-acetyl-l-cysteine | X. laevis | rOAT1 | 31 | 31 |
| N-Acetyl-l-cysteine-Hg2+ | MDCK II | hOAT1 | 44 | 12 |
TABLE 2.
Effect of HgCl2 (4 mg/kg of body weight, intraperitoneal) in rats at 18 h after treatment on body weight, kidney weight, and plasma BUN levels
Results are expressed as means ± S.E.
| Control (n = 4) | HgCl2-treated (n = 4) | |
|---|---|---|
| Body weight (g) | 341 ± 2 | 351 ± 7 |
| Kidney weight (g) | 2.22 ± 0.04 | 2.90 ± 0.06a |
| BUN (mg/dl) | 16 ± 1 | 93 ± 6a |
a p < 0.05 versus control rats.
FIGURE 1.
Effect of HgCl2 (4 mg/kg of body weight, intraperitoneal) in adult rats at 18 h after treatment (n = 4). A, ratio of kidney weight to body weight. B, urine volume collected for 18 h after HgCl2 treatment. C, creatinine clearance. *, p < 0.05 versus control rats.
Tubular Damage following HgCl2 Exposure
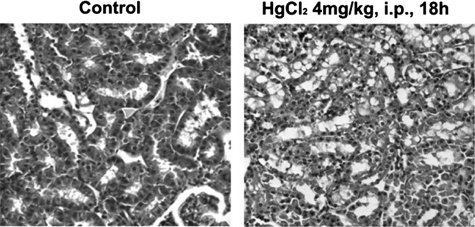
To confirm that the physiological disturbance observed was of renal tubular origin, kidneys from both the HgCl2-treated and control rats were examined histologically. Examination of hematoxylin/eosin-stained rat kidney slices 18 h after HgCl2 treatment revealed dilated tubular lumina, disrupted tubular basement membranes, sloughing of cells, and intratubular cast formation (Fig. 2), suggesting early stages of tubular necrosis that are consistent with acute kidney injury. When the relative diameter of the lumen was measured, significant increases in the width of the lumen in tubules was found in HgCl2-treated rats compared with vehicle-treated controls. Thus, histopathological data are consistent with pathophysiological analyses showing that a single dose of 4 mg of HgCl2/kg of body weight is sufficient to cause proximal tubular injury within 18 h.
FIGURE 2.
Representative micrographs of hematoxylin/eosin-stained rat kidney sections at 18 h after treatment with vehicle or HgCl2 (4 mg/kg of body weight, intraperitoneal). Shown are detailed views of healthy and damaged proximal tubules in control and treated kidneys, respectively. i.p., intraperitoneal.
Significantly Reduced Renal Injury and Insufficiency in Oat1 Knock-outs after HgCl2 Exposure
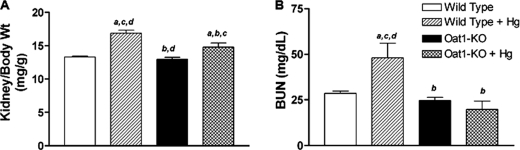
The kidney is a primary target organ of mercury injury. Studies in vitro have suggested that mercury-induced renal injury could be mediated by organic anion transporters (10–12). We thus sought to confirm this in vivo by treating mice deficient in Oat1 with HgCl2 in a manner similar to that described above for rats. The Oat1 coding sequences in mice and rats are extremely similar (17), and it is known that similar substrates are transported (18). As in the case of the rat, treatment of wild-type mice with 4 mg of HgCl2/kg of body weight (intraperitoneal) led to a significant increase in the ratio of kidney weight to body weight (Fig. 3A). In addition, significant increases in plasma BUN levels were also observed in wild-type (C57/BL6) mice following HgCl2 treatment (Fig. 3B). These data are consistent with the phenotypes observed in rats upon similar treatment.
FIGURE 3.
Effect of HgCl2 (4 mg/kg of body weight, intraperitoneal) in wild-type and Oat1−/− mice (n = 4) at 18 h after treatment. A, ratio of kidney weight to body weight. B, renal function in terms of elevation of plasma BUN levels. a–d, p < 0.05 versus control WT, HgCl2-treated WT, control Oat1−/−, and HgCl2-treated Oat1−/−, respectively.
To obtain direct evidence regarding the role of Oat1 in the renal injury induced by mercury, Oat1/Slc22a6 knock-outs (backcrossed to C57/BL6) were subjected to treatment with 4 mg of HgCl2/kg of body weight (intraperitoneal; same conditions as for the wild-type mice; see above). Following treatment with HgCl2, there was a slight increase in the ratio of kidney weight to body weight in Oat1-deficient mice compared with vehicle-treated wild-type mice. However, this increase was much smaller than that observed in HgCl2-treated wild-type mice (Fig. 3A).
In contrast to wild-type mice treated with HgCl2, no significant increase in the plasma BUN level was observed in Oat1−/− mice treated with HgCl2 (Fig. 3B). These data suggest that mercury-induced renal insufficiency is markedly reduced in mice deficient in Oat1/Slc22a6.
Reduced Tubular Histopathological Damage in Oat1−/− Kidneys after HgCl2 Exposure
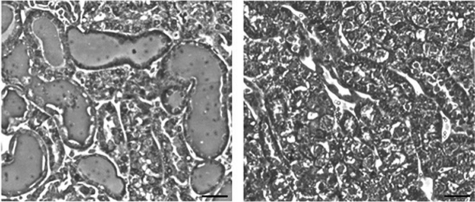
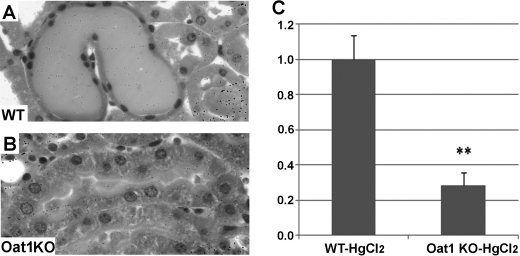
Histopathological damage as assessed by hematoxylin and eosin staining of kidney sections revealed a marked difference in the pattern of renal injury between wild-type and Oat1 knock-out mice (Fig. 4). The wild-type kidneys showed proximal tubular necrosis characterized by dilated tubular lumina with retention of urine and loss of the epithelial brush border with pyknotic nuclei. These signs of renal injury were virtually undetectable in the Oat1 knock-out kidneys (Figs. 4 and 5). Our functional and histopathological data indicate that Oat1/Slc22a6 is a direct mediator of mercury renal injury and that its deletion in Oat1−/− mice is responsible for the remarkable reduction in HgCl2-induced proximal tubular necrosis (Fig. 6).
FIGURE 4.
Representative micrographs of hematoxylin/eosin-stained sections of WT (left panel) and Oat1 knock-out (right panel) mouse kidneys at 18 h following treatment with vehicle control or HgCl2 (4 mg/kg of body weight, intraperitoneal), respectively. Scale bar, 100 μm.
FIGURE 5.
A and B, high magnification hematoxylin and eosin images demonstrating tubular structures in WT (A) and Oat1 knock-out (B) kidneys after HgCl2 treatment. C, bar graph of the relative width of the tubular lumen derived from measurements in hematoxylin/eosin-stained sections of WT and Oat1 knock-out (Oat1KO) kidneys at 18 h after treatment with a single dose of HgCl2 (4 mg/kg body weight, intraperitoneal). **, p < 0.01.
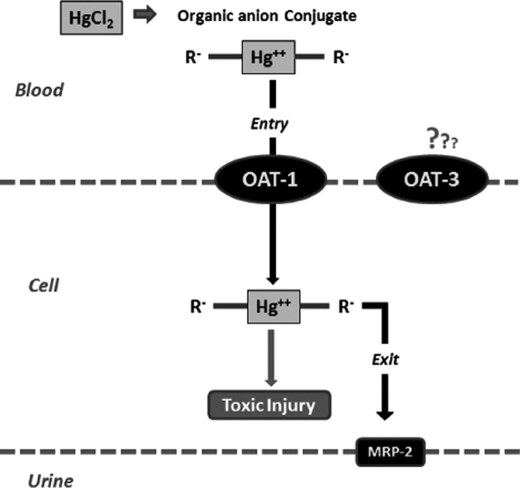
FIGURE 6.
Diagram showing the flow of mercury from blood to urine in the form of an organic anion conjugate, which enters the proximal tubular cell via the Oat1 transporter and can then be excreted into the urine via Mrp-2.
DISCUSSION
Mercury accumulates preferentially in kidneys and can cause kidney injury (19). In this study, a single injury dose of HgCl2 induced significant renal impairment in rodents. This HgCl2-induced renal insufficiency is caused by proximal tubular injury mediated mainly by the renal organic anion transporter Oat1/Slc22a6.
Inorganic mercury has been recognized as a hazardous environmental pollutant; nevertheless, it is still used in some batteries and continues to be an essential component of fluorescent light bulbs (20). People can be exposed to mercury through contaminated water and food (21). Moreover, different mercury species (vapor, inorganic mercury, and methyl mercury) have been associated with human contamination in gold-mining areas (22, 23).
Renal proximal tubular cells represent the primary target site where highly reactive mercuric ions rapidly accumulate and induce cell injury by binding to and inhibiting various sulfhydryl-containing enzymes (4). Complex factors may account for this susceptibility, including the selective presence of specific transport systems involved in the uptake of mercuric ions by particular cell populations (6). The extraction of mercury from renal tubular cells does not appear to involve glomerular filtration but rather a direct secretory process whereby mercury moves from peritubular blood into the tubular lumen (24). Mercury is believed to access proximal tubular cells primarily via the organic anion transporters found on the basolateral plasma membrane (12, 13, 16); however, direct in vivo evidence for this has not been available so far.
Oat1 is expressed in the proximal tubules of the kidneys, where it plays an important role in the elimination of numerous organic anions of physiological (25), pharmacological, and toxicological (9, 17, 26–30) relevance. Although we have detected inhibition by HgCl2 of the transport of 6-carboxyfluorescein (an Oat1 substrate), the in vivo physiological relevance of this finding and whether inorganic mercury is a true Oat1 substrate are not clear (data not shown). The bulk of published evidence supports the view that inorganic mercury is converted to an organic form before being transported by Oat1. In vitro assays indicate that Oat1 mediates the uptake of a number of mercuric conjugates, including homocysteine, cysteine, N-acetylcysteine, and glutathione (Table 1) (10–12, 31).
In this study, we have provided direct evidence that Oat1 is a mediator of HgCl2 renal injury in vivo. For example, the observed increase in kidney weight (presumably due to edema) (27, 32–34) and BUN levels seen in rats and wild-type mice following HgCl2 treatment was lessened dramatically in the Oat1−/− mice, suggesting a role for Oat1 in the uptake of mercuric conjugates from the blood and into the renal epithelial cells. In addition, other parameters of renal function perturbed by HgCl2 treatment were also less affected in the Oat1 knock-out mice. For example, BUN levels were largely similar to control levels, whereas histological parameters (35–37) were largely unchanged in the Oat1−/− kidney following HgCl2 treatment. These differences in renal injury are striking and indicate that Oat1 is the main transporter responsible for HgCl2-induced renal injury. This is also one of the most impressive phenotypes reported in the Oat1 knock-out.
It is worth noting that the expression of Oat3 is somewhat reduced in the Oat1 knock-out kidney, but this appears to be physiologically insignificant based upon analysis of Oat3-selective substrates in knock-outs (7, 38, 39). Although Oat3 transport of mercury in vitro has been reported, it remains possible, although unlikely, that some of the observed protection was due to somewhat decreased Oat3 expression. The expression of other Slc22 family members is unchanged (40). Moreover, in vitro evidence suggests that Oat1 is the primary mediator of proximal tubular basolateral uptake of mercuric conjugates. On the other hand, several transporters, including multidrug resistance-associated proteins of the ABCC gene family, have been suggested to mediate the mercury excretion at the apical membrane of the proximal tubules. However, our previous analysis of ABCC gene family expression did not reveal significant alterations in the Oat1 knock-out kidneys (40).
In summary, in the study, we demonstrated that a single dose of HgCl2 can cause acute kidney injury in both rats and mice, which is mediated mainly by injury to the proximal tubule. This injury is directly linked to the organic anion transporter Oat1 based on in vivo and in vitro studies. The fact that mercury-induced acute kidney injury is directly linked to Oat1 expression may be useful for developing biological protection against toxic effects exerted by mercury or by specific forms of mercury. Pharmacological modulation of the expression and/or function of Oat1 (such as the use of specific inhibitors) might be an effective therapeutic strategy for reducing kidney injury due to mercury.
Acknowledgment
We acknowledge the editorial contribution of Megan Bettilyon.
This work was supported, in whole or in part, by National Institutes of Health Grants DK079784 and GM88824 (to S. K. N.). This work was also supported by Fund for Scientific and Technological Research (FONCYT) Grant PICT 00966 and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Grant PIP 01665 (to A. M. T.) and the Society of Toxicology and Colgate-Palmolive (to A. V. D.).
- BUN
- blood urea nitrogen.
REFERENCES
- 1. Lopez-Nieto C. E., You G., Bush K. T., Barros E. J., Beier D. R., Nigam S. K. (1997) J. Biol. Chem. 272, 6471–6478 [DOI] [PubMed] [Google Scholar]
- 2. Lee R., Middleton D., Caldwell K., Dearwent S., Jones S., Lewis B., Monteilh C., Mortensen M. E., Nickle R., Orloff K., Reger M., Risher J., Rogers H. S., Watters M. (2009) Environ. Health Perspect. 117, 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bose-O'Reilly S., McCarty K. M., Steckling N., Lettmeier B. (2010) Curr. Probl. Pediatr. Adolesc. Health Care 40, 186–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zalups R. K. (2000) Pharmacol. Rev. 52, 113–143 [PubMed] [Google Scholar]
- 5. Bridges C. C., Zalups R. K. (2005) Toxicol. Appl. Pharmacol. 204, 274–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bridges C. C., Zalups R. K. (2010) J. Toxicol. Environ. Health B Crit. Rev. 13, 385–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eraly S. A., Vallon V., Vaughn D. A., Gangoiti J. A., Richter K., Nagle M., Monte J. C., Rieg T., Truong D. M., Long J. M., Barshop B. A., Kaler G., Nigam S. K. (2006) J. Biol. Chem. 281, 5072–5083 [DOI] [PubMed] [Google Scholar]
- 8. Pavlova A., Sakurai H., Leclercq B., Beier D. R., Yu A. S., Nigam S. K. (2000) Am. J. Physiol. Renal Physiol. 278, F635–F643 [DOI] [PubMed] [Google Scholar]
- 9. Wu W., Dnyanmote A. V., Nigam S. K. (2011) Mol. Pharmacol. 79, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zalups R. K., Ahmad S. (2005) Kidney Int. 68, 1684–1699 [DOI] [PubMed] [Google Scholar]
- 11. Zalups R. K., Ahmad S. (2004) J. Am. Soc. Nephrol. 15, 2023–2031 [DOI] [PubMed] [Google Scholar]
- 12. Aslamkhan A. G., Han Y. H., Yang X. P., Zalups R. K., Pritchard J. B. (2003) Mol. Pharmacol. 63, 590–596 [DOI] [PubMed] [Google Scholar]
- 13. Lungkaphin A., Chatsudthipong V., Evans K. K., Groves C. E., Wright S. H., Dantzler W. H. (2004) Am. J. Physiol. Renal Physiol. 286, F68–F76 [DOI] [PubMed] [Google Scholar]
- 14. Zalups R. K., Barfuss D. W. (2002) Toxicol. Appl. Pharmacol. 182, 234–243 [DOI] [PubMed] [Google Scholar]
- 15. Vanwert A. L., Srimaroeng C., Sweet D. H. (2008) Mol. Pharmacol. 74, 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Giusto G., Anzai N., Ruiz M. L., Endou H., Torres A. M. (2009) Arch. Toxicol. 83, 887–897 [DOI] [PubMed] [Google Scholar]
- 17. Wu W., Baker M. E., Eraly S. A., Bush K. T., Nigam S. K. (2009) Physiol. Genomics 38, 116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanWert A. L., Gionfriddo M. R., Sweet D. H. (2010) Biopharm. Drug Dispos. 31, 1–71 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka-Kagawa T., Suzuki M., Naganuma A., Yamanaka N., Imura N. (1998) J. Pharmacol. Exp. Ther. 285, 335–341 [PubMed] [Google Scholar]
- 20. Clarkson T. W. (1993) Environ. Health Perspect. 100, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magos L., Clarkson T. W. (2006) Ann. Clin. Biochem. 43, 257–268 [DOI] [PubMed] [Google Scholar]
- 22. Steckling N., Boese-O'Reilly S., Gradel C., Gutschmidt K., Shinee E., Altangerel E., Badrakh B., Bonduush I., Surenjav U., Ferstl P., Roider G., Sakamoto M., Sepai O., Drasch G., Lettmeier B., Morton J., Jones K., Siebert U., Hornberg C. (2011) Sci. Total Environ. 409, 994–1000 [DOI] [PubMed] [Google Scholar]
- 23. Drasch G., Böse-O'Reilly S., Beinhoff C., Roider G., Maydl S. (2001) Sci. Total Environ. 267, 151–168 [DOI] [PubMed] [Google Scholar]
- 24. Zalups R. K. (1998) J. Toxicol. Environ. Health A 53, 615–636 [DOI] [PubMed] [Google Scholar]
- 25. Wikoff W. R., Nagle M. A., Kouznetsova V. L., Tsigelny I. F., Nigam S. K. (2011) J. Proteome Res. 10, 2842–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burckhardt B. C., Burckhardt G. (2003) Rev. Physiol. Biochem. Pharmacol. 146, 95–158 [DOI] [PubMed] [Google Scholar]
- 27. Di Giusto G., Anzai N., Endou H., Torres A. M. (2008) Pharmacology 81, 127–136 [DOI] [PubMed] [Google Scholar]
- 28. Endou H. (1998) Toxicol. Lett. 102–103, 29–33 [DOI] [PubMed] [Google Scholar]
- 29. Nigam S. K., Bush K. T., Bhatnagar V. (2007) Nat. Clin. Pract. Nephrol. 3, 443–448 [DOI] [PubMed] [Google Scholar]
- 30. Mónica Torres A., Mac Laughlin M., Muller A., Brandoni A., Anzai N., Endou H. (2005) Biochim. Biophys. Acta 1740, 29–37 [DOI] [PubMed] [Google Scholar]
- 31. Koh A. S., Simmons-Willis T. A., Pritchard J. B., Grassl S. M., Ballatori N. (2002) Mol. Pharmacol. 62, 921–926 [DOI] [PubMed] [Google Scholar]
- 32. Villar S. R., Brandoni A., Anzai N., Endou H., Torres A. M. (2005) Kidney Int. 68, 2704–2713 [DOI] [PubMed] [Google Scholar]
- 33. Green B. T., Umana E. (2000) South. Med. J. 93, 721–723 [PubMed] [Google Scholar]
- 34. Brady W. J., Ferguson J. D., Ullman E. A., Perron A. D. (2004) Emerg. Med. Clin. North Am. 22, 865–885 [DOI] [PubMed] [Google Scholar]
- 35. Di Giusto G., Torres A. M. (2010) Arch. Toxicol. 84, 741–749 [DOI] [PubMed] [Google Scholar]
- 36. Stacchiotti A., Borsani E., Ricci F., Lavazza A., Rezzani R., Bianchi R., Rodella L. F. (2006) Toxicol. Lett. 166, 168–177 [DOI] [PubMed] [Google Scholar]
- 37. Nava M., Romero F., Quiroz Y., Parra G., Bonet L., Rodríguez-Iturbe B. (2000) Am. J. Physiol. Renal Physiol. 279, F910–F918 [DOI] [PubMed] [Google Scholar]
- 38. Nagle M. A., Truong D. M., Dnyanmote A. V., Ahn S. Y., Eraly S. A., Wu W., Nigam S. K. (2011) J. Biol. Chem. 286, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Truong D. M., Kaler G., Khandelwal A., Swaan P. W., Nigam S. K. (2008) J. Biol. Chem. 283, 8654–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vallon V., Rieg T., Ahn S. Y., Wu W., Eraly S. A., Nigam S. K. (2008) Am. J. Physiol. Renal Physiol. 294, F867–F873 [DOI] [PubMed] [Google Scholar]