Abstract
Invasion of hepatocytes by Plasmodium sporozoites deposited by Anopheles mosquitoes, and their subsequent transformation into infective merozoites is an obligatory step in the initiation of malaria. Interactions between the sporozoites and hepatocytes lead to a distinct, complex and coordinated cellular and systemic host response. Little is known about host liver cell response to sporozoite invasion, or whether it is primarily adaptive for the parasite, for the host, or for both. Our present study used gene expression profiling of human HepG2-A16 liver cells infected with Plasmodium falciparum sporozoites to understand the host early cellular events and factors influencing parasite infectivity and sporozoite development. Our results show that as early as 30 min following wild-type, non-irradiated sporozoite exposure, the expressions of at least 742 genes was selectively altered. These genes regulate diverse biological functions, such as immune processes, cell adhesion and communications, metabolism pathways, cell cycle regulation, and signal transduction. These functions reflect cellular events consistent with initial host cell defense responses, as well as alterations in host cells to sustain sporozoites growth and survival. Irradiated sporozoites gave very similar gene expression pattern changes, but direct comparative analysis between liver gene expression profiles caused by irradiated and non-irradiated sporozoites identified 29 genes, including glypican-3, that were specifically up-regulated only in irradiated sporozoites. Elucidating the role of this subset of genes may help identify the molecular basis for the irradiated sporozoites inability to develop intrahepatically, and their usefulness as an immunogen for developing protective immunity against pre-erythrocytic stage malaria.
Keywords: Gene Expression, Liver, Microarray, Parasite, Transcription, HepG2-A16, Plasmodium falciparum, Liver Cells, Malaria, Sporozoites
Introduction
Malaria transmission occurs when Plasmodium sporozoites from the salivary glands of female Anopheles mosquitoes are inoculated into vertebrate hosts during a blood meal. Sporozoites quickly reach the liver through the circulation and traverse through several hepatocyte cells by membrane disruption before invading and settling down in a final hepatocyte for their liver stage development. Exo-erythrocytic forms of the parasite grow within hepatocytes to produce several thousand merozoites, which exit the infected hepatocytes and invade erythrocytes to initiate clinical malaria.
Infection by a pathogen triggers a complex and distinct set of cellular and systemic events, some of which may be orchestrated by the parasite to support development, and others representing a host defense response. Interactions between host and pathogens are diverse and are regulated in specific patterns by unique molecules and mechanisms involving activation of transcriptional events of innate and adaptive immunity (1–4). In malaria, this complex interaction between pathogen and host is a critical factor in determining the progression and outcome of the development in liver of the parasite. Understanding global changes that occur both at the host and parasite transcriptome level will allow better understanding of the various host and parasite factors influencing infectivity and parasite development in mammalian hosts. Little is known about host liver cell response to sporozoite invasion, although considerable progress have been made in elucidating the parasite genes and proteins that are important for liver infection (5–13). The few other studies on this area so far were focused on specific factors, such as hepatocyte growth factor (14) and CD81 (15), whereas studies using genome-wide microarray approaches were predominantly performed using non-human host/sporozoite pairings. Because the biology of non-human host/sporozoite combinations differs from those of human, the relevance of the findings from these studies remain to be confirmed.
The development of an effective malaria vaccine has been an area of intense research in recent years. Attenuated whole sporozoites, their constituent proteins, or the genes encoding them are useful candidates for development of a malaria vaccine. These studies were aimed at discovering specific potential targets for development of a pre-erythrocytic vaccine or drug for malaria. Limited studies have been conducted on changes in hepatocyte transcriptome or proteome on the initial encounter with malaria sporozoites. The liver is a critical gateway for invading sporozoites that can be targeted for intervention before the development of malaria sporozoites into disease producing blood-stage parasites. The various hepatic factors influencing binding and invasion of sporozoites into hepatocytes have being studied in mice using Plasmodium yoelii and Plasmodium berghei sporozoites, but the biology of these sporozoites is different from human malaria parasites, such as Plasmodium falciparum or Plasmodium vivax.
We used a cell culture model of HepG2-A16 hepatoma cells (16, 17) infected with isolated P. falciparum sporozoites, and evaluated global hepatic gene expression changes by high-density microarray profiling. Microarrays can identify genome-wide transcriptional events that underlie liver responses to sporozoite invasion, and provide insights into molecular events and hepatic factors related to sporozoite invasion and development. The in vitro liver cell culture system allows evaluation of much earlier and well defined stages of sporozoite invasion that are not possible in human subject studies. Although genome-wide microarray analyses have been used to study malaria sporozoite-hepatocyte interactions (18–20), these studies are limited to either transcriptome profiling of the sporozoites or of non-human host cells/parasite combinations. Our present study was aimed at specifically identifying changes in human hepatocyte transcripts differentially regulated following early P. falciparum sporozoites infection. We therefore used the HepG2-A16 human hepatoma cells that have been extensively used in P. falciparum sporozoite invasion assay studies (21–24) and to which P. falciparum sporozoites are known to attach and invade without further exoerythrocytic stage development. Differential effects on liver gene expression profiles caused by wild-type and radiation-attenuated sporozoites were also determined.
EXPERIMENTAL PROCEDURES
Preparation of Malaria Sporozoites
Sporozoites were isolated from the same batch of Anopheles stephensi mosquitoes infected with either irradiated or non-irradiated P. falciparum (NF 54 strain) using a modified microcentrifugation technique described previously (25). Briefly, mosquitoes were placed on a glass slide, head and thoraxes were separated, and the abdomens discarded. Up to 50 heads and thoraxes were placed on a 0.5-ml microcentrifuge tube previously perforated with a 25-gauge needle and plugged with glass wool. This tube was placed in a 1.5-ml microcentrifuge tube, and 50 μl of buffer (PBS, pH 8, 1.5% glucose, 5% fetal bovine serum) was added and the tubes were centrifuged for 2 min at 10,000 × g in an Eppendorf microcentrifuge. Filtrates were collected, and centrifugation was repeated after the addition of another 50 μl of buffer. Pooled filtrate was adjusted to 1 ml with buffer and layered on top of a DEAE-cellulose (DE-52, Whatman) column to remove mosquito debris and microbial contaminants (26). The column was prepared using a 25-ml pipette plugged with glass wool and packed with 10 ml of a 50% DEAE slurry. Sporozoites were eluted from the DEAE column by gravity using 20 ml of buffer, and the initial 14–15 ml of eluate was collected and centrifuged to pellet the sporozoites. Isolated sporozoites were resuspended in DMEM and counted using a hemocytometer. Because there is the possibility that residual contaminating salivary gland proteins in the sporozoite preparations may contribute to differential gene expressions, a mock sporozoite preparation from non-infected but the same batch of mosquitoes was also made using similar scaled-down procedures. This non-sporozoite containing salivary gland extract was used at the same dilution as the sporozoite preparations.
Irradiation of Malaria Sporozoites
Anopheles stephensi mosquitoes infected with P. falciparum (NF54 strain) sporozoites were exposed to 150 gray dose of γ-radiation using a 60Co source. The time of exposure of infected mosquitoes to achieve the target radiation doses was based on the calibration of the irradiator by dosimetry and the half-life of 60Co.
Infection of HepG2-A16 Cells in Vitro
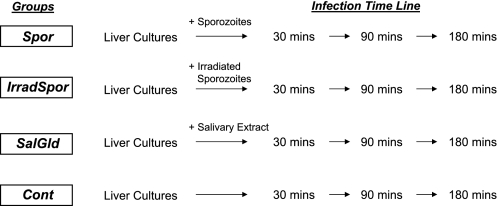
HepG2-A16 human liver cells were maintained in complete Dulbecco's modified Eagle's medium (DMEM) buffered with bicarbonate and supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 environment. For infection by P. falciparum sporozoites, 1 × 106 cells were cultured in DMEM in 6-well plates at 80% confluence and exposed to freshly prepared 1 × 106 sporozoites. The use of equivalent numbers of sporozoites to HepG2-A16 cells is to ensure uniformity and consistency of infectivity across all samples. Cells were exposed to sporozoites for different time periods (30, 90, and 180 min), after which the sporozoite-containing medium was aspirated from each well. Cells were washed three times with complete DMEM medium to ensure removal of free sporozoites on the cell surfaces, and harvested for RNA extraction. For controls, uninfected cell cultures, and cell cultures exposed to salivary gland extract prepared from non-infected mosquitoes were used. All infection experiments were performed in duplicate; however, cells from these two experiments were later pooled together prior to RNA extraction to obtain sufficient hepatic RNAs for microarray analysis. In each experiment there were 4 sample groups (Fig. 1); wild-type sporozoite-infected HepG2-A16 liver cells (Spor), irradiated sporozoite-infected liver cells (IrradSpor), non-infected control liver cells (Control), and liver cells exposed to salivary gland extract from uninfected mosquitoes (SalGld). The 90-min pooled sample for IrradSpor was lost on storage, and was not included in the microarray analysis.
FIGURE 1.
Experimental design to determine the early liver responses to malarial sporozoite infection. Cultured HepG2-A16 cells were treated with wild-type sporozoites (Spor), radiation-attenuated sporozoites (IrradSpor), or non-sporozoite containing salivary gland extract (SalGld) for 30, 90, and 180 min, following which cells were washed and lysed for RNA extraction. Non-treated cells (Control) were also included as comparison.
RNA Isolation, Amplification, Labeling, and Hybridization
Following infection, cell cultures were washed with PBS, lysed in 4.5 m guanidine-hydrochloride lysis buffer, and the total RNA extracted using High PureTM RNA isolation kit (Roche Applied Science). For precaution against possible RNA degradation, all extracted RNAs were treated with 50 units of ribonuclease inhibitor (RNasin, Promega, Madison, WI) and stored at −80 °C. Extracted RNAs were quantitated by absorbance measurements at 260 nm using the NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA fidelity was verified using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and graded using RNA integrity number to assess integrity and ensure consistency across all samples. Only RNAs that conformed to the recommended quality control metrics were used for probe preparation and array hybridization. Probe labeling was performed using standard Affymetrix approved RT-PCR, in vitro transcription, and fragmentation methods. Array hybridization was performed using instrumentation consistent with the Affymetrix Integrated GeneChip System, and the Affymetrix GeneChip Operating System software was used for probe intensity measurements. Probe labeling, array hybridization, and intensity measurements were performed by Asuragen (Austin, TX).
Gene expression profiling was made using Affymetrix Human Genome 133 Plus 2.0 GeneChip array. This microarray contains over 54,000 probe sets representing over 47,000 transcripts and variants, including 38,500 well characterized human genes.
Analysis of Microarray Data
Intensity signals from the hybridized GeneChips were analyzed using Affymetrix MAS 5.0 software to generate CEL (fluorescence intensity) files. The quality of hybridization signals was assessed using the array outlier percentage as determined by the dChip (version 1.3) software (27). In addition, microarray signals were evaluated for the presence of 60 liver-specific genes (Gene Enrichment Profiler) as well as six short half-life RNA transcripts (SMAD7, IL10RA, CXCR4, GATA3, MYC, RB1) to ensure that the microarray signals were not compromised by selective loss of liver-specific transcripts. CEL files were normalized at the probe level by using the robust multichip average method (28). This method fits a robust linear model to the probe level data, analyzing each hybridized chip within the context of other chips in the experiment. The average intensity of each probe was expressed as log2. Statistical analysis of gene expression data as well as gene set comparison analysis were performed using the R statistics-based BRB Array Tools (version 3.8) developed by Dr. Richard Simon and Amy Peng Lam (29), and GeneSpring software (Agilent Technologies, CA).
RESULTS
Effects of Treatment and Post-infection Time on HepG2-A16 Gene Expression
Initial filtering and subsetting of gene expression data were performed based on a 1.5-fold change from the median in at least 25% of the arrays and showing a log intensity variation of p < 0.001. A total of 1570 probe sets, which represents 1115 genes, passed the filtering criteria and were retained for subsequent statistical analysis. Regression analysis of the time series (i.e. 30, 90, and 180 min) expression data among the four liver cell culture groups (Control, Spor (wild-type sporozoites), IrradSpor (radiation-attenuated sporozoites), and SalGld (salivary gland)) indicated that effects of sporozoite exposure time on gene expression changes within each group were minimal, and that the effects of different treatments (i.e. Control, SalGld, Spor, and IrradSpor) were the predominant contributor to gene expression changes. Therefore, in subsequent statistical analysis, we grouped datasets from the three time points for each treatment group together, and treated them as replicates within each treatment group.
Hierarchical clustering analysis using these genes showed that study samples can be grouped into three broad aggregated clusters representative of treatment conditions: Control, SalGld, and Spor with IrradSpor together, indicating that gene expression changes caused by Spor and IrradSpor exposure were very similar (Fig. 2A). Gene expression changes, at least until 180 min post-infection (the longest time point studied), indicated that hepatic cell responses to malaria sporozoite infection were nearly identical, whether sporozoites were irradiated or not. Principal component analysis of the data (Fig. 2B) also clustered study samples into three well defined groups, with Spor and IrradSpor grouping together. These analyses highlighted the close similarity between liver gene transcriptional responses to Spor and IrradSpor, and their significant differences from Control and SalGld groups.
FIGURE 2.
Clustering analysis of 951 DEGs from 11 HepG2-A16 cell culture samples representing four study groups. A, hierarchical clustering of samples using clustered correlation with average linkage. B, principal component analysis of samples; Control (▴), non-infected; Spor (*), wild-type sporozoite infected; IrradSpor (●), irradiated sporozote infected; SalGld (■), mosquito salivary gland extract exposed.
Identification of Genes Associated with Sporozoite Infection
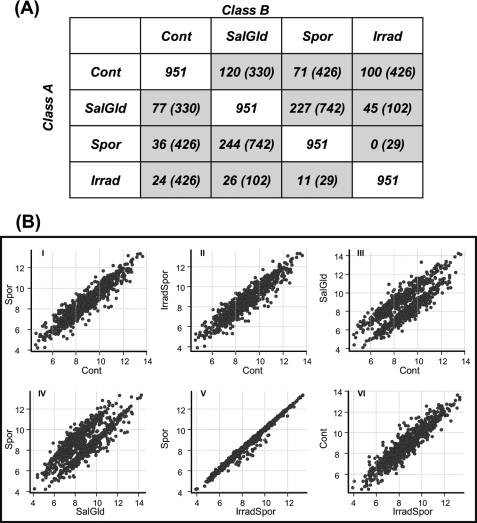
Altogether, a total of 951 genes that were differentially expressed among the four treatment classes were identified using a univariate random variance model F-test. A post hoc pairwise comparison was then used to identify the subset of genes differentially regulated between different pairs of infection groups, and results are summarized in Fig. 3A. Of the 426 genes altered in the Spor group (compared with Control), 107 genes were regulated with a >2-fold change in either direction, with 36 genes up-regulated and 71 genes down-regulated. In the IrradSpor group (compared with Control), 426 genes were differentially expressed, of which 24 genes were up-regulated and 100 genes down-regulated. Sporozoite-infected groups and the SalGld group had more down-regulated genes than up-regulated genes when compared with the Control group. This showed that non-sporozoite containing salivary gland extract alone (SalGld) can induce gene expression changes when added to HepG2-A16 cell cultures (Scatterplot of 951 differentially expressed genes (DEGs)3 for each pairwise comparison; Fig. 3B, panel III). Gene expression changes induced by Spor and IrradSpor treatments were almost identical (Fig. 3B, panel V).
FIGURE 3.
Distribution of differentially expressed genes across the four study groups. A, number of genes that are differentially expressed among the four study groups. A univariate random model F-test (p < 0.001) was used to determine the set of genes differentially expressed across the four study groups. A post hoc pairwise comparison was then used to identify the subset of genes differentially regulated between different pairs of study groups. Each box shows the number of genes statistically different in a pairwise group to group comparison. Total number of DEGs analyzed (951 genes) is shown in the white boxes. Up-regulated (>2-fold) genes in a Class A versus Class B comparisons are shown in gray boxes. Numbers in parentheses within each box indicates the total number of genes differentially regulated in each pairwise comparison. B, scatter plots of pairwise comparison among the four study groups were plotted using the 951 DEGs.
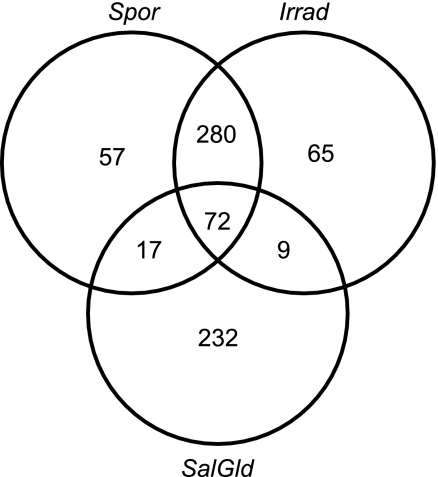
DEGs from pairwise comparisons identified regulated genes (relative to Control) that were unique to each of the three treatment groups (Spor, IrradSpor, and SalGld). Fig. 4 demonstrates that 57 genes were uniquely regulated in the Spor group (relative to Control), compared with 65 and 232 genes for IrradSpor and SalGld, respectively.
FIGURE 4.
Venn diagram showing co-regulatory relationships of the DEGs among the three study groups. DEGs from pairwise comparison among each of the three study groups (Spor, IrradSpor, and SalGld) relative to control were intersected with each other to show the number of common or unique genes that are regulated.
Pairwise comparison between the Spor and SalGld groups identified 742 selectively altered genes from wild-type sporozoite infection alone (supplemental Table S1), 244 genes up-regulated and 227 genes down-regulated at >2-fold following sporozoite infection (Fig. 5A). DEGs were defined using Gene Ontology annotations, to infer the biological relevance of these DEGs to sporozoite invasion of liver cells, and the major Gene Ontology categories involved were those associated with mitotic cell cycle regulation, chromatin packaging and remodeling, immunity and defense, protein proteolysis, and intracellular protein trafficking. DEGs were also mapped to KEGG (30) and BioCarta pathways (supplementary Table S2) to understand their differential regulation within the context of biological pathways and functional inter-relationships. The functional families involved were those associated with antigen processing and presentation, ubiquitin-mediated proteolysis, glycerolipid metabolism, extracellular matrix-receptor interaction, cell communication, MAPK-signaling pathway, fatty acid metabolism, cell adhesion molecules, xenobiotics metabolism by cytochrome P450 and pathways associated with immune system and defense, as well as cell cycle and DNA replication pathways (Table 1).
FIGURE 5.
Volcano plot of 951 DEGs comparing the size of the fold-change to statistical significance level in the pairwise comparison between (A) Spor to SalGld, and (B) Spor to Irrad. Vertical and horizontal dotted lines indicate DEGS with >2-fold expression change and p < 0.001 value cutoff, respectively. A random variance model two-sample t test was used. Fold-change was calculated for Spor relative to SalGld (panel A) or Irrad (panel B). Seven up-regulated and three down-regulated genes showing unusually high significance (p < 10−7) in the Spor versus SalGld comparison are annotated (panel A). Twenty nine DEGs are found to be down-regulated in Spor versus Irrad (panel B), and are listed in Table 2.
TABLE 1.
Functional classification of genes differentially expressed genes between the Spor and SalGld groups using KEGG and Biocarta pathways
Gene expression changes were expressed as fold-change between Spor to SalGld, and represent the ratio of the geometric mean intensities of Spor to that of SalGld. Datasets representing the three time points within each experimental group have been grouped together and were regarded as experimental replicates.
| Pathway | Pathway description | Number of genes | p value | |
|---|---|---|---|---|
| 1 | hsa04612 | Antigen processing and presentation | 13 | 0.000013 |
| 2 | hsa04120 | Ubiquitin mediated proteolysis | 5 | 0.0000203 |
| 3 | hsa00561 | Glycerolipid metabolism | 6 | 0.0000244 |
| 4 | hsa04110 | Cell cycle | 26 | 0.0001556 |
| 5 | hsa04512 | Extracellular matrix-receptor interaction | 7 | 0.000186 |
| 6 | hsa01430 | Cell communication | 6 | 0.000171 |
| 7 | hsa04010 | MAPK signaling pathway | 13 | 0.0000013 |
| 8 | hsa00071 | Fatty acid metabolism | 5 | 0.000015 |
| 9 | hsa04940 | Type I diabetes mellitus | 5 | 0.0004183 |
| 10 | hsa04514 | Cell adhesion molecules (CAMs) | 8 | 0.0001145 |
| 11 | hsa00010 | Glycolysis/gluconeogenesis | 8 | 0.0000349 |
| 12 | hsa04610 | Complement and coagulation cascades | 11 | 0.0001723 |
| 13 | hsa04810 | Regulation of actin cytoskeleton | 7 | 0.0000267 |
| 14 | hsa04650 | Natural killer cell-mediated cytotoxicity | 5 | 0.0004625 |
| 15 | hsa00240 | Pyrimidine metabolism | 9 | 0.0000175 |
| 16 | hsa04640 | Hematopoietic cell lineage | 6 | 0.00001 |
| 17 | hsa04060 | Cytokine-cytokine receptor interaction | 12 | 0.0000817 |
| 18 | hsa00230 | Purine metabolism | 7 | 0.0000133 |
| 19 | hsa04510 | Focal adhesion | 7 | 0.0001511 |
| 20 | hsa04540 | Gap junction | 5 | 0.0002494 |
| 21 | hsa04350 | TGF-β signaling pathway | 8 | 0.0004184 |
| 22 | hsa00564 | Glycerophospholipid metabolism | 6 | 0.0005068 |
| 23 | hsa04310 | Wnt signaling pathway | 7 | 0.0000095 |
| 24 | h_g1Pathway | Cell cycle: G1/S checkpoint | 6 | 0.0002942 |
| 25 | h_mcmPathway | CDK regulation of DNA replication | 8 | 0.0003691 |
| 26 | h_pparaPathway | Mechanism of gene regulation by peroxisome proliferators via PPARα | 8 | 0.0003646 |
Expressions of 18 genes were highly differentially regulated (>5-fold change) between Spor and SalGld (Fig. 5A and supplemental Table S1). Three (NDC80, SPC25, NCAPG) of the four Spor up-regulated genes encode nuclear proteins involved in the regulation of the cell cycle phase during mitosis. The other up-regulated gene (KIF20A) encodes for a kinesin-like motor protein that interacts with the GTP-bound forms of RAB6A and RAB6B to regulate transport of Golgi vesicles along microtubules. Interestingly, a recent study on 62 Gabonese children with varying severity of malaria (31) found that 10 genes, including KIF20A, located in chromosomal region 5q31–33, were strongly correlated with malaria caused by parasites expressing specific subtypes of P. falciparum erythrocyte surface protein 1. Significantly, KIF20A expression was increased and positively correlated with the upsA P. falciparum erythrocyte surface protein 1 var. gene subtype.
Of the 14 highly down-regulated genes, 10 were for secreted proteins that were either cytokines/chemokines (GDF15, CTGF, CCL20, CXCL2) or binding proteins (S100P, CP, IGFBP1, TTR, AZGP1). Another gene, SERPINE1, encodes for the serine protease inhibitor (plasminogen activator inhibitor type 1) of tissue plasminogen activator and urokinase, and functions principally in fibrinolysis, cell adhesion, and cell migration. In addition, plasminogen activator inhibitor type 1 has an important anti-parasitic effector function and plasminogen activator inhibitor type 1-deficient mice showed increased susceptibility to P. chabaudi blood-stage malaria (32).
Other highly down-regulated genes were CYP1A1, MAFF, TUBB2B, and ITIH3. The cytochrome P450 enzymes are monooxygenases that catalyze many reactions involved in drug metabolism and the synthesis of cholesterol, steroids, and other lipids. Expression and activities of some of these liver enzymes are known to be differentially regulated in malaria. For example, cytochrome P450 family 1, subfamily A enzymes (CYP1A) are reduced in mouse malaria models 2–6 days following infection corresponding to the period of peak parasitemia (33, 34). In our human liver infection model, down-regulation of liver CYP1A1 expression occurred very early (<30 min) after sporozoite infection, and is isoform-specific, because expression of CYP1A2 and CYP2B6 remained unchanged. This discrepancy may be due to species differences in malaria parasite-host interactions.
Tubulin, the product of the TUBB2B gene, is an important constituent of microtubules that act as scaffolds to determine cell shape and as a backbone for cell organelles and vesicles to move. ITIH3 is part of the inter-α-trypsin inhibitor family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization, and may act as a carrier of hyaluronan in serum or as a binding protein between hyaluronan and other matrix proteins to regulate the localization, synthesis, and degradation of hyaluronan. MAFF is a basic leucine zipper transcription factor that lacks a transactivation domain and, because it can both homodimerize with itself and heterodimerize with other leucine-zipper transcription factors, it is a transcriptional regulator. Its role in malaria is unknown.
Differential Effects of Wild-type and Irradiated Sporozoites on Hepatocyte Gene Expression
A pairwise comparison between the Spor and IrradSpor groups were made using the list of 951 DEGs obtained from the initial analysis of variance analysis (Fig. 5B), to determine the subset of hepatocyte genes whose expressions were differentially affected by wild-type sporozoites compared with irradiated sporozoites. Twenty-nine genes were differentially affected by irradiation of malarial sporozoites (Table 2); all were up-regulated in IrradSpor compared with Spor (supplemental Fig. S1). Seven of those genes (ATAD2, ZNF83, SMARCA4, TOP1, RBM39, THOC2, and SART3) are involved in nucleic acid binding, suggesting that DNA replication and RNA transcription/processing is the main functional difference between Spor and IrradSpor infections of the liver. Other predominant biological processes represented by these 29 genes were macromolecule localization (7 genes), protein transport and localization (10 genes), and morphogenesis of anatomical structures (6 genes).
TABLE 2.
Liver genes whose expression is differentially altered upon exposure to radiation-attenuated sporozoite (IrradSpor) compared to wild type sporozoite (Spor)
Fold-change indicated represents the ratio of the geometric means of IrradSpor to Spor. Datasets representing the different time points within each experimental group have been grouped together and were regarded as experimental replicates.
| Gene symbol | Description | Parametric p value | Fold-change |
|---|---|---|---|
| TPR | Translocated promoter region (to activated MET oncogene) | 1.92e-05 | 1.74 |
| DKFZP434L187 | Hypothetical LOC26082 | 2.75e-05 | 1.94 |
| AP3D1 | Adaptor-related protein complex 3, delta 1 subunit | 8.21e-05 | 1.83 |
| SLC25A36 | Solute carrier family 25, member 36 | 8.76e-05 | 1.79 |
| RIF1 | RAP1 interacting factor homolog (yeast) | 9.62e-05 | 2.02 |
| TOP1 | Topoisomerase (DNA) I | 0.0001056 | 2.46 |
| SDCCAG10 | Serologically defined colon cancer antigen 10 | 0.000139 | 1.8 |
| SART3 | Squamous cell carcinoma antigen recognized by T cells 3 | 0.0001545 | 1.95 |
| GPC3 | Glypican 3 | 0.0001556 | 1.8 |
| C1orf104 | Chromosome 1 open reading frame 104 | 0.000156 | 1.89 |
| EHBP1 | EH domain binding protein 1 | 0.0001801 | 2.27 |
| RASEF | RAS and EF-hand domain containing | 0.0001905 | 2.03 |
| THOC2 | THO complex 2 | 0.0002131 | 1.54 |
| KCNAB1 | Potassium voltage-gated channel, shaker-related subfamily, β member 1 | 0.0002981 | 1.81 |
| CDC27 | Cell division cycle 27 homolog (Saccharomyces cerevisiae) | 0.0003108 | 2.17 |
| NARG1 | NMDA receptor regulated 1 | 0.0003196 | 1.83 |
| BAT2D1 | BAT2 domain containing 1 | 0.0004393 | 1.77 |
| MAP4 | Microtubule-associated protein 4 | 0.0004831 | 2.46 |
| PCYOX1 | Prenylcysteine oxidase 1 | 0.0005171 | 1.73 |
| PTPLB | Protein-tyrosine phosphatase-like (proline instead of catalytic arginine), member b | 0.0005192 | 2.77 |
| SPG7 | Spastic paraplegia 7 (pure and complicated autosomal recessive) | 0.000659 | 1.73 |
| RBM39 | RNA binding motif protein 39 | 0.0006645 | 2.51 |
| ATAD2 | ATPase family, AAA domain containing 2 | 0.0006707 | 1.54 |
| HIST1H4C | Histone cluster 1, H4c | 0.0006731 | 1.58 |
| SMARCA4 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 | 0.0007271 | 1.47 |
| ZNF83 | Zinc finger protein 83 | 0.0008767 | 2.45 |
| ANKRD36B | Ankyrin repeat domain 36B | 0.0008908 | 1.88 |
| ANKRD36 | Ankyrin repeat domain 36 | 0.0009445 | 2.11 |
| RSRC1 | Arginine/serine-rich coiled-coil 1 | 0.000971 | 1.62 |
DISCUSSION
The liver is the site of development of infectious merozoites from pre-erythrocytic Plasmodium sporozoites, but little is known about the liver hepatocyte responses in the early stages of infection with P. falciparum sporozoites. Our study documented the early gene expression profile changes with infection of hepatocytes. Sporozoite infection of liver cells is rapid and the in vitro inhibition of sporozoite invasion assay and the inhibition of liver stage development assay using cultured hepatocytes normally require a maximum of 180 min for maximal sporozoites binding/invasions (35, 36). Therefore, in our studies, we used a time series experimental design to determine hepatic gene expression changes at 30, 90, and 180 min after adding the P. falciparum sporozoites to our HepG2-A16 cultures. However, the different exposure times we used had no significant effects on gene expression changes. Hepatic gene transcriptional responses were rapid and observed within 30 min following sporozoite exposure.
Many of the differentially expressed genes identified regulate diverse biological functions, such as antigen processing and presentation, cell adhesion, metabolism pathways, and cell cycle regulation. Although liver stage malaria is clinically asymptomatic, a number of sporozoite ligand-liver receptor interactions occur and enable sporozoites to develop within the liver cells without initiating a local inflammatory response (37). Plasmodium circumsporozoite protein (CSP) is the most prominent antigen covering the sporozoite surface and is critical for sporozoite function and hepatocyte invasion. Interaction of CSP with hepatocytes inhibits target cell protein synthesis (38), and alters the expression of a large number of genes involved with various metabolic processes, as well as down-regulating genes controlled by NF-κβ (37). Our studies demonstrate that the majority of hepatic genes are down-regulated following sporozoite invasion. Similarly, Singh et al. (37) documented that forced expression of CSP in HeLa cells down-regulated 10 times as many genes as were up-regulated. Of the 742 differentially expressed genes we identified, 96 genes were similar to those reported by Singh et al. (37) suggesting that the altered expression of this subset of genes can be attributable to the interaction of CSP with liver cells alone, independent of the sporozoites functional metabolic processes. These genes were predominantly involved with general responses to stimuli and wounding, protein localization, and intracellular transport, processes consistent with the role of CSP.
GSTA2, the gene for glutathione S-transferase A2, was up-regulated by exposure of HepG2-A16 cells to P. falciparum sporozoites (supplemental Fig. S2). Glutathione S-transferase A2, a member of the α class glutathione S-transferases (GST-α), is the most abundantly expressed GST-α in liver and is released rapidly in large quantities into the bloodstream during hepatocellular damage (39). Besides metabolizing bilirubin and anti-cancer drugs in the liver, GST-α exhibit glutathione peroxidase activity, which protects cells from reactive oxygen intermediates and peroxidation products. Unlike the progression of malaria in infected humans where circulating glutathione S-transferase levels were decreased (40), the induced expression of GSTA2 in sporozoite-infected HepG2-A16 cells may be indicative of oxidative stress contributing to the cells' inability to support the complete intrahepatic development of P. falciparum sporozoites into infectious merozoites. Because the half-life of GST-α in plasma is short (∼1 h) (41), its concentration in plasma will better reflect changes in hepatocellular damage than the two widely used hepatic markers, aspartate aminotransferase (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2), which have plasma half-lives of ∼17 and 47 h, respectively (39). GST-α may, therefore, be one suitable biomarker for early detection of P. falciparum infection.
Radiation attenuated whole sporozoites have proven to be very effective in conferring sterile protection against malaria infection (42). Attenuated sporozoites are metabolically active and their invasion into hepatocytes and subsequent transformation into trophozoites is similar to that observed for wild-type sporozoites (43). However, unlike non-irradiated sporozoites, trophozoites from irradiated sporozoites persist within the cells and do not transform into schizonts (43). Although HepG2-A16 cells do not support the transformation of either wild-type or irradiated P. falciparum sporozoites into infectious exoerythrocytic forms, we did observe a subset of genes that were differentially expressed during the early phase of host-pathogen interactions between the Spor and IrradSpor groups. Pairwise comparison of genes between the two sporozoites groups identified 29 differentially expressed genes, all up-regulated in IrradSpor. Functionally, these genes were predominantly involved in DNA metabolic processes, binding, localization, and transport systems that are consistent with the ability of irradiated sporozoites to influence modifications of hepatocytes cellular processes, which enable them to reside intracellularly for longer periods of time.
Two major surface proteins of P. falciparum sporozoites, CSP and sporozoite surface protein 2 (SSP2/TRAP), are known to have high affinity binding for heparin sulfate proteoglycans (HSPGs) present on hepatocytes (44) to facilitate the invasion of sporozoites into hepatocytes. However, the class of HSPGs to which they bind is not known. Through binding with CSP, malarial sporozoites exploit the sulfation level of host HSPGs to navigate within the mammalian host (45). Cells expressing low sulfated HSPGs, such as those in skin and endothelium, permit sporozoite migration, whereas cells with highly sulfated HSPGs, such as hepatocytes, inhibit migration and promote invasion by sporozoites (45). One important observation of our present study is that the glypican-3 gene, GPC3, is up-regulated in HepG2-A16 cells infected with irradiated but not wild-type sporozoites. Glypican-3 is a heparin sulfate proteoglycan (46) secreted in the plasma of hepatocellular carcinoma patients, and regarded as a diagnostic serum marker for hepatocellular carcinoma (47–50). Unlike wild-type sporozoites, irradiated sporozoites are believed to invade and persist within hepatocytes for some time and do not transform into blood stage parasites. Up-regulation of GPC3 in irradiated sporozoite-infected cells could alter the nature of the interaction between the sporozoites and host cells to make the hepatocyte environment more conductive for their long-term survival without further transformative development. Immunohistopathology of benign human liver tissues infected with hepatitis C virus (HCV) showed strong staining for glypican-3 (51), and yeast two-hybrid assays revealed that glypican-3 interacts with CD81, a member of the tetraspanin family reported to be involved in HCV infection and cell proliferation (52). Indeed, hepatocyte CD81 has also been shown to be required for Plasmodium sporozoite invasion of hepatocytes (7, 53), although the specific signaling pathways for CD81 interaction with sporozoites is unknown. Recently, a prospective longitudinal study of a cohort of 319 individuals showed that HCV infection leads to the slower emergence of P. falciparum parasites in blood (54). It is possible that glypican-3 and CD81 could provide the functional link between HCV and P. falciparum infections, and that increased glypican-3 caused by HCV infection may retard the transformation of P. falciparum sporozoites into mature schizonts. In addition, given the current lack of a suitable biochemical marker for assessing “vaccine take” for whole sporozoite-based malaria vaccines, glypican-3 is a promising candidate for diagnostic and therapeutic use in the management of malaria. The mechanism of protection conferred by attenuated whole sporozoite-based malaria vaccines is not yet fully understood. The presence of liver stage parasites in the hepatocytes seems to be important for maintenance of protection conferred by attenuated sporozoite vaccines (55–57) via CD8+ T cells. However, the mechanism by which antigen(s) of the existing parasites in the hepatocytes are processed and presented to CD8+ T-cells is not clearly understood as liver is not particularly enriched with immune effector cells other than Kupffer cells. Expression of glypican-3 is known to stimulate the recruitment of macrophages into human hepatocellular carcinoma tissues (58, 59). A possible similar mechanism of recruitment of macrophages by elevated glypican-3 during sporozoite infection of hepatocytes may occur to process and present liver stage antigens to CD8+ T-cells to confer protection from malaria with whole sporozoite-based vaccines. This should be studied further as a possible mechanism of protection with attenuated whole sporozoite malaria vaccines.
A subset of the Ras family of genes (RHOA, RHOB, RAB4B, RAB22A, RASD1) was down-regulated upon sporozoite (both wild-type and irradiated) infection, with only RASEF being up-regulated (supplemental Fig. S3). The Ras family proteins are small GTPases involved in signal transduction pathways that regulate cell growth, differentiation, and survival. Down-regulation of Ras family genes arrests cell growth and increases apoptosis. Up-regulation of glypican-3 and down-regulation of Ras family genes in the HepG2-A16 hepatocarcinoma cells suggest that P. falciparum sporozoite infection slows down cellular growth and differentiation in hepatocytes. An earlier study reported that sporozoite infection induced apoptosis in hepatocytes and that apoptotic Plasmodium-infected hepatocytes provide antigens to liver dendritic cells (60). It remains to be determined if apoptosis in plasmodium sporozoite-infected hepatocytes is induced by interactions among sporozoites, up-regulated glypican-3, or down-regulated Ras genes.
The HepG2-A16 liver cell line used in our study is not permissive for functional development of P. falciparum sporozoites into mature exoerythrocytic stage schizonts, although using low growth density, this cell line has been shown to support the exoerythrocytic stage development of P. vivax (54). Development of exoerythrocytic stage forms in vitro has only been demonstrated in the cytoplasm of primary human hepatocytes (61, 62), HHS-102 hepatoma cells (63) and, recently, the HC-04 liver cell line (17). Presently, there is no human hepatocyte cell line available in which a high level of sporozoite infection and formation of parasitophorous vacuole during transformation of sporozoites to exoerythrocytic stage of P. falciparum have been demonstrated. Because the goal of our study was to identify early (<3 h) changes in host gene expression following the initial infection by P. falciparum sporozoites rather than mapping host gene changes leading to the exoerythrocytic stage development, the in vitro infection model of HepG2-A16 cells was used. This hepatoma line has been extensively used in P. falciparum sporozoite invasion assay studies (21–24) in which P. falciparum sporozoites have been shown to attach and invade these cells but do not develop into mature exoerythrocytic stage schizonts. As this cell line does not support P. falciparum exoerythrocytic stage development, this study can serve as a starting point for future comparative differential analysis of genome-wide transcriptomic changes between other permissive cells (such as primary hepatocytes and HC-04 cells) to help identify genes and pathways that are specifically required for P. falciparum transformation and development, including the formation of a parasitophorous vacuole.
One limitation of our study is the small sample size, due in part to the difficulties in obtaining sufficient amounts of fresh sporozoites and the limited amounts of RNAs obtained from infected cultures. Although we have not performed post-analysis experimental validation (i.e. using alternative assay techniques such as RT-PCR) of our microarray results (which, when performed on the same samples, would only verify microarray methodologies), our results showed concordance with those obtained in an earlier study (37). Of the 742 genes whose expressions were selectively altered when infected with wild-type P. falciparum sporozoites (Spor versus SalGld), ∼13% were found to be similar to the subset of genes specifically induced by malaria circumsporozoite protein (37). This agreement of genes from our results to those reported previously (37) not only provides functional verification of our results and validates the general performance of our study but also provides confidence in the overall data obtained (64).
In summary, using global gene expression profiling, we have documented a list of genes that are differentially altered in human liver cells following early infection by P. falciparum sporozoites. Detailed analysis of the functional significance of these genes will help provide further insights on the biological mechanisms by which the liver influences infectivity and transformation of sporozoites into infectious exoerythrocytic forms. As the liver is likely the only site for pre-erythrocytic development of malaria sporozoites, it remains an important gateway for invading sporozoites that can be targeted for intervention before their development into symptomatic parasites.
Supplementary Material
This work was supported by Department of Defense Small Business Innovative Research Grant W81XWH-05-C-0030 (to S. H. P., Y. M., E. W. F., and G. T. O.) and the work between Sun BioMedical Technologies and the Malaria Program, Naval Medical Research Center was conducted through a CRADA collaboration Grant LP-CRADA-NMRC-05-2107 and was supported by Grant 6000.RAD1.F.A0309 (to R. C., P. V., and T. L. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1 and S2.
- DEG
- differentially expressed gene
- CSP
- circumsporozoite protein
- HSPG
- heparin sulfate proteoglycan
- HCV
- hepatitis C virus.
REFERENCES
- 1. Beutler B. (2001) Biochem. Soc. Trans. 29, 853–859 [DOI] [PubMed] [Google Scholar]
- 2. Brodsky I. E., Medzhitov R. (2009) Nat. Cell Biol. 11, 521–526 [DOI] [PubMed] [Google Scholar]
- 3. Maglione P. J., Chan J. (2009) Eur. J. Immunol. 39, 676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen S. B., Reinert L. S., Paludan S. R. (2009) APMIS 117, 323–337 [DOI] [PubMed] [Google Scholar]
- 5. Westenberger S. J., McClean C. M., Chattopadhyay R., Dharia N. V., Carlton J. M., Barnwell J. W., Collins W. E., Hoffman S. L., Zhou Y., Vinetz J. M., Winzeler E. A. (2010) PLoS Negl. Trop. Dis. 4, e653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams C. T., Azad A. F. (2010) PLoS One 5, e10267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silvie O., Rubinstein E., Franetich J. F., Prenant M., Belnoue E., Rénia L., Hannoun L., Eling W., Levy S., Boucheix C., Mazier D. (2003) Nat. Med. 9, 93–96 [DOI] [PubMed] [Google Scholar]
- 8. Torgler R., Bongfen S. E., Romero J. C., Tardivel A., Thome M., Corradin G. (2008) J. Immunol. 180, 3990–3999 [DOI] [PubMed] [Google Scholar]
- 9. Moreira C. K., Marrelli M. T., Jacobs-Lorena M. (2004) Int. J. Parasitol. 34, 1431–1440 [DOI] [PubMed] [Google Scholar]
- 10. Wang Q., Brown S., Roos D. S., Nussenzweig V., Bhanot P. (2004) Mol. Biochem. Parasitol. 137, 161–168 [DOI] [PubMed] [Google Scholar]
- 11. Zhou Y., Ramachandran V., Kumar K. A., Westenberger S., Refour P., Zhou B., Li F., Young J. A., Chen K., Plouffe D., Henson K., Nussenzweig V., Carlton J., Vinetz J. M., Duraisingh M. T., Winzeler E. A. (2008) PLoS One 3, e1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser K., Matuschewski K., Camargo N., Ross J., Kappe S. H. (2004) Mol. Microbiol. 51, 1221–1232 [DOI] [PubMed] [Google Scholar]
- 13. Kappe S. H., Gardner M. J., Brown S. M., Ross J., Matuschewski K., Ribeiro J. M., Adams J. H., Quackenbush J., Cho J., Carucci D. J., Hoffman S. L., Nussenzweig V. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9895–9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carrolo M., Giordano S., Cabrita-Santos L., Corso S., Vigário A. M., Silva S., Leirião P., Carapau D., Armas-Portela R., Comoglio P. M., Rodriguez A., Mota M. M. (2003) Nat. Med. 9, 1363–1369 [DOI] [PubMed] [Google Scholar]
- 15. Silvie O., Goetz K., Matuschewski K. (2008) PLoS Pathog. 4, e1000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollingdale M. R., Collins W. E., Campbell C. C., Schwartz A. L. (1985) Am. J. Trop. Med. Hyg. 34, 216–222 [DOI] [PubMed] [Google Scholar]
- 17. Sattabongkot J., Yimamnuaychoke N., Leelaudomlipi S., Rasameesoraj M., Jenwithisuk R., Coleman R. E., Udomsangpetch R., Cui L., Brewer T. G. (2006) Am. J. Trop. Med. Hyg. 74, 708–715 [PubMed] [Google Scholar]
- 18. Albuquerque S. S., Carret C., Grosso A. R., Tarun A. S., Peng X., Kappe S. H., Prudêncio M., Mota M. M. (2009) BMC Genomics 10, 270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siau A., Silvie O., Franetich J. F., Yalaoui S., Marinach C., Hannoun L., van Gemert G. J., Luty A. J., Bischoff E., David P. H., Snounou G., Vaquero C., Froissard P., Mazier D. (2008) PLoS Pathog. 4, e1000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarun A. S., Peng X., Dumpit R. F., Ogata Y., Silva-Rivera H., Camargo N., Daly T. M., Bergman L. W., Kappe S. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollingdale M. R., Nardin E. H., Tharavanij S., Schwartz A. L., Nussenzweig R. S. (1984) J. Immunol. 132, 909–913 [PubMed] [Google Scholar]
- 22. Rathore D., Hrstka S. C., Sacci J. B., Jr., de la Vega P., Linhardt R. J., Kumar S., McCutchan T. F. (2003) J. Biol. Chem. 278, 40905–40910 [DOI] [PubMed] [Google Scholar]
- 23. Rathore D., Nagarkatti R., Jani D., Chattopadhyay R., de la Vega P., Kumar S., McCutchan T. F. (2005) J. Biol. Chem. 280, 20524–20529 [DOI] [PubMed] [Google Scholar]
- 24. Rathore D., Sacci J. B., de la Vega P., McCutchan T. F. (2002) J. Biol. Chem. 277, 7092–7098 [DOI] [PubMed] [Google Scholar]
- 25. Ozaki L. S., Gwadz R. W., Godson G. N. (1984) J. Parasitol. 70, 831–833 [PubMed] [Google Scholar]
- 26. Moser G., Brohn F. H., Danforth H. D., Nussenzweig R. S. (1978) J. Protozool. 25, 119–124 [DOI] [PubMed] [Google Scholar]
- 27. Li C., Hung Wong W. (2001) Genome Biol. 2, RESEARCH0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irizarry R. A., Bolstad B. M., Collin F., Cope L. M., Hobbs B., Speed T. P. (2003) Nucleic Acids Res. 31, e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simon R., Lam A., Li M. C., Ngan M., Menenzes S., Zhao Y. (2007) Cancer Inform. 3, 11–17 [PMC free article] [PubMed] [Google Scholar]
- 30. Kanehisa M., Goto S., Kawashima S., Okuno Y., Hattori M. (2004) Nucleic Acids Res. 32, D277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalmbach Y., Rottmann M., Kombila M., Kremsner P. G., Beck H. P., Kun J. F. (2010) J. Infect. Dis. 202, 313–317 [DOI] [PubMed] [Google Scholar]
- 32. Krücken J., Dkhil M. A., Braun J. V., Schroetel R. M., El-Khadragy M., Carmeliet P., Mossmann H., Wunderlich F. (2005) Infect. Immun. 73, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carvalho R. S., Friedrich K., De-Oliveira A. C., Suarez-Kurtz G., Paumgartten F. J. (2009) Eur. J. Pharmacol. 616, 265–269 [DOI] [PubMed] [Google Scholar]
- 34. De-Oliveira A. C., Carvalho R. S., Paixão F. H., Tavares H. S., Gueiros L. S., Siqueira C. M., Paumgartten F. J. (2010) Malar. J. 9, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rénia L., Vigário A. M., Belnoue E. (2002) Methods Mol. Med. 72, 507–516 [DOI] [PubMed] [Google Scholar]
- 36. Sacci J. B., Jr. (2002) Methods Mol. Med. 72, 517–520 [DOI] [PubMed] [Google Scholar]
- 37. Singh A. P., Buscaglia C. A., Wang Q., Levay A., Nussenzweig D. R., Walker J. R., Winzeler E. A., Fujii H., Fontoura B. M., Nussenzweig V. (2007) Cell 131, 492–504 [DOI] [PubMed] [Google Scholar]
- 38. Frevert U., Galinski M. R., Hügel F. U., Allon N., Schreier H., Smulevitch S., Shakibaei M., Clavijo P. (1998) EMBO J. 17, 3816–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beckett G. J., Hayes J. D. (1993) Adv. Clin. Chem. 30, 281–380 [DOI] [PubMed] [Google Scholar]
- 40. Sohail M., Kaul A., Raziuddin M., Adak T. (2007) Clin. Biochem. 40, 377–382 [DOI] [PubMed] [Google Scholar]
- 41. Beckett G. J., Dyson E. H., Chapman B. J., Templeton A. J., Hayes J. D. (1985) Clin. Chim. Acta 146, 11–19 [DOI] [PubMed] [Google Scholar]
- 42. Hoffman S. L., Goh L. M., Luke T. C., Schneider I., Le T. P., Doolan D. L., Sacci J., de la Vega P., Dowler M., Paul C., Gordon D. M., Stoute J. A., Church L. W., Sedegah M., Heppner D. G., Ballou W. R., Richie T. L. (2002) J. Infect. Dis. 185, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 43. Sigler C. I., Leland P., Hollingdale M. R. (1984) Am. J. Trop. Med. Hyg. 33, 544–547 [DOI] [PubMed] [Google Scholar]
- 44. Ejigiri I., Sinnis P. (2009) Curr. Opin. Microbiol. 12, 401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coppi A., Tewari R., Bishop J. R., Bennett B. L., Lawrence R., Esko J. D., Billker O., Sinnis P. (2007) Cell Host. Microbe 2, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu H. C., Cheng W., Lai P. L. (1997) Cancer Res. 57, 5179–5184 [PubMed] [Google Scholar]
- 47. Capurro M., Wanless I. R., Sherman M., Deboer G., Shi W., Miyoshi E., Filmus J. (2003) Gastroenterology 125, 89–97 [DOI] [PubMed] [Google Scholar]
- 48. Hippo Y., Watanabe K., Watanabe A., Midorikawa Y., Yamamoto S., Ihara S., Tokita S., Iwanari H., Ito Y., Nakano K., Nezu J., Tsunoda H., Yoshino T., Ohizumi I., Tsuchiya M., Ohnishi S., Makuuchi M., Hamakubo T., Kodama T., Aburatani H. (2004) Cancer Res. 64, 2418–2423 [DOI] [PubMed] [Google Scholar]
- 49. Nakatsura T., Yoshitake Y., Senju S., Monji M., Komori H., Motomura Y., Hosaka S., Beppu T., Ishiko T., Kamohara H., Ashihara H., Katagiri T., Furukawa Y., Fujiyama S., Ogawa M., Nakamura Y., Nishimura Y. (2003) Biochem. Biophys. Res. Commun. 306, 16–25 [DOI] [PubMed] [Google Scholar]
- 50. Wang X. Y., Degos F., Dubois S., Tessiore S., Allegretta M., Guttmann R. D., Jothy S., Belghiti J., Bedossa P., Paradis V. (2006) Hum. Pathol. 37, 1435–1441 [DOI] [PubMed] [Google Scholar]
- 51. Abdul-Al H. M., Makhlouf H. R., Wang G., Goodman Z. D. (2008) Hum. Pathol. 39, 209–212 [DOI] [PubMed] [Google Scholar]
- 52. Liu B., Paranjpe S., Bowen W. C., Bell A. W., Luo J. H., Yu Y. P., Mars W. M., Michalopoulos G. K. (2009) Am. J. Pathol. 175, 717–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yalaoui S., Huby T., Franetich J. F., Gego A., Rametti A., Moreau M., Collet X., Siau A., van Gemert G. J., Sauerwein R. W., Luty A. J., Vaillant J. C., Hannoun L., Chapman J., Mazier D., Froissard P. (2008) Cell Host. Microbe 4, 283–292 [DOI] [PubMed] [Google Scholar]
- 54. Ouwe-Missi-Oukem-Boyer O., Ndouo F. S., Ollomo B., Mezui-Me-Ndong J., Noulin F., Lachard I., Ndong-Atome G. R., Makuwa M., Roques P., Branger M., Preux P. M., Mazier D., Bisser S. (2011) PLoS One 6, e16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Berenzon D., Schwenk R. J., Letellier L., Guebre-Xabier M., Williams J., Krzych U. (2003) J. Immunol. 171, 2024–2034 [DOI] [PubMed] [Google Scholar]
- 56. Mueller A. K., Deckert M., Heiss K., Goetz K., Matuschewski K., Schlüter D. (2007) Am. J. Pathol. 171, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scheller L. F., Azad A. F. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4066–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takai H., Ashihara M., Ishiguro T., Terashima H., Watanabe T., Kato A., Suzuki M. (2009) Cancer Biol. Ther. 8, 2329–2338 [DOI] [PubMed] [Google Scholar]
- 59. Takai H., Kato A., Kato C., Watanabe T., Matsubara K., Suzuki M., Kataoka H. (2009) Liver Int. 29, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 60. Leiriao P., Mota M. M., Rodriguez A. (2005) J. Infect. Dis. 191, 1576–1581 [DOI] [PubMed] [Google Scholar]
- 61. Mazier D., Beaudoin R. L., Mellouk S., Druilhe P., Texier B., Trosper J., Miltgen F., Landau I., Paul C., Brandicourt O., et al. (1985) Science 227, 440–442 [DOI] [PubMed] [Google Scholar]
- 62. Smith J. E., Meis J. F., Ponnudurai T., Verhave J. P., Moshage H. J. (1984) Lancet 2, 757–758 [DOI] [PubMed] [Google Scholar]
- 63. Karnasuta C., Pavanand K., Chantakulkij S., Luttiwongsakorn N., Rassamesoraj M., Laohathai K., Webster H. K., Watt G. (1995) Am. J. Trop. Med. Hyg. 53, 607–611 [DOI] [PubMed] [Google Scholar]
- 64. Chuaqui R. F., Bonner R. F., Best C. J., Gillespie J. W., Flaig M. J., Hewitt S. M., Phillips J. L., Krizman D. B., Tangrea M. A., Ahram M., Linehan W. M., Knezevic V., Emmert-Buck M. R. (2002) Nat. Genet. 32, (suppl.) 509–514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.